Abstract
Purpose
To investigate long-term swallowing function in oropharyngeal cancer patients treated with IMRT, and to identify novel dose-limiting criteria predictive for dysphagia.
Methods and Materials
Thirty-one patients with stage IV oropharyngeal squamous carcinoma enrolled on a phase II trial were prospectively evaluated by modified barium swallow studies at baseline, and 6, 12, and 24 months post-radiation. Candidate dysphagia-associated organs-at-risk (OARs) were retrospectively contoured into original treatment plans. Twenty-one (68%) cases were base of tongue, and 10 (32%) were tonsil. Stage distribution was T1 (12), T2 (10), T3 (4), T4 (2), and TX (3), and N2 (24), N3 (5), and NX (2). Median age was 52.8 years (Range: 42–78). Thirteen (42%) received concurrent chemotherapy during IMRT. Thirteen (42%) were former smokers. Mean dose to glottic larynx for the cohort was limited to 18 Gy (range: 6–39 Gy) by matching IMRT to conventional low neck fields.
Results
Dose-volume constraints (V30 < 65% and V35 < 35% for anterior oral cavity and V55 < 80% and V65 < 30% for high superior pharyngeal constrictors) predictive for objective swallowing dysfunction were identified by univariate and multivariate analyses. Aspiration and feeding tube dependence were observed in only one patient at 24 months.
Conclusions
In the context of glottic laryngeal shielding, we describe candidate oral cavity and superior pharyngeal constrictor OARs and dose-volume constraints associated with preserved long-term swallowing function; these constraints are currently undergoing prospective validation. Strict protection of the glottic larynx via beam-split IMRT techniques promises to make chronic aspiration an uncommon outcome.
Keywords: Dysphagia, IMRT, radiation, head and neck cancer, dose-volume constraints, toxicity, swallowing
INTRODUCTION
Head and neck cancer severely impacts quality of life. Although aggressive radiotherapy and chemoradiotherapy regimens have improved survival outcomes for this disease (1), this success has proven costly (2–4). Intensity modulated radiotherapy (IMRT) techniques permit protection of normal tissues adjacent to tumors (5). It has been assumed that reducing dose to the uninvolved larynx and pharyngeal axis improves post-treatment rehabilitation. However, there are limited data to support this premise or to guide radiation oncologists to avoid specific organs-at-risk (OARs) important to swallowing. Eisbruch and colleagues (6, 7) have recently associated dose to the superior pharyngeal constrictors and supraglottic larynx with post-radiation aspiration. Despite such early progress, data remains sparse for post-radiation swallowing outcomes past one year, and published series lack quantifiable measures for swallowing function.
The purpose of this study was to identify specific candidate structures and dose-volume constraints for dysphagia-sparing oropharyngeal IMRT planning. Unique features of this data include 1) uniform disease presentation and IMRT techniques, 2) prospective collection of longitudinal functional outcomes beyond 12 months and 3) utilization of modified barium swallow outcome measures to objectively assess swallowing function in a quantitative fashion.
METHODS
Forty-eight patients with stage IV head and neck squamous cell carcinoma were enrolled onto an IRB-approved institutional phase II chemoradiotherapy trial (Figure 1). Swallowing outcomes were prospectively evaluated as an exploratory aim of the phase II trial. We analyzed swallowing function in a subgroup of 31 patients with tonsil or base of tongue primary disease in whom candidate dysphagia-associated organs-at-risk (OARs) were retrospectively contoured.
Figure 1.
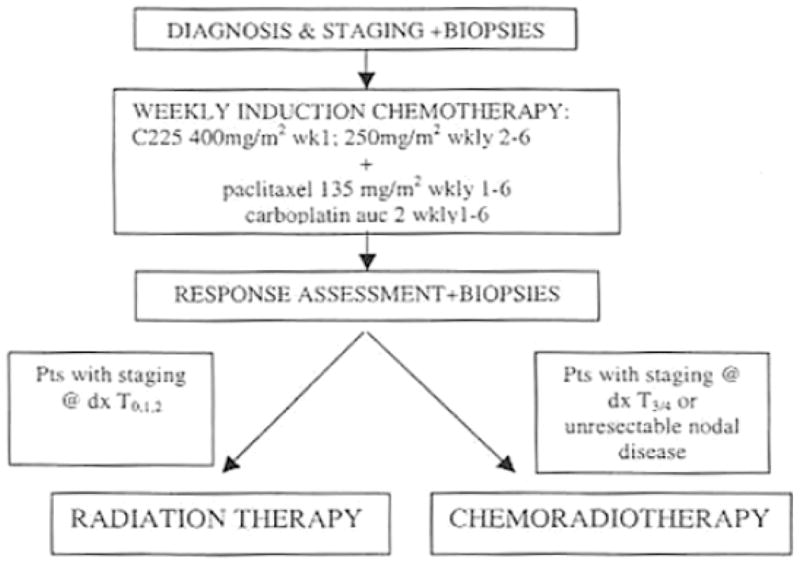
Protocol treatment pathway for study cohort
IMRT was delivered via a step-and-shoot, multileaf collimation through a static treatment gantry using a mono-isocentric technique using a Pinnacle3 system (version 6.2b or later, Philips Medical Systems, Andover, MA). Treatment plans incorporated a clinical target volume 1 (CTV1, gross disease and high risk regions requiring high dose) target treated to 66–70 Gy in 30–33 daily fractions, CTV2 (intermediate-risk nodal levels or soft tissues) treated to 60–63 Gy in 30–33 daily fractions, and CTV3 (prophylactic nodal coverage) target treated to 54–57 Gy in 30–33 daily fractions.
