Abstract
Background
Changing trends in head and neck cancer (HNC) merit an understanding of late effects of therapy, but few studies examine dysphagia beyond 2 years of treatment.
Methods
A case series was examined to describe the pathophysiology and outcomes in dysphagic HNC survivors referred for modified barium swallow (MBS) studies ≥5 years after definitive radiotherapy or chemoradiotherapy (01/2001–05/2011). Functional measures included the Penetration-Aspiration Scale (PAS), Performance Status Scale-Head and Neck (PSS-HN), Swallowing Safety Scale (NIH-SSS), and MBSImp.
Results
Twenty-nine patients previously treated with radiotherapy (38%) or chemoradiotherapy (62%) were included (median years post-treatment: 9, range: 5–19). The majority (86%) had oropharyngeal cancer; 52% were never smokers. Seventy-five percent had T2-T3 disease; 52% were N+. Median age at diagnosis was 55 (range: 38–72). Abnormal late examination findings included: dysarthria/dysphonia (76%), cranial neuropathy (48%), trismus (38%), and radionecrosis (10%). MBS studies confirmed pharyngeal residue and aspiration in all dysphagic cases owing to physiologic impairment (median PAS: 8; median NIH-SSS: 10; median MBSImp: 18) whereas stricture was confirmed endoscopically in 7 (24%). Twenty-five (86%) developed pneumonia, half requiring hospitalization. Swallow postures/strategies helped 69% of cases, but no patient achieved durable improvement across functional measures at last follow-up. Ultimately 19 (66%) were gastrostomy dependent.
Conclusions
Although functional organ preservation is commonly achieved, severe dysphagia represents a challenging late effect that may develop or progress years after radiation-based therapy for HNC. These data suggest that novel approaches are needed to minimize and better address this complication that is commonly refractory to many standard dysphagia therapies.
Keywords: deglutition disorders, radiotherapy, head and neck cancer, toxicity, late effect
INTRODUCTION
Dysphagia has been acknowledged as a potentially dose-limiting toxicity of radiotherapy-based treatments of head and neck cancer (HNC).1 Swallowing function may be impaired due to a number of normal tissue changes including edema, neuropathy, and fibrosis. Acute toxicities such as mucositis and edema commonly disrupt normal swallowing during treatment, but improve substantially in the months following radiotherapy or chemoradiotherapy in a majority of patients. In contrast, neuropathy and fibrosis of the oral, laryngeal, and pharyngeal musculature may develop or persist long after the completion of treatment.2,3 These late effects ultimately impair the range of motion of key swallowing structures and have been implicated as the primary mechanisms of long-term dysphagia in HNC survivors. In severe cases of late dysphagia, dietary restrictions and malnutrition necessitate lifelong gastrostomy tube dependence and aspiration poses risk for potentially life-threatening pneumonia.
Changing demographics and survival trends in HNC merit a clear understanding of late toxicities that affect upper aerodigestive tract functioning.4 The increasing proportion of pharyngeal cancers attributable to human papillomavirus and decreased prevalence of tobacco use in the United States have shifted the profile of HNC in recent decades, and led to an increase in younger survivors with more favorable prognosis.5,6 This subgroup of young HNC survivors has the potential to live decades with the sequelae of their disease, but few authors have examined dysphagia in survivors beyond 2 years of treatment. The primary objective of this case series was to describe the clinical history, pathophysiology, and outcomes of late dysphagia after definitive radiotherapy or chemoradiotherapy in long-term (≥5 years) HNC survivors.
METHODS
Study Design and Eligibility Criteria
A case series was examined to describe dysphagia in long-term HNC survivors. Inclusion criteria were: 1) history of oropharyngeal, hypopharyngeal, or laryngeal cancer, 2) referred for a modified barium swallow (MBS) study at The University of Texas MD Anderson Cancer Center (MDACC, 01/2001–05/2011) ≥5 years after definitive radiotherapy or chemoradiotherapy, and 3) pharyngeal dysphagia per MBS. Exclusion criteria were: 1) recurrent local or regional disease or second primary malignancy; 2) history of head and neck surgery (exception biopsy, tonsillectomy, or non-radical neck dissection); or 3) nasopharyngeal cancer. We queried an institutional MBS database to identify eligible subjects. Only 1 dysphagia referral ≥5 years post-treatment had no evidence of pharyngeal dysphagia, rather this patient presented with Zenker’s diverticulum and was excluded. Long-term HNC survivors are not routinely evaluated with MBS studies unless they are symptomatic with dysphagia. Hence, our sampling method identified only dysphagic cases precluding the ability to estimate the prevalence of late dysphagia. The study was approved by the Institutional Review Board at MDACC. A waiver of informed consent was obtained.
