Abstract
Although glucocorticoid receptors are highly expressed in the prefrontal cortex, the hippocampus remains the predominant focus in the literature examining relationships between cortisol and brain. We examined phenotypic and genetic associations of cortisol levels with the thickness of prefrontal and anterior cingulate cortex regions, and with hippocampal volume in a sample of 388 middle-aged male twins who were 51–59 years old. Small but significant negative phenotypic associations were found between cortisol levels and the thickness of left dorsolateral (superior frontal gyrus, left rostral middle frontal gyrus) and ventrolateral (pars opercularis, pars triangularis, pars orbitalis) prefrontal regions, and right dorsolateral (superior frontal gyrus) and medial orbital frontal cortex. Most of the associations remained significant after adjusting for general cognitive ability, cardiovascular risk factors, and depression. Bivariate genetic analyses suggested that some of the associations were primarily accounted for by shared genetic influences; that is, some of the genes that tend to result in increased cortisol levels also tend to result in reduced prefrontal cortical thickness. Aging has been associated with reduced efficiency of hypothalamic-pituitary-adrenal function, with frontal lobe shrinkage, and with increases in health problems, but our present data do not allow us to determine the direction of effects. Moreover, the degree or the direction of the observed associations and the extent of their shared genetic underpinnings may well change as these individuals age. Longitudinal assessments are underway to elucidate the direction of the associations and the genetic underpinnings of longitudinal phenotypes for changes in cortisol and brain morphology.
Keywords: heritability, magnetic resonance imaging (MRI), hippocampus, HPA axis structure, genetic correlation
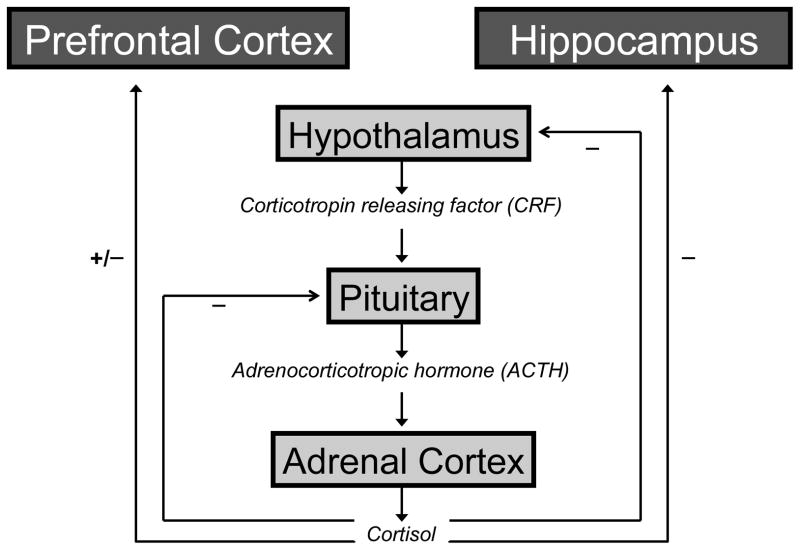
The human neuroendocrine stress response is initiated and coordinated by the activation of brain corticotropin releasing factor (CRF) neurotransmission and the release of hypothalamic paraventricular CRF which, in turn, binds to anterior pituitary CRF1 receptors to trigger secretion of adrenocorticotropic hormone (ACTH); ACTH, in turn, binds to its receptor in the adrenal cortex to stimulate secretion of cortisol (Hauger et al., 2006). Although activation of the CRF-ACTH-cortisol cascade is critical for survival in the context of internal or external threats to homeostasis, rapid counter-regulation of this process is equally important for reestablishing physiological homeostasis upon threat termination. As illustrated in Figure 1, this regulation takes place, in part, via feedback from target brain regions. The most well known of these is the hippocampus. Indeed, since the seminal study of McEwen and colleagues (1968) showed that the highest concentration of receptors for corticosteroids in the rodent brain was in the hippocampus, cognitive and brain research on cortisol regulation has focused overwhelmingly on hippocampal dysfunction and hippocampal-dependent episodic memory. Later cellular and molecular research identified two corticosteroid receptor subtypes: the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) (Lupien and Lepage, 2001). Interestingly, the prefrontal cortex in primates was found to be a major site of low affinity glucocorticoid receptor expression, thereby indicating that prefrontal cortical neurons are also an important target of cortisol (Lupien and Lepage, 2001; Mizoguchi et al., 2003; Sánchez et al., 2000; Sarrieau et al., 1986). In his evolving work, McEwen (2007) too has noted the importance of other brain regions, including prefrontal cortex, in the adaptation to stress.
Figure 1. Schematic representation of the hypothalamic-pituitary-adrenal (HPA) axis.
Beginning with the perception of a stressor, the hypothalamus releases CRF which ultimately leads to the secretion of cortisol by the adrenal gland and its delivery to target systems in the brain and body. Minus signs indicate negative (inhibitory) feedback; plus sign indicates positive (excitatory) feedback.
The high expression of GRs in the prefrontal cortex may also be an important factor in brain aging. Although evidence for increased cortisol dysregulation with age is mixed, the predominant view is that aging is associated with reduced efficiency on the hypothalamic-pituitary-adrenal (HPA) axis (Bergendahl et al., 2000; Deuschle et al., 1997; Ferrari et al., 2001; Inglis et al., 1999; Lupien et al., 1994; Seeman et al., 2001; Van Cauter et al., 1996; Van Cauter et al., 2000). Multiple studies also suggest that the frontal lobes undergo greater age-related shrinkage than other brain tissue regions (Raz, 2000; Raz and Rodrigue, 2006), and there is evidence that both the frontal cortex and the underlying white matter of the frontal lobes are disproportionately affected by aging (Jernigan et al., 2001). Aging is also associated with reductions in prefrontal dopamine (Wenk et al., 1989). Moreover, one of the predominant models of normative aging in humans is that it consists largely of frontal-subcortical changes in contrast to the predominant medial temporal changes associated with Alzheimer’s disease (Buckner, 2004; Hedden and Gabrieli, 2004; Pugh and Lipsitz, 2002).
Other evidence suggests that chronic cortisol exposure—perhaps due to stress or allostatic load—could have an impact on the structure of prefrontal regions. Chronic corticosteroid exposure in animal studies has been associated with both dopaminergic and serotonergic changes (Piazza et al., 1996; Stenfors et al., 2004), dendritic remodeling (Shansky et al., 2009; Wellman, 2001), and asymmetries of brain activity (Kalin et al., 1998). Because age-related brain changes in humans appear to preferentially affect prefrontal-subcortical circuit components, these effects of corticosteroid exposure might also increase with age.