In all cases, IMRT was matched at the superior aspect of the arytenoid cartilages to a conventional AP bilateral low neck field with a 3 × 3 cm larynx block, treated to 50 Gy in 25 daily fractions (Figure 2). This 3 cm wide larynx block was subsequently extended inferiorly to the lower border of the AP low neck field to become a full length midline spinal cord block after 40–42 Gy. Coned-down midneck AP photon and/or en face electron boost fields were used to boost low cervical neck nodal stations adjacent to or directly involved with nodal disease to 60–66 Gy in 2 Gy daily fractions. Routine dose-limiting structures included ipsilateral and contralateral parotid glands, spinal cord, brainstem, mandible, and glottic larynx. A planning goal was to limit mean dose (Dmean) to at least one parotid gland to < 26 Gy, V30Gy to < 50%, or V20Gy to < 20cc. An anterior oral avoidance volume was used when permitted by disease extent to limit Dmean to < 30–40 Gy. We routinely limited larynx Dmean to 25 Gy or lower, though the glottic larynx and a portion of the inferior constrictors were shielded and not included in the IMRT volumes.
Figure 2. Candidate dysphagia-specific OAR volumes.
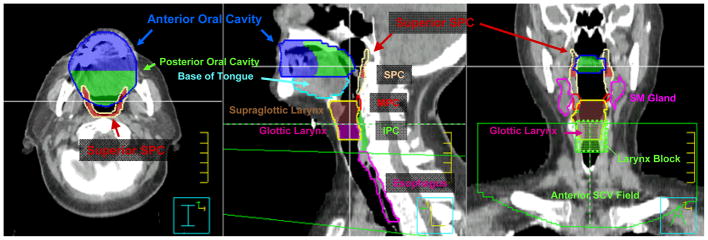
The superior pharyngeal constrictor (SPC) volume (tan) is defined from the skull base to superior edge of the hyoid bone. The SPC volume (dark red) residing above the inferior edge of C1 is defined as superior SPC. The middle pharyngeal constrictor (MPC) volume (red) extends from superior hyoid to the inferior edge of the hyoid. The inferior pharyngeal constrictor (IPC) volume (green) extends from below the hyoid to the level of the cricopharyngeus muscle at the inferior edge of the cricoid cartilage. The oral cavity is subdivided into equal anterior (blue) and posterior (green) halves for subregional analysis. The glottic larynx (magenta) is distinguished from supraglottic larynx (brown) as laryngeal volume residing between the top of the arytenoid cartilages and the bottom of the cricoid cartilage. For all patients, IMRT was matched at the superior aspect of the arytenoids to a conventional AP SCV field with a 3 × 3 cm larynx block, treated to 50 Gy in 25 daily fractions. The larynx block was extended inferiorly to the lower border of the AP SCV field to become a full length midline spinal cord block after 40–42 Gy. SCV = AP supraclavicular field, SM gland = submandibular gland.
Additional candidate normal tissue structures at risk for dysphagia-related morbidity were contoured retrospectively into each study subject’s actual radiotherapy treatment plan. These volumes were not used as formal dose constraints for IMRT planning. These volumes were modeled after previous reports (6, 7) and included: right and left-sided submandibular salivary glands, oral cavity (including all of the mucosal surfaces of the hard/soft palate extending back and including the uvula as a landmark, oral tongue located at or above the floor of mouth, gingiva, buccal mucosa, and floor of mouth), tongue base below the floor of mouth down to the insertion of geniohyoid to the hyoid, pharyngeal constrictor muscles, and cervical esophagus below the inferior edge of the cricoid cartilage (demarcating the cricopharyngeus muscle) down to the level of the manubrium. The oral cavity was subdivided into equal anterior and posterior halves for subregional analysis. Pharyngeal constrictor muscles were subdivided into superior, middle, and inferior constrictors by the cranial and caudal surfaces of the hyoid bone, respectively. The superior pharyngeal constrictor was subdivided further into superior and inferior subregions at the inferior edge of the C1 vertebral body. The entire larynx was contoured from the level of the superior edge of the hyoid bone down to the bottom of the cricoid. Within this OAR, the glottic larynx was demarcated from the top of the arytenoid cartilages to the bottom of the cricoid. Parotid gland volumes were included in our analysis. Representative dysphagia-specific OAR volumes, including specific illustrations of laryngeal, superior pharyngeal constrictor, and oral cavity OAR volumes are illustrated in Figure 2.
Study subjects were scheduled to be evaluated by a speech-language pathologist at baseline, and 6, 12, and 24 months after IMRT. Modified barium swallow (MBS) studies (8) were recorded on the Kay Elemetrix Digital Swallowing Workstation. Frame-by-frame analysis was performed by 4 trained clinicians blinded to patient cases. Oropharyngeal swallowing efficiency (OPSE) measures the entire process of swallowing from the lips to cervical esophagus. OPSE has been validated across head and neck cancer sites (9) and serves as a standard objective measure of swallowing function. We obtained OPSE score components, including oral transit time (OTT), pharyngeal delay time (PDT), and pharyngeal transit time (PTT), to calculate OPSE by dividing percentage of bolus swallowed (100 - % residue - % aspirated) by bolus transit time (seconds) from the oral cavity through the cricopharyngeus (OTT + PDT + PTT). We calculated OPSE for swallows of three bolus types: 10 mL thin liquid, 1 tsp. pudding, and ¼ cracker with barium paste. The OPSE scores for each consistency (liquid, pudding, and cracker) were averaged to provide an overall global swallowing efficiency score. All clinicians met standard target levels of reliability for temporal analysis previously published by Logemann (8). Aspiration was graded using the Penetration-Aspiration scale (10). Scores are determined by depth of bolus invasion into the airway and the patient’s response. A Penetration-Aspiration Scale score ≥ 6 was considered an aspiration event. The M. D. Anderson Dysphagia Inventory (MDADI) has been validated for content, criterion, and construct validity (11). The MDADI is considered useful for determining the impact of swallowing function on quality of life in patients with head and neck cancer. MDADI and the Performance Status Scale for Head and Neck Cancer Patients (PSS-HN) were administered at the time of the MBS study. Diet levels were recorded using the PSS-HN Normalcy of Diet subscale, in which 0 indicates non-oral nutrition and 100 represents full oral nutrition without restrictions (12). Questionnaires were mailed to patients who missed examinations.