Modified Barium Swallow Studies
All patients underwent an MBS study at the time of their late dysphagia referral. MBS studies were conducted using standard methods.7 Results were uniformly collected in an institutional database. Varibar® thin liquid barium was administered to all subjects in measured volumes (5-mL and/or 10-mL); additional consistencies and compensatory strategies were attempted at the clinician’s discretion. MBS video-recordings were analyzed retrospectively by a single rater (KAH) using the Penetration-Aspiration Scale (PAS), Swallowing Safety Scale (NIH-SSS), and MBSImp. Test re-test correlations indicated acceptable intra-rater reliability for these measures (r=0.87–1.00). Diet levels were recorded at the time of MBS using the Performance Status Scale-Head and Neck Cancer (PSS-HN) Normalcy of Diet subscale, in which 0 indicates non-oral nutrition and 100 represents full oral nutrition without restrictions.8
Penetration-Aspiration Scale (PAS)
The PAS is a validated 8-point ordinal scale that ranks swallowing safety by the depth of bolus entry into the airway and the patient’s response (0=no airway entry, 8=silent aspiration).9 The maximum PAS score for measured thin liquid trials was calculated for each patient.
Swallowing Safety Scale (NIH-SSS)
The NIH-SSS quantifies swallowing safety on the basis of 7 observations from the MBS study (residue, laryngeal penetration, aspiration, aspiration response, maximal esophageal entry, and multiple swallows) and has demonstrated high reliability (intra- and inter-rater ICC >0.95) in dysphagic patients.10 The NIH-SSS was calculated for measured thin liquid trials. The NIH-SSS provides a continuous score (0=normal, no ceiling) with higher scores indicating greater impairment.
MBSImp
The MBSImp is a validated, standardized measure that rates physiologic components of the oropharyngeal swallow. To evaluate the pathophysiology of dysphagia, MBSImp ratings for pharyngeal phase components were calculated by review of the entire MBS video recording and included: soft palate elevation, laryngeal elevation, anterior hyoid excursion, epiglottic movement, laryngeal vestibule closure, pharyngeal stripping wave, pharyngeal contraction, pharyngoesophageal segment opening, tongue base retraction, and pharyngeal residue. Each component is rated using a 3 to 5-point ordinal scale in which 0 indicates no impairment. An overall pharyngeal impairment MBSImp score was calculated as the sum of the 10 component measures, providing a continuous score (range: 0 to 28) for which higher ratings indicate greater physiologic impairment.11
Retrospective Medical Record Review
Demographic and treatment data were extracted from the electronic medical record. Data points included demographic characteristics, tobacco/alcohol exposure, tumor site, tumor staging according to TNM classification, and treatment history. The primary treatment modality was reviewed including method of radiotherapy (conventional 3D conformal fields or intensity modulated radiotherapy [IMRT]), radiotherapy fractionation schedule (standard or accelerated), total radiotherapy dose (Gy), number of fractions, timing of chemotherapy (none, induction, concurrent), agent, and neck dissection (type, levels, side). Vital status and disease status at last follow-up were collected.
Functional data points collected at the time of late referral and final follow-up included: feeding tube, diet level, tracheostomy, MBS findings, laryngeal videostroboscopy findings, clinical cranial nerve examination findings, motor speech examination findings (i.e., dysphonia, dysarthria, resonance disturbance), radionecrosis (confirmed radiographically), trismus (<35mm or 3-finger widths interincisal opening), oxygen dependence, and pneumonia history. Pneumonia events were rated categorically: <1, 2, 3–5, or > 5 per year.12 Adverse events associated with pneumonia were reviewed and included: hospitalization, endotracheal intubation, and tracheotomy.
Rehabilitation efforts and treatment response were reviewed. Rehabilitation methods prescribed and attempted at MDACC or outside facilities included: compensatory swallowing strategies, swallowing exercise, neuromuscular electrical stimulation, esophageal dilation, and vocal fold medialization. Final outcomes were assessed prior to elective total laryngectomy, if it occurred.
Statistical Methods
Descriptive statistics were calculated to describe the treatment history, examination findings, and outcomes in late dysphagia cases. Our sampling method precluded the ability to estimate the prevalence of late dysphagia. Statistical differences between paired data were analyzed using the nonparametric sign-rank test. Statistical significance was considered α-level 0.05. Statistical analyses were performed using the STATA data analysis software, version 10.0 (StataCorp LP, College Station, TX).
