Abstract
Clinical observations suggest that post-menopausal women have a higher incidence of aneurysmal rupture than premenopausal women. We hypothesize that a relative deficiency in estrogen may increase the risks for aneurysmal growth and subarachnoid hemorrhage in post-menopausal women. We assessed the effects of estrogen and selective estrogen receptor subtype agonists on the development of aneurysmal rupture in ovariectomized female mice. We utilized an intracranial aneurysm mouse model that recapitulates the key features of human intracranial aneurysms, including spontaneous rupture. Ten- to twelve-week-old ovariectomized female mice received treatment with estrogen, non-selective estrogen receptor antagonist, estrogen receptor-α agonist, or estrogen receptor-β agonist starting 6 days after aneurysm induction so that the treatments affected the development of aneurysmal rupture without affecting aneurysmal formation. Estrogen significantly reduced the incidence of ruptured aneurysms and rupture rates in ovariectomized mice. Non-selective estrogen receptor antagonist abolished the protective effect of estrogen. Though estrogen receptor-α agonist did not affect the incidence of ruptured aneurysms or rupture rates, estrogen receptor-β agonist prevented aneurysmal rupture without affecting the formation of aneurysms. The protective role of estrogen receptor-β agonist was abolished by the inhibition of nitric oxide synthase. We showed that estrogen prevented aneurysmal rupture in ovariectomized female mice. The protective effect of estrogen appeared to occur through the activation of estrogen receptor-β, a predominant subtype of estrogen receptor in human intracranial aneurysms and cerebral arteries.
Keywords: Intracranial aneurysm rupture, estrogen, menopause, animal model
Introduction
Clinical observations suggest that post-menopausal women have a higher incidence of aneurysmal subarachnoid hemorrhage than pre-menopausal women.1 In addition, hormone replacement regimens that contain estrogen appear to reduce the risk for subarachnoid hemorrhage in post-menopausal women.2 These epidemiological observations suggest the potentially protective role of estrogen against the development of aneurysmal rupture in post-menopausal women.1, 3 Experimental studies using a rat model of intracranial aneurysms indicate the protective effect of estrogen against the formation of aneurysms.4, 5 However, no experimental study has sought to establish a direct link between estrogen and the prevention of aneurysmal rupture.
In this study, we assessed the effects of estrogen and selective estrogen receptor subtype agonists on the development of aneurysmal rupture in ovariectomized female mice. Ovariectomized female mice were used to mimic the conditions of post-menopausal women. We sought to investigate the receptor subtype and the underlying mechanisms responsible for the potentially protective effect of estrogen against the development of aneurysmal subarachnoid hemorrhage in post-menopausal women. We utilized an intracranial aneurysm mouse model that recapitulates the key features of human intracranial aneurysms, including spontaneous rupture.6–8
Methods
Experiments were conducted in accordance with the guidelines approved by the University of California, San Francisco, Institutional Animal Care and Use Committee. We combined induced systemic hypertension (deoxycorticosterone acetate-salt hypertension) and a single injection of elastase into the cerebrospinal fluid at the right basal cistern as previously described.6–8. Bilateral ovariectomy or sham ovariectomy was performed one week prior to aneurysm induction. Detailed methods are presented in Online Data Supplements.