For statistical analysis, post-RT OPSE measurements for each patient were normalized to baseline values. All clinical and dosimetric factors were initially tested for associations with OPSE and MDADI using random-effects linear regression, to take into account the multiple observations per patient. For each candidate OAR, a comprehensive investigation of potentially relevant dose-volume constraints was performed as previously described (13–16). For each choice of dose (D) and relative volume (V%), normalized post-RT OPSE values were compared in the patient groups with VD > V% versus VD ≤ V%, where VD denotes the relative volume of the OAR exposed to doses > D Gy. Bonferroni corrections were applied to adjust significance level. Results were plotted as grid plots (Figure 3) to show level of predictive significance across all dose-volume combinations. A multivariate analysis was performed using a forward stepwise procedure to identify up to two independently significant dose-volume constraints. The first factor selected for inclusion in our model had the smallest p-value for which % OPSE was smaller when VD > V%. The second factor selected for inclusion was the one which, when added to the first factor, resulted in the smallest p-value, while at the same time having a coefficient consistent with % OPSE smaller when VD > V% and not causing the first factor to lose significance or to change sign. Because of the large number of OARs and dose factors considered (n = 589), two additional analyses were performed to assess stability and potential reproducibility of multivariate results. First, we performed a leave-one-out cross-validation procedure, in which a model based on a candidate dose-volume constraint (e.g. V30 ≤ 65% versus V30 > 65% for anterior oral cavity) was fitted to the OPSE data repeatedly, each time omitting one patient. For each fit, the squared error was computed as the squared difference between the predicted and observed average OPSE for the omitted patient, and the mean squared error was computed by averaging the squared errors over all patients. Second, we did a bootstrap analysis in which we sampled from the patient population with replacement and repeated the multivariate analysis 1000 times, each time identifying up to 2 significant factors.
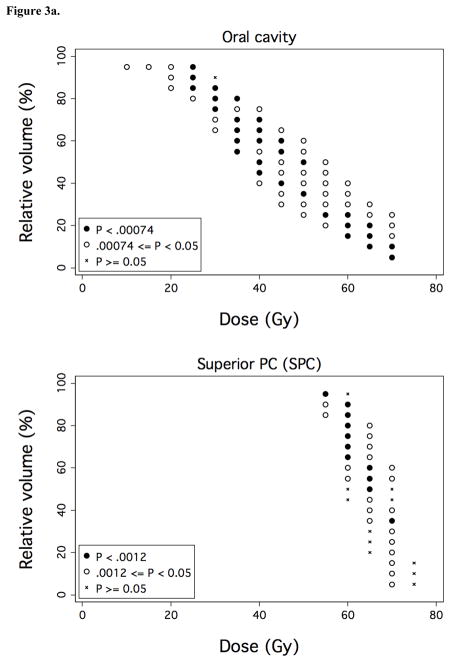
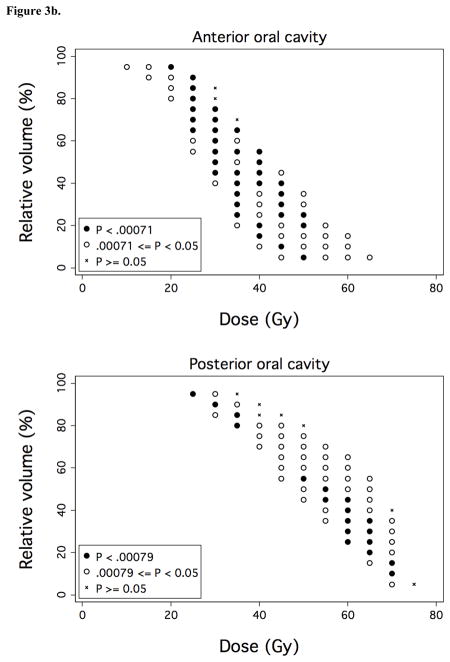
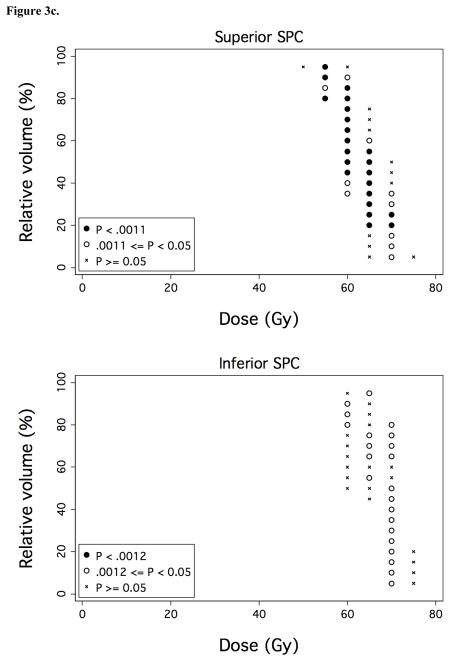
Figure 3. Associations between OAR dose-volume thresholds and OPSE outcomes.