RESULTS
Sample Characteristics
Twenty-nine disease-free HNC survivors with late dysphagia previously treated with definitive radiotherapy or chemoradiotherapy for primary tumors of the oropharynx, hypopharynx, or supraglottis were included. No late dysphagia cases were referred after treatment for glottic laryngeal primaries. Most dysphagia cases (62%) were treated with combined regimens of chemotherapy and radiotherapy (total dose: 70–74 Gy), 6 (21%) of whom received accelerated radiotherapy concurrent with chemotherapy. Three cases (14%) received conventional fractionated radiotherapy alone (total dose: 66–70 Gy). The median total dose of radiation therapy was 72 Gy (range: 66–77) and median number of fractions was 44 (range: 30–64). Characteristics of the dysphagic sample are summarized in Table 1.
Table 1.
Characteristics of Sample with Late Dysphagia
| N (%) | |
|---|---|
| Sex | |
| Male | 25 (86%) |
| Female | 4 (14%) |
| Age | |
| Median (range) | 55 (38–72) |
| Tumor site | |
| Oropharynx | 25 (86%) |
| Hypopharynx | 1 (3%) |
| Supraglottic larynx | 3 (10%) |
| T-classification | |
| T1 | 2 (7%) |
| T2 | 7 (24%) |
| T3 | 14 (48%) |
| T4 | 3 (10%) |
| Unknown | 3 (10%) |
| N-classification | |
| N0 | 0 (24%) |
| N1 | 2 (7%) |
| N2 | 9 (31%) |
| N3 | 4 (14%) |
| NX | 4 (14%) |
| Unknown | 3 (10%) |
| Smoking status | |
| Never | 15 (52%) |
| Former | 6 (21%) |
| Current | 8 (28%) |
| Therapeutic combination | |
| Radiation alone | 11 (38%) |
| Induction + RT | 6 (21%) |
| Concurrent chemo + RT | 12 (41%) |
| RT type | |
| 3D conformal/conventional | 23 (79%) |
| IMRT | 2 (7%) |
| Unknown | 4 (14%) |
| RT schedule | |
| Standard fractionation | 9 (31%) |
| Accelerated fractionation | 16 (55%) |
| Unknown | 4 (14%) |
| Neck dissection | |
| None | 20 (69%) |
| Unilateral | 7 (24%) |
| Bilateral | 2 (7%) |
| Time to late referral after HNC treatment | |
| Median yrs. (range) | 9 (5–19) |
| Total Sample | 29 |
Functional Status at Time of Late MBS Referral
Cases were referred with late dysphagia a median of 9 years (range: 5–19) after treatment. Functional status at the time of late referral is described in Table 2. Six cases (21%) were feeding tube dependent, 2 cases (7%) were oxygen dependent, and none had tracheostomy.
Table 2.
Functional Status Indicators at the Time of Late Referral and Last Follow-up
| Functional Status Indicators | Late Referral | Final | p-value |
|---|---|---|---|
| Tracheostomy status | |||
| No | 29 (100%) | 27 (93%) | |
| Yes | 0 (0%) | 2 (7%) | 0.014 |
| Feeding tube status | |||
| No feeding tube | 23 (79%) | 10 (34%) | |
| Feeding tube | 6 (21%) | 19 (66%) | <0.001 |
| PSS-HN Normalcy of Diet | |||
| Full, no restriction (PSS-HN 100) | 0 (0%) | 1 (3%) | |
| Full, liquid assist (PSS-HN 90) | 9 (31%) | 3 (10%) | |
| All meat (PSS-HN 80) | 0 (0%) | 0 (0%) | |
| Raw carrots, celery (PSS-HN 70) | 1 (3%) | 2 (7%) | |
| Dry breads, crackers (PSS-HN 60) | 2 (7%) | 0 (0%) | |
| Soft, chewable (PSS-HN 50) | 9 (31%) | 5 (17%) | |
| Soft, nonchewable (PSS-HN 40) | 0 (0%) | 0 (0%) | |
| Pureed (PSS-HN 30) | 1 (30%) | 0 (0%) | |
| Warm liquids (PSS-HN 20) | 1 (3%) | 3 (10%) | |
| Cold liquids (PSS-HN 10) | 1 (3%) | 1 (3%) | |
| Non-oral (PSS-HN 0) | 4 (14%) | 13 (45%) | |
| PO, unspecified | 1 (3%) | 1 (3%) | 0.002 |
Abbreviations: MDADI, MD Anderson Dysphagia Inventory; PO, per oral; PSS-HN, Performance Status Scale for Head and Neck Cancer Normalcy of Diet Scale
Pathophysiology of Late Dysphagia
Abnormal late examination findings included: dysarthria or dysphonia (22/29, 76%), cranial neuropathy (14/29, 48%), trismus (11/29, 38%), mandibular osteoradionecrosis (3/29, 10%), and laryngeal chondroradionecrosis (1/29, 4%). Cranial nerves X (7/29, 24%) and XII (9/29, 31%) were most commonly impaired, and 6 patients (21%) had evidence of multiple cranial nerve impairments on clinical examination. Baseline examinations were available in 10 of 14 patients with late cranial neuropathy and found intact cranial nerve function prior to cancer therapy.