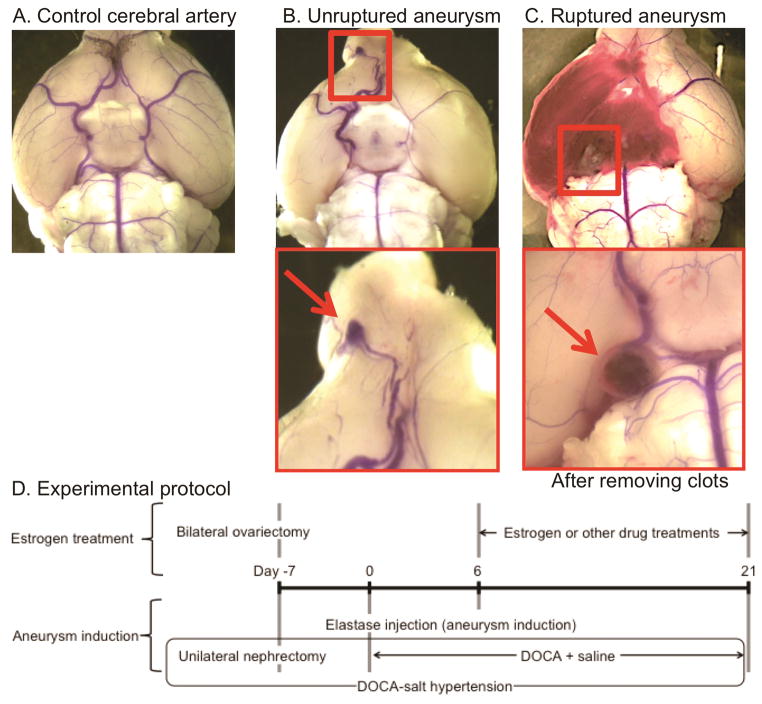
To detect aneurysmal rupture, two blinded observers performed daily neurological examination as previously described.7 Neurological symptoms were scored as follows: 0: normal function; 1: reduced eating or drinking activity demonstrated by a weight loss greater than two grams of body weight (approximately 10% weight loss) over 24 hours; 2: flexion of the torso and forelimbs upon lifting the whole animal by the tail; 3: circling to one side with a normal posture at rest; 4: leaning to one side at rest; and 5: no spontaneous activity. Mice were euthanized when they developed neurological symptoms (score 1–5). All asymptomatic mice were euthanized 21 days after aneurysm induction. The brain samples were perfused with phosphate-buffered saline, followed by a gelatin containing blue dye to visualize cerebral arteries. Aneurysms were defined as a localized outward bulging of the vascular wall, whose diameter was greater than the parent artery diameter.6, 8 Figures 1A–1C show a representative mouse with normal cerebral arteries, an unruptured aneurysm from a mouse that was asymptomatic throughout the experimental period, and a ruptured aneurysm with subarachnoid hemorrhage from a mouse that became symptomatic 10 days after aneurysm induction.
Figure 1.
A–C. Representative intracranial aneurysms in mice. A: Normal cerebral artery. B: Unruptured aneurysm in the anterior cerebral artery. C: Ruptured aneurysm with subarachnoid hemorrhage. D. Experimental protocol to study the protective role of estrogen against the development of aneurysmal rupture. DOCA: deoxycorticosterone acetate
Our previous study found that aneurysm formation occurs during the first 6 days after aneurysm induction in this model and that aneurysmal rupture begins to occur approximately 7 days after the aneurysm induction.7 Therefore, in this study, the treatments with estrogen (17β-estradiol, 0.17/mg/kg/day), non-selective estrogen receptor antagonist (ER antagonist: ICI-182780: 3 mg/kg/day),9 propyl pyrazole triol (estrogen receptor-α agonist: PPT, 0.17/mg/kg/day), or diarylpropionitrile (estrogen receptor-β agonist: DPN, 0.17/mg/kg/day) were started 6 days after aneurysm induction so that the treatments affected the development of aneurysmal rupture without affecting aneurysmal formation (Figure 1D). Dosages of estrogen and ER agonists, and ER antagonist were chosen based on previous publications.10–15
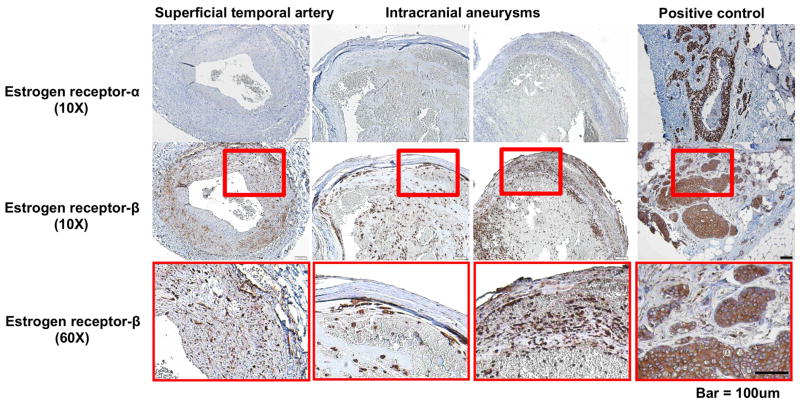
Human intracranial aneurysm and superficial temporal artery tissues were collected according to the protocol approved by the University of Iowa Institutional Review Board. Three aneurysm tissues and one superficial temporal artery tissues were collected and stained with anti-estrogen receptor-α antibody (1D5, Dako, Carpinteria, CA, USA) or anti-estrogen receptor-β antibody (Ab3577, Abcam, Cambridge, MA, USA). Two blinded observers counted estrogen receptor positive cells in randomly chosen areas. Semi-quantitative analysis of the slides was performed based on the immunostained positive cell counts per high-power field (40X): grade 0 = 0–10 cells; grade 1 = 10–20 cells; grade 2 = 20–30 cells; grade 3 = greater than 30 cells.