Over a comprehensive range of dose (D) and relative volume (V%), normalized post-RT OPSE values were compared in the patient groups with VD > V% versus VD ≤ V%, where VD denotes the relative volume of the OAR exposed to doses > D Gy. Results are plotted as grid plots to indicate statistical significance of each dose-volume combination received by cohort patients. Dose-volume constraints significantly associated with OPSE outcomes following Bonferroni correction are designated by closed circles (●). a) Oral cavity and SPC. b) Anterior and posterior oral cavity subregion. c) Superior and inferior SPC subregion.
RESULTS
The patient, tumor, and treatment characteristics of our cohort (n = 31) are presented in Table 1 and summary statistics for OAR radiation doses are listed in Table 2.
Table 1.
Patient Characteristics
| N (%) | |
|---|---|
| Sex | |
| Male | 22 (71) |
| Female | 9 (29) |
| Primary Site | |
| Base of Tongue | 21 (68) |
| Tonsil | 10 (32) |
| T-Stage | |
| 1 | 12 (39) |
| 2 | 10 (32) |
| 3 | 4 (13) |
| 4 | 2 (7) |
| X | 3 (10) |
| N-Stage | |
| 2 | 1 (3) |
| 2b | 17 (55) |
| 2c | 6 (19) |
| 3 | 5 (16) |
| X | 2 (7) |
| Concurrent Chemotherapy | |
| No | 18 (58) |
| Yes | 13 (42) |
| Smoking Status at Diagnosis | |
| Never | 18 (58) |
| Quit | 11 (35) |
| Current | 2 (7) |
Table 2.
OAR Dose Summary
| IMRT doses | Median | Range |
|---|---|---|
| Whole Larynx | ||
| Mean dose | 44 Gy | 26–60 Gy |
| V40 | 57% | 25–80% |
| Glottic Larynx | ||
| Mean dose | 18 Gy | 6–39 Gy |
| Supraglottic Larynx | ||
| Mean dose | 63 Gy | 23–74 Gy |
| Oral Cavity | ||
| Mean dose | 46 Gy | 33–63 Gy |
| V30 | 79% | 51–100% |
| V50 | 39% | 18–83% |
| Superior Pharyngeal Constrictors | ||
| Mean dose | 63 Gy | 54–73 Gy |
| V60 | 64% | 0–100% |
| Ipsilateral Parotid | ||
| Mean dose | 29 Gy | 20–54 Gy |
| Contralateral Parotid | ||
| Mean dose | 21 Gy | 14–41 Gy |
Swallowing Outcomes
Post-treatment swallowing assessments, planned at 6, 12, and 24 months after the completion of radiation therapy, were performed on average 6.6, 12.7, and 24.5 months after treatment (Table 3). Mean OPSE scores were 13.2 points lower than baseline 6 months after treatment (p = 0.029) without significant improvement thereafter. Composite MDADI scores were significantly lower than baseline at all post-IMRT test intervals (6 months, p < 0.0001; 12 months, p < 0.001; 24 months, p < 0.0001). Aspiration after treatment was uncommon; one patient (4%) had dysphagia with aspiration at 6 months that subsequently resolved. An additional patient was found to have late-onset dysphagia with aspiration that was identified 12 months after radiation treatment and persisted at 24 months. At baseline, 84% (26/31) of patients tolerated a full oral diet without restrictions (PSS-HN normalcy of diet scale = 100). After treatment, chronic feeding tube dependence was observed in 8% (2/24) of patients at 12 months and 4% (1/26) at 24 months.
Table 3.
Swallowing Outcomes
| Baseline | 6 mo.* | 12 mo.* | 24 mo.* | |
|---|---|---|---|---|
| n (MBS) | 31 | 27 | 21 | 17 |
| n (Questionnaire)* | 31 | 29 | 24 | 26 |
| Mean (range) mos. post-IMRT | -- | 6.6 (4.3, 9.4) | 12.7 (9.6, 18.7) | 24.5 (21.8, 29.5) |
| OPSE, mean (SD) | ||||
| 10 mL liquid | 102.5 (20.6) | 89.9 (31.3) | 94.2 (29.9) | 87.4 (27.1) |
| Pudding | 92.9 (29.9) | 84.6 (34.9) | 69.7 (28.8) | 71.9 (40.4) |
| Cracker | 90.2 (35.2) | 74.0 (38.8) | 67.4 (35.9) | 81.1 (44.0) |
| All consistencies | 95.2 (16.1) | 82.0 (28.1) | 76.9 (23.5) | 78.8 (31.1) |
| Aspiration, n patients (%) | ||||
| 10 mL liquid | 0 (0) | 1 (3.7) | 1 (4.8) | 1 (5.8) |
| Pen-Asp Scale, median (range) | ||||
| 10 mL liquid | 1 (1–2) | 1 (1–6) | 1 (1–7) | 1 (1–7) |
| MDADI, mean (SD) | ||||
| Global score | 89.7 (19.2) | 76.5 (26.2) | 82.6 (24.3) | 84.8 (24.0) |
| Composite score | 89.1 (14.7) | 75.6 (15.7) | 81.5 (16.2) | 82.4 (13.2) |
| Feeding Tube, n patients (%) | ||||
| No | 30 (96.8) | 26 (89.7) | 22 (91.7) | 25 (96.1) |
| Yes | 1 (3.2) | 3 (10.3) | 2 (8.3) | 1 (3.8) |
| PSS-HN Normalcy of Diet, n patients (%) | ||||
| Full diet (no restriction) | 26 (83.9) | 6 (20.7) | 4 (16.7) | 8 (30.8) |
| Full diet (liquid assist) | -- | 5 (17.2) | 11 (45.8) | 14 (53.9) |
| All meat | -- | 2 (6.9) | -- | -- |
| Raw carrots, celery | -- | 3 (10.3) | 1 (4.2) | -- |
| Dry bread and crackers | 1 (3.2) | 1 (3.5) | 2 (8.3) | -- |
| Soft, chewable foods | 3 (9.7) | 10 (34.5) | 4 (16.7) | 3 (11.5) |
| Soft, non-chewable foods | 1 (3.2) | 1 (3.5) | 1 (4.2) | -- |
| Pureed foods | -- | -- | -- | 1 (3.9) |
| Warm liquids | -- | -- | -- | -- |
| Cold Liquids | -- | 1 (3.5) | -- | -- |
| Non-oral feeding (NPO) | -- | -- | 1 (4.2) | -- |
Questionnaires mailed to patients who missed MBS
Clinical Factors
On univariate analysis, the only factor significantly associated with OPSE was tumor site; patients with tonsil disease had 12–24 month post-treatment OPSE scores that were on average 70% of baseline, compared to 93% of baseline for patients with base-of-tongue tumors (p = 0.019). Although smoking status was not significantly correlated with OPSE outcomes, all never smokers (compared with 64% of ever smokers) were eating a full diet (PSS-HN score 90 or 100) at 24 months (borderline significance at p = 0.07). No significant difference was found in OPSE in patients who received induction therapy followed by concurrent chemoradiotherapy compared with those who received induction therapy followed by IMRT alone (p = 0.376). No factor was significantly associated with MDADI scores.
