Abstract
The European Union lacks a comprehensive framework to address the threats posed by the introduction and spread of marine non-indigenous species (NIS). Current efforts are fragmented and suffer substantial gaps in coverage. In this paper we identify and discuss issues relating to the assessment of spatial and temporal patterns of introductions in European Seas (ES), based on a scientifically validated information system of aquatic non-indigenous and cryptogenic species, AquaNIS. While recognizing the limitations of the existing data, we extract information that can be used to assess the relative risk of introductions for different taxonomic groups, geographic regions and likely vectors. The dataset comprises 879 multicellular NIS. We applied a country-based approach to assess patterns of NIS richness in ES, and identify the principal introduction routes and vectors, the most widespread NIS and their spatial and temporal spread patterns. Between 1970 and 2013, the number of recorded NIS has grown by 86, 173 and 204% in the Baltic, Western European margin and the Mediterranean, respectively; 52 of the 879 NIS were recorded in 10 or more countries, and 25 NIS first recorded in European seas since 1990 have since been reported in five or more countries. Our results highlight the ever-rising role of shipping (commercial and recreational) as a vector for the widespread and recently spread NIS. The Suez Canal, a corridor unique to the Mediterranean, is responsible for the increased introduction of new thermophilic NIS into this warming sea. The 2020 goal of the EU Biodiversity Strategy concerning marine Invasive Alien Species may not be fully attainable. The setting of a new target date should be accompanied by scientifically robust, sensible and pragmatic plans to minimize introductions of marine NIS and to study those present.
Keywords: non-indigenous species, introduction patterns, environmental regulation, management, European seas, Suez Canal, invasion vectors
INTRODUCTION
Introduction of non-indigenous species (NIS) has strongly impacted the conservation of biodiversity, structure and function of ecosystems and sustainable exploitation of natural resources, and may negatively impact industries and pose threats to human health (Carlton 2002; Bax et al. 2003; Simberloff 2011).
Recognition of the significant threats posed by NIS is evident in the European Union Marine Strategy Framework Directive (MSFD) and Biodiversity Strategy (EC 2012, 2014). NIS are one of the 11 descriptors that constitute the basis for the evaluation of ‘Good Environmental Status’ (GES) of marine ecosystems in the MSFD (EC 2008). The Commission Decision (EC 2010) established two criteria and three indicators for assessing progress towards GES relevant to NIS. Moreover, the EU Biodiversity Strategy (EC 2011) aims that ‘ By 2020, Invasive Alien Species (IAS) and their pathways are identified and prioritised, priority species are controlled or eradicated, and pathways are managed to prevent the introduction and establishment of new IAS’. However, a recently published proposal for a Regulation of the European Parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species EC (2013) states that though ‘The impact of IAS on biodiversity is significant … one of the major, and growing, causes of biodiversity loss and species extinction…. the European Union currently lacks a comprehensive framework to address the threats posed by IAS …’ (p. 1).
Until such time as monitoring programs for assessing the environmental status of marine waters are established and implemented by Member States, as mandated by Article 11 of the MSFD, the EU depends on existing NIS datasets for basic information on trends in abundance, temporal occurrence and spatial distribution of NIS, in particular for invasive NIS, their routes and vectors (Ojaveer et al. 2014). One of the problems facing the designers of roadmaps, programs and management measures of NIS is the standardisation of terminology and metrics to describe the status of biological invasions, influenced, in turn, by quality, validity and potential bias of the underlying data (e.g. Pyšek et al. 2008).
In this paper, we identify and discuss issues relating to the assessment of spatial and temporal patterns of NIS invasions in European Seas. Our analyses are based on carefully checked data reflecting the current state of knowledge about invasion patterns in European seas. While recognizing the limitations of the existing data, we extract information that can be used to assess the relative risk of invasions for different taxonomic groups, geographic regions and vectors. This account demonstrates the value of a scientifically validated database when used to establish the ecological status of a given country and/or its regional seas in terms of the numerical incidence of NIS present in coastal areas.
A sound scientific basis is needed in order to set up those administrative procedures needed to manage within ES, where ‘Efforts are fragmented, with substantial gaps in species coverage, and are often poorly coordinated … this fragmented approach can lead to action in one Member State being undermined by a lack of action in neighboring Member States …’ (EESC 2014). The countries bordering ES form a mosaic of cultures and values which need to be considered when applying uniform management measures.
Spatial patterns of NIS spread predicate ecological patterns, and as such are crucial for setting efficient management options. Analysis of the appropriate data should aid the implementation of the EU MSFD and the Biodiversity Strategy. We apply a country-based approach to: (1) assess patterns of NIS richness in European Seas, (2) identify the principal invasion vectors and (3) identify the most widespread NIS and the patterns of their spatial and temporal spread.
METHODS
Our account comprises NIS in three European Seas (ES) (sensu Narayanaswamy et al. 2013): the Baltic Sea (including the Kattegat), the Western European Margin (WEM, comprising the Iberian coast, Celtic Seas, North Sea, Skagerrak, Norwegian Sea, Barents Sea, Iceland shelf), and the Mediterranean. The records of introduction events document the occurrence of NIS in a country. For bicoastal countries (e.g. France-Mediterranean, France-Atlantic) introduction records are listed separately for each seacoast. We include EU Member States and non-EU countries, such as Russia-Baltic Sea, Norway, Iceland, and the Levantine and North-African countries of the Mediterranean Sea. We exclude records from the Black and Caspian Seas and Macaronesia. We have sought natural biogeographic rather than administrative units. Country-based records (or regional records in bi-coastal countries regions) were merged into the three ES.
The NIS follow the definition set out in Annex 1 of MSFD: ‘Non-indigenous species introduced by human activities’ (EC 2008). We use the term ‘invasive’ to denote an NIS whose population has proliferated and is rapidly extending its range (Occhipinti-Ambrogi & Galil 2004). We record NIS as ‘widespread’ when recorded in 10 or more countries in the ES, and as ‘post-1990 widespread’ when recorded and spread to five or more countries since 1990.
Species undergoing climate-shifted population distributions, but no human-assisted spread, are not considered to be NIS (Pederson et al. 2011). Cryptogenic species, sensu Carlton (1996), were excluded, as we focus herein on NIS. Cryptogenic species in European coastal waters will be addressed by Gollasch et al. (in prep.).