Overall, human research provides a mixed picture regarding associations between chronic cortisol exposure and brain structure. Mixed results may be due, in part, to the fact that most of the studies had small sample sizes. When significant results were observed, they were often in comparisons of extreme groups such as normal controls versus patients with Cushing’s disease, Alzheimer’s disease, or depression, or low- versus hyper-secreters (O’Brien et al., 1996, Sheline, 1996 #2326, Lupien, 1998 #760; Sapolsky et al., 2000). Small correlations have been reported between cortisol levels and prefrontal or anterior cingulate regions, but often within the context of mixed results in which even the direction of association was not always consistent (Gold et al., 2005; MacLullich et al., 2005; MacLullich et al., 2006). Results based on extreme groups or people with specific disorders may not extrapolate to the general population. Several findings were also based on challenges such as dexamethasone suppression tests. Although these constitute valid and useful ways to assess HPA axis dysregulation, acute response to challenge may not necessarily parallel the effects of chronic hyperactivity of the HPA axis.
Individual differences in both human cortisol output (Bartels et al., 2003; Kirschbaum et al., 1992; Levene et al., 1972; Meikle et al., 1988), and brain structure (Schmitt et al., 2007) are influenced by genetic factors to varying degrees, but we are unaware of any examination of the genetic relationship between cortisol and brain structure. In the present study, we sought to examine the relationship of cortisol levels and brain structure in a large sample that is representative of U.S. men in the age range under study. In addition, our twin data allowed us to address the key issue of the extent to which any significant phenotypic correlations between cortisol levels and brain structure are account for by underlying genetic or environmental factors.
Based on knowledge of GR concentrations and the relatively small amount of existing MRI research, our primary focus was on prefrontal cortex. However, previous reports also led us to examine the anterior cingulate cortex and hippocampus as well. We tested whether cortisol levels would be associated with reductions in the size of these brain regions. Because prefrontal cortex is large and heterogeneous, we examined specific prefrontal cortical parcellation units rather than simply measuring the frontal lobes as a whole.
All of the previous reports used volume measures of brain regions. We also used volume measures for the hippocampus, but for regions across the cortical ribbon we examined measures of average thickness and total surface area. The volume of a cortical region is essentially the product of its thickness and surface area. These two measures reflect relatively independent processes in brain development as articulated in Rakic’s (1988, 1995, 2007) radial unit hypothesis. Elsewhere we have shown that cortical thickness and surface area are each influenced by different, independent sets of genes (Panizzon et al., 2008). When volume is measured, these two different processes are combined and it is not possible to determine whether one or the other or both is accounting for size differences. Furthermore, thickness and surface area might counteract one another to yield no correlation with an external variable, if both are associated independently but in opposite directions. Based on the radial unit hypothesis, we would expect that cortisol would be related primarily to cortical thickness rather than surface area.
Finally, the cortisol awakening response (CAR)—the pronounced cortisol increase within 30–60 minutes after awakening—is also of interest, in part, because it is significantly associated with self-reported stress (Pruessner et al., 1999). The CAR has been associated with hippocampal volume in young adult men, although the direction of association may differ in younger and older individuals (Pruessner et al., 2007). To our knowledge, the association between the CAR and the thickness of prefrontal cortical regions has not been examined.
Methods
Participants
A total of 1237 twins participated in wave 1 of the longitudinal Vietnam Era Twin Study of Aging (VETSA) project, an overview of which can be found elsewhere (Kremen et al., 2006). Participants were randomly selected from an earlier study of twins in the Vietnam Era Twin Registry study (Tsuang et al., 2001). Registry members are male-male twin pairs who both served in the United States military between 1965 and 1975. The Registry is neither a VA nor a patient sample, and the large majority was not exposed to combat. The VETSA MRI Study and the VETSA Cortisol Study were begun after the parent VETSA project was underway. We began the VETSA MRI study in the third year of the primary VETSA study. At the time of this report there were 474 individual VETSA participants with analyzable MRI data; 241 were scanned in San Diego and 231 were scanned in Boston. The VETSA Cortisol Study began sometime after the start of the MRI study. The present analyses are based on 388 individuals (86 monozygotic [MZ] pairs, 78 dizygotic [DZ] pairs, and 60 unpaired twins) who participated in both the VETSA MRI and Cortisol studies, and had data on all of the variables that were included in the statistical models. Zygosity was initially classified according to questionnaire and blood group information. These classifications are being updated on the basis of 25 microsatellite markers. To date, 56% of the MRI study participants have DNA-determined zygosity. Consistent with the overall VETSA project, 95% of the questionnaire-based classifications were in agreement with the DNA-based classifications; when differences occurred, we used the DNA-based classifications.
Participants live throughout the United States and were given the option of traveling to San Diego or Boston for a day-long series of assessments. The MRI session was typically the day after the in-lab evaluation. Only 6% of VETSA participants who were eligible to undergo MRI declined to participate; 59% were included. The remaining participants were excluded for reasons such as possible metal in the body (7%), claustrophobia (3%), testing being conducted in the twins’ hometown (5%), scanner problems (8%), co-twin being excluded (9%), and other reasons (3%).
Basic demographic and health characteristics of the VETSA sample are comparable to U.S. census data for similarly aged men (Centers for Disease Control and Prevention, 2003; National Center for Disease Statistics, 2003). Mean age of the MRI participants was 55.8 (2.6) years (range: 51–59), mean years of education was 13.9 (SD=2.1), and 85.2% were right-handed. Most participants were employed full-time (74.9%), 4.2% were employed part-time, and 11.2% were retired. There were 88.3% non-Hispanic white, 5.3% African-American, 3.4% Hispanic, and 3.0% “other” participants. Self-reported overall health status was as follows: excellent (14.8%); very good (36.5%); good (37.4%); fair (10.4%); and poor (0.9%). These demographic characteristics did not differ from the entire VETSA sample, nor were there significant differences between MZ and DZ twins.
Salivary Cortisol
Saliva Collection
On the day of testing, saliva samples were obtained at awakening, 30 minutes post-awakening, 10:00 am, 3:00 pm, and 9:00 pm or bedtime. The first two morning samples and the evening sample were provided by participants at the hotel where they stayed for the study. Twins brought the morning samples with them to the laboratory and dropped off the evening sample the next morning at the hotel front desk. If necessary, participants chewed original Trident gum to stimulate saliva production and removed the gum prior to providing the sample. Previous testing by one of the investigators (SM) showed that this particular gum does not alter cortisol concentrations.
Cortisol Assays
Samples were sent to the laboratory of Dr. Mendoza at the University of California, Davis where all assays were performed. Prior to conducting the assays, samples were centrifuged at 3000 rpm for 20 minutes to separate the aqueous component from mucins and other suspended particles. Salivary concentrations of cortisol were estimated in duplicate using commercial radioimmunoassay kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Assay procedures were modified to accommodate overall lower levels of cortisol in human saliva relative to plasma as follows: 1) standards were diluted to concentrations ranging from 2.76 to 345 nmol/L; 2) sample volume was increased to 200 μl, and 3) incubation times were extended to 3 hours. Serial dilution of samples indicates that the modified assay displays a linearity of 0.98 and an assay sensitivity (least detectable dose) of 1.385 nmol/L. Intra- and inter-assay coefficients of variation are 3.96% and 5.66%. Samples from each participant were analyzed in the same assay; one to three individuals were included in the same assay batch. When three people were included in a batch, it typically included two co-twins and one unrelated twin. Comparison of related and unrelated twins revealed significant batch effects. Therefore, cortisol levels are adjusted for batch in all analyses. Cortisol assays were always performed without knowledge of the zygosity of the participant.