Identification of Dysphagia-Associated OARs
We performed dose-volume analyses to identify candidate dysphagia-associated OARs predictive for OPSE scores 6–24 months post-treatment. No OAR was predictive for MDADI outcomes; thus, analysis was limited to dose-volume associations with OPSE outcomes. Mean whole larynx dose for the cohort was 44 Gy (range: 26–60 Gy). However, mean dose to glottic larynx in the study cohort was limited to 18 Gy (range: 6–39 Gy) through matching of conventional low neck fields with IMRT, resulting in a narrow range of volumetric dose (VD) values for this structure. Accordingly, laryngeal VD did not associate with OPSE outcomes. In the context of laryngeal dose sparing, VD for two OARs, the oral cavity and superior pharyngeal constrictors (SPC), associated strongly with OPSE outcomes (Figure 3a). VD values for BOT, submandibular and parotid glands, middle/inferior constrictors, and esophagus did not predict for OPSE outcomes in this cohort following Bonferroni correction. To better identify regions within the oral cavity OAR which could be selectively contoured for sparing by IMRT planning, we subdivided the oral cavity OAR into equal anterior and posterior halves. VD values for anterior oral cavity (encompassing anterior oral tongue, buccal mucosa, hard palate, and floor of mouth) predominantly drove the predictive power of oral cavity VD values (Figure 3b). Likewise, we subdivided the superior pharyngeal constrictors into two subvolumes located above (superior SPC) and below (inferior SPC) the inferior edge of the C1 vertebral body. VD values for superior SPC were responsible for all of the predictive power of SPC VD values for OPSE outcomes (Figure 3c).
OAR Dosimetric Constraints Predictive for Long-Term Dysphagia
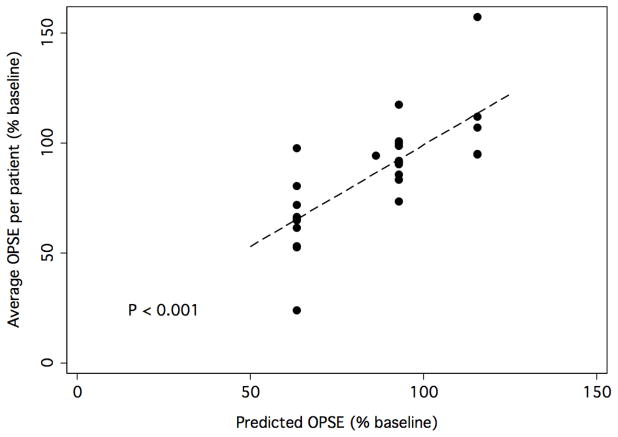
We next attempted to identify a subset of independent dose-volume constraints specific to these or other OARs. A forward stepwise multivariate analysis of factors from the grid analysis of OPSE (% change from baseline) for all candidate OARs was performed. Two threshold values, 1) V30 > 65% for anterior oral cavity and 2) V55 > 80% for superior SPC, were found to be significantly and independently associated with an increased risk for reduced OPSE scores. Figure 4 illustrates the relationship between predicted and observed OPSE (% of baseline, average per patient) for the model incorporating these two dose-volume constraints.
Figure 4. Illustration of the model of OPSE as a function of dose-volume constraints for anterior oral cavity and superior SPC.
Forward stepwise multivariate analysis found two threshold values to be significantly and independently associated with an increased risk for reduced OPSE scores: 1) V30 > 65% for anterior oral cavity and 2) V55 > 80% for superior SPC. The graph demonstrates the relationship between predicted and observed OPSE (% of baseline, average per patient) for the model incorporating these two dose-volume constraints.
Because of the large number of dose-volume constraints considered and the limited number of sampled cases, we further investigated dose-volume factors associated with OPSE using a leave-one-out cross-validation (LOOCV) approach. The dose-volume threshold with the smallest mean squared error for predicting OPSE scores was V30 > 65% for anterior oral cavity, i.e., the same as the most significant threshold from previous analyses. Other thresholds with small mean squared errors by LOOCV were V25 > 85% and V35 > 35% for anterior oral cavity, V65 < 30% for superior SPC, V35 > 70% for oral cavity and tongue base combined, and V60 > 70% for all pharyngeal constrictors combined. This suggests that structures other than anterior oral cavity and superior SPC might be of significance, but may also simply indicate dose correlations across OARs. Many of our dose-volume constraints correlated tightly with one another and defined similar patient groups; for example, the group defined by V60 > 70% for PC differ from the group defined by V30 > 65% for anterior oral cavity by only 3 of 28 patients. Bootstrap analysis of simulated studies of the same size echoed these findings. The OAR selected most often (30% of bootstrap replicates) for strongest association with OPSE scores was, again, anterior oral cavity, followed by superior SPC, the combined oral cavity and BOT OAR, and entire pharyngeal constrictor OAR.
