Abstract
The current paper presents a novel approach to understanding and treating addiction. Drawing from work in behavioral economics and developments in the new field of neuroeconomics, we describe addiction as pathological patterns of responding resulting from the persistently high valuation of a reinforcer and/or an excessive preference for the immediate consumption of that reinforcer. We further suggest that, as indicated by the competing neurobehavioral decision systems theory, these patterns of pathological choice and consumption result from an imbalance between two distinct neurobehavioral systems. Specifically, pathological patterns of responding result from hyperactivity in the evolutionarily older impulsive system (which values immediate and low-cost reinforcers) and/or hypoactivity in the more recently evolved executive system (which is involved in the valuation of delayed reinforcers). This approach is then used to explain five phenomena that we believe any adequate theory of addiction must address.
Keywords: Addiction, Treatment, Pathology, Behavioral economics, Neuroeconomics, Neural systems, Theory, Impulsivity, Reinforcer, Executive function, Human
“Science is facts; just as houses are made of stone, so is science made of facts; but a pile of stones is not a house, and a collection of facts is not necessarily science.”
Jules Henri Poincaré
“About thirty years ago there was much talk that geologists ought only to observe and not theorize; and I well remember someone saying that at this rate a man might well go into a gravel-pit and count the pebbles and describe the colours. How odd it is that anyone should not see that all observation must be for or against some view to be of any service.”
Charles Darwin (1861), letter to Henry Fawcett
Introduction
The above two quotes suggest that science requires a dynamic, interdependent dance between data and theory. Either one alone leaves much to be desired. A theory without facts may only be wishful thinking, while facts without a theory may only be similar to Poincaré’s pile of stones. The strong interplay of data and theory would be useful in the study of addiction. The field on occasion has collected facts and proposed theories that have been orthogonal to each other. One research domain in which that interplay of data and theory has made substantial contributions has been the application of economics to the phenomena of addiction. First, behavioral economics, the integration of concepts from psychology and economics, has provided important data about how addiction is behaviorally expressed. It has shown that addicts demonstrate extreme valuation of their addictive reinforcer and preference for receiving it in the short term. We refer to these patterns of reinforcer consumption as reinforcer pathology. Second, neuroeconomics, the integration of neuroscience, psychology, and economics, has provided a new conceptual model of addictive behavior that explains reinforcer pathology and provides novel insights into the etiology and treatment of addiction.
The overall purpose of this paper is to elucidate this concept of reinforcer pathology as it pertains to addiction. More specifically, we first fully elucidate the two general features of reinforcer pathology as they are empirically observed in addiction. Note that in the process of explicating the notion of reinforcer pathology, we do not systematically review or explicate the concepts of behavioral economics, as they are available elsewhere [1••], and that is beyond the scope and space limitations of this paper. Rather we only use those concepts that help explain the findings of studies that we think illuminate the concept of reinforcer pathology. Second, we explain a theory derived from the emerging field of neuroeconomics that provides an explanation of reinforcer pathology. Third and before concluding, we explore how this new theory can handle some of the cardinal aspects of addiction that we would expect an effective theory of addiction to address.
Two General Characteristics of Reinforcer Pathology
Reinforcer pathology, as we define it, refers to the presence of two distinct but likely interacting repertoires that tend to be at the extremes of the distribution of behaviors. More specifically, we define reinforcer pathologies as resulting from 1) the persistently high valuation of a reinforcer, broadly defined to include tangible commodities and experiences; and/or 2) the excessive preference for the immediate acquisition or consumption of a commodity despite long-term negative outcomes [1••]. Prototypical examples of reinforcer pathology include overconsumption of food and drugs either legal (e.g., cigarettes or alcohol) or illegal (e.g., cocaine or heroin). These two features of reinforcer pathology are assessed via procedures derived from behavioral economics [2, 3].
Research on the first feature of reinforcer pathology, persistently high valuation of a reinforcer, uses the sophisticated quantitative concepts and procedures of 1) own-price elasticity of demand, and 2) cross-price elasticity of demand. Own-price elasticity of demand refers to the sensitivity of a person’s consumption of a commodity (reinforcer) to the price of the commodity [4–6]. Procedurally, participants are allowed to consume, or hypothetically purchase, as much of a commodity as they choose at a given price. Typically, the effects of a variety of prices on consumption are measured, and from those effects, sensitivity of consumption to price can be discerned. This can indicate the extent to which the person values the commodity. If sensitivity to price is very limited (i.e., amount of consumption declines little in the face of rising prices, or is highly defended), then this could reflect a pathological valuation of the commodity. Cross-price elasticity of demand refers to the impact of changes in the price of one commodity on the consumption of another fixed-price commodity [7–9]. These interactions can result in substitution, in which the increases in price of one commodity (e.g., one brand of cola) increase the consumption of the other commodity (e.g., consumption of another brand of cola increases), or can result in a complementary interaction in which the increase in the price of one commodity (e.g., soup) decreases the consumption of another commodity (e.g., soup crackers). In addictions, some treatments are based on substitution (e.g., nicotine patch and cigarettes), or some patterns of pathological consumption can be related to complementary interactions (e.g., concurrent use of cocaine and alcohol).