Cortisol Measures
We used the mean of the five samples as the primary cortisol measure for three reasons. The primary reason was that the mean output on the day of testing was the most heritable cortisol measure, and we were interested in determining the genetic overlap between cortisol and brain structure (see Statistical Analysis). Second, the mean output on the day of testing provides a good index of overall output. Third, if there is an association between cortisol levels and brain structure, it is likely to be a function of chronic rather than acute effects. Although the mean will, in part, reflect overall responsivity to the sustained testing, it is more likely to reflect chronic cortisol exposure as compared with specific responsivity measures such as the cortisol awakening response (CAR) or cortisol slope. We also examined AUC for the five samples as another index of overall output. The AUC is often considered the best overall output measure, but it was not as highly heritable as the mean. Moreover, it was very highly correlated with the mean (r=.87, p<.001) and yielded similar results. Finally, we examined the AUC for the cortisol awakening response (CAR), defined as the AUC for the awakening and 30 minutes post-awakening time points.
Cortisol values above 50 nmol were converted to missing. This value corresponds approximately to average maximum values for the participants plus 3 standard deviations. The cortisol measures had skewed distributions and were log transformed to normalize the distributions. If data were missing for one of the five samples, we imputed the missing values based, in part, on individual profiles. For example, if time point 5 was missing, we calculated the average decrease from time point 4 to 5. We then decreased an individual’s own time point 4 by that amount in order to impute his time point 5 value. If two or more time points were missing, the mean and AUC values were treated as missing because it was felt that there was an insufficient percentage of data present.
MRI Acquisition
Images were acquired on Siemens 1.5 Tesla scanners (241 at University of California, San Diego; 233 at Massachusetts General Hospital). Sagittal T1-weighted MPRAGE sequences were employed with a TI=1000ms, TE=3.31ms, TR=2730ms, flip angle=7degrees, slice thickness=1.33mm, voxel size 1.3×1.0×1.3mm. Raw DICOM MRI scans (including two T1-weighted volumes per case) were downloaded to the MGH site. These data were reviewed for quality, registered, and averaged to improve signal-to-noise.
MRI Processing
Volume Measures
Volumetric measures were created for hippocampus using volumetric segmentation (Fischl et al., 2002; Fischl et al., 2004a) and cortical surface reconstruction (Dale et al., 1999; Dale and Sereno, 1993; Fischl et al., 2002; Fischl et al., 2004a; Fischl et al., 1999; Fischl et al., 2004b) methods based on the publicly available FreeSurfer software package. The automated, fully 3D whole-brain segmentation procedure uses a probabilistic atlas and applies a Bayesian classification rule to assign a neuroanatomical label to each voxel (Fischl et al., 2002; Fischl et al., 2004a). A widely used training atlas has been shown to be comparable to that of expert manual labeling and is sensitive to subtle brain changes in Alzheimer’s disease and normal aging (Fischl et al., 2002; Fischl et al., 2004a). However, we created a new manually-derived training set from 20 unrelated, randomly selected VETSA participants. This VETSA-specific atlas was created by the same laboratory at the MGH Center for Morphometric analysis using the same neuroanatomic criteria and the same reliability standards as original atlas. Our rationale was that the VETSA-specific atlas would be more representative of the VETSA sample, thereby yielding more accurate measurements. Comparison of the atlases did show that the VETSA-specific measures were closest to the “gold standard” manually-derived measures (available upon request). Estimated total intracranial volume, derived according to the method of Buckner et al. (2004), is also provided in FreeSurfer and was used to control for differences in head size. Hippocampal volumes were, therefore, adjusted for intracranial volume.
Cortical Thickness and Surface Area Measures
Using semi-automated cortical surface reconstruction methods (Dale et al., 1999; Dale and Sereno, 1993; Fischl and Dale, 2000; Fischl et al., 1999; Fischl et al., 2004b) based on the publicly available FreeSurfer software package, we measured thickness at each surface location, or vertex. Intensity variations due to magnetic field inhomogeneities are corrected, a normalized intensity image is created, and the skull (non-brain) is removed from the normalized image. The preliminary segmentation is partitioned using a connected components algorithm, with connectivity not allowed across the established cutting planes that separate the cerebral hemispheres and disconnect brainstem and cerebellum. Any interior holes in the components representing white matter are filled, resulting in a single filled volume for each cortical hemisphere. The resulting surface is covered with a triangular tessellation and smoothed to reduce metric distortions. After the initial surface model has been constructed, a refinement procedure is applied to obtain a representation of the gray/white boundary. This surface is subsequently deformed outwards to obtain an explicit representation of the pial surface.
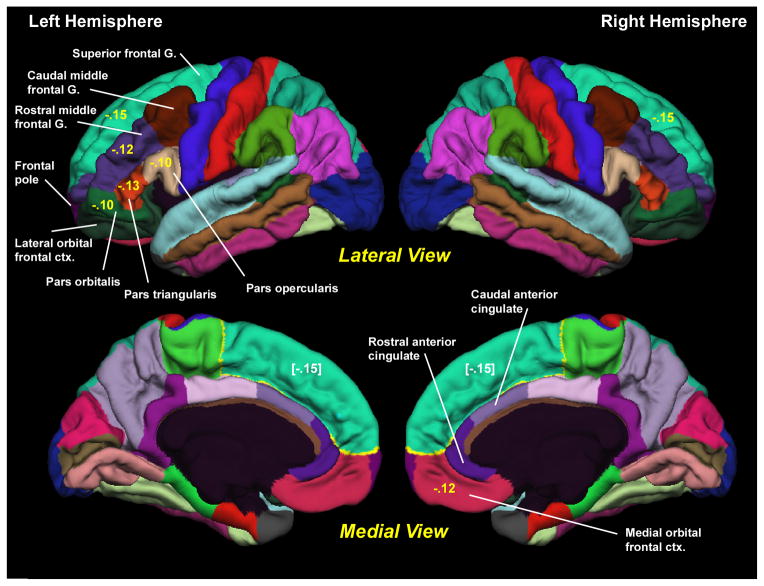
The surface was then divided into distinct cortical regions of interest (ROIs) (Fischl et al., 2004b). Each surface location, or vertex, was assigned a neuroanatomical label based on 1) the probability of each label at each location in a surface-based atlas space, based on a manually parcellated training set; 2) local curvature information; and 3) contextual information, encoding spatial neighborhood relationships between labels (conditional probability distributions derived from the manual training set). The parcellation scheme labels cortical sulci and gyri (Desikan et al., 2006), which form the basis for defining ROIs. In the present analyses, we examined the thickness and surface area of the nine prefrontal and two anterior cingulate ROIs in each hemisphere (Figure 2). Cortical thickness was defined as the average distance between the gray-white boundary and the pial surface within each ROI. Surface area was defined as the sum of the areas of each triangular tesselation falling within a given ROI in each individual’s native space.