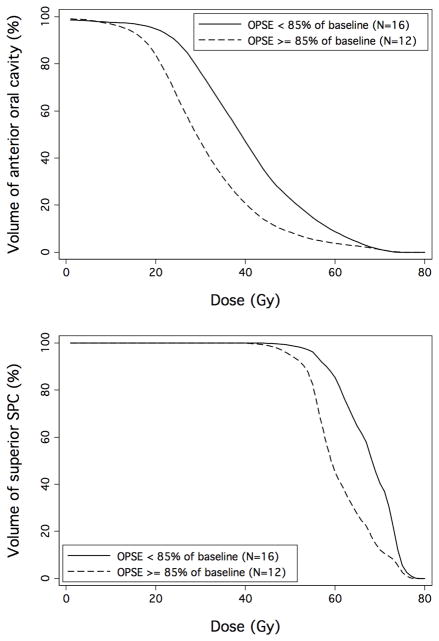
Finally, we generated averaged anterior oral cavity and superior SPC DVH curves for patients dichotomized according to OPSE scores < or ≥ 85% of baseline, as shown in Figure 5. These curves provide a summarized illustration of the association between volumetric dose exposure to these OARs and long-term swallowing outcomes, and suggest a range of dose-volume constraints appropriate for follow-up validation.
Figure 5.
Average anterior oral cavity and superior SPC DVH curves for patients dichotomized according to post-treatment OPSE scores < or ≥ 85% of baseline
DISCUSSION
Dysphagia is a key determinant of quality of life in patients treated with radiation for advanced head and neck cancer (3, 4), and is potentially more important than xerostomia (17). Efforts to study post-radiotherapy dysphagia have been complicated by a diverse spectrum of head and neck cancer presentations and treatment regimens, as well as the recent transition from conventional to IMRT techniques. Recently published data specific to IMRT has implicated high dose treatment of pharyngeal constrictor muscles and/or larynx with post-radiation aspiration (7, 18). However, long-term swallowing outcomes have not been published and several challenging issues remain unaddressed:
First, which anatomic structures critical to oropharyngeal swallowing function can be realistically spared high dose treatment?
When certain key swallowing structures, such as the tongue base and soft palate, cannot be spared due to disease involvement, are there alternative avoidance structures that can be feasibly spared dose for functional preservation? To address this question, it is important to consider baseline swallowing physiology. The oropharyngeal swallow consists of three discrete phases. Phase 1 (oral preparatory phase) mechanically prepares food by chewing and salivary lubrication to become a bolus. This bolus is pushed by the anterior oral tongue back towards the oropharynx during the phase 2 (oral phase). Phase 3 (pharyngeal phase) begins when the swallow trigger is initiated. The pharyngeal constrictor muscles propel the bolus, and the larynx closes the airway while the bolus is transported around it towards the cervical esophagus (19). Given this sequence of events, our a priori hypothesis was that, in addition to the larynx, candidate dysphagia OARs would be located in the oral cavity and along the pharyngeal constrictor axis. This is consistent with our results.
We found a strong correlation between anterior oral cavity dose and objective measures of post-treatment swallowing motility (OPSE scores). We propose that this is a function of the role the anterior oral tongue plays in the generation of the initial lingual pressure needed to transport food through the oropharyngeal swallow (19), as well as lubrication provided by the sublingual glands and minor salivary glands along the anterior oral mucosa (20). Because the anterior oral cavity is frequently well separated from high dose oropharyngeal target volumes and is directly involved in food propagation, it is an ideal candidate OAR.
We also found a significant relationship between OPSE and the volumetric dose delivered to the region of high pharyngeal musculature (Figure 3a). This region includes portions of the superior and middle pharyngeal constrictors, longitudinal muscles, and palatopharyngeal folds. The pharyngeal phase of swallowing depends on a precise interaction of these muscles with tongue base movement, pharyngeal contraction, and hyolaryngeal excursion (6, 19). Our findings are consistent with those of Levendag, et. al. (21), who associated dose to pharyngeal constrictor muscles with dysphagia. We add to this by demonstrating that dose to the highest pharyngeal musculature above the inferior edge of the C1 vertebral body drives this association. This region is frequently separated from gross primary and nodal disease, making dose sparing feasible. We would not expect more caudally situated pharyngeal musculature to serve as feasible candidate OARs since high bystander doses would typically be required for disease target coverage.
At our institution, we are currently using the following candidate dose-volume constraints for prospective validation: 1) anterior oral cavity: V30 < 65% and V35 < 35%, and 2) superior SPC: V55 < 80% and V65 < 30%. Representative anterior oral cavity and superior SPC OARs, and their geographical relationship to typical IMRT dose distributions, are demonstrated in Figures 2 and 6. Our experience has shown that tight sparing of these structures can be accomplished in the absence of bulky retropharyngeal nodes or extensive disease involvement of the nasopharynx/oral cavity. Given the distance separating anterior oral cavity and superior SPC OARs from parotids and larynx, we would not anticipate significant trade-offs in dose sparing to these established OARs, which would take precedence. However, dosimetric trade offs with other normal structures, such as brain and posterior neck, are an important consideration which will require direct prospective evaluation. We anticipate that the utility of our candidate OARs will be most closely related to balancing dose sparing with high dose delivery to target volumes. Our institutional policy is to never sacrifice coverage of disease target volumes to spare noncritical avoidance structures. Nonetheless, our candidate dysphagia constraints should prove geographically feasible in many cases and can serve as a starting point for further optimization in individual patients.