Statistical Analysis
We used Fisher’s exact test to analyze the incidence of ruptured intracranial aneurysms and the rupture rate (number of mice with ruptured aneurysm/number of mice with ruptured or unruptured aneurysms). As an exploratory analysis, the survival analysis was performed using the Log-rank test. Mice that did not develop aneurysms were excluded from the survival analysis. All of the results were expressed as the mean ± SD. P < 0.05 was considered statistically significant.
Results
Expression of estrogen receptors in human intracranial aneurysms
We collected three human intracranial aneurysm tissues and a control artery (superficial temporal artery) from female patients underwent aneurysms clipping. Positive control tissues exhibited strong expression of both estrogen receptor-α and estrogen receptor-β (Figure 2). In the control artery, estrogen receptor-β was expressed in the smooth muscle layers, but estrogen receptor-α was almost absent (Figure 2). Similarly, there was no significant expression of estrogen receptor-α in the aneurysmal wall. Estrogen receptor-β was expressed in the smooth muscle layers and organized thrombus of the intracranial aneurysms (Figure 2). Supplementary table S1 shows the clinical characteristics and the semi-quantitative grading of estrogen receptor-α and estrogen receptor-β positive cells.
Figure 2. Estrogen receptors in intracranial aneurysms and control arteries in humans.
Though estrogen receptor-α was almost absent in both human intracranial aneurysms and superficial temporal arteries, estrogen receptor-β was highly expressed in smooth muscle cell layers of the human intracranial aneurysms and superficial temporal arteries.
Effects of estrogen on the development of aneurysmal rupture in ovariectomized mice
To test whether estrogen is protective against the development of aneurysmal rupture in ovariectomized mice (i.e., post-menopausal mice), we treated ovariectomized mice with estrogen (17β estradiol), vehicle, or estrogen + non-selective estrogen receptor antagonist (ICI-182780) starting at 6 days after aneurysm induction. The treatments were continued for 2 weeks.
Treatments with estrogen or estrogen + non-selective estrogen receptor antagonist started 6 days after aneurysm induction did not significantly affect the formation of aneurysms, as demonstrated by no difference in the total incidence of aneurysms (i.e., the incidence of both ruptured and unruptured aneurysms) among the three groups (Figure 3A). However, compared to the vehicle treatment, the estrogen treatment significantly reduced both the incidence of ruptured aneurysms (Figure 3A; vehicle control vs. estrogen 63% vs. 18%, P < 0.01) and the rupture rate (Figure 3B; vehicle control vs. estrogen 86% vs. 23%, P < 0.01) in ovariectomized mice. For the purpose of exploratory analysis, a symptom-free curve (Kaplan-Meier analysis curve) was plotted after excluding mice that did not have aneurysms (Figure 3C). A log-rank test revealed a significant reduction of aneurysmal rupture with the estrogen treatment (P < 0.01).
Figure 3. Estrogen protected against the development of aneurysmal rupture in ovariectomized female mice.