MBS study findings in dysphagic cases are described in Table 3. A range of impairment was identified across measures, however, all dysphagic cases had pharyngeal residue and aspiration (median PAS: 8 “silent aspiration”; median NIH-SSS: 10, median MBSImp pharyngeal residue scores: 3 “majority of contrast within or on pharyngeal structures”). Physiologic impairment was documented by MBSImp in all cases whereas stricture was confirmed endoscopically in 7 patients (24%). Specifically, laryngeal elevation, epiglottic deflection, tongue base retraction, and pharyngeal contraction MBSImp scores were abnormal in all cases, and anterior hyoid excursion MBSImp scores were abnormal in all but one case. Ceiling scores indicating the highest level of impairment were observed for at least one MBSImp component in 86% (25/29) of cases; these included absent laryngeal elevation (17%, 5/29), absent anterior hyoid excursion (38%, 11/29), absent epiglottic movement (86%, 25/29), no laryngeal vestibule closure (66%, 19/29), absent bilateral pharyngeal contraction (39%, 10/29), and absent tongue base retraction (17%, 5/29). Complete distributions for these MBSImp components are summarized in Table 4.
Table 3.
Overview of Late MBS Results
| No. (%) | |
|---|---|
| Penetration Aspiration Scale (PAS) | |
| Aspiration w/ non-productive response (PAS=7) | 6 (21%) |
| Silent aspiration (PAS=8) | 23 (82%) |
| Swallowing Safety Scale (NIH-SSS) | |
| Median (range), thin liquid | 10 (2–14) |
| MBSImp | |
| Median (range), Overall Pharyngeal Impairment | 18 (5–24) |
Table 4.
Selected Physiologic Impairments on Late MBS per MBSImp
| No. (%) | |
|---|---|
| MBSImp 8 - Laryngeal elevation | |
| 0 = Full elevation | 0 (0%) |
| 1 = Partial elevation | 14 (48%) |
| 2 = Minimal elevation | 10 (35%) |
| 3 = No elevation | 5 (17%) |
| MBSImp 9 - Anterior hyoid excursion | |
| 0 = Complete excursion | 1 (3.5%) |
| 1 = Partial excursion | 17 (59%) |
| 2 = No excursion | 11 (38%) |
| MBSImp 10 - Epiglottic movement | |
| 0 = Full inversion | 0 (0%) |
| 1 = Partial inversion | 4 (14%) |
| 2 = No inversion | 25 (86%) |
| MBSImp 11 – Laryngeal vestibule closure | |
| 0 = Complete closure | 0 (0%) |
| 1 = Incomplete closure | 10 (35%) |
| 2 = No closure | 19 (66%) |
| MBSImp13 – Pharyngeal contraction | |
| 0 = Complete | 0 (0%) |
| 1 = Incomplete bilaterally | 10 (39%) |
| 2 = Absent unilaterally | 6 (23%) |
| 3 = Absent bilaterally | 10 (39%) |
| Antero-posterior view not evaluable | 3 (10%) |
| MBSImp 15 – Tongue base retraction | |
| 0 = Full retraction | 0 (0%) |
| 1 = Reduced retraction (trace column BOT to PW) | 1 (3%) |
| 2 = Partial retraction (narrow column BOT to PW) | 7 (24%) |
| 3 = Minimal retraction (wide column BOT to PW) | 16 (55%) |
| 4 = No retraction | 5 (17%) |
Pneumonia associated with Late Dysphagia
Twenty-five late dysphagia cases (86%) developed pneumonia in the follow-up period, and 18 (62%) experienced recurrent pneumonias. Fifty-two percent (15/29) required hospitalization due to pneumonia, among whom 4 (14%) required endotracheal intubation and ultimately tracheotomy.