The identification of an NIS is dependent upon its known regional and global distribution. Surveys of marine biota in ES, especially molluscs and fish, had been conducted by the 19th century, allowing for a reasonable measure of confidence in separating NIS from the indigenous biota in some major taxa. The historical tracking of NIS is based on the tradition of classification of biota. Country-based records were used to ensure data reliability and validity across the study area. Criteria for determining an NIS are (modified after Wolff 2005): (1) conspicuous arrival, (2) geographical discontinuity, (3) highly localised occurrence, (4) insufficient natural dispersal to account for presence, (5) rapid population expansion, (6) association with vector, (7) dependent on another NIS, (8) molecular similarity to spatially distant populations and (9) belonging to a spatially distant taxon. In this account we limit ourselves to multicellular organisms as the identity and origin of many single-celled organisms are confused and in doubt (Gómez 2008). The date of introduction is rarely known and we use the first record within each country (the date of collection or, when missing, the date of publication), noting the date of collection may be some years after the actual date of introduction.
A ‘route’ is defined as the geographic path over which a species is transported from donor to target area, a ‘vector’ as the transport mechanism or physical means by which an NIS was transported (after Carlton & Ruiz 2005). A route may accommodate several vectors (i.e. ‘Culture activities’ comprises ‘aquaculture equipment’, ‘associated water & packaging material’, ‘intercontinental stock movement’, ‘regional stock movement’ and ‘unintentional release and escapees’). With the exception of documented intentional introductions (i.e. culture and stocking activities, introduced live food), only rarely are the pathways and vectors of introduction known from direct evidence. Mostly they are deduced from the biology and ecology (if known) of the species, the habitats and localities it occupies in the native and introduced range, and its pattern of dispersal (if known), i.e. for a fouling species frequently recorded from ports, shipping is assumed to be the most probable vector. Inference from one case of introduction to another may be fraught with uncertainty, as routes and vectors may differ between regions and between primary and secondary introductions. We list the vector where it is known from direct evidence [e.g. removed from a fouled vessel), or associated with a vector or route (e.g. found in, or adjacent to, ports (vessels), shellfish-farms (culture), or ‘stepping stones’ records from the Levant (Suez Canal)1]. NIS considered to have been introduced by more than a single vector are listed accordingly. Where two or more vectors may possibly operate, but we don't know which one(s) are in play for the NIS in question, it is referred to as polyvectic.
Data were extracted from the AQUANIS, a new pan-European aquatic non-indigenous and cryptogenic species information system (AquaNIS 2013, version 2.3) and augmented with published records to 2013.
RESULTS
Geographical patterns of NIS
NIS richness differed among the ES, and was substantially greater for the Mediterranean than the WEM or Baltic Sea. Of the 879 multicellular NIS reported in the ES, 95 were found in the Baltic, 237 along the WEM and 680 from the Mediterranean. The dataset comprises 2867 introduction events, of which 118 had been recorded prior to 1900. Post-1900, 249, 630 and 1870 primary country records were reported along the Baltic, WEM and the Mediterranean, whereas post-1990, 105, 304 and 982 primary country records were reported in the Baltic, WEM and the Mediterranean, respectively. The number of NIS recorded in the ES increased: over the period 1970–2013, their numbers grew by 86, 173 and 204% in the Baltic, WEM and the Mediterranean, respectively (Fig. 1).
Fig. 1.
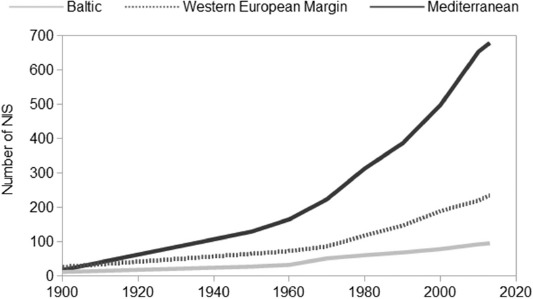
— Cumulative number of non-indigenous species (NIS) recorded in the Baltic Sea, Western European Margin and Mediterranean Sea.
Our data by country show strong geographical patterns in NIS richness. In the Baltic the numbers of NIS are similar between countries, whereas the countries along the WEM, and those in the Mediterranean, had widely differing NIS numbers (Fig. 2). The countries with the greatest number of NIS cluster in the eastern Mediterranean.
Fig. 2.
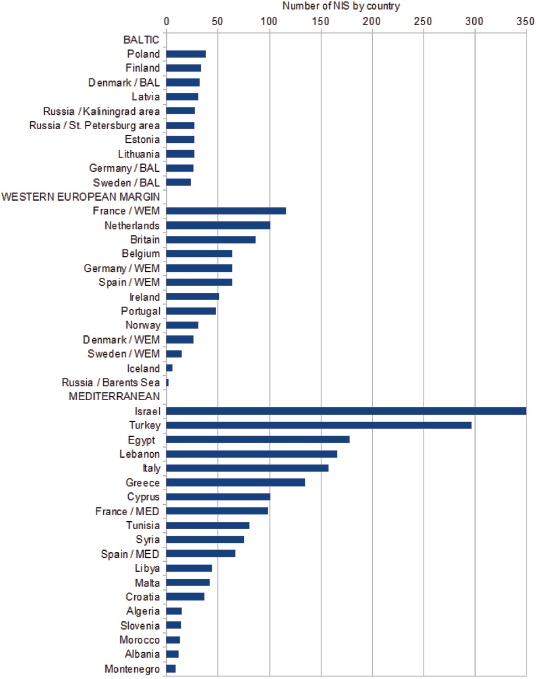
— Number of non-indigenous species (NIS) recorded by country in the Baltic Sea (BAL), Western European Margin (WEM) and Mediterranean Sea (MED).
Almost 6% of the NIS (52 of the 879) were recorded in 10 or more countries, and 25 NIS first recorded since 1990 have been found in five, or more, countries.
The earliest record in each ES was taken to be the primary record, and the vector of that record, as far as can be deduced (see above), is considered the primary vector. The most common vectors in the Baltic are likely culture (47%) and vessels (39%), in the WEM vessels (45%) and culture (35%), and in the Mediterranean, the Suez Canal (53%) and vessels (24%) (Fig. 3a), though the relative importance of vectors varies among individual countries (Fig. 4). A higher percentage of vessel-introduced NIS is noticeable among the most widespread NIS: 50, 49 and 26% in the Baltic, WEM and the Mediterranean, respectively (Fig. 3b), and post-1990 widespread NIS − 80, 77 and 26% in the Baltic, WEM and the Mediterranean, respectively (Fig. 3c).
Fig. 3.