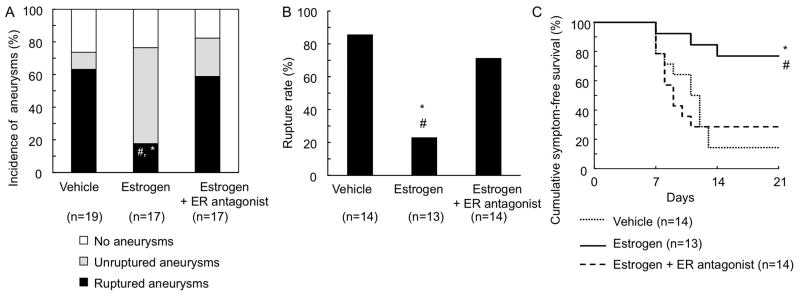
A: Incidence of ruptured and unruptured aneurysms. B: Rupture rate. C: Survival curve. Survival curves were constructed after excluding those mice that did not have aneurysms so that the curves mimic clinical settings. * vs vehicle, # vs Estrogen + ER antagonist. ER: estrogen receptor.
Furthermore, the protective effect of estrogen against the development of aneurysmal rupture was abolished by treatment with non-selective estrogen receptor antagonist (Figures 3A and 3B; incidence of intracranial aneurysms: estrogen vs. estrogen + estrogen receptor antagonist 18% vs. 59%, P < 0.05; rupture rate: estrogen vs. estrogen + estrogen receptor antagonist 23% vs. 71%, P < 0.05), confirming that the protective effect of estrogen was mediated by the activation of estrogen receptors.
Stimulation of estrogen receptor-β, but not of estrogen receptor-α, protected against the development of aneurysmal rupture
To identify the estrogen receptor subtype that was responsible for the protective role of estrogen against the development of aneurysmal rupture, we treated the ovariectomized female mice with estrogen receptor-α agonist (PPT) or estrogen receptor-β agonist (DPN).
Neither estrogen receptor-α agonist nor estrogen receptor-β agonist affected the overall incidence of aneurysms. However, the treatment with estrogen receptor-β agonist significantly reduced both the incidence of ruptured aneurysms (Figure 4A; vehicle control vs. receptor-β agonist 63% vs. 13%, P < 0.01) and the rupture rate (Figure 4B; vehicle control vs. receptor-β agonist 86% vs. 18%, P < 0.01). In contrast, estrogen receptor-α agonist did not have any significant effects on the aneurysmal formation or rupture (Figures 4A and 4B). Log-rank tests revealed a significant reduction of aneurysmal rupture with the estrogen receptor-β agonist treatment (P < 0.01), but not with the estrogen receptor-α agonist treatment (Figure 4C).
Figure 4. Stimulation of estrogen receptor-β, but not of estrogen receptor-α, protected against the development of aneurysmal rupture.
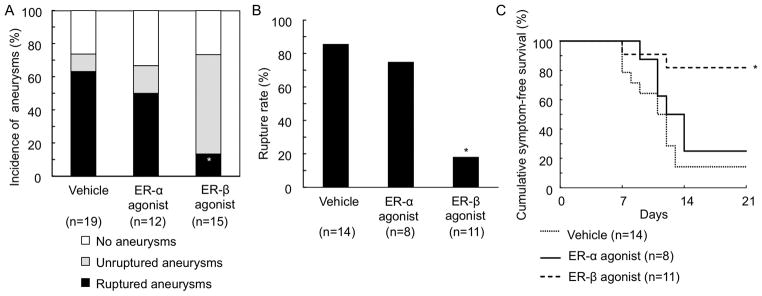
A: Incidence of ruptured and unruptured aneurysms. B: Rupture rate. C: Survival curve. *vs vehicle
ER: estrogen receptor
The protective effect of estrogen receptor-β stimulation depends on nitric oxide production
Estrogen can exert various effects on vasculature by increasing the production and availability of nitric oxide.16 The stimulation of estrogen receptor-β by estrogen can upregulate both inducible and endothelial nitric oxide synthases, and nitric oxide causes s-nitrosylation of various target proteins that are important for tissue remodeling.17, 18 Therefore, we tested whether the protective effect of estrogen receptor-β stimulation against the development of aneurysmal rupture was dependent on the production of nitric oxide.