The second feature of reinforcer pathology is studied in research on delay discounting, which refers to the observation that the value of rewards decreases as a function of delay until the time of their receipt. People intuitively understand this notion, as nearly anybody will choose to receive $1,000 now rather than 1 year from now; that is, the immediate amount has greater value and is selected because the later amount is discounted. To measure the precise extent of discounting entails varying the immediate amount until preference shifts from the immediate to the later constant amount. Thus, for example, when the immediate amount is reduced to $800, if the choice then shifts from the immediate to $1,000 option available 1 month later, we could infer that this participant discounts a $1,000 reward by 20% in 1 month. If we repeat this process across a variety of time frames, we will obtain a function describing discounting across time. The empirical results suggest that discounting is hyperbolic in form and that discounting rates may vary across individuals or commodities. As we review below, addicts excessively discount the future, and this contributes to their pathology.
Demand and Reinforcer Pathology
Studies of reinforcer consumption as a function of price (i.e., analysis of demand) have revealed important and sometimes nuanced relationships between elasticity of demand and consumption of pathological reinforcers. Saelens and Epstein [10] studied obese and nonobese women and found that consumption of food in the obese group was relatively more reinforcing compared with the nonobese group. The reinforcing-value-of-food measure also predicted the greater energy intake in the experiment by the obese group compared with the nonobese group. This predictive relationship was confirmed in a study by Epstein et al. [11]. In that study, the hedonic ratings of different foods were also studied, and reinforcing value was found to be a better predictor of food intake than the hedonic ratings [12].
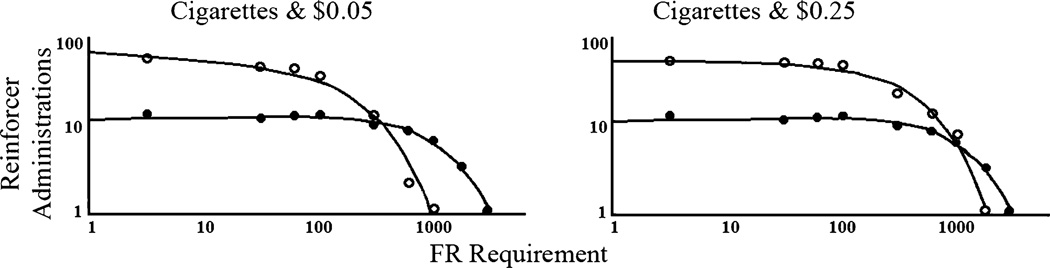
In our view, excessively high valuation of a given reinforcer and severity of reinforcer pathology are both defined by lack of diminution of consumption of the reinforcer with increases in the price of the reinforcer. Notably, individuals suffering from reinforcer pathologies typically show this insensitivity of consumption to price changes (i.e., demand inelasticity) for the substances that they abuse, but not for nonpathological reinforcers. For example, Johnson and Bickel [6] studied participants’ consumption of cigarette puffs versus monetary reinforcers. As shown in Fig. 1smokers’ consumption of cigarette puffs was considerably less elastic (less price sensitive) than either of the monetary reinforcers [9, 13, 14] These findings highlight the pathological valuation of addictive—but not nonaddictive—substances and show that price increases have differential effects on consumption of reinforcers that differ in levels of pathological severity.
Fig. 1.
Demand data for cigarette puffs (solid circles) and monetary awards (open circles) plotted on the same graph. Note the double-logarithmic axes. Consumption (self-administrations) is plotted as a function of price (fixed ratio [FR], schedule requirement). For the left panelthe monetary award amount self-administered per each reinforcer that was earned by completing an FR schedule requirement was $0.05. For the right panelthis amount was $0.25. The same cigarette puff data are plotted in the left and right panels. Greater elasticity of demand for the monetary award reinforcers compared with the cigarette puff reinforcers is illustrated by the more precipitous decline in consumption of the monetary award reinforcers to zero in spite of the greater consumption of those reinforcers at lower prices. The duplication of the cigarette puffs curve in both panels serves as a common frame of reference that reveals a greater elasticity of demand for the $0.05 reinforcers than for the $0.25 reinforcers. This illustrates the comparatively greater reinforcer value of the $0.25 reinforcers and the greater extent to which that reinforcing commodity might be described as pathological. (From Johnson MW, Bickel WK: Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav 2006, 85, 73–93. Copyright 2006 by the Society for the Experimental Analysis of Behavior, Inc.)
Discounting and Reinforcer Pathology
The science of delay discounting has been applied to the understanding of reinforcer pathology in general, and addiction in particular, and results generally suggest that the rate of delay discounting predicts several important behavioral patterns regarding pathological reinforcers. One category of results in delay discounting research bears upon the external validity of other research in the field and should be considered first. The most commonly used procedures pose to human participants several series of choice trials. In each trial, they select between two alternatives that are both hypothetical outcomes (participants know they will not actually receive any of their chosen alternatives). The use of hypothetical amounts makes it practical for the experiments to assess the discounting of rewards consisting of commodities (e.g., illicit drugs) and in large amounts (e.g., worth $1,000) typical of the real world, outside the experimental laboratory. Indeed, delay discounting studies commonly use human participants and are not constrained by these factors because they use hypothetical rewards. However, do delay discounting rates determined from choices between hypothetical reward amounts have a scientifically valid relation to discounting rates for rewards that when chosen are actually received? This issue has been explored, and the answer is affirmative. Some studies compared two types of procedures: 1) those in which all choice alternatives are hypothetical rewards, and 2) those in which the participant will receive a selection he or she makes in a single choice trial randomly selected from all choice trials in the procedure (a “potentially real rewards” procedure). The participant should in theory treat all choice trials as if they involve real rewards, as they cannot predict which choice is not hypothetical. In these studies, the assessed discounting rates from procedures using all hypothetical rewards were comparable to those from the potentially real procedures [15–20]. Moreover, Lagorio and Madden [21] used a procedure with these different conditions: 1) participants chose between immediate or delayed rewards that were all hypothetical; and 2) participants chose between alternatives that were all real, consumable rewards to be delivered immediately or after a delay. Lagorio and Madden [21] found no systematic effect of reward type (real or hypothetical). The results across these studies have led to the conclusion that assessment of delay discounting using hypothetical rewards is a practical procedure that yields valid data [15–21].