Rehabilitation and Final Outcomes
Dysphagia rehabilitation was individualized on the basis of late MBS findings. Swallowing therapy was not exclusively provided at MDACC; 15 cases had attempted swallowing therapy at outside facilities (i.e., exercise, neuromuscular electrical stimulation, or dilation) prior to their referral at our institution. All but 2 dysphagic cases were prescribed targeted swallowing exercises; esophageal dilation was performed in 11 (38%). Six (21%) cases ultimately elected to receive total laryngectomy to prevent aspiration.
Final outcomes were assessed at last follow-up (median: 10 months, range: 0–78); 7 cases did not have long-term follow-up after presenting for a second-opinion after therapy at other institutions. Response to swallowing therapy varied in degree and duration during the review period, but no dysphagic patient achieved durable improvement across functional measures at last follow-up despite rehabilitative efforts. Swallow strategies improved pharyngeal transit and/or airway protection in 20 dysphagic cases (69%) and were used as compensatory techniques to maintain oral intake and prevent aspiration compromise when possible. Ultimately 19 (66%) of cases were gastrostomy dependent due to pneumonia or nutritional compromise. All cases requiring gastrostomy developed pneumonia, and the majority (79%) had persistent aspiration despite some benefit from swallowing strategies. Final functional status is described in Table 2.
DISCUSSION
Radiotherapy with or without concurrent chemotherapy is the workhorse for organ-preservation in the treatment of many pharyngeal and laryngeal cancers. In most cases, radiotherapy and chemoradiotherapy offer high rates of locoregional control with good potential for functional organ preservation. However, the functional toxicities of radiation-based treatment can be significant and dysphagia is commonly cited as a potentially dose-limiting toxicity within the first two years of treatment.1,13,14 With remarkable progress and refinements in the delivery of radiotherapy, the advent of targeted molecular therapy, and the changing etiology of HNC, the number of long-term HNC survivors is increasing.5,6,15,16 Thus, it is critical to understand the functional consequences of therapy beyond the initial years of survivorship. Previous authors have evaluated late normal tissue effects of radiotherapy in long-term (>5 year) HNC survivors,17 but to our knowledge, none have focused on dysphagia. Herein, we describe severe dysphagia as late complication of radiotherapy-based treatment in long-term (≥5 year) HNC survivors.
The number of cases included in this 10-year case series is small, suggesting that severe, late dysphagia is uncommon. However, our comprehensive analysis of MBS studies indicates that the level of dysfunction in these cases is often extreme. Silent aspiration and profound pharyngeal residue were the norm in HNC survivors with late radiation-associated dysphagia, and the vast majority of cases (86%) ultimately developed aspiration pneumonia as a consequence of their dysphagia. While many cases benefited from swallowing strategies, these gains were not always sufficient to sustain oral intake without aspiration compromise and 66% of cases were gastrostomy-dependent at last follow-up. Moreover, these data suggest that severe, late dysphagia is refractory to standard, non-surgical therapies given that, despite transient successes, no single case in this series had durable improvement across objective swallowing-related outcome measures in the follow-up period. The pathophysiology of late dysphagia might help to explain the lack of durable treatment response. Chronic dysphagia was not typically the result of stricture or structural change that might respond to dilation. Rather, impaired range of motion of the hyolaryngeal complex, pharyngeal constrictors, and tongue base was evident in all cases (per standardized MBSImp ratings) likely as a result of postradiation fibrosis, muscular atrophy, and cranial neuropathies. The process of fibrosis after radiotherapy is commonly attributed to aberrations in normal wound healing that prompt an overproduction of transforming growth factor β (TFG-β1).2 Once activated, the fibrotic process is thought to be self-inducing and difficult to halt. Translational studies are needed to improve our understanding of the mechanisms that underlie progressive neuromuscular dysfunction long after radiotherapy so that novel therapies can be developed to address the associated functional complications.