We treated mice with a non-specific nitric oxide synthase inhibitor, N-nitro-L-arginine methyl ester (L-NAME). Although L-NAME alone did not affect the incidence of ruptured aneurysms or the rupture rate in ovariectomized mice, it abolished the protective effect of estrogen receptor-β agonist (DPN) (Figures 5A and 5B; incidence of ruptured aneurysms: L-NAME + DPN vs. DPN alone = 69% vs. 13%, P < 0.01; rupture rate: L-NAME + DPN vs. DPN alone = 79% vs. 18%, respectively, P < 0.01), indicating that the protective effect of estrogen receptor-β stimulation against the development of aneurysmal rupture depends on the production of nitric oxide by nitric synthase.
Figure 5. The protective effect of estrogen receptor-β stimulation was dependent on nitric oxide production.
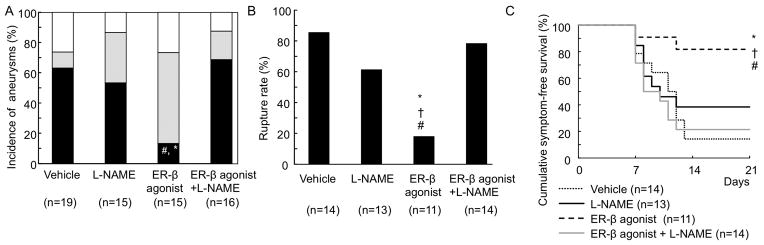
A: Incidence of ruptured and unruptured aneurysms. B: Rupture rate. C:Survival curve. * p vs vehicle, † vs L-NAME, # vs ER-β+L-NAME.
In our study, the L-NAME did not significantly affect the blood pressure. This is probably because blood pressure in our model was already raised by the DOCA-salt treatment. DOCA-salt induced hypertension probably masked the blood pressure augmentation effect of L-NAMA that is normally observed in normotensive animals. Similarly, estrogen or estrogen receptor agonist did not affect the blood pressure. Again, this is probably due to the hypertensive effect of DOCA-salt treatment that negated the effects from estrogen or estrogen receptor agonist (Supplementary Table S2).
Discussion
In this study, we showed that estrogen prevented aneurysmal rupture in ovariectomized female mice, consistent with the epidemiological studies.1, 2 The protective effect of estrogen appeared to occur through the activation of estrogen receptor-β, a predominant subtype of estrogen receptor in human intracranial aneurysms and cerebral arteries. Furthermore, the protective effect of estrogen receptor-β stimulation was dependent on the production of nitric oxide. These findings support the causal and mechanistic link between the stimulation of estrogen receptor-β by estrogen and the prevention of aneurysmal rupture.
Estrogen receptor-α and estrogen receptor-β regulate different sets of genes and mediate different cellular and tissue effects.19 For example, in vascular smooth muscle cells, estrogen receptor-β mediates the upregulation of nitric oxide synthase, but estrogen receptor-α exerts the opposite effect.20 We found that estrogen receptor-β is a predominant form of estrogen receptor in human intracranial aneurysms and superficial temporal artery samples, leading us to speculate that estrogen’s effects on cerebral arteries and intracranial aneurysms are mainly mediated by estrogen receptor-β.
In ovariectomized female mice (i.e., menopausal mice), we found that the protective effect of estrogen receptor-β activation was dependent on the production of nitric oxide. Similar to our findings, estrogen’s cardioprotective effects can be mediated by estrogen receptor-β in a nitric oxide-dependent manner.18, 21 The activation of estrogen receptor-β can stimulate not only the expression of both inducible and endothelial nitric oxide synthases but also the production of nitric oxide.17 Both inducible and endothelial nitric oxide synthase are involved in acute and chronic inflammation, processes that are emerging as integral parts of the pathophysiology of intracranial aneurysms.22 The s-nitrosylation of various proteins by nitric oxide can prevent the oxidative modification of cysteine residues,18, 21 thereby potentially reducing the excessive tissue remodeling that can leads to aneurysm rupture.
There are a number of limitations of our study. In our experiment, it is not clear which type of nitric oxide synthase is responsible for the protective effect of estrogen receptor-β or which proteins undergo s-nitrosylation. Future studies should utilize isoform-specific nitric oxide synthase inhibitors and isoform-specific nitric oxide synthase knockout mice.