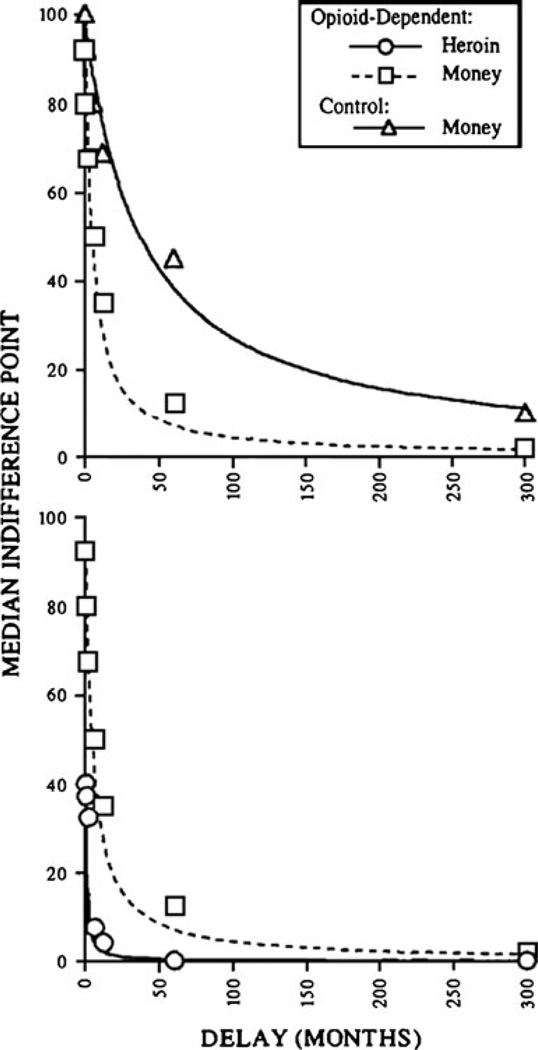
Research using delay discounting assessment procedures has determined that individuals who suffer from reinforcement pathologies discount delayed rewards more than matched controls. Figure 2 (top graph) is an illustration of this. It shows that opioid-dependent individuals discount hypothetical money more than community, nondependent, matched controls. This result has been found for opioid-dependent individuals [22, 32]; obese women [23]; pathological gamblers [24, 25]; and individuals addicted to cocaine [26, 27], alcohol [28, 29], and cigarettes [15, 18, 30]. Furthermore, evidence suggests that severity of reinforcer pathology is positively related to rates of discounting. Vuchinich and Simpson [29] observed this association in alcohol users, Bickel and colleagues [18, 30] observed it across groups of individuals with different levels of cigarette smoking activity, and Alessi and Petry [24] found it across groups assessed to have different levels of the pathological gambling malady. Although individuals’ rates of discounting for various commodities appear to be correlated [27, 31], evidence also suggests that those affected by reinforcement pathologies discount their pathological reinforcer to a greater extent than they discount other commodities. This can be seen in Fig. 2 (lower panel), in which opioid addicts are discounting a hypothetical reward of $1,000 worth of heroin at a faster rate than a hypothetical $1,000 monetary reward. This conclusion has been suggested by comparisons of discounting rates for drugs versus money conducted with participants who were opioid dependent [22, 32], cocaine dependent [26], and nicotine dependent [15, 30].
Fig. 2.
Median indifference points from hypothetical choices between large delayed and small immediate heroin and monetary rewards. The top graph shows data from opioid-dependent and control participants’ choices between monetary rewards. The bottom graph shows data from opioid-dependent participants’ choices between monetary rewards and between heroin amounts. The indifference points reflect the present value of larger, more delayed rewards (i.e., the value of delayed rewards stated in immediate-reward units). So that heroin and monetary rewards could be compared on a common axis, the vertical axis shows the percentage of the large delayed reward. Higher discounting rates are characterized by steeper overall slopes and closer proximity of the fitted curve to the axes. The top graph illustrates a greater discounting rate for monetary rewards by opioid-dependent participants than controls. The bottom graph illustrates a greater discounting rate for heroin rewards than for monetary rewards by opioid-dependent participants. (From Madden GJ, Petry NM, Badger GJ, Bickel WK: Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and Clinical Psychopharmacology 1997, 5, 256–262. Published by the American Psychological Association. Reprinted with permission.)