The proportion of oropharyngeal cancers attributable to human papillomavirus (HPV) has risen to the majority since the 1980’s.5 HPV-positive oropharyngeal cancers represent a distinct subgroup of HNCs that are associated with favorable survival and are diagnosed at a younger age than tobacco-related oropharyngeal cancers.5,18 HPV status was not available for cases in this study, but half of the late dysphagia cases in this series were never smokers with a history of oropharyngeal cancer who might have had HPV-attributable disease if considering tobacco exposure as a surrogate measure. Radiotherapy combined with systemic treatment is considered the standard of care for many advanced-stage oropharyngeal carcinomas,19 but the precise combination of agents and the methods of radiotherapy (i.e., fields, fractionation, and dose) are not standard across institutions. The highest proportion of late dysphagic cases in this series were treated with concurrent chemoradiotherapy and/or acceleration fractionation schedules. However, we also identified cases with late, refractory dysphagia who had received single modality conventional radiotherapy alone and two cases in this series were treated with IMRT. These data suggest that this severe late effect is not exclusive to combined chemoradiotherapy regimens that have previously been found to have a high prevalence (43%) of late grade 3–4 laryngopharyngeal toxicity,20 but may occur after diverse therapeutic techniques and combinations. The potential for late dysphagia after various radiation-based regimens will be an important consideration when planning trials that consider de-escalation of therapy in HPV-positive tumors with favorable prognosis, and should be considered as an endpoint measure.
The total dose of radiotherapy likely impacts the occurrence of severe late toxicity, but a threshold dose for late dysphagia is not clear on the basis of published studies. Larson and colleagues previously found a high prevalence (30% to 67%) of late grade IV mucosal or bone toxicity in disease-free tonsil cancer survivors (≥5 years after radiotherapy alone) regardless of total dose (60–75 Gy, once daily 2-Gy/fraction).17 In contrast, all but one dysphagic case in our study received a total dose of 70-Gy or more (range: 66–77 Gy) suggesting that late dysphagia may be more likely at the current standard dose of 70-Gy or higher. At present, treatment factors that predispose to late dysphagia are unknown, but it is likely a complex interplay between patient factors, baseline function, tumor burden, target volumes, total dose, fractionation schedules, and systemic treatments. Comparative conclusions cannot be drawn from this small case series, and predictive factors for late dysphagia should be a priority area for research.
Dysphagia-organ sparing radiotherapy techniques hold promise to reduce late dysphagia by constraining dose to critical structures such as the pharyngeal constrictors, larynx, oral cavity, and esophageal inlet.21,22 The use of a novel split-beam approach that matches IMRT for oropharyngeal cancers with a conventional supraclavicular laryngeal block (3 × 3 cm) using AP bilateral low neck fields has been shown to achieve a lower laryngeal and esophageal inlet dose in comparison to full IMRT fields, and has been used at our institution since 2000.21,22 Notably, only one case of severe late dysphagia in this series was treated with the split beam IMRT approach. The benefit of prophylactic swallowing therapy has also been demonstrated in recent years. Prophylactic swallowing therapy encourages persistent use of the swallowing musculature during treatment by avoidance of NPO periods and use of targeted preventive swallowing exercise. Favorable outcomes associated with preventive swallowing exercises include superior swallowing-related quality of life scores,23,24 better base of tongue retraction and epiglottic inversion,25 larger post-radiotherapy muscle mass and T2 signal intensity of the genioglossus, mylohyoid, and hyoglossus,26 and shorter duration of gastrostomy dependence.27 Thus, it is currently our practice to prescribe preventive swallowing exercise to all patients prior to definitive radiotherapy or chemoradiotherapy for HNC. This study was not designed to ascertain the effect of preventive dysphagia rehabilitation efforts on late dysphagia. Future studies will be needed to determine potential long-term benefits of these regimens, and the required dose and duration of exercise adherence to achieve this goal. Preventive efforts will also be optimized by early identification of high risk patients through systematic evaluation using instrumental examinations and complementary patient-reported outcome measures.
This study presents novel findings that shed insight into a rare, but devastating late complication of nonsurgical organ preservation. Unfortunately, current clinical practices are not sufficient to identify the prevalence of late dysphagia using retrospective, observational study methods. As such, the primary objective of this study was to provide preliminary descriptive observations of this likely under-reported late complication of therapy. Our inclusion of patients referred for MBS studies allowed for a comprehensive evaluation of the pathophysiology of dysphagia in this subpopulation of HNC survivors, but our sampling frame did not provide a denominator to estimate the prevalence of the problem. In the era of HPV-driven oropharyngeal cancers that will lead to younger, working survivors at risk of long-term functional toxicity, it will be critical for prospective trials to include systematic, functional assessments to adequately account for late dysphagia. Without these prospective data, it is tempting to consider using the rate of chronic feeding tube dependence as a surrogate measure of dysphagia, but chronic feeding tube rates do not sufficiently identify these cases. Only 21% of our cases were feeding tube dependent at the time of the late dysphagia referral despite evidence of (often significant) physiologic impairment and aspiration on MBS studies in all cases. Another potential limitation of this study is the lack of pre-treatment MBS studies in all cases to rule-out baseline, tumor-related dysphagia as a confounding factor that influenced long-term outcomes. Baseline dysphagia is most likely in individuals with advanced -stage primary tumors or hypopharyngeal disease.13,28 T4 tumors and hypopharyngeal disease were rare in this study accounting for only 3 cases, all of whom had normal cranial nerve examinations and tolerated a regular diet before treatment, with normal baseline MBS studies available in 2 of the 3 cases. Hence, it is unlikely that dysphagia was primarily influenced by baseline neuropathy or tumor burden in these cases, and instead represents an unanticipated late sequelae of treatment. Longitudinal studies with baseline and long-term follow-up will be required to thoroughly evaluate the relative contributions of tumor burden, cancer treatment, and confounding patient factors to late dysphagia in HNC survivors.