Figure 2. Cortical Parcellation.
The cortical parcellation units are generated by Freesurfer and are based on the parcellation system of Desikan et al. (2006). All of the parcellation units are shown. The 9 prefrontal and 2 anterior cingulate regions examined in the present study are labeled. G.=gyrus. ctx.=cortex. Numbers in yellow are the phenotypic correlations between mean cortisol level and thickness of the region. Bracketed numbers in white repeat the correlations with the superior frontal gyrus, but are shown on the medial surface.
MRI Quality Control
Of the 493 scans available at the time of these analyses, quality control measures excluded 0.6% (3 cases) due to scanner artifact and 3% (16 cases) due to inadequate image processing results (e.g., poor contrast caused removal of non-brain to fail). Scans are visually inspected—blind to participant characteristics—and manually edited by trained technicians. In conjunction with the Morphometry Biomedical Informatics Research Network (BIRN; http://www.nbirn.net/research/morphometry/index.shtm), which is sponsored by the National Institutes of Health and the National Center for Research Resources, the reliability and validity of these image acquisition and processing methods across sites and scanners has been demonstrated (Dickerson et al., 2008; Fennema-Notestine et al., 2007; Han et al., 2006; Jovicich et al., 2006; Jovicich et al., 2009). Studies have also demonstrated a high correlation of automatic and manual measures in vivo and ex vivo (Fischl and Dale, 2000; Walhovd et al., 2005).
Statistical Analysis
Non-Twin Analyses
These analyses examining the phenotypic relationship between cortisol levels and brain structure by means of multiple regression SAS Proc Glimmix (SAS Institute, 2000). Clustering of twins pairs was included in the models as a random effect. All models were adjusted for batch effects of the cortisol assays; batch was included as a random effect. Site effects were included as fixed effects in order to adjust for possible scanner differences at the two sites.
In a second set of analyses, the effects of several demographic and health characteristics that might be associated with the size of brain structures or with cortisol levels were included as covariates and treated as fixed effects in addition to the adjustments for batch and site effects. These included age, young adult general cognitive ability, hypertension, cardiovascular illness, depression, diabetes, smoking, alcohol consumption, and site. Descriptive statistics for these variables are shown in Table 1. The index of young adult cognitive ability was the score on the Armed Forces Qualification Test (AFQT). The AFQT was given to VETSA participants just prior to induction into the military at average age 20. It is highly correlated (r=.81–.84) with Wechsler IQ and provides a good g measure that is not confounded with aging effects (Grafman et al., 1988; McGrevy et al., 1974). AFQT scores are percentiles, and these were log transformed to reflect a normal distribution. AFQT provides a measure of general cognitive ability that is more precise than the proxy variable of education, and it is also not confounded by later aging effects. Hypertension was a dichotomous variable based on whether the participant had in-laboratory systolic blood pressure over 140, diastolic blood pressure over 90, or was taking antihypertensive medications. Cardiovascular illness was dichotomous variable that was rated “yes” if a participant had a history of heart surgery, heart catheterization, stroke, heart attack, heart failure, or peripheral vascular disease. Depression was based on the Center for Epidemiological Studies Depression Scale (CES-D). CES-D scores were log transformed in order to obtain a more normal distribution. Smoking was defined based on whether or not a participant was a current smoker. Alcohol consumption was based on a 4-point scale for drinking during the past two weeks: 0=non-drinker; 1=up to 1 drink per day on average; 2=more than 1 and up to 2 drinks per day on average; 3=more than 2 drinks per day on average. A similar scale has been used in large epidemiological studies (Paul et al., 2008).
Table 1.
Sample Characteristics
| Measure | Mean | SD | ||
|---|---|---|---|---|
| Cortisol (nmol/L) | ||||
| Mean | 1.68 | .41 | ||
| Area under the curve | 23.81 | 7.00 | ||
| Cortisol awakening response | 1.19 | .52 | ||
| Prefrontal Cortical Thickness (mm) | ||||
| L superior frontal gyrus | 2.19 | .11 | ||
| R superior frontal gyrus | 2.20 | .11 | ||
| L Rostral middle frontal gyrus | 1.85 | .10 | ||
| R Rostral middle frontal gyrus | 1.81 | .10 | ||
| L Caudal middle frontal gyrus | 2.03 | .13 | ||
| R Caudal middle frontal gyrus | 2.04 | .14 | ||
| L Pars orbitalis | 2.19 | .17 | ||
| R Pars orbitalis | 2.19 | .16 | ||
| L Pars triangularis | 1.91 | .13 | ||
| R Pars triangularis | 1.92 | .13 | ||
| L Pars opercularis | 2.04 | .14 | ||
| R Pars opercularis | 2.05 | .15 | ||
| L Frontal pole | 2.38 | .26 | ||
| R Frontal pole | 2.34 | .25 | ||
| L Lateral orbital frontal cortex | 2.11 | .14 | ||
| R Lateral orbital frontal cortex | 2.07 | .13 | ||
| L Medial orbital frontal cortex | 1.85 | .16 | ||
| R Medial orbital frontal cortex | 1.84 | .15 | ||
| L Rostral anterior cingulate cortex | 2.01 | .25 | ||
| R Rostral anterior cingulate cortex | 2.00 | .27 | ||
| L Caudal anterior cingulate cortex | 2.07 | .33 | ||
| R Caudal anterior cingulate cortex | 2.20 | .27 | ||
| Hippocampal Volume (mm3) | ||||
| L Hippocampus | 3991.75 | 390.98 | ||
| R Hippocampus | 4225.29 | 431.40 | ||
| Covariates | ||||
| General cognitive ability in early adulthood (AFQT) | 61.11 | 22.83 | ||
| Depression (CES-D) | 7.95 | 7.81 | ||
|
| ||||
| % Yes | % No | |||
|
| ||||
| Hypertension | 54.6% | 45.4% | ||
| Cardiovascular illness | 15.2% | 84.8% | ||
| Diabetes | 7.6% | 92.4% | ||
| Current smoking | 23.6% | 76.4% | ||
|
| ||||
| % L0 | % L1 | % L2 | % L3 | |
|
| ||||
| Alcohol use (level [L])a | 40.9% | 44.1% | 6.3% | 8.7% |
L= Left. R=Right. AFQT=Armed Forces Qualification Test. CES-D=Center for Epidemiological Studies Depression Scale.