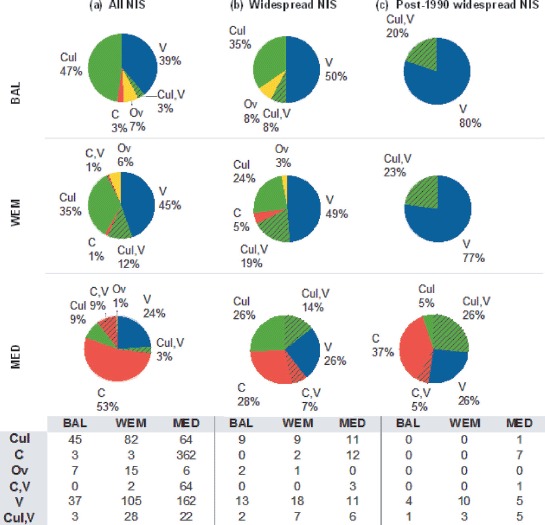
— Likely vectors of introduction of non-indigenous species (NIS) in the Baltic Sea (BAL: upper panel), Western European Margin (WEM: intermediate panel) and Mediterranean Sea (MED: lower panel). (a) All NIS; (b) widespread NIS; (c) post-1990 widespread NIS. C = Canals; C, V = Canals, Vessels; V = Vessels, Cul, V = Culture, Vessels; Cul = Culture; Ov = Other vectors.
Fig. 4.
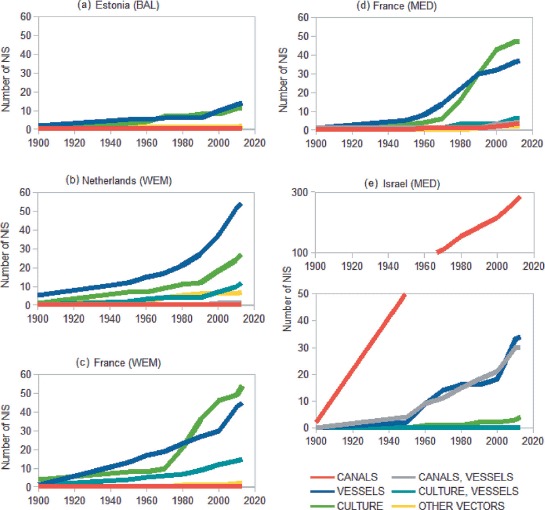
— Cumulative number of non-indigenous species (NIS) by likely vector and country: (a) Estonia (Baltic Sea), (b) Netherlands (Western European Margin), (c) France (Western European Margin), (d) France (Western Mediterranean Sea), (e) Israel (Eastern Mediterranean Sea).
Widespread NIS
Of the 879 NIS recorded, 52 species are considered to be ‘widespread’: 26, 37 and 43 respectively in the Baltic, WEM and the Mediterranean. Seventeen of these ‘widespread’ NIS are common to all three ES (Table 1). The most widespread species is the Pacific oyster, Crassostrea gigas, introduced from the 1960s onwards for cultivation and now occurring from Norway to North Africa (Wrange et al. 2010). Widespread distribution of the algae Colpomenia peregrina, Neosiphonia harveyi and Sargassum muticum most probably reflects the stock movements of oysters (Verlaque 2001); other widespread species, such as Ruditapes philippinarum and Crepidula fornicata, have been introduced intentionally and unintentionally, respectively. Some of the NIS present in all three ES likely originate from historic ship-mediated introductions: the serpulid polychaete Ficopomatus enigmaticus was described from the Canal de Caen, Normandy, in 1921 though reported even earlier in the Mediterranean (FAUVEL 1923; LINDEGG 1934). Nine widespread NIS are common to the Baltic and the WEM but are absent from the Mediterranean, 11 are recorded in the WEM and the Mediterranean and are absent from the Baltic, whereas 15 widespread NIS were recorded in the Mediterranean alone. Introduction records of ‘widespread’ NIS ranged from 80 in the Baltic, (27% of all introduction events), 251 in the WEM (37% of all introduction events) and 351 events in the Mediterranean countries (constituting only 19% of all introduction events) (Fig. 5).
Table 1.
List of widespread non-indigenous species (NIS) in European Seas. The second column lists NIS recorded from 10 or more countries; the third column lists NIS first recorded after 1990 and reported from five or more countries. Columns 4 to 6, the number of countries in each region (BAL = Baltic, WEM = Western European Margin, MED = Mediterranean) where the NIS was recorded. In the last column the likely vectors of introduction are given; C = Canals; Cul = Culture, V = Vessels.
| Species name | Widespread NIS | Post-1990 widespread NIS | BAL | WEM | MED | Pathways |
|---|---|---|---|---|---|---|
| Acartia (Acanthacartia) tonsa Dana 1849 | x | 9 | 8 | 3 | V | |
| Anguillicoloides crassus (Kuwahara, Niimi & Itagaki 1974) | x | 9 | 9 | Cul | ||
| Asparagopsis armata Harvey 1855 | x | 6 | 10 | Cul (WEM) + V (MED) | ||
| Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon 1845 | x | 1 | 9 | Cul, V (WEM) + C, V (MED) | ||
| Austrominius modestus (Darwin 1854) | x | 9 | 1 | V | ||
| Bonnemaisonia hamifera Hariot 1891 | x | 1 | 11 | 4 | V (WEM, BAL) + Cul, V (MED) | |
| Botrylloides violaceus Oka 1927 | x | 7 | 1 | V | ||
| Brachidontes pharaonis (P. Fischer 1870) | x | 11 | C | |||
| Bursatella leachii Blainville 1817 | x | 12 | C | |||
| Callinectes sapidus Rathbun 1896 | x | 2 | 7 | 14 | V | |
| Caprella mutica Schurin 1935 | x | 9 | V | |||
| Caprella scaura Templeton 1836 | x | x | 2 | 8 | Cul, V | |
| Caulerpa racemosa var. cylindracea (Sonder) | x | x | 14 | V | ||
| Verlaque, Huisman & Boudouresque 2003 | ||||||
| Cercopagis (Cercopagis) pengoi (Ostroumov 1891) | x | 8 | V | |||
| Cerithium scabridum Philippi 1848 | x | 10 | C | |||
| Chama asperella Broderip 1835 | x | 5 | C | |||
| Codium fragile fragile (Suringar) Hariot 1889 | x | 1 | 8 | 11 | Cul, V | |
| Colpomenia peregrina Sauvageau 1927 | x | 1 | 9 | 7 | Cul | |
| Corella eumyota Traustedt 1882 | x | 5 | V | |||
| Crassostrea gigas (Thunberg 1793) | x | 2 | 11 | 11 | Cul | |
| Crepidula fornicata (Linnaeus 1758) | x | 1 | 10 | 4 | Cul | |
| Didemnum vexillum Kott 2002 | x | 4 | 1 | Cul, V | ||
| Dreissena polymorpha (Pallas 1771) | x | 6 | 6 | V | ||
| Ensis directus (Conrad 1843) | x | 2 | 8 | V | ||
| Ergalatax junionae Houart 2008 | x | 6 | C | |||
| Eriocheir sinensis H. Milne Edwards 1853 | x | 9 | 11 | 3 | V (WEM, BAL) + C, V (MED) | |