Another major limitation of the study is that we used relatively young female mice (8–10 weeks-old). Female mice become reproductive approximately at 7 weeks, and menopause occurs at approximately 12–14 months.23 The majority of studies that assessed roles of estrogen during the post-menopausal period used ovariectomy in 8–10 week-old mice.14, 24 However, ovariectomy in relatively young, pre-menopausal mice may not completely simulate physiological menopause that normally occurs in much older female mice.25 Ovariectomy may result in an abrupt loss of ovarian hormones and bypass the peri-menopausal stage that can be characterized by a gradual loss and fluctuation of estrogen.25 Effects of ovariectomy and estrogen therapy may be different between the early stage and late stage of reproductive age, because of the age-related changes in various tissues and their response.25, 26 Moreover, there appears to be significant differences in the expression levels, sensitivity, and response of estrogen receptors between early and late stage of menopause27, 28 Further studies using older female mice may be needed to confirm the protective effect of estrogen receptor-β activation against the development of aneurysmal rupture in post-menopausal female mice.
Menopause causes a loss of estrogen and progesterone. Progesterone can improve outcomes after ischemic and traumatic brain injury in animals partly through the modulation of inflammation and oxidative stress.29 There seem to be interactions between estrogen and progesterone in neuroprotection.29 In addition, testosterone can augment inflammation by modulating oxidative stress or activating pro-inflammatory cytokines.29 Vascular inflammation in the brain may be influenced by the balance among estrogen, testosterone, and progesterone.
In our study, we chose dosages of 17β estradiol, DPN and PPT based on previous publications.9–15 While we used the previously established dosages of these agents, we did not establish the dose-dependent effects of each agent in this study. Although DPN is highly selective for estrogen receptor-β, it still possesses a weak agonistic effect on estrogen receptor-α (estrogen receptor-α vs. estrogen receptor-β = 1:170).30 PPT, a selective estrogen receptor-α, also has a weak agonistic activity on estrogen receptor-β (estrogen receptor-α vs. estrogen receptor-β = 1000:1).30
We used the uterine weight as a bioassay of estrogen receptor stimulation to confirm the efficacy of ovariectomy and drug treatment. Uterus is an estrogen sensitive organ of which weight can be augmented by estrogen receptor stimulation. Uterine weight has been successfully used to verify the biological activity of estrogen in mice, and the uterine weight closely correlates with the plasma estrogen levels.9, 13–15, 24 However, the lack of the direct measurement of blood estrogen levels remains a limitation of this study.
Perspectives
In this study, we found the protective effect of estrogen, mainly through estrogen receptor-β, against the development of aneurysmal rupture. Estrogen’s unwanted effects, such as increased risks for breast cancer and endometrial cancer in certain populations, are often attributed to its agonistic activity without tissue specificity. To avoid the unwanted effects of estrogen, selective estrogen receptor modulators (SERMs) that exert agonistic or antagonistic actions on estrogen receptors in a tissue specific fashion are under vigorous investigation. Our findings may become the basis for testing SERMs with a favorable tissue specificity profile to prevent the growth and rupture of intracranial aneurysms in humans, particularly in post-menopausal women.
Supplementary Material
Novelty and Significance.
What Is New?
Using a mouse model of intracranial aneurysm, we found that estrogen can protect against the development of intracranial aneurysm rupture in ovariectomized mice through the activation of estrogen receptor-β.
What Is Relevant
Our data is relevant to the development of the new pharmacological treatment for the prevention of aneurysmal subarachnoid hemorrhage in post-menopausal women who are at the high risk for aneurysmal subarachnoid hemorrhage.
Summary.
Estrogen receptor-β appears to play critical role in protective effects of estrogen against intracranial aneurysmal rupture in estrogen deficient state.
Acknowledgments
Sources of Funding
The project described was supported by Grant Number R01NS055876 (TH), R01NS082280 (TH), and K08NS082363 (DMH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS), and the Brain Aneurysm Foundation Shirley Dudek Demmer Chair of Research (KS).