0=non-drinker; 1=> 0 and ≤ 1 drink per day; 2=> 1 and ≤ 2 drinks per day; 3=> 2 drinks per day.
Twin Analyses
The standard twin (“ACE”) model estimates the proportion of phenotypic variance due to additive genetic effects (A), shared environmental effects (C), and individual-specific environmental effects (E) (Eaves et al., 1978; Neale and Cardon, 1992). Shared environmental influences are those that make twins similar; individual-specific environmental influences are those that make twins different. Because measurement error is assumed to be random, it is uncorrelated within twin pairs; consequently, it forms part of the individual-specific environmental variance.
In the basic univariate ACE model: 1) additive genetic factors correlate 1.0 for MZ twins and 0.5 for DZ twins; 2) shared environmental factors correlate 1.0 across twins regardless of zygosity; 4) individual-specific environmental factors are uncorrelated across twins; and 5) the variance of the underlying latent genetic and environmental factors is fixed at 1.0. If MZ twin pairs are more highly correlated than DZ pairs, it suggests that genetic factors account for some of the individual differences in a phenotype.
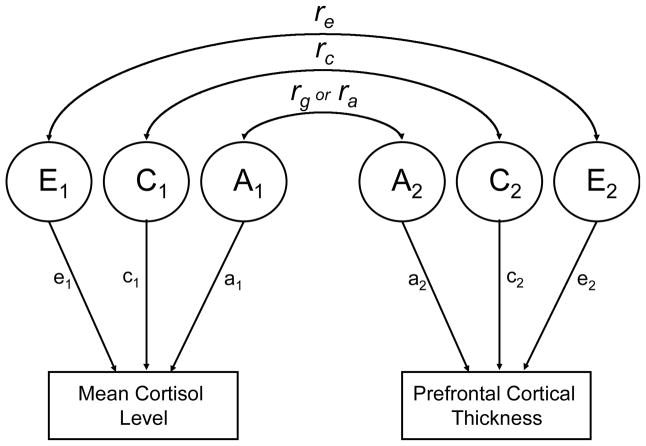
We have previously reported the heritabilities of the individual measures, but our interest in this article was in the relationships between measures. The ACE model is easily extended to a bivariate model that includes the genetic, shared and individual-specific environmental covariances between two measures. Figure 3 shows a bivariate correlated factors model, but for ease of presentation, only one twin is shown. Bivariate twin models were examined when there were statistically significant phenotypic correlations between mean cortisol level and brain structure measures. Genetic correlations (rg), shared environmental correlations (rc), and individual-specific environmental correlations (re) are derived from the bivariate models. A genetic correlation is like a standard phenotypic correlation except that it is based on genetic covariances only, i.e., the genetic covariance between two variables divided by the square root of the product of their genetic variances (Neale and Cardon, 1992). For the most part, genetic correlations indicate the amount of genetic overlap between measures. Similarly, shared environmental correlations reflect only the overlap between shared environmental influences on each measure, and individual-specific environmental correlations reflect only the overlap between individual-specific environmental correlations. All three sets of influences contribute to observed (phenotypic) correlations, but it is not possible to determine which of these factors accounts for the observed correlations in non-twin studies. The twin models were tested formally with Mx, a maximum-likelihood-based structural equation modeling program (Neale et al., 2003).
Figure 3. Bivariate Correlated Factors Model.
A=Additive genetic influences. C=Shared or common environmental influences. E=Nonshared or individual-specific environmental influences. rg or ra=Genetic correlation. rc=Shared environment correlation. re=Unique environment correlation. Arrows from A1, C1, and E1 to mean cortisol level represent parameter estimates for the contribution of those components to that variable. The same is true for arrows from A2, C2, and E2 to prefrontal cortical thickness. Squaring these parameter estimates provides the proportion of variance accounted for by each component. For ease of presentation, only one twin is represented.
Results
Phenotypic Associations
Table 2 shows the results for mean cortisol level and Figure 2 illustrates the regions. Higher mean cortisol level was significantly associated with thinner cortex in seven prefrontal regions: left and right superior frontal gyrus; left rostral middle frontal gyrus; left pars opercularis; left pars triangularis; left pars orbitalis; and right medial orbital frontal cortex. After adjusting for the covariates, the associations between mean cortisol level and cortical thickness remained significant in five of these seven prefrontal regions: left and right superior frontal gyrus; left pars opercularis, left pars triangularis; and right medial orbital frontal cortex. Significance was reduced to trend levels for left rostral middle frontal gyrus and left pars orbitalis. The significant correlations ranged from −.10 to −.15 (ps=.05 to .004) when adjusted for batch and site effects only, and from −.11 to −.13 (ps=.04 to .01) after adjusting for all of the covariates.
Table 2.
Association of cortisol level and prefrontal cortical thickness: Mixed model results and phenotypic correlations for mean cortisol level
| Region of Interest | Batch/Site Only (n=388)a | All Covariates (n=383)a | ||||||
|---|---|---|---|---|---|---|---|---|
| df | t | rb | P | df | t | r | p | |
| Left superior frontal gyrus | 114 | −2.94 | −.15 | .004 | 102 | −2.40 | −.12 | .02 |
| Right superior frontal gyrus | 114 | −2.94 | −.15 | .004 | 102 | −2.49 | −.13 | .01 |
| Left rostral middle frontal gyrus | 114 | −2.45 | −.12 | .02 | 102 | −1.79 | −.09 | .08 |
| Left pars opercularis | 114 | −1.99 | −.10 | .05 | 102 | −2.15 | −.11 | .03 |
| Left pars triangularis | 114 | −2.61 | −.13 | .01 | 102 | −2.38 | −.12 | .02 |
| Left pars orbitalis | 114 | −2.03 | −.10 | .04 | 102 | −1.91 | −.10 | .06 |
| Right medial orbital frontal cortex | 114 | −2.44 | −.12 | .02 | 102 | −2.16 | −.11 | .03 |
Batch/Site Only=Analyses adjusted for batch and site effects only. All Covariates=Analyses adjusted for batch and site effects plus demographic and health covariates. Associations that were not significant at the .05 level in either analysis are not shown. Associations that were significant at the .05 level are shown in bold; associations that were significant at trend levels (p<.10) are shown in italics.
All analyses were based on mixed models that account for non-independence of observations within twin pairs.
The results were similar for cortisol AUC (Table 3). Thinner cortex in eight prefrontal regions was associated with larger AUC values: left and right superior frontal gyrus; left rostral middle frontal gyrus; left pars opercularis; left pars triangularis; right lateral orbital frontal cortex; and left and right medial orbital frontal cortex (rs=−.10 to −.15; ps=.05 to .004). After adjusting for covariates, the associations remained significant between four of these prefrontal regains and cortisol AUC: left pars opercularis, right lateral orbital frontal cortex; and left and right medial orbital frontal cortex (rs=−.10 to −.13; ps=.05 to .01). The area under the curve for the CAR was not significantly associated with any of these brain regions. There were no significant associations between cortisol level and the surface area of the prefrontal and anterior cingulate ROIs, and there were no significant associations between any of the cortisol measures and hippocampal volumes.