Interactions Between Demand and Discounting Approaches
Going forward, the recognition of the related nature of the two scientific approaches to reinforcer pathology should prompt studies and conceptualizations that integrate the two approaches [3]. The theoretical nexus of these two approaches derives from the notion of substitutes, as noted earlier. The elasticity of demand (sensitivity of consumption to price) is determined in part by the availability of substitutes. For instance, an individual might choose to not buy one commodity at a higher price if he or she can get a substitute elsewhere at a lower price. Such substitution would result in greater elasticity of demand for the first commodity. One source of substitution is intertemporal (e.g., the individual might not choose to purchase a commodity today at a higher price if he knows he can get it next week at a lower price). Now, if the consumer of a commodity excessively discounts the future, then this would functionally decrease the availability of intertemporal substitutes, which in turn should result in less price sensitivity. This synergistic interaction between these two processes may drive the price insensitivity to a point that characterizes reinforcer pathology. This interplay between immediate reinforcers and their potential substitutes merits exploration via the use of measures of demand and discounting in the same study. For example, MacKillop et al. [34] obtained measures of alcohol demand and discounting rates for delayed rewards among their sample of heavy drinkers. They found that the measures of demand intensity (a particular demand analysis measure) and delay discounting were correlated. MacKillop and Tidey [31] also used measures from both approaches in a study of nicotine dependence among schizophrenics. They determined that demand measures distinguished schizophrenics from controls, while delay discounting rates did not. In contrast, Rollins et al. [35] found that the relative reinforcing value of food predicted ad libitum eating in high discounters, but not low discounters. These studies are examples of methodologically closing the gap between the two approaches to reinforcement pathology, and only additional data will suggest whether the theoretical relationship outlined above is empirically supported.
The Competing Neurobehavioral Decision Systems Theory
During the past 20 years, major technological advances have refined the tools available to neuroscientists. Harnessing these tools, neuroscientists have ventured into new research areas, expanding our understanding of many phenomena. For example, the development of functional MRI has enabled the precise measurement of brain activity as humans perform experimental tasks [36].
These refined methods in part birthed neuroeconomicsa discipline that melds the concepts and tools of economics, psychology, and neuroscience to explore the neural substrates of economic choices [37]. Neuroeconomic data may be central to understanding the choices made by individuals suffering from reinforcement pathologies. For example, McClure and colleagues [33, 38] found that when participants completed delay discounting tasks, choices for the smaller, more immediate reinforcer were associated with relatively high levels of activation in parts of the limbic system (e.g., paralimbic cortex), whereas choices for the larger, later reinforcer were associated with relatively high levels of activation in parts of the prefrontal cortex (e.g., lateral prefrontal cortex, posterior parietal cortex).
These findings [33, 38] further enabled the development of the competing neurobehavioral decision systems theory (hereafter referred to as competing decision systems [1••, 40, 41, 41••]). The competing decision systems view posits that choices between immediate and delayed reinforcers are related to the regulatory balance of activation in two neural systems. The evolutionarily older impulsive system, which consists of portions of the limbic and paralimbic areas, is primarily involved in the valuation of immediate rewards. In contrast, the more recently developed executive system, which consists of portions of the prefrontal cortices, is involved in the consideration of the future and the selection of delayed rewards. The most straightforward and widely studied manifestation of these competing decision systems has involved performance on delay discounting tasks [16, 33, 38, 42, 43] (reviewed in [44]); however, demand also appears to be associated with activation in the impulsive system [45]. According to the competing decision systems view, the rate that an individual discounts delayed rewards reflects the relative strength of these two systems. Thus, the patterns of responding associated with reinforcer pathologies (e.g., drug addiction [1••, 41••, 44]) are related to a hyperactive impulsive and/or a hypoactive executive system.
Support for the Competing Decision Systems Theory
Evidence of the Impulsive System's Role in Valuation
Data suggest that a hyperactive impulsive system is involved in the pathological valuation of immediate and/ or low-cost reinforcers. Like McClure et al. [33, 38], several studies have found that activation in the impulsive system is associated with the valuation of reinforcers [16, 42, 43, 46, 47], and that this activation is diminished as reinforcers are delayed [42, 43]. A similar pattern of activation is seen for low-versus high-cost reinforcers. For example, Croxson and colleagues [45] found more activation in the impulsive system when participants were presented with cues indicating that they would earn reinforcers at a lower unit price than at a higher unit price.
Evidence of the Executive System's Role in Valuation
A convergence of multiple types of evidence suggests that a hypoactive executive system is involved in pathological reinforcer choice. First, also like McClure and colleagues [33, 38], functional neuroimaging studies have found that the valuation of delayed reinforcers is related to activity in the executive system [46, 47]. Second, like decisions between immediate and delayed reinforcers, decisions between reinforcers available at different costs result in elevated levels of activation in the executive system [48, 49]. Third, the executive systems of individuals suffering from reinforcer pathologies such as drug addiction typically have lower cortical volume and gray matter density than do controls [50, 51]. Finally, lesions in the executive system result in substantial impairments in decision making, such as the inability to change future behavior based on the negative feedback provided for previous behavior [52].
Manipulation of Competing Decision Systems
The competing decision systems theory is further supported by studies that experimentally manipulate activity in these brain regions. For example, subjective reports of drug craving are associated with elevated levels of activation of the impulsive system [53]. Not surprisingly, opioid addicts discount delayed reinforcers at higher rates during periods of deprivation than when they have had access to buprenorphine [54]. Thus, the elevated impulsive system activation associated with deprivation disrupts the balance between these competing decision systems, resulting in elevated rates of discounting.
Manipulating the executive system can have similar effects on delay discounting rates. For example, Hinson and colleagues [55] found that discounting rates were higher when study participants were required to concurrently complete a working memory task. Thus, an executive system taxed through its concurrent use in other tasks ineffectively competes with the impulsive system, resulting in elevated rates of discounting.