Conclusion
Although rare, severe dysphagia is an extremely challenging late effect of radiation-based therapy for HNC in which physiologic impairments lead to profound pharyngeal residue and a tendency for silent aspiration. Chronic aspiration led to pneumonia in a majority of cases examined in this series, and ultimately necessitated long-term gastrostomy dependence for most cases despite rehabilitative efforts. These data suggest that novel approaches are needed to prevent and manage this potentially progressive complication that is commonly refractory to many standard dysphagia therapies.
Figure 1.
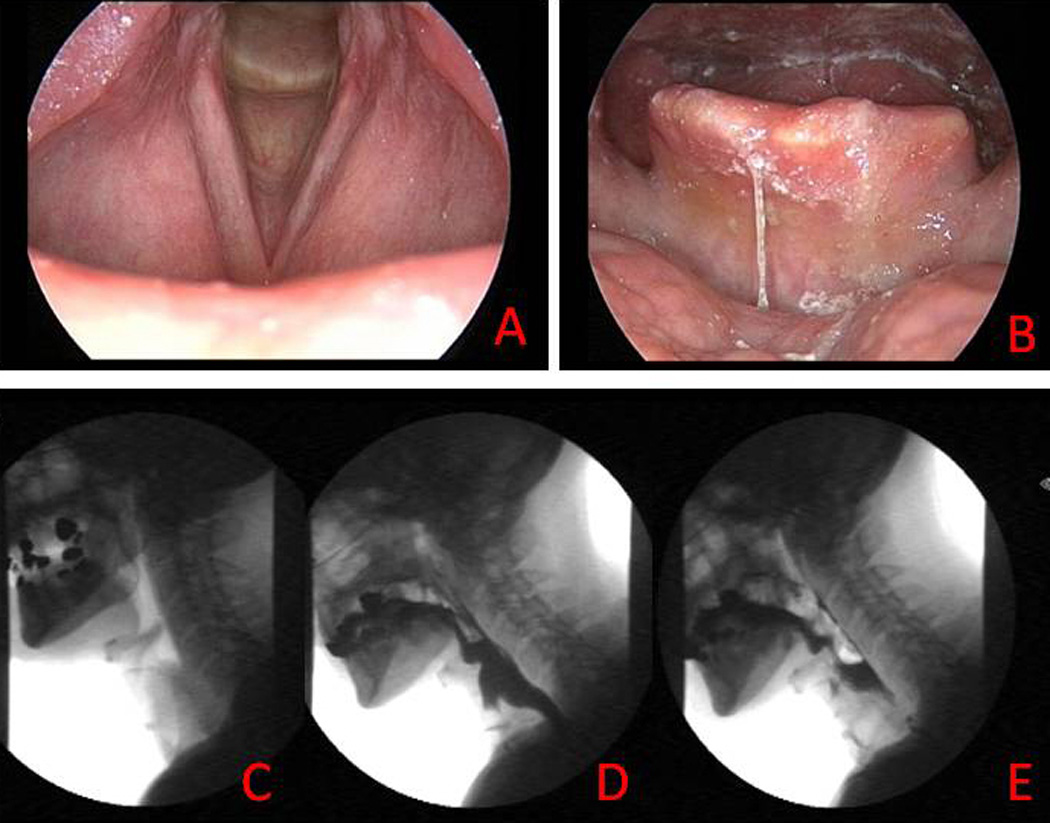
Case example of chronic dysphagia after radiotherapy. Endoscopic examination of glottic larynx (a) and laryngopharynx (b). Lateral radiograph of laryngopharynx at rest prior to swallowing (c), maximally contracted during the swallow (d), and residual barium after the swallow (e). Note minimal bolus clearance through the pharynx due to bilateral hypoglossal palsy, absent tongue retraction, absent pharyngeal contraction, and impaired hyolaryngeal excursion despite adequately preserved larynx 22 years after single modality radiotherapy (72 Gy, 42 fractions, concomitant boost schedule).