Table 3.
Association of cortisol and prefrontal cortical thickness: Mixed model results and phenotypic correlations for cortisol area under the curve
| Region of Interest | Batch/Site Only (n=387)a | All Covariates (n=382)a | ||||||
|---|---|---|---|---|---|---|---|---|
| df | t | rb | P | df | t | r | p | |
| Left superior frontal gyrus | 113 | −2.12 | −.11 | .04 | 101 | −1.55 | −.08 | .13 |
| Right superior frontal gyrus | 113 | −2.07 | −.10 | .04 | 101 | −1.60 | −.08 | .11 |
| Left rostral middle frontal gyrus | 113 | −2.20 | −.11 | .03 | 101 | −1.55 | −.08 | .12 |
| Left pars opercularis | 113 | −1.95 | −.10 | .05 | 101 | −1.95 | −.10 | .05 |
| Left pars triangularis | 113 | −2.16 | −.11 | .03 | 101 | −1.90 | −.10 | .06 |
| Right lateral orbital frontal cortex | 113 | −2.04 | −.10 | .04 | 101 | −1.95 | −.10 | .05 |
| Left medial orbital frontal cortex | 113 | −2.07 | −.10 | .04 | 101 | −2.00 | −.10 | .05 |
| Right medial orbital frontal cortex | 113 | −2.91 | −.15 | .004 | 101 | −2.63 | −.13 | .01 |
Batch/Site Only=Analyses adjusted for batch and site effects only. All Covariates=Analyses adjusted for batch and site effects plus demographic and health covariates. Associations that were not significant at the .05 level in either analysis are not shown. Associations that were significant at the .05 level are shown in bold; associations that were significant at trend levels (p<.10) are shown in italics.
All analyses were based on mixed models that account for non-independence of observations within twin pairs.
Bivariate Twin Analyses
We performed bivariate twin analyses for the ROIs that were significantly correlated with mean cortisol levels at the phenotypic level. These analyses were limited to mean cortisol levels because, as noted in the introduction, mean cortisol had the highest heritability of the cortisol measures, and the brain regions with significant associations in the other analyses were largely consistent with those for mean cortisol levels. In previous univariate analyses, the heritability of mean cortisol level during the study was .43, but it was only .18 for the AUC (submitted manuscript). Heritabilities for the relevant prefrontal regions were: left superior frontal gyrus (.75); right superior frontal gyrus (.68); left rostral middle frontal gyrus (.45); left pars opercularis (.62); left pars triangularis (.44); left pars orbitalis (.37) and right medial orbital frontal cortex (.39) (submitted manuscript).
Table 4 shows the results of the model fitting for the mean cortisol level and left superior frontal gyrus. In the full model, genetic and environmental factors are allowed to covary between the two measures. The reduced models were compared to the full model to determine whether dropping particular parameters would result in a significant reduction in the fit of the model. This procedure is used to establish the most parsimonious explanation of the covariance. As shown in Table 4, Model 6—in which both the shared environmental (rc) and the unique environmental (re) correlations are dropped—was the most parsimonious model. In other words, the covariance between mean cortisol level and left superior frontal gyrus thickness can be explained by the genetic correlation alone without a significant reduction in model fit. In this model, rg=−.61 (95% CI=−1.00 to −0.17). Similarly, for the right superior frontal gyrus, rc and re could be dropped from the model. Here again, the genetic correlation alone provided an adequate explanation of the data (rg=−.70; (95% CI=−1.00 to −.14).
Table 4.
Model fitting results for bivariate genetic analysis of mean cortisol level and superior frontal gyrus thickness
| −2LL | df | LRT | Δdf | p | AIC | |
|---|---|---|---|---|---|---|
| 1 Full Model | 2285.938 | 851 | - | - | - | - |
| 2 Drop rg | 2301.032 | 852 | 15.095 | 1 | <.001 | 13.095 |
| 3 Drop rc | 2286.869 | 852 | 0.931 | 1 | .335 | −1.069 |
| 4 Drop re | 2287.988 | 852 | 2.051 | 1 | .152 | 0.051 |
| 5 Drop rg and rc | 2301.044 | 853 | 15.107 | 2 | .001 | 11.107 |
| 6 Drop rc and re | 2288.491 | 853 | 2.554 | 2 | .279 | −1.446 |
| 7 Drop rg and re | 2301.337 | 853 | 15.399 | 2 | <.001 | 11.399 |
| 8 Drop all covariance parameters | 2326.287 | 854 | 40.349 | 3 | <.001 | 34.349 |
−2LL=−2 log-likelihood; LRT=likelihood ratio chi-square test; Δdf=change in df; AIC=Akaike information criterion; rg=genetic correlation; rc=shared (common) environmental correlation; re=individual-specific environmental correlation.
Models 2–8 are tested against the full (Cholesky) model. The most parsimonious (best-fitting) model is shown in boldface type; it indicates that the phenotypic correlation between the two measures can be accounted for almost entirely by genetic factors.
In the full models for left pars opercularis, left pars triangularis, and right medial orbital frontal cortex, the genetic correlations ranged from −.23 to −.68. These genetic correlations were not significant in the full model, but rc and re could still be dropped from the models without a significant reduction in fit. In the full models for left rostral middle frontal gyrus and left pars orbitalis, rg and rc could be dropped without a significant loss of fit. In the full models, neither shared environmental (rc) nor unique environmental (re) correlations between an ROI and a given cortisol measure were statistically significant
Discussion
We found that higher salivary cortisol levels were associated with thinner cortex in prefrontal regions in a large-scale study of middle-aged men. Significant associations were observed for mean cortisol level and AUC, but not CAR. Neither cortisol index was significantly associated with cortical surface measures. The significant phenotypic correlations were of small magnitude, but they can be considered reliable given our large sample size. In our effort to have a representative sample, we did not exclude participants on the basis of health measures. However, adjusting for the effects of age, general cognitive ability, depression, cardiovascular risk factors, diabetes, smoking, and alcohol use had relatively little impact on the overall results. There were no significant associations of hippocampal volumes with any cortisol measures. As seen in Figure 2, the significant correlations between cortisol level and prefrontal ROIs were in partially contiguous regions. This outcome suggests a meaningful pattern rather than a handful of chance results. Our data showing that higher salivary cortisol levels were significantly correlated with prefrontal cortical thinning, and that this association may be genetically regulated may provide insight into the susceptibility of cognitive function to the dysregulation of cortisol secretion during aging.