| Evadne anonyx G.O. Sars 1897 | x | 5 | V | |||
| Ficopomatus enigmaticus (Fauvel 1923) | x | 1 | 8 | 10 | V | |
| Fistularia commersonii Rüppell 1838 | x | x | 16 | C | ||
| Fulvia (Fulvia) fragilis (Forsskål in Niebuhr 1775) | x | 10 | C, V | |||
| Gammarus tigrinus Sexton 1939 | x | 7 | 6 | V | ||
| Goniobranchus annulatus (Eliot 1904) | x | 5 | C | |||
| Gracilaria vermiculophylla (Ohmi) Papenfuss 1967 | x | x | 2 | 8 | 1 | Cul, V |
| Halophila stipulacea (Forsskål) Ascherson 1867 | x | 10 | C, V | |||
| Hemigrapsus sanguineus (De Haan 1835) | x | 4 | 2 | V | ||
| Hemigrapsus takanoi Asakura & Watanabe 2005 | x | 6 | Cul, V | |||
| Hemimysis anomala G. O. Sars 1907 | x | 6 | 5 | Cul (BAL) + C (ATL) | ||
| Hemiramphus far (Forsskål 1775) | x | 11 | C | |||
| Heterosiphonia japonica Yendo 1920 | x | 1 | 8 | 2 | Cul (WEM+MED); Cul, V (BAL) | |
| Hydroides dianthus (Verrill 1873) | x | 2 | 9 | V | ||
| Hydroides elegans (Haswell 1883) | x | 2 | 10 | V | ||
| Lagocephalus sceleratus (Gmelin 1789) | x | 7 | C | |||
| Lophocladia lallemandii (Montagne) F. Schmitz 1893 | x | 12 | C | |||
| Marenzelleria viridis (Verrill 1873) | x | 3 | 7 | V | ||
| Marsupenaeus japonicus (Spence Bate 1888) | x | 4 | 10 | C (MED) + Cul (WEM) | ||
| Mnemiopsis leidyi A. Agassiz 1865 | x | x | 4 | 6 | 8 | V |
| Neogobius melanostomus (Pallas 1814) | x | x | 9 | 2 | V | |
| Neosiphonia harveyi (J.W. Bailey) M.-S. Kim, H.-G. Choi, Guiry & G.W. Saunders 2001 | x | 1 | 9 | 6 | Cul | |
| Oculina patagonica de Angelis 1908 | x | 10 | V | |||
| Oncorhynchus mykiss (Walbaum 1792) | x | 9 | 8 | Cul | ||
| Palaemon macrodactylus Rathbun 1902 | x | 6 | 1 | V | ||
| Paraleucilla magna Klautau, Monteiro & Borojevic 2004 | x | 1 | 4 | Cul, V | ||
| Percnon gibbesi (H. Milne Edwards 1853) | x | x | 11 | V | ||
| Petricolaria pholadiformis (Lamarck 1818) | x | 2 | 7 | 1 | Cul | |
| Pinctada imbricata radiata (Leach 1814) | x | 1 | 14 | C (MED) + V (WEM) | ||
| Potamopyrgus antipodarum (J.E. Gray 1843) | x | 7 | 8 | Cul, V | ||
| Pseudodiaptomus marinus Sato 1913 | x | 4 | 1 | V | ||
| Pteragogus pelycus Randall 1981 | x | 6 | C | |||
| Rhithropanopeus harrisii (Gould 1841) | x | 8 | 7 | 3 | V | |
| Ruditapes philippinarum (Adams & Reeve 1850) | x | 8 | 6 | Cul | ||
| Sargassum muticum (Yendo) Fensholt 1955 | x | 1 | 10 | 2 | Cul | |
| Saurida macrolepis Tanaka 1917 | x | 10 | C | |||
| Sepioteuthis lessoniana Lesson 1830 | x | 5 | C | |||
| Siganus luridus (Rüppell 1829) | x | 13 | C | |||
| Siganus rivulatus Forsskål & Niebuhr 1775 | x | 10 | C | |||
| Sphyraena flavicauda Rüppell 1838 | x | 5 | C | |||
| Stephanolepis diaspros Fraser-Brunner 1940 | x | 12 | C | |||
| Styela clava Herdman 1881 | x | 1 | 9 | 1 | Cul, V | |
| Tricellaria inopinata d'Hondt & Occhipinti Ambrogi 1985 | x | 8 | 2 | Cul, V | ||
| Zoobotryon verticillatum (Delle Chiaje 1822) | x | 2 | 9 | V |
Fig. 5.
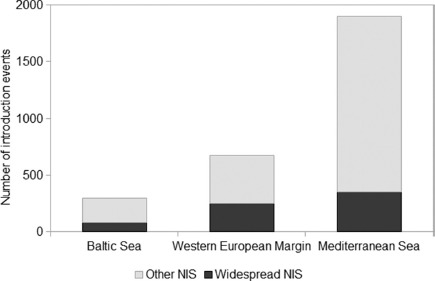
— Number of introduction events in the Baltic Sea, Western European Margin and Mediterranean Sea showing the proportion of records of widespread non-indigenous species (NIS).
A subset of the widespread NIS expanded in at least 10 countries after the year 1990: Caprella scaura, Caulerpa racemosa var. cylindracea, Fistularia commersonii, Gracilaria vermiculophylla, Mnemiopsis leidyi, Neogobius melanostomus and Percnon gibbesi (Table 1). The recent expansion of these species indicates that they are in a phase of active colonization and should be followed with the utmost attention. In addition, 25 NIS that were first recorded later than 1990 are now present in at least five countries (Table 1); their spread too should be accorded special attention. The majority of the post-1990 introductions occur in the Mediterranean, underscoring the dynamics in this nearly enclosed sea.
Of the 879 multicellular NIS in ES, molluscs (26.8%), crustaceans (19.7%), fish (16.8%) and macroalgae (14.1%) make the largest contributions to the number of documented NIS, comprising nearly 78% of NIS richness (Table 2). The relative frequency of taxonomic groups shifts somewhat when only widespread NIS are polled: a disproportionate percentage is comprised of macrophytes (23.1%), while mollusc contribution decreases (21.2%). Among the post-1990 widespread NIS, the relative frequency of taxonomic groups shifts dramatically, macroalgae contribute only 8%, whereas crustaceans and fish dominate, with 36 and 20%, respectively, of the total, comprising ship-mediated introductions such as the skeleton shrimps Caprella mutica and C. scaura, and fast spreading Erythraean fish Fistularia commersonii and Lagocephalus sceleratus.