Types of Dysregulation
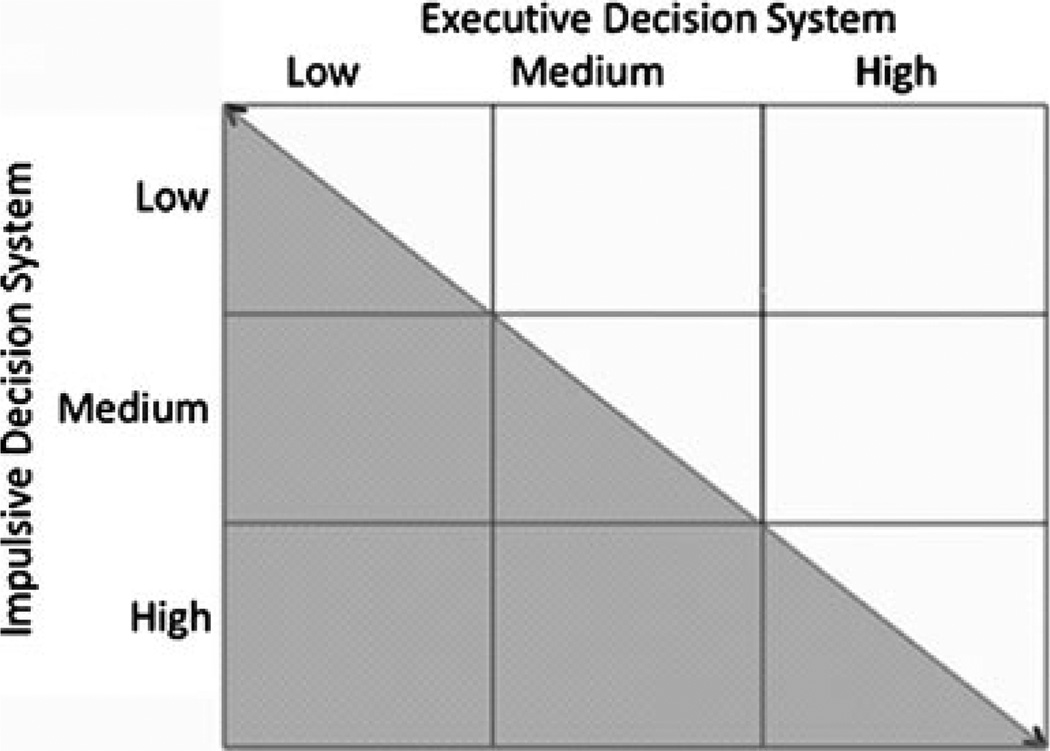
Individuals are at risk of developing reinforcer pathologies whenever the strength of the impulsive system exceeds that of the executive system [41••], as illustrated by the shaded regions of Fig. 3. There are, however, several ways that the executive and impulsive systems can be out of regulatory balance. For example, an individual with a weak executive and a weak impulsive system (upper left corner) runs the same risk of developing reinforcer pathologies as an individual with a strong executive and a strong impulsive system (lower right corner). The specifics of these types of dysregulation, however, suggest different courses of action. Specifically, the individual with weak executive and impulsive systems would benefit from strengthening the executive system [56••], whereas weakening the impulsive system may be more beneficial for the individual with strong executive and impulsive systems.
Fig. 3.
Potential interactions between differing strengths of the impulsive and executive decision systems. Note that the shaded area indicates the risk of addiction. (From Bickel WK, Mueller ET, Jarmolowicz DP: What is addiction? In Addictions: A Comprehensive Guidebook, Second Edition. Edited by McCrady B, Epstein E. 2011 (in press) Copyright 2011 by and by permission of Oxford University Press, Inc. [http://www.oup.com].)
Critiques of the Competing Decision Systems View
Some researchers disagree with the competing decision systems view [42, 43, 57], contending that the impulsive system values all reinforcers, immediate or delayed. For example, Kable and Glimcher [42] had 12 individuals complete three 1-hour delay discounting sessions wherein the individuals made a series of choices between responding to receive a variable but specified amount of money (i.e., $20.25–$110.00) at a variable but specified delay (i.e., 180 s–6 h), or omitting a response to receive $20 immediately. The 10 individuals who demonstrated stable rates of discounting during these initial sessions then participated in a functional MRI session wherein they completed the same behavioral task. They found that activation in the impulsive system tracked reinforcer value at all delays but found little support for the executive system. These findings were replicated in a follow-up study [43] that measured brain activation as 22 participants (4 of whom came from the previous experiment [42]) completed a modified version of the behavioral task that required them to choose actively between immediate or delayed rewards [43]. The studies by Kable and Glimcher [42, 43], however, entailed procedural choices that may have obscured the influence of the executive system. In their first study, only the delayed option was presented, leaving participants to indicate if they were willing to forgo the implicit immediate amount (i.e., $20) in favor of the delayed amount [42]. This may have lessened the influence of the executive system by discouraging the consideration of the value of each reward. Furthermore, only participants who exhibited stable discounting participated in the imaging component of the study. Because stable responding may be somewhat automatic, this procedural choice may have underplayed the role of the executive system. Their follow-up paper [43] not only used stable discounters but also selectively invited the participants with the highest discounting rates during the previous study [42] to participate. Consistent with the competing decision systems theory, the executive system had little influence on these individuals’ high rates of discounting.
New Theory and Addiction
Several persistent questions remain in the study of addiction. Any effective theory of addiction should answer the following questions [41••, 58, 59]:
Why are some commodities addictive, whereas others are not?
Why do people who excessively use addictive substances have difficulty reducing their use or becoming abstinent even though they recognize that their addiction is problematic?