As noted in the introduction, previous studies have frequently focused on selected participants, and many of the associations between cortisol levels and hippocampal volume were found in relatively extreme groups. In contrast, the VETSA sample is representative of men in this age range. It may be that associations are of greater magnitude at the extremes (e.g., disease states such as Alzheimer’s or Cushing’s; high vs. low dexamethasone suppression response; hypertensive vs. normotensive), but are of lesser magnitude in our non-patient sample.
To our knowledge, this was also the first study to examine the underlying genetic and environmental influences on the existing relationships between cortisol levels and brain structure. There were significant genetic correlations in two of the seven bivariate twin models. Those genetic correlations of −.61 for left and −.70 for right superior frontal gyrus, respectively, indicate that most of the correlations were accounted for by common genes that influence both cortisol level and superior frontal gyrus thickness. Some of the other genetic correlations were moderate but they were not significant. None of the shared or unique environmental correlations was significant. For three prefrontal regions—pars opercularis, left pars triangularis, and right medial orbital frontal cortex—the environmental correlations could be dropped from the models even though the genetic correlations in the full model were not significant. This result suggests that common genes were still important determinants of the cortisol-prefrontal correlations, but even with our relatively large sample size, the study was underpowered to demonstrate that conclusively. Like the phenotypic correlations, the genetic correlations were negative, indicating that genes that result in increased cortisol levels also tend to result in thinner prefrontal cortex.
Several previous studies have examined prefrontal cortex as a single region, but significant phenotypic associations in the present study were found in selected prefrontal subregions. In the left hemisphere these regions showed a partial gradient from superior to inferior frontal gyri that included dorsolateral (Brodmann areas [BA] 9 and 46) and ventrolateral (BA 47, 45, and 44; inferior frontal gyrus) regions. In the right hemisphere the regions included dorsolateral (BA 9 and 46) and orbital-medial (BA 10, 11 and 12) prefrontal cortex. Decreased glucose metabolism in BA 9 and 10 has been associated with cortisol increases during a 25-minute stress test (Kern et al., 2008). This regional overlap with some of our structural findings supports the link between these regions and cortisol output.
The original impetus for examining relationships between cortisol levels and prefrontal brain regions was the fact that glucocorticoid receptors are highly expressed in prefrontal cortex but the links have less well studied compared with the hippocampus. We considered average cortisol level and AUC for the day of testing as indices reflecting long-term corticosteroid exposure. It seems mostly likely that the association of cortisol with brain structure would reflect long-term exposure. At least two factors can account for high levels of cortisol: 1) responsivity that occurs in response to stressors or challenges in which the system is activated and rapidly returns to basal levels; and 2) dysregulation of the system that leads to chronic elevations even in the absence of challenge. It is important to note that responsivity does not imply a pathological process as it occurs naturally daily in response to awakening (CAR). Persistently high levels, however, may be pathological and may preclude the unfolding of processes that are inhibited by cortisol and may even cause retraction of dendritic arborization and loss of neurons (e.g., Shansky et al., 2009). Change in the CAR in response to an acute challenge may reflect responsivity, but without a clear before-and-after comparison, the CAR may also reflect long-term response to stress in the present study. It is also difficult to explain how the response to stressors on a given day could measurably affect brain structure. Given that the other factors we examined did not appear to mediate the association between cortisol levels and brain structure, it seems reasonable to conclude that the thinning of some prefrontal cortical regions may be related to HPA dysregulation associated with chronic secretion of glucocorticoids.
Our results cannot shed light on the direction of this relationship. Chronically elevated glucocorticoids have been related to brain volume reductions and cognitive impairment in humans (Lupien et al., 1999), and chronic stress and exogenous glucocorticoids results in brain atrophy in rats (Cerqueira et al., 2005; Cook and Wellman, 2004). On the other hand, factors causing prefrontal cortical thinning might result in disruptions of HPA axis functioning considering that studies indicate the prefrontal cortex exerts an inhibitory action on stress-sensitive neurons in the extended amygdala. Prefrontal lesions in humans can result in elevated morning cortisol levels (Tchiteya et al., 2003), and animal studies have shown that medial prefrontal lesions result in HPA axis dysregulation (Gerrits et al., 2003). Genetic factors have received little or no attention when researchers have described these prefrontal-cortisol links. As the VETSA participants age, these different factors may also combine to have a significant mediating or moderating effect on the association between cortisol and brain structure.
Additional evidence suggests that the direction of associations between cognition and hippocampal volume are opposite in younger and older individuals (Pruessner et al., 2007). Thus, it is possible that even the direction of the relationship between cortisol and prefrontal cortical structure might change with age. Given the fact that aging has been associated with both HPA axis dysfunction and frontal lobe shrinkage, it is entirely possible that there are bidirectional effects such that both processes increasingly affect each other as a person ages. A goal of the VETSA follow-up assessments, which are now underway, is to shed light on the genetic influences underlying changes in both brain structure and HPA axis functioning. Whether or not One way to assess directionality would be to see if genes that influence HPA axis functioning (e.g., CRF gene) are associated with prefrontal cortical thinning over time.
Strengths of the study include the large sample size, use of cortisol measurements that were based on multiple (5) time points, and the ability to examine the genetic underpinnings of the associations between cortisol and brain structure. Some limitations should be noted as well. Like each of the studies cited that examined cortisol and prefrontal or cingulate cortex structure, our sample included only men. Therefore, these relationships need to be examined in women. We do not know whether these associations are the same at different ages, and it is not possible to determine the direction of effect in these cross-sectional analyses. It is a goal of the VETSA to follow participants as they age, which would enable us to address the issue of changes in mid-to-later life.
In sum, consistent with theory and previous evidence, prefrontal regions were associated with cortisol level. We observed small, but significant negative correlations of cortisol level with thickness in left dorsolateral and ventrolateral prefrontal cortex, and right dorsolateral and orbital-medial frontal cortex. There were no significant associations with anterior cingulate cortex thickness or hippocampal volume, but given the small size of the significant associations with prefrontal regions, it may be best to think of these associations as being different in degree rather than present versus absent. For the most part, the associations were not due to general cognitive ability, cardiovascular risk factors, or depression. The results suggest that at least some of the associations were primarily accounted for by shared genetic influences, but the study was underpowered to draw a definitive conclusion in this regard. Stress in animal models has been shown to decrease dendritic arborization and spine density of prefrontal cortex neurons (Shansky et al., 2009). Furthermore, attentional control and prefrontal cortical processing were disrupted in young individuals exposed to experimental conditions with increased psychosocial stress (Liston et al., 2009). It is likely that alteration in prefrontal cortex functioning in both paradigms resulted from increased cortisol secretion. We hypothesize that stress-cortisol-prefrontal cortex interactions may be even more pronounced in humans as they age. Moreover, genetic factors may increase an individual’s sensitivity to the detrimental impact of glucorticoids on prefrontal cortex during aging. Alternatively, genetic factors may confer “resilience” to cortisol-prefrontal cortex interactions. Longitudinal analysis could shed light on the direction or on changes in the degree of the associations with age. The demographic and health factors that we assessed may also begin to have greater impacts on the association of cortisol levels and brain structure as these study participants age.