Table 2.
Taxa composition of all non-indigenous species (NIS), widespread NIS and post-1990 widespread NIS in European Seas.
| Taxa | All NIS | Widespread NIS | Post-1990 widespread NIS |
|---|---|---|---|
| Molluscs | 26.8% | 21.2% | 16.0% |
| Crustaceans | 19.7% | 21.2% | 36.0% |
| Fish | 16.8% | 15.4% | 20.0% |
| Macroalgae | 14.1% | 23.1% | 8.0% |
| Annelid worms | 9.3% | 7.7% | – |
| Other taxa | 13.2% | 11.5% | 20.0% |
DISCUSSION
Terminology
The study of biological invasions resulted in a glut of terms, liable to misapplication and error. Occhipinti-Ambrogi & Galil (2004) aimed to clarify the terminology as a consensual set of terms and definitions regarding invasions in order to facilitate discourse among the science, policy and management communities. Yet the EC's own documents utilize assorted terms and confusing definitions.
Annex I of MSFD (EC 2008) concerns ‘Non-indigenous species introduced by human activities’, when Annex III of the same document lists ‘… non-indigenous exotic species’. The Commission Decision on criteria and methodological standards on Good Environmental Status of marine waters (EC 2010) uses both ‘Non-indigenous species introduced by human activities’ (their, L 232/19), and ‘invasive non-indigenous species’ – the latter term is associated with consequences: ‘Impacts of non-indigenous invasive species at the level of species, habitats and ecosystem’ (L 232/19).
A recent proposal for a regulation of the European Parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species (EC 2013) uses a different term: ‘Alien species are species that are transported as a result of human action outside of their natural range across ecological barriers’, and defined ‘Invasive Alien Species’ (IAS) as those with ‘a significant negative impact on biodiversity as well as serious economic and social consequences’, but declined to clarify the levels of disturbance to be considered significant and serious. A companion document, Commission Staff Working Document, Impact Assessment (EC SWD 2013, Annex VIII: 80), defines IAS as ‘alien species whose introduction or spread has been found, through risk assessment, to threaten biodiversity and ecosystem services, or to have a negative impact on the environment, society and the economy’, again, without delineation of threat levels. These documents utilize both ‘pathway’ and ‘vector’ [as in ‘some alien species are introduced … in transport vectors’ (3) but only the former term is defined as ‘the routes and mechanisms of biological invasion’ (16)]. We follow the concepts of CARLTON & RUIZ (2005) who laid out a detailed framework for vector terminology.
A uniform, unambiguous terminology must underpin EU and EC documents. NIS (synonyms: alien, exotic, introduced, non-native) is defined as a species, subspecies or lower taxon introduced intentionally or accidentally by a human-mediated vector outside its natural range (past or present) and outside its natural dispersal potential (OLENIN et al. 2013). Since quantitative, or even qualitative, levels of NIS impacts on biodiversity, economy and society have not been delineated, let alone discussed and agreed upon, it is best at the present time to adhere to plain, transparent and uncomplicated terms, clearly defined. Therefore, we use the term ‘invasive’ for clearly quantifiable ‘wide spread’2 (NIS recorded in 10 or more countries in ES), and ‘post-1990 widespread’ (NIS first recorded in ES in 1990 or later, and reported in five or more countries) to describe NIS populations sustained without human assistance and spreading beyond introduction sites.
Routes and vectors
The MSFD (EC 2008) places emphasis on the ‘trends in abundance, temporal occurrence and spatial distribution in the wild of non-indigenous species … in relation to the main vectors and pathways’. A recent proposal for the ‘regulation concerning the prevention and management of the introduction and spread of invasive alien species’ (EC 2013: 11) acknowledges the essential role of vectors in biological invasions and considers it ‘crucial to manage the pathways of unintentional introduction’. The general provisions ask Member States to ‘carry out a comprehensive analysis of the pathways of unintentional introduction and spread of invasive alien species in their territory and identify the pathways which require priority action [“priority pathways”], because of the volume of species or of the damage caused by the species entering the Union through them’ (Article 11, p. 22).
Identifying the major introduction vectors is a first step to prioritize vector management efforts with the aim to reduce new species arrivals. For both dominating vectors, i.e. shipping and culture, mechanisms have been identified. In shipping, a voluntary exchange of ballast water at sea applies for vessels arriving with ballast water originating from outside Europe (DAVID & GOLLASCH 2008; HELCOM 2008). Intra ES ballast water movements are not addressed; stricter standards are considered once the International Maritime Organization (IMO) Ballast Water Management Convention enters into force. Biofouling of recreational and commercial vessels was considered by IMO, resulting in voluntary recommendations (IMO 2004; IMO MEPC 2011, 2012). For culture imports, quarantine measures are recommended by the European Council Regulation No 708/2007 of 11 June 2007, concerning use of alien and locally absent species in aquaculture. This regulation applies to NIS introductions or locally absent species (translocations) for aquaculture use and covers all aquatic species (exemptions apply) (EC 2007).
The relative importance of shipping and culture activities as the principal vectors in the Baltic and WEM is apparent in the numbers of NIS and widespread NIS. The Mediterranean is strikingly different; there the Suez Canal is the main route (GALIL 2006; 2012). Eleven widespread NIS restricted to the Mediterranean have been introduced through the Suez Canal. An analysis of the temporal and spatial patterns of introduction events, such as the one performed through AQUANIS, provides supporting evidence to legislation and management in their efforts to prevent new introductions into ES and to manage the spread of NIS already present.
Some NIS, once introduced, may spread by natural dispersal mechanisms, i.e. currents or transport by another organism of a different species (Armonies & Reise 1998; Wasson et al. 2001; Ribeiro et al. 2012). This spread, although widely recognised, requires further assessment since these natural processes may impede management efforts.