ACKNOWLEDGEMENT
The authors acknowledge the valuable support of Janet Hampton.
FUNDING SOURCES: Dr. Hutcheson acknowledges funding from the UT Health Innovation for Cancer Prevention Research Fellowship, The University of Texas School of Public Health – Cancer Prevention and Research Institute of Texas (CPRIT) grant #RP101503.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors made no disclosures.
Publisher's Disclaimer: DISCLAIMER: The content is solely the responsibility of the authors and does not necessarily represent the official views of the CPRIT.
REFERENCES
- 1.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 2.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 3.Martin S, Chung B, Bratlund C, et al. Movement trajectories during percutaneous stimulation at rest of the hyolaryngeal muscles in head and neck cancer patients treated with radiation therapy. Dysphagia. 2010;25:358. [Google Scholar]
- 4.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31:748–758. doi: 10.1002/hed.21022. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logemann J, editor. Manual for the Videofluorgraphic Study of Swallowing. 2nd ed. Austin, TX: Pro-Ed; 1993. [Google Scholar]
- 8.List MA, D'Antonio LL, Cella DF, et al. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale. A study of utility and validity. Cancer. 1996;77:2294–2301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 10.Ludlow CL, Sonies BC, Humbert IA, Crujido L, Martin S, Lowell SY. Validity and reliability of the swallowing safety scale. Dysphagia. 2007;22:1. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment-MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takano Y, Suga M, Sakamoto O, Sato K, Samejima Y, Ando M. Satisfaction of patients treated surgically for intractable aspiration. Chest. 1999;116:1251–1256. doi: 10.1378/chest.116.5.1251. [DOI] [PubMed] [Google Scholar]
- 13.Logemann JA, Rademaker AW, Pauloski BR, et al. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28:64–73. doi: 10.1002/hed.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheson KA, Barringer DA, Rosenthal DI, May AH, Roberts DB, Lewin JS. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134:178–183. doi: 10.1001/archoto.2007.33. [DOI] [PubMed] [Google Scholar]
- 15.Eisbruch A, Levendag PC, Feng FY, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys. 2007;69:S40–S42. doi: 10.1016/j.ijrobp.2007.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 17.Larson DL, Lindberg RD, Lane E, Goepfert H. Major complications of radiotherapy in cancer of the oral cavity and oropharynx. A 10 year retrospective study. Am J Surg. 1983;146:531–536. doi: 10.1016/0002-9610(83)90247-7. [DOI] [PubMed] [Google Scholar]
- 18.Gillison M. HPV and tts effect on head and neck cancer prognosis. Clin Adv Hematol Oncol. 2010;8:3. [PubMed] [Google Scholar]
- 19.Pfister DG, Kie-Kian A, Brizel DM, et al. Head and neck cancers. J Natl Compr Canc Netw. 2011;9:596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 20.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 24.Shinn E, Lewin JS, Barringer DB, Hutcheson KA, Alvarez CP, Baum G. The effect of adherence to swallowing exercises on swallowing outcomes in head and neck cancer patients treated with Radiotherapy. Dysphagia. 2011;26:443. [Google Scholar]
- 25.Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118:39–43. doi: 10.1097/MLG.0b013e31815659b0. [DOI] [PubMed] [Google Scholar]
- 26.Carnaby-Mann G, Crary M, Amdur R, Schmalfuss I. Preventative exercise for dysphagia following head/neck cancer. Dysphagia. 2007;22:381. [Google Scholar]
- 27.Bhayani M, Hutcheson KA, Barringer DA, Roberts DB, Lewin JS, Lai S. Gastrostomy tube placement in patients with hypopharyngeal cancer treated with chemoradiotherapy: factors affecting placement and dependence. Dysphagia. 2011;26:471. doi: 10.1002/hed.23199. [DOI] [PubMed] [Google Scholar]
- 28.Pauloski BR, Rademaker AW, Logemann JA, et al. Pretreatment swallowing function in patients with head and neck cancer. Head Neck. 2000;22:474–482. doi: 10.1002/1097-0347(200008)22:5<474::aid-hed6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