Footnotes
Conflict of interest/Disclosure
None
References
- 1.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372. doi: 10.1136/jnnp.2007.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D Australasian Cooperative Research on Subarachnoid Hemorrhage Study G. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: An international population-based, case-control study. Stroke. 2001;32:606–612. doi: 10.1161/01.str.32.3.606. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen TH, Bartek J, Jr, Andresen M, Springborg JB, Romner B. Modifiable risk factors for aneurysmal subarachnoid hemorrhage. Stroke. 2013;44:3607–3612. doi: 10.1161/STROKEAHA.113.001575. [DOI] [PubMed] [Google Scholar]
- 4.Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M, Nagahiro S. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens. 2009;27:1284–1292. doi: 10.1097/HJH.0b013e328329d1a7. [DOI] [PubMed] [Google Scholar]
- 5.Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Kuwayama K, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part ii: Experimental study of the effects of hormone replacement therapy in rats. J Neurosurg. 2005;103:1052–1057. doi: 10.3171/jns.2005.103.6.1052. [DOI] [PubMed] [Google Scholar]
- 6.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T. Pharmacological stabilization of intracranial aneurysms in mice: A feasibility study. Stroke. 2012;43:2450–2456. doi: 10.1161/STROKEAHA.112.659821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama T, Galea GL, Lanyon LE, Price JS. Mechanical loading-related bone gain is enhanced by tamoxifen but unaffected by fulvestrant in female mice. Endocrinology. 2010;151:5582–5590. doi: 10.1210/en.2010-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rayner K, Sun J, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O’Brien ER. Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: Role of selective estrogen receptor beta modulation. Arterioscler Thromb Vasc Biol. 2009;29:1751–1756. doi: 10.1161/ATVBAHA.109.193656. [DOI] [PubMed] [Google Scholar]
- 11.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in apoe−/− mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgin JB, Knowles JW, Kim HS, Smithies O, Maeda N. Interactions between endothelial nitric oxide synthase and sex hormones in vascular protection in mice. J Clin Invest. 2002;109:541–548. doi: 10.1172/JCI14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate alzheimer-like changes in female 3xtg-ad mice. Endocrinology. 2008;149:2607–2611. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res. 2009;104:265–275. doi: 10.1161/CIRCRESAHA.108.190397. 211p following 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess DT, Foster MW, Stamler JS. Assays for s-nitrosothiols and s-nitrosylated proteins and mechanistic insights into cardioprotection. Circulation. 2009;120:190–193. doi: 10.1161/CIRCULATIONAHA.109.876607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuedling S, Karas RH, Mendelsohn ME, Katzenellenbogen JA, Katzenellenbogen BS, Meyer R, Vetter H, Grohe C. Activation of estrogen receptor beta is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Lett. 2001;502:103–108. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in s-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitman DC, Paruthiyil S, Vivar OI, Saunier EF, Herber CB, Cohen I, Tagliaferri M, Speed TP. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr Opin Pharmacol. 2010;10:629–636. doi: 10.1016/j.coph.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsumi S, Zhang X, Takata K, Takahashi K, Karas RH, Kurachi H, Mendelsohn ME. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J Endocrinol. 2008;199:267–273. doi: 10.1677/JOE-07-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased s-nitrosylation of the l-type ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 22.Hasan DM, Mahaney KB, Brown RD, Jr, Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC International Study of Unruptured Intracranial Aneurysms I. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver LM. Mouse genetics: Concepts and applications. New York: Oxford University Press; 1995. [Google Scholar]
- 24.Toutain CE, Filipe C, Billon A, Fontaine C, Brouchet L, Guery JC, Gourdy P, Arnal JF, Lenfant F. Estrogen receptor alpha expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arterioscler Thromb Vasc Biol. 2009;29:1543–1550. doi: 10.1161/ATVBAHA.109.192849. [DOI] [PubMed] [Google Scholar]
- 25.Mayer LP, Dyer CA, Eastgard RL, Hoyer PB, Banka CL. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Thromb Vasc Biol. 2005;25:1910–1916. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- 26.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 27.Pinna C, Cignarella A, Sanvito P, Pelosi V, Bolego C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension. 2008;51:1210–1217. doi: 10.1161/HYPERTENSIONAHA.107.106807. [DOI] [PubMed] [Google Scholar]
- 28.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovascular research. 2002;53:605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 29.Cheng J, Hurn PD. Sex shapes experimental ischemic brain injury. Steroids. 2009 doi: 10.1016/j.steroids.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (er)alpha and erbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.