Risk and prediction of possible NIS impact
Commission Decision (EC 2010, Annex I, Part b, L 232/19) which establishes criteria and methodological standards for Good Environmental Status relevant for Directive 2008/56/EC, calls for assessment of ‘Trends in abundance, temporal occurrence and spatial distribution in the wild of non-indigenous species, particularly invasive non-indigenous species, notably in risk areas’. Our data distinguish countries with several times more NIS than others in the same sea (Fig. 2). A large number of NIS unquestionably indicates heightened risk. Yet it is accepted that we grossly underestimate the total number of NIS. As a matter of fact, data are entirely absent for many of the small-sized invertebrate phyla because of limited search efforts and erosion of taxonomic expertise. The NIS unicellular biota is underreported, though it is well established that anthropic dispersal and redistribution of propagules in ballast water and sediments and shellfish transplantation facilitate range expansions. These encompass harmful algal bloom (HAB) forming species and other microalgae, as well as other microbial loop components, including viruses, algicidic bacteria and microbial loop grazers such as protoperidinians, ciliates and other protists (Hallegraeff & Bolch 1992; Galil & Hülsmann 1997; Pierce et al. 1997; Smayda 2007). The magnitude of the gap is difficult to assess as research efforts vary greatly among taxa, habitats and locations. Data are rarely if ever gathered through standardized surveys specifically designed to detect NIS. Poorly-studied NIS taxa, NIS in poorly-studied habitats and regions, small-bodied species and additional lacunae impede our understanding of NIS diversity (Carlton 2009).
Our understanding of the impacts of marine bioinvasions is extremely limited, as most have not been quantitatively or experimentally studied over a sufficiently long term, and thus no conclusions about the percentage of NIS impacts on the marine environment can yet be drawn. The proportion of marine NIS with ‘significant negative impact’ is low because our understanding of marine ecosystems functions is constrained due to lack of study, and unless impacts are conspicuous, they fail to elicit research funding. Studies show NIS marine herbivores and predators transforming community composition and ecosystem properties through trophic cascades and changed nutrient cycling (e.g. Kornis et al. 2012), whereas ‘engineering’ NIS build structures, altering erosion regimes and changing habitat suitability for other species (Wallentinus & Nyberg 2007). However, cumulative impacts at the ecosystem level may not be readily detected and NIS populations may remain innocuous for many years before spreading and turning invasive (Crooks 2011; Essl et al. 2011).
The MSFD recognizes that ‘There is still only limited knowledge about the effects of the non-indigenous species on the environment’ (EC 2010: L 232/19); at present, we lack the knowledge to ascertain the magnitude of impacts of the great majority of marine NIS in ES either from direct observation or accurate quantitative risk assessment processes. The EC does recognize ‘that costs per IAS tend to increase in line with their spread … that many IAS are continuing their expansion and, consequently, it is reasonable to expect that the average damage per IAS will increase’ (EC SWD 2013: 321,19).
Attempts at predicting which species are more likely to be successfully introduced and thus candidates for the keenest monitoring and surveillance have been made (e.g. Catford et al. 2012). Thus far, the most reliable indicator is past performance: widespread NIS are likely to disperse further. Therefore, for the purpose of this study, we chose the geographic spread of NIS populations across ES as a proxy measurement for potential impact. About 5% of the NIS recorded in ES may be characterized as widespread, having been recorded in 10 or more countries. We are aware of the shortcomings of this measure, as some NIS may exert ‘significant negative impact on biodiversity as well as serious economic and social consequences’ (EC SWD 2013:2) but have limited spread. We argue that close monitoring of NIS richness, abundance and spatial extent may indicate whether management is effective and sufficient.
Climate-driven changes
Climate-driven changes in marine ecosystems, through modifications in physical, chemical and biological components and processes, challenge management to provide effective preparation and response. Rising temperatures and other climate-driven changes have already shifted geographic distributions of marine species by boosting thermophilic NIS (Occhipinti-Ambrogi 2007; Aronson et al. 2009; Van der Putten et al. 2010). Climate change effects on marine organisms and ecosystems are set to increase with projected changes in temperature, salinity, acidification, circulation, stratification and other parameters, though due to the complexity of marine ecosystems and non-linear interactions with climatic and non-climatic stressors, the change is far from predictable (Griffis & Howard 2013).
Climate-related changes in shipping routes (i.e. opening the Arctic route) and commercial patterns, and choice of species for mariculture will affect the diversity and frequency of introduced propagules. Climate-induced shifts in the distribution of thermophilic NIS raise the possibility of driving these NIS, now mostly restricted to tropical and subtropical seas, into temperate regions. As the world is exposed to climate change, policy and management are urgently needed to reduce non-climatic marine stressors, including bioinvasions, that may augment the resilience of regional ecosystems. It is impossible to predict whether some environmental changes may be beyond human control; however, unabated bioinvasions further constrain the choices available to us.
CONCLUSIONS
The present study provides the first comprehensive analysis of NIS data in ES based on scientifically validated data. Our results highlight the ever-rising role of shipping (commercial and recreational) as a vector for the widespread and recently spread NIS. The Suez Canal continues to act as a route enabling some Indo-Pacific and Erythraean biota to enter the warming Mediterranean Sea.
The use of the first introduction event as the basic information unit has provided a first-hand measurable metric to assess the magnitude of the NIS invasion phenomenon across biogeographic and administrative borders. It is also suggested here that the number of NIS per country (or better, the combination of country and regional sea) may provide a robust estimate of the ecological status and may be used as a baseline for future monitoring schemes and evaluating the success of species introductions management measures.
The unification of invasion terminology as well as clarification of invasion vectors requires urgent attention. In order to provide decision makers with adequate scientific knowledge, further research needs include risk evaluation methods, identification of the Good Environmental Status (including impact assessments) and effective prevention measures.
We conclude by pointing out that at the time of writing, the 2020 goal of the EU Biodiversity Strategy concerning marine Invasive Alien Species may not be fully achievable. Current knowledge regarding several important aspects related to the agreed targets is insufficient. Therefore, one of the urgent needs is to institute scientifically robust, NIS-targeting standardized quantitative monitoring. The resulting data is essential for documenting the full extent of marine bioinvasions, and for providing information for the development of competent and pragmatic management plans and effective conservation policies.
ACKNOWLEDGEMENTS
The authors thank J.T. Carlton for his much-appreciated erudite comments on an earlier version of the manuscript. The research leading to these results received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under Grant Agreement No. 266445 for the Vectors Change in Oceans and Seas marine Life, Impact on Economics Sectors (VECTORS). Contribution of HO was also supported by the Estonian Ministry of Education and Research (Grant SF0180005s10). Stephan Gollasch (VECTORS Champion for Invasive Alien Species) is thanked for the co-contribution to the WEM datasets considered in this manuscript.
NOTES
NIS may also be introduced through the Suez Canal with shipping, likely in fouling.
The proposal for Regulation of the European Parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species (EC 2013) defines ‘widely spread’ as ‘an invasive alien species whose population has gone beyond the naturalization stage, in which a population maintains a self-sustaining population, and has spread to colonize a large part of the potential range where it can survive and reproduce’ (Article 3, p. 16).