Why does addiction follow a predictable developmental course consisting of an increase in the consumption of addictive commodities during adolescence and a decrease as individuals age (commonly called maturing out)?
Why does addiction tend to commonly co-occur with a range of other unhealthy behaviors?
Does this theory directly inform effective treatment?
Bickel and colleagues [41••] maintain that any theory that cannot explain all these observations in addiction is incomplete. The competing decision systems theory answers all the above questions. First, reinforcers with high addiction potential generally have a rapid onset of effects and a short time to peak effect [1••, 41••]. These sorts of reinforcers typically result in high levels of activation in the impulsive decision system and its associated brain regions [60].
Second, individuals suffering from drug addiction tend to continue their excessive use of harmful substances despite the recognition of problematic consequences. The competing decisions system view posits that this occurs because of an ongoing disruption in the regulatory balance between the executive and impulsive systems. Specifically, the relatively strong impulsive decision system drives continued consumption, whereas the relatively weak executive system fails to value deferred outcomes associated with abstinence. This perspective is supported by studies showing that excessive discounting among smokers predicts relapse in a laboratory setting [61] and poorer response in smoking cessation treatment [62], and also by studies that demonstrate that cognitive deficits among cocaine addicts predict treatment attrition [63].
Third, the competing decision systems view accounts for the developmental course observed in addiction wherein individuals typically try drugs in their teens and often “mature out” of drug abuse as they get older. Specifically, the impulsive system matures in the early- to mid-teens, whereas the executive system does not typically reach full maturity until the mid-20s [64]. Consistent with this uneven development in these brain systems, discounting rates tend to be higher in adolescence than in early-adulthood. Because elevated discounting rates are associated with drug abuse [15, 22, 25–30, 32], it is not surprising that many individuals first try drugs during this vulnerable period [65]. Conversely, the tendency of individuals to stop using drugs as they get older (i.e., mature out) is consistent with the observed age-related decline in impulsive system function [66] and the tendency of discounting rates to decrease as individuals age [67].
Fourth, the competing decision systems theory explains why individuals suffering from one addiction often suffer from other forms of reinforcement pathology (i.e., gambling [25]). The Shaffer et al. [68] syndrome model of addiction posits that like other syndromes, addictions are a manifestation of an abnormal underlying condition. Hence, pathological consumption of multiple problematic reinforcers (i.e., comorbidities) is due to this underlying condition. The competing decision systems theory holds that this abnormal underlying condition is an imbalance between the executive and impulsive systems. Similarly, Bickel and Mueller [69] suggested that excessive discounting represents a trans-disease process (i.e., a process operates across multiple disorders [e.g., addictions], making findings from one disorder relevant to other disorders), rendering pathological consumption of various addictive reinforcers as manifestations of this trans-disease process. According to the competing decision systems viewpoint, this underlying process is a disruption in the regulatory balance between the executive and impulsive systems.
Lastly, Bickel and colleagues [56] recently published preliminary evidence suggesting that the competing decisions system theory may be a robust theory of addiction, particularly with respect to how the theory may directly inform therapeutic approaches. Specifically, they examined efforts to improve aspects of the executive system among the addicted. In this study, stimulant addicts receiving treatment at a local facility were randomly assigned to receive active working memory training or control training. Delay discounting rates were assessed before and after working memory training. The interesting results of this study included significant decreases in delay discounting in the group that received active working memory training, but no significant changes in delay discounting in the group that received control training. In addition, the findings report a negative correlation between discounting rates and performance scores on the memory training measures. From the viewpoint of the competing decisions systems theory, these preliminary findings are encouraging in terms of identifying and testing new therapeutic approaches.
The above findings suggest an innovative intervention for changing the valuation of delayed reinforcers. This was the first study to report that neurocognitive training of working memory yielded significant decreases in delay discounting. Higher discounting rates reliably observed when addicts are compared to matched control individuals fit with the hypothesis that addiction is the result of hypoactive executive and hyperactive impulsive systems. Furthermore, this new finding that decreased delay discounting was observed only after active working memory training is consistent with the notion of an increase in the relative activation of the executive function system. These results, however, must be interpreted with caution given the preliminary nature of the above study; future studies examining the impact of neurocognitive training on substance use outcomes will prove informative.
The reinforcement pathologies resulting from dysregulation in the competing decision systems can predict treatment outcomes and may inform new therapeutic approaches. Consistent with the notion that rates of delay discounting reflect the regulation of the executive and impulsive systems [1••, 39–41••, 44], discounting rates are predictive of treatment outcomes [11, 62, 63, 70, 71]. For example, low rates of discounting predict higher rates of success in substance abuse treatment programs [62, 63] as well as engagement in treatment program activities such as voucher redemption [70]. Conversely, excessive discounting is predictive of unhealthy behavioral patterns such as relapse [71]. Similarly, demand for a given reinforcer can also predict the consumption of that reinforcer. For example, demand for food predicts the amount that participants will eat when given free access to food [11]. Thus, just as pathological discounting of delayed reinforcers predicts unhealthy choices, pathological valuation of reinforcers also predicts consumption of these reinforcers.
As noted above, a recent study found that these two aspects of reinforcement pathology interact to predict responding. Rollins et al. [35] found that their participants’ motivation to eat, as measured by demand for edible reinforcers, interacted with their rate of discounting to predict the amount of food that they would eat on an ad libitum eating task. Specifically, demand for edible reinforcers predicted the amount of food that high discounters would eat when given free access to food, but not participants who discounted at a lower rate. This study represents an interesting step toward integrating the study of these two aspects of reinforcement pathology.