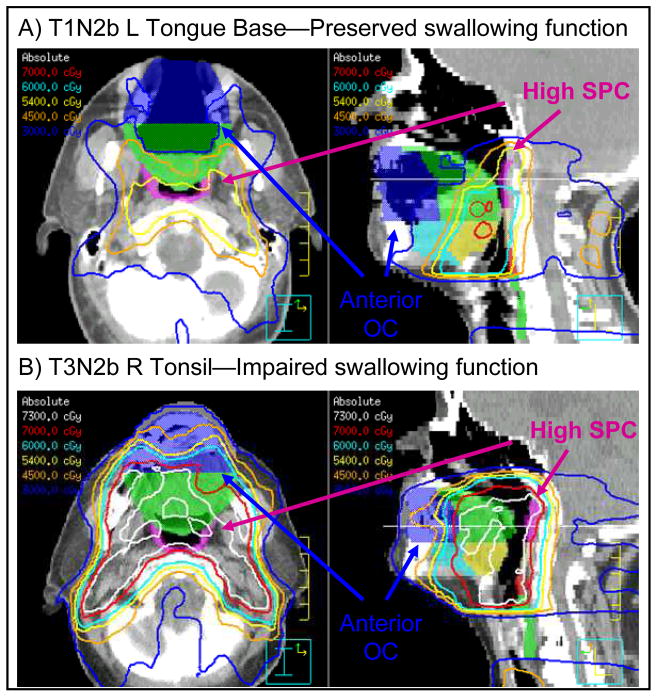
Figure 6. Representative examples of candidate dysphagia OAR volumes within context of IMRT treatment plans.
Oral cavity OAR has been subdivided into equal anterior (dark blue) and posterior (green) halves. The SPC has been divided into superior (magenta) and inferior (tan) subregions at the inferior edge of the C1 vertebral body. Additional OARs can be seen demarcating anterior (light blue) and posterior (yellow) tongue base, MPC (red), and IPC (green). OARs are demonstrated within IMRT treatment plans for two patients with equivalent larynx dose sparing and divergent swallowing function outcomes. A) T1N2b disease of low left tongue base. Anterior oral cavity and high SPC dose sparing can be appreciated. An 84% improvement in OPSE from baseline was measured at 24 months. B) Bulky T3N2b right tonsillar carcinoma with extension into soft palate and tongue base, necessitating high dose coverage of anterior oral cavity and high SPC. A 40% decrease in OPSE from baseline was observed at 24 months.
Second, can appropriate dose sparing prevent post-radiotherapy aspiration?
Previous data (7) have associated radiation dose to pharyngeal constrictor muscles with aspiration. However, the physiologic role the pharyngeal constrictors play in airway protection is minimal relative to the larynx, which is responsible for preventing swallowed material from entering the trachea. Post-radiotherapy aspiration should most closely associate with disrupted normal laryngeal excursion, glottic closure, and cricopharyngeal relaxation. Thus, prevention of post-radiotherapy aspiration should primarily focus on protecting the larynx.
Earlier reports identify aspiration in 40–80% of patients treated with conventional radiotherapy, with or without concurrent chemotherapy (22–24). We document a 4% aspiration rate in our study cohort, which benefited from strict shielding of the arytenoids, inferior larynx, and cricopharyngeal inlet by matching IMRT with conventional low neck fields with larynx blocking. Notably, the previously cited reports either do not provide any laryngeal dosimetric measures, or cite a significantly higher mean laryngeal dose of 55 Gy for their study cohort (7).
The relative merits of full neck IMRT versus isocentric matching of IMRT with conventional low neck treatment continue to be debated. Our published experience from M.D. Anderson Cancer Center has demonstrated a marked improvement in potential laryngeal dose sparing (18.7 Gy vs. 47 Gy, p = 0.001) with low field matching, especially for patients with high oropharyngeal primary disease such as tonsillar cancer (25). Published results (44% aspiration) using larynx dose constraints as part of full neck IMRT delivery (6, 7) remain inferior to the outcomes we achieved in this series.
Finally, are dysphagia-associated OARs relevant to current trends in head and neck radiotherapy?
There is growing appreciation for the improved treatment outcomes (26, 27) of oropharyngeal disease associated with high-risk human papillomavirus (HPV) infection. A controversial concept worthy of investigation is the pursuit of treatment de-escalation strategies in this patient population. Validated dysphagia-associated OAR dose constraints would help justify this approach, and would geographically define where best to limit dose. Also, the location, shape, and size of disease and normal anatomy change due to positioning and physiological factors during treatment. Adaptive radiotherapy (ART) is a novel approach to correct for daily anatomic variations through automated, online or offline modification of baseline IMRT target volumes and plans. We are studying ART prospectively at our institution, with a focus on parotid sparing. Identification of dysphagia-associated OARs promises to further refine ART technique.