REFERENCES
- AquaNIS. World Wide Web electronic publication. 2013. Information system on aquatic non-indigenous and cryptogenic species. (Available at http://www.corpi.ku.lt/databases/aquanis) version 2.3 (Accessed 25/09/2013). [Google Scholar]
- Armonies W., Reise K. On the population development of the introduced razor clam Ensis americanus near the island of Sylt (North Sea) Helgoländer Meeresuntersuchungen. 1998;52:291–300. doi: 10.1007/BF02908903.. [DOI] [Google Scholar]
- Aronson R.B., Moody R.M., Ivany L.C., Blake D.B., Werner J.E., Glass A. Climate change and trophic response of the Antarctic bottom fauna. PLoS ONE. 2009;4(2):e4385. doi: 10.1371/journal.pone.0004385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax N., Williamson A., Aguero M., Gonzalez E., Geeves W. Marine invasive alien species: a threat to global biodiversity. Marine Policy. 2003;27:313–323. doi: 10.1016/S0308-597X(03)00041-1. [DOI] [Google Scholar]
- Carlton J.T. Biological invasions and cryptogenic species. Ecology. 1996;77(6):1653–1655. doi: 10.2307/2265767.. [DOI] [Google Scholar]
- Carlton J.T. Bioinvasion ecology: assessing invasion impact and scale. In: Leppakoski E., et al., editors. Invasive aquatic species of Europe: distribution, impacts and management. Dordrecht: Kluwer Academic Publishers; 2002. pp. 7–19. [Google Scholar]
- Carlton J.T. Deep invasion ecology and the assembly of communities in historical time. In: Rilov G., Crooks J.A., editors. Biological invasions in marine ecosystems. Berlin: Springer-Verlag; 2009. pp. 13–56. [Google Scholar]
- Carlton J.T., Ruiz G.M. Vector science and integrated vector management in bioinvasion ecology: conceptual frameworks. In: Mooney H.A., editor. Invasive alien species: a new synthesis. Covelo: Island Press; 2005. pp. 36–58. [Google Scholar]
- Catford J.A., Vesk P.A., Richardson D.M., Pyšek P. Quantifying levels of biological invasion: towards the objective classification of invaded and invasible ecosystems. Global Change Biology. 2012;18:44–62. doi: 10.1111/j.1365-2486.2011.02549.x.. [DOI] [Google Scholar]
- Crooks J.A. Lag times. In: Simberloff D., Rejmánek M., editors. Encyclopedia of biological invasions. Berkeley: University of California Press; 2011. pp. 404–410. [Google Scholar]
- David M., Gollasch S. EU shipping in the dawn of managing the ballast water issue. Marine Pollution Bulletin. 2008;56:1966–1972. doi: 10.1016/j.marpolbul.2008.09.027.. [DOI] [PubMed] [Google Scholar]
- EC. Council Regulation (EC) No 708/2007 of 11 June 2007 concerning use of alien and locally absent species in aquaculture. Official Journal of the European Union. 2007;168:1–17. [Google Scholar]
- EC. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive) Official Journal of the European Union. 2008;L 164:19–40. [Google Scholar]
- EC. Decision on criteria and methodological standards on good environmental status of marine waters. Decision 2010/477/EU. Official Journal of the European Union. 2010;L 232:14–24. [Google Scholar]
- EC. Communication from the Commission to the European Parliament, the Council, the Economic and Social Committee and the Committee of the Regions. 2011. Our Life Insurance, Our Natural Capital: an EU Biodiversity Strategy to 2020. COM/2011/244. [Google Scholar]
- EC. A marine strategy directive to save Europe's seas and oceans. Available at: http://ec.europa.eu/environment/water/marine/directive_en.htm; 2012. [Google Scholar]
- EC. Proposal for a Regulation of the European parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species. 2013. COM(2013) 620 final. 2013/0307 (COD). [Google Scholar]
- EC. EU biodiversity strategy to 2020 – towards implementation. 2014. Available at: http://ec.europa.eu/environment/nature/biodiversity/comm2006/2020.htm. [Google Scholar]
- EC SWD. Commission Staff Working Document. 2013. Executive Summary of the Impact Assessment accompanying the document ‘Proposal for a regulation of the European Parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species’. Swd (2013) 321 final. [Google Scholar]
- EESC. Opinion of the European Economic and Social Committee on the Proposal for a regulation of the European Parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species. 2014. CoM(2013) 620 final – 2013/0307 (COD). NAT/623 EESC-2013 – 06354-00-00-AC-TRA-2013/0307(COD). [Google Scholar]
- Essl F., Dullinger S., Rabitsch W., Hulme P.E., Hülber K., Jarošík V., Kleinbauer I., Krausmann F., Kühn I., Nentwig W., Vilà M., Genovesi P., Gherardi F., Desprez-Loustau M.-L., Roques A., Pyšek P. Socioeconomic legacy yields an invasion debt. Proceedings of the National Academy of Sciences. 2011;108:203–207. doi: 10.1073/pnas.1011728108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel P. Un nouveau serpulien d'eau saumatre Merceriella n.g., enigmatica n.sp. Bulletin de la Société Zoologique de France. 1923;47:424–430. [Google Scholar]
- Galil B.S. The marine caravan – The Suez Canal and the Erythrean invasion. In: Gollasch S., et al., editors. Bridging divides – Maritime canals as invasion corridors. Dordrecht: Springer; 2006. pp. 207–289. Monographiae Biologicae 83. [Google Scholar]
- Galil B.S. Truth and consequences: the bioinvasion of the Mediterranean Sea. Integrative Zoology. 2012;7:299–311. doi: 10.1111/j.1749-4877.2012.00307.x.. [DOI] [PubMed] [Google Scholar]
- Galil B.S., Hülsmann N. Protist transport via ballast water – biological classification of ballast tanks by food web interactions. European Journal of Protistology. 1997;33:244–253. doi: 10.1016/S0932-4739(97)80002-8.. [DOI] [Google Scholar]
- Gómez F. Phytoplankton invasions: comments on the validity of categorizing the non-indigenous dinoflagellates and diatoms in European Seas. Marine Pollution Bulletin. 2008;56:620–628. doi: 10.1016/j.marpolbul.2007.12.014.. [DOI] [PubMed] [Google Scholar]
- Griffis R., Howard J., editors. Oceans and marine resources in a changing climate: a technical input to the 2013 national climate assessment. Washington D.C.: Island Press; 2013. [Google Scholar]