Conclusions
In this review, we have shown how behavioral economics and neuroeconomics have contributed to a new conceptual–empirical understanding of addiction. We reviewed how behavioral economics has identified important empirical features of addictive behavior that we term reinforcement pathologies. More specifically, we reviewed the two constituent processes of reinforcement pathology: 1) the persistent high valuation of a commodity or substance, and 2) the preference for the immediate acquisition and/or consumption of that commodity despite long-term negative outcomes [1••]. Additionally, on the basis of our economic understanding of these two processes, we expect that they act in a way that may be synergistic, and perpetuate addictive features. The notion of reinforcer pathologies provides a means to organize and illustrate commonalities across addictions that may demonstrate the importance of trans-disease processes [69].
We also showed how neuroeconomics has provided an understanding of the conceptual model that underlies the behavior associated with reinforcer pathology. That model, referred to as the competing neurobehavioral decision systems approachprovides an understanding of addiction that allows it to address and account for at least five features of addiction that we would expect any conceptual or theoretical model to address. Moreover, by suggesting an important role of a hypoactive executive decision system in addiction, it has suggested a new approach to the treatment of addiction: executive function therapy. The first limited exploration of that approach conducted with working memory training has suggested that such training can result in greater valuation of the future. Additional findings will be necessary to replicate and extend those observations, but perhaps the most important test of the utility of this approach is related to treatment. Can the use of these new procedures truly enhance the outcomes associated with the treatment of addiction? If so, then the utility of this new approach will be supported. Answering this and many other important questions will permit a deeper understanding of the significance and power of behavioral economics and neuroeconomics for addiction and could suggest that they have an important role in the prevention and treatment of reinforcement pathologies.
Acknowledgments
Dr. Bickel has received grant support from the National Institutes of Health/National Institute on Drug Abuse (grant nos. 5 R01 DA012997-11 [principal investigator], 1 R01 DA024080-01A1 [principal investigator], 1 R01 DA026817-01A1 [co–principal investigator], and 1 R01 DA022386-01A1 [co-investigator]) and the National Institute on Alcohol Abuse and Alcoholism (grant no. 3R01DA024080-02S1).
Footnotes
Disclosure Dr. Bickel has received honoraria for presentations made at the Providence Regional Medical Center’s 13th Annual Fundamentals of Addiction Medicine Conference, the Duke Institute for Brain Sciences Addiction Research Group Seminar Series, the Association for Behavioral Analysis International Behavioral Economics Conference, and at Texas A&M University (colloquia speaker).
Dr. Jarmolowicz, Dr. Mueller, and Ms. Gatchalian reported no potential conflicts of interest relevant to this article.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1. Bickel WK, Jarmolowicz DP, MacKillop J, et al. The behavioral economics of reinforcement pathologies. In: Shaffer HJ, editor. Addiction syndrome handbook. Washington, DC: American Psychological Association; 2011. This chapter provides an extended description of the concept of reinforcer pathologies as applied to addiction.
- 2.Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rachlin H. Diminishing marginal value as delay discounting. J Exp Anal Behav. 1992;57:407–415. doi: 10.1901/jeab.1992.57-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel WK, DeGrandpre RJ, Higgins ST. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend. 1993;33:173–192. doi: 10.1016/0376-8716(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 5.Bickel WK, DeGrandpre RJ, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. II. A unit-price analysis of cigarette smoking. J Exp Anal Behav. 1991;55:145–154. doi: 10.1901/jeab.1991.55-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav. 2006;85:73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology. 1995;118:250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- 8.DeGrandpre RJ, Bickel WK, Higgins ST, Hughes JR. A behavioral economics analysis of concurrently available money and cigarettes. J Exp Anal Behav. 1994;64:191–201. doi: 10.1901/jeab.1994.61-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MW, Bickel WK. The behavioral economics of cigarette smoking: the concurrent presence of a substitute and an independent reinforcer. Behav Pharmacol. 2003;14:137–144. doi: 10.1097/00008877-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 11.Epstein LH, Wright SM, Paluch RA, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav. 2004;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Petry NM. Effects of increasing income on polydrug use: a comparison of heroin, cocaine and alcohol abusers. Addiction. 2000;95:705. doi: 10.1046/j.1360-0443.2000.9557056.x. [DOI] [PubMed] [Google Scholar]
- 13.Bickel WK, Madden GJ, DeGrandpre RJ. Modeling the effects of combined behavioral and pharmacological treatment on cigarette smoking: behavioral-economic analyses. Exp Clin Psychopharmacol. 1997;5:334–343. doi: 10.1037//1064-1297.5.4.334. [DOI] [PubMed] [Google Scholar]
- 14.Shahan TA, Bickel WK, Badger GJ, Giordano LA. Sensitivity of nicotine-containing and de-nicotinized cigarette consumption to alternative non-drug reinforcement: a behavioral economic analysis. Behav Pharmacol. 2001;12:277–284. doi: 10.1097/00008877-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- 16.Bickel WK, Pitcock JA, Yi R, Angtuaco EJC. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol. 2007;15:187–194. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- 19.Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- 20.Madden GJ, Raiff BR, Laforio CH, et al. Delay disocounting of potentially real and hypothetical rewards: II. Between-and within-subject comparison. Exp Clin Psychopharmacol. 2004;12:251–256. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- 21.Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behav Process. 2005;69:173. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 23.Weller RE, Cook III EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Process. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 25.Petry NM, Casarella R. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug and Alcohol Depend. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 26.Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Bickel WK, Christensen DR, Landes RD, et al. : Single and cross commodity among cocaine addicts: the commodity and its temporal location determine discounting rate. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2272-x. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 29.Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 30.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 31.MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology. 2011;216:91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- 33.McClure SM, Ericson KM, Laibson DI, et al. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKillop J, Miranda R, Monti PM, et al. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol. 2010;119:106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raichle ME. A brief history of human brain mapping. Trends Neurosci. 2009;32:118–126. doi: 10.1016/j.tins.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Glimcher PW, Camerer C, Poldrack RA, Fehr E. Neuro-economics decision making and the brain. London: Academic; 2008. [Google Scholar]
- 38.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 39.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 40.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 41. Bickel WK, Mueller ET, Jarmolowicz DP. In: What is addiction? In Addictions: A Comprehensive Guidebook. Second Edition. McCrady B, Epstein E, editors. 2011. 2011. This chapter provides an extended description of the competing neurobehavioral decisions systems theory, its genesis, and its adequacy in explaining relevant features of addiction.