Despite such promise, we wish to stress the limitations of this pilot study. The significant dose-volume constraints identified by our study are strictly candidate planning constraints which will require prospective corroboration. It is possible that our findings result from correlations with dose to additional structures which will require future identification. For example, classification of patients by anterior oral cavity doses coincided with classification of patients by pharyngeal constrictors doses in 89% (25/28) of our patients. Also, the most clinically relevant threshold reduction of OPSE from baseline remains unknown and needs to be established. We selected a 15% reduction in OPSE from baseline as our threshold cutoff (Figure 5) based on our experience showing an average 10–15 point range decrease in OPSE scores after treatment, mirrored by similar findings from other groups (28, 29). Our effort lacked sialometry data to correlate OAR dose-volume data and OPSE scores with objective functional salivary outcomes. Xerostomia may indirectly impact OPSE scores adversely due to reduced food lubrication and mucosal irritation, but such a mechanism remains unconfirmed. We should note that we achieved parotid gland dose sparing goals in all study patients, which should reduce the potential confounding influence of xerostomia on OPSE outcomes. Finally, patient perceptions of dysphagia in our study cohort, as defined by MDADI self-assessment scores, were highly variable and correlated poorly with OPSE. Previous reports have demonstrated poor correlation between perceptual and instrumental measures of dysphagia (30, 31). MDADI is a perceptual tool measuring patients’ interpretation of how well they swallow. OPSE objectively measures the efficiency of neuromuscular swallowing function. Given radiotherapy’s recognized impact on neuromotor function, it is not surprising that subtle OAR dosimetric differences were associated only with physiologic measures (OPSE). Nonetheless, decreases in OPSE scores in our cohort following IMRT were closely reflected by parallel decreases in MDADI scores and dietary tolerance (Table 3), supporting OPSE as a measure of swallowing outcomes and an indicator of clinically significant dysphagia. It will be critical for future work to better clarify the relationship between perceptual and instrumental measures of dysphagia.
Acknowledgments
Supported by NCI grant CA132281. We acknowledge Dr. Avraham Eisbruch for his assistance with the design of our candidate dysphagia-associated OARs.
Footnotes
Conflict of Interest Notification: The authors report no conflict of interest.
Presented in part at the annual meeting of the American Society of Therapeutic Radiology and Oncology, Boston, MA, September, 2008.
References
- 1.Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 3.Rademaker AW, Vonesh EF, Logemann JA, et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: a 12-month follow-up study accounting for dropout. Head Neck. 2003;25:1034–1041. doi: 10.1002/hed.10317. [DOI] [PubMed] [Google Scholar]
- 4.Mittal BB, Pauloski BR, Haraf DJ, et al. Swallowing dysfunction--preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiat Oncol Biol Phys. 2003;57:1219–1230. doi: 10.1016/s0360-3016(03)01454-8. [DOI] [PubMed] [Google Scholar]
- 5.Chao KS, Wippold FJ, Ozyigit G, et al. Determination and delineation of nodal target volumes for head-and-neck cancer based on patterns of failure in patients receiving definitive and postoperative IMRT. Int J Radiat Oncol Biol Phys. 2002;53:1174–1184. doi: 10.1016/s0360-3016(02)02881-x. [DOI] [PubMed] [Google Scholar]
- 6.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 8.Logemann J. Manual for the Videofluorgraphic Study of Swallowing. 2. Austin, TX: Pro-Ed; 1993. [Google Scholar]
- 9.Rademaker AW, Pauloski BR, Logemann JA, et al. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res. 1994;37:314–325. doi: 10.1044/jshr.3702.314. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 11.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–876. [PubMed] [Google Scholar]
- 12.List MA, D’Antonio LL, Cella DF, et al. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale. A study of utility and validity. Cancer. 1996;77:2294–2301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Hartford AC, Niemierko A, Adams JA, et al. Conformal irradiation of the prostate: estimating long-term rectal bleeding risk using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1996;36:721–730. doi: 10.1016/s0360-3016(96)00366-5. [DOI] [PubMed] [Google Scholar]
- 14.Boersma LJ, van den Brink M, Bruce AM, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70–78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1998;41:83–92. doi: 10.1016/s0360-3016(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 15.Koper PC, Heemsbergen WD, Hoogeman MS, et al. Impact of volume and location of irradiated rectum wall on rectal blood loss after radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1072–1082. doi: 10.1016/j.ijrobp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Tucker SL, Liu HH, Liao Z, et al. Analysis of radiation pneumonitis risk using a generalized Lyman model. Int J Radiat Oncol Biol Phys. 2008;72:568–574. doi: 10.1016/j.ijrobp.2008.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 18.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- 20.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 21.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 23.Langerman A, Maccracken E, Kasza K, et al. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1289–1295. doi: 10.1001/archotol.133.12.1289. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen NP, Frank C, Moltz CC, et al. Aspiration rate following chemoradiation for head and neck cancer: an underreported occurrence. Radiother Oncol. 2006;80:302–306. doi: 10.1016/j.radonc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Dabaja B, Salehpour MR, Rosen I, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys. 2005;63:1000–1005. doi: 10.1016/j.ijrobp.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 26.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 27.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 28.Campbell BH, Spinelli K, Marbella AM, et al. Aspiration, weight loss, and quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg. 2004;130:1100–1103. doi: 10.1001/archotol.130.9.1100. [DOI] [PubMed] [Google Scholar]
- 29.Pauloski BR, Rademaker AW, Logemann JA, et al. Swallow function and perception of dysphagia in patients with head and neck cancer. Head Neck. 2002;24:555–565. doi: 10.1002/hed.10092. [DOI] [PubMed] [Google Scholar]
- 30.Barringer DA, Hutcheson KA, Sturgis EM, et al. Effect of induction chemotherapy on speech and swallowing function in patients with oral tongue cancer. Head Neck. 2009;31:611–617. doi: 10.1002/hed.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillespie MB, Brodsky MB, Day TA, et al. Laryngeal penetration and aspiration during swallowing after the treatment of advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2005;131:615–619. doi: 10.1001/archotol.131.7.615. [DOI] [PubMed] [Google Scholar]