- Hallegraeff G.M., Bolch C.J. Transport of diatom and dinoflagellate resting spores in ships’ ballast water: implications for plankton biogeography and aquaculture. Journal of Plankton Research. 1992;14:1067–1084. doi: 10.1093/plankt/14.8.1067.. [DOI] [Google Scholar]
- HELCOM. General guidance on the voluntary interim application of the D-1 ballast water exchange standard in the north east Atlantic and the Baltic Sea. 2008. p. 6. Agenda Item 2 HELCOM Baltic Sea Action Plan. Document code 2/8, from 26.2.2008. Submitted by the Executive Secretary. In: Proceedings of the HELSINKI COMMISSION, Baltic Marine Environment Protection Commission, Twenty-ninth Meeting, Helsinki, Finland, March 5–6, 2008. [Google Scholar]
- IMO [International Maritime Organization] International convention for the control and management of ships’ ballast water and sediments. 2004. p. 36. London: International Maritime Organization. [Google Scholar]
- IMO MEPC. MEPC.207(62). Guidelines for the control and management of ships’ biofouling to minimize the transfer of Invasive aquatic species. 2011. p. 25. London: International Maritime Organization. MEPC 62/24/Add.1, Annex 26, [Google Scholar]
- IMO MEPC. MEPC.1/Circ.792. Guidance for minimizing the transfer of invasive aquatic species as biofouling (hull fouling) for recreational craft. 2012. p. 5. London: International Maritime Organization. MEPC 64/23 (see Annex 5 of document BLG 16/16), [Google Scholar]
- Kornis M.S., Mercado-Silva N., Vander Zanden M.J. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology. 2012;80:235–285. doi: 10.1111/j.1095-8649.2011.03157.x.. [DOI] [PubMed] [Google Scholar]
- Lindegg G. La “Mercierella enigmatica” Fauvel nello stagno di Cabras in Sardegna. Natura, Milano. 1934;25:135–145. [Google Scholar]
- Narayanaswamy B.E., Coll M., Danovaro R., Davidson K., Ojaveer H., Renaud P.E. Synthesis of knowledge on marine biodiversity in European Seas: from census to sustainable management. PLoS ONE. 2013;8(3):e58909. doi: 10.1371/journal.pone.0058909.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhipinti-Ambrogi A. Global change and marine communities: alien species and climate change. Marine Pollution Bulletin. 2007;55:342–352. doi: 10.1016/j.marpolbul.2006.11.014.. [DOI] [PubMed] [Google Scholar]
- Occhipinti-Ambrogi A., Galil B.S. A uniform terminology on bioinvasions: a chimera or an operative tool? Marine Pollution Bulletin. 2004;49:688–694. doi: 10.1016/j.marpolbul.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ojaveer H., Galil B.S., Minchin D., Olenin S., Amorim A., Canning-Clode J., Chainho P., Copp G., Gollasch S., Jelmert A., Lehtiniemi M., McKenzie C., Mikuš J., Miossec L., Occhipinti-Ambrogi A., Pećarević M., Pederson J., Quilez-Badia G., Wijsman J., Zenetos A. Ten recommendations for advancing the assessment and management of non-indigenous species in marine ecosystems. Marine Policy. 2014;44:160–165. doi: 10.1016/j.marpol.2013.08.019.. [DOI] [Google Scholar]
- Olenin S., Narššius A., Minchin D., Galil B., Gollasch S., Marchini A., Occhipinti-Ambrogi A., Ojaveer H., Zaiko A. Biological Conservation. 2013. Making non-indigenous species information systems practical for management and useful for research: an aquatic perspective. (Available at: online 24 August 2013 http://dx.doi.org/10.1016/j.biocon.2013.07.040). [Google Scholar]
- Pederson J., Mieszkowska N., Carlton J.T., Gollasch S., Jelmert A., Minchin D., Occhipinti-Ambrogi A., Wallentinus I. Climate change and non-native species in the North Atlantic. In: Reid P.C., Valdés L., editors. ICES Cooperative Research Report. 2011. pp. 174–190. No. 310. ICES status report on climate change in the North Atlantic. [Google Scholar]
- Pierce R.W., Carlton J.T., Carlton D.A., Geller J.B. Ballast water as a vector for tintinnid transport. Marine Ecology Progress Series. 1997;149:295–297. doi: 10.3354/meps149295.. [DOI] [Google Scholar]
- Pyšek P., Richardson D.M., Pergl J., Jarošík V., Sixtová Z., Weber E. Geographical and taxonomic biases in invasion ecology. Trends in Ecology & Evolution. 2008;23:237–244. doi: 10.1016/j.tree.2008.02.002.. [DOI] [PubMed] [Google Scholar]
- Ribeiro S., Amorim A., Andersen T.J., Abrantes F., Ellegaard M. Reconstructing the history of an invasion: the toxic phytoplankton species Gymnodinium catenatum in the Northeast Atlantic. Biological Invasions. 2012;14:969–985. doi: 10.1007/s10530-011-0132-6.. [DOI] [Google Scholar]
- Simberloff D. How common are invasion-induced ecosystem impacts? Biological Invasions. 2011;13:1255–1268. doi: 10.1007/s10530-011-9956-3.. [DOI] [Google Scholar]
- Smayda T.J. Reflections on the ballast water dispersal-harmful algal bloom paradigm. Harmful Algae. 2007;6:601–622. doi: 10.1016/j.hal.2007.02.003.. [DOI] [Google Scholar]
- Van der Putten W.M., Macel M., Visse M.E. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philosophical Transactions of the Royal Society (B: Biological Sciences) 2010;365:2025–2034. doi: 10.1098/rstb.2010.0037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaque M. Checklist of the macroalgae of Thau Lagoon (Hérault, France), a hot spot of marine species introduction in Europe. Oceanologica Acta. 2001;24:29–49. doi: 10.1016/S0399-1784(00)01127-0.. [DOI] [Google Scholar]
- Wallentinus I., Nyberg C.D. Introduced marine organisms as habitat modifiers. Marine Pollution Bulletin. 2007;55:323–332. doi: 10.1016/j.marpolbul.2006.11.010.. [DOI] [PubMed] [Google Scholar]
- Wasson K., Zabin C., Bedinger L., Diaz M., Pearse J. Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biological Conservation. 2001;102:143–153. doi: 10.1016/S0006-3207(01)00098-2.. [DOI] [Google Scholar]
- Wolff W.J. Non-indigenous marine and estuarine species in the Netherlands. Zoologische Mededelingen. 2005;79:1–116. [Google Scholar]
- Wrange A.-L., Valero J., Harkestad L.S., Strand Ø., Lindegarth S., Christensen H.T., Dolmer P., Kristensen P.S., Mortensen S. Massive settlements of the Pacific oyster, Crassostrea gigas in Scandinavia. Biological Invasions. 2010;12:1145–1152. doi: 10.1007/s10530-009-9535-z.. [DOI] [Google Scholar]