- 42.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kable JW, Glimcher PW. An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. J Neurophysiol. 2010;103:2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bickel WK, Miller ML, Yi R, et al. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90S:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croxson PL,Walton ME, O’Reilly JX, et al. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monterosso JR, Ainslie G, Xu JS, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L, Liang ZY, Wang K, et al. Neural mechanism of intertemporal choice: from discounting future gains to future losses. Brain Res. 2009;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 48.Prevost C, Pessiglione M, Metereau E, et al. Separate valuation subsystems for delay and effort decision costs. J Neurosci. 2010;30:14080–1490. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wunderlich K, Rangel A, O’Doherty JP. Neural computations underlying action-based decision making in the human brain. Proc Natl Acad Sci USA. 2009;106:17199–17204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pezawas LM, Fischer G, Diamant K, et al. Cerebral CT findings in male opioid-dependent patients: stereological, planimetric and linear measurements. Psychiatr Res. 1998;83:139–147. doi: 10.1016/s0925-4927(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Hao Y, Kaneko Y, et al. Frontal and cingulate gray matter volume reduction in heroin dependence: optimized voxel-based morphometry. Psychiatry Clin Neurosci. 2009;63:563–568. doi: 10.1111/j.1440-1819.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 52.Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]
- 53.Risinger RC, Salmeron BJ, Ross TJ, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 54.Giordano L, Bickel WK, Loewenstein G, et al. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- 55.Hinson JM, Jameson TL,Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- 56. Bickel WK, Yi R, Landes RD, et al. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatr. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. This recent paper provides an example of a novel treatment approach derived from the competing neurobehavioral decisions systems appraoch to addiction.
- 57.Monterosso JR, Luo S. An argument against dual valuation system competition: cognitive capacities supporting future orientation mediate rather than compete with visceral motivation. Journal of Neuroscience, Psychology, and Economics. 2010;3:1–14. doi: 10.1037/a0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards G, Lader M. The nature of drug dependence. Oxford: Oxford University Press; 1990. [Google Scholar]
- 59.Peele S. What treatment for addiction can do and what it can’t; what treatment for addiction should do and what it shouldn’t. J Subst Abuse Treat. 1985;2:225–228. doi: 10.1016/0740-5472(85)90005-4. [DOI] [PubMed] [Google Scholar]
- 60.Volkow ND, Fowler JS, Wang GJ, et al. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 61.Mueller ET, Bickel WK, Landes RD. In 72nd Annual Meeting of The College on Problems of Drug Dependence. Scottsdale, Arizona: 2010. Smoker’s delay discounting indifference points are associated with changes in opportunity-cost- informed price. [Google Scholar]
- 62.MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aharonovich E, Hasin DS, Brooks AC, et al. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 65.Johnson RA, Gerstein DR. Initiation of use of alcohol, cigarettes, marijuana, cocaine, and other substances in US birth cohorts since 1919. Am J Public Health. 1998;88:27–33. doi: 10.2105/ajph.88.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hintzen AK, Cramer J, Karagulle D, et al. Does alcohol craving decrease with increasing age? Results from a cross-sectional study. J Stud Alcohol Drugs. 2011;72:158–162. doi: 10.15288/jsad.2011.72.158. [DOI] [PubMed] [Google Scholar]
- 67.Green L, Myerson J, Lichtman D, et al. Temporal discounting in choice between delayed rewards: the role of age and income. Psychol Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- 68.Shaffer HJ, LaPlante DA, LaBrie RA, et al. Toward a syndrome model of addiction: multiple expressions, common etiology. Harv Rev Psychiatr. 2004;12:367–374. doi: 10.1080/10673220490905705. [DOI] [PubMed] [Google Scholar]
- 69.Bickel WK, Mueller ET. Toward the study of trans-disease processes: a novel approach with special reference to the study of co-morbidity. Journal of Dual Diagnosis. 2009;5:131–138. doi: 10.1080/15504260902869147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bickel WK, Jones BA, Landes RD, et al. Hypothetical inter-temporal choice and real economic behavior: delay discounting predicts voucher redemptions during contingency-management procedures. Exp Clin Psychopharmacol. 2010;18:546–552. doi: 10.1037/a0021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller ET, Landes RD, Kowal BP, et al. Delay of smoking gratification as a laboratory model of relapse: effects of incentives for not smoking, and relationship with measures of executive function. Behav Pharmacol. 2009;20:461–473. doi: 10.1097/FBP.0b013e3283305ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]