Acknowledgments
Funding
Funded by National Institute on Aging (AG022982, AG022381, AG018384, AG018386); National Center for Research Resources (P41-RR14075; NCRR BIRN Morphometric Project BIRN002); National Institute for Biomedical Imaging and Bioengineering ( R01EB006758); National Institute for Neurological Disorders and Stroke (R01 NS052585-01); Mental Illness and Neuroscience Discovery (MIND) Institute, part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149. Additional support was provided by The Autism & Dyslexia Project funded by the Ellison Medical Foundation. The U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
References
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJ. Heritability of cortisol levels: Review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28:121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Iranmanesh A, Mulligan T, Veldhuis JD. Impact of age on cortisol secretory dynamics basally and as driven by nutrient-withdrawal stress. Journal of Clinical Endocrinology and Metabolism. 2000;85:2203–2214. doi: 10.1210/jcem.85.6.6628. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Public health and aging: Trends in aging--United States and worldwide. MMWR CDC Surveillance Summaries. 2003;52:101–106. [Google Scholar]
- Cerqueira JJ, Catania C, Sotiropoulos I, Schubert M, Kalisch R, Almeida OF, Auer DP, Sousa N. Corticosteroid status influences the volume of the rat cingulate cortex: A magnetic resonance imaging study. Journal of Psychiatric Research. 2005;39:451–460. doi: 10.1016/j.jpsychires.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I: Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sciences. 1997;61:2239–2246. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behavior. Heredity. 1978;41:249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, Buckner R, Killiany R, Blacker D, Dale AM, Fischl B, Dickerson B, Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte SB, Fioravanti M, Cuzzoni G, Ponitggia B, Magri F. Age-related changes of the hypothalamic-pituitary-adrenal axis: Pathophysiological correlates. European Journal of Endocrinology. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gerrits M, Westenbroek C, Fokkema DS, Jongsma ME, Den Boer JA, Ter Horst GJ. Increased stress vulnerability after a prefrontal cortex lesion in female rats. Brain Research Bulletin. 2003;61:627–635. doi: 10.1016/j.brainresbull.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Rogers K, Bayoumy A, McHugh PF, Convit A. Hypertension and hypothalamo-pituitary-adrenal axis hyperactivity affect frontal lobe integrity. Journal of Clinical Endocrinology and Metabolism. 2005;90:3262–3267. doi: 10.1210/jc.2004-2181. [DOI] [PubMed] [Google Scholar]
- Grafman J, Jonas BS, Martin A, Salazar AM, Weingartner H, Ludlow C, Smutok MA, Vance SC. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111:169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS and Neurological Disorders - Drug Targets. 2006;5:453–749. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Inglis GC, Ingram MC, Holloway CD, Swan L, Birnie D, Hillis WS, Davies E, Fraser R, Connell JMC. Familial pattern of corticosteroids and their metabolism in adult human subjects -The Scottish Adult Twin Study. Journal of Clinical Endocrinology and Metabolism. 1999;84:4132–4137. doi: 10.1210/jcem.84.11.6146. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale AM. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behavioral Neuroscience. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. Journal of Clinical Endocrinology and Metabolism. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Levene RZ, Schwartz B, Workman PL. Heritability of plasma cortisol. Archives of Ophthalmology. 1972;87:389–391. doi: 10.1001/archopht.1972.01000020391004. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the Nationall Academy of Sciences U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NPV, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. The Journal of Neuroscience. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Nair NPV, Briere S, Maheu F, Tu MT, Lemay M, McEwen BS, Meaney MJ. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia in later life. Reviews in Neurosciences. 1999;10:117–139. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Deary IJ, Starr JM, Ferguson KJ, Wardlaw JM, Seckl JR. Plasma cortisol levels, brain volumes and cognition in healthy elderly men. Psychoneuroendocrinology. 2005;30:505–515. doi: 10.1016/j.psyneuen.2004.12.005. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. Jouranl of Clinical Endocrinology and Metabolism. 2006;91:1591–1594. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weis JM, Schwartz LS. Selective retention of corticosterone by limbic structure in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGrevy DF, Knouse SB, Thompson RA. Personnel Research Division, Air Force Human Resources Laboratory Technical Report, AFHRL-TR-74-25. Brooks Air Force Base; TX: 1974. Relationships among an individual intelligence test and two air force screening and selection tests. [Google Scholar]
- Meikle AW, Stringham JD, Woodward MG, Bishop DT. Heritability of variation of plasma cortisol levels. Metabolism: Clinical and Experimental. 1988;37:514–517. doi: 10.1016/0026-0495(88)90164-3. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: Involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- National Center for Disease Statistics. Health, United States, 2003. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2003. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Department of Psychiatry, Medical College of Virginia; Richmond, VA: 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1992. [Google Scholar]
- O’Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer’s disease. British Journal of Psychiatry. 1996;168:679–687. doi: 10.1192/bjp.168.6.679. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Prom-Wormley E, Neale MC, Jacobson KC, Lyons MJ, Franz CE, Fischl B, Seidman LJ, Dale AM, Kremen WS. Is cortical volume the correct phenotype for studying the aging brain? Behavior Genetics Association; Louisville, KY: 2008. [Google Scholar]
- Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, Wolf PA. Association of alcohol consumption with brain volume in the Framingham study. Archives of Neurology. 2008;65:1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Fraga, MF. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Pruessner JC, Hellhammer DH, Bruce Pike G, Lupien SJ. The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Research: Neuroimaging. 2007;155:1–10. doi: 10.1016/j.pscychresns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiology of Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends in Neuroscience. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. The radial edifice of cortical architecture: From neuronal silhouettes to genetic engineering. Brain Research Review. 2007;55:204–219. doi: 10.1016/j.brainresrev.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Erlbaum; Hillsdale, NJ: 2000. pp. 1–90. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30 doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinology Review. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sarrieau AS, Dussaillant M, Agid F, Moguilewsky M, Philibert D, Agid Y, Rostene WH. Radioautographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. Journal of Steroid Biochemistry. 1986;25:717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide. Vol. 8. SAS Institute; Carey, NC: 2000. [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Research and Human Genetics. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors C, Hallerback T, Larsson LG, Wallsten C, Ross SB. Pharmacology of a novel selective 5-hydroxytryptamine1B receptor antagonist, AR-A000002. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2004;369:330–337. doi: 10.1007/s00210-004-0866-0. [DOI] [PubMed] [Google Scholar]
- Tchiteya BM, Lecours AR, Elie R, Lupien SJ. Impact of a unilateral brain lesion on cortisol secretion and emotional state: Anterior/posterior dissociation in humans. Psychoneuroendocrinology. 2003;28:674–686. doi: 10.1016/s0306-4530(02)00050-1. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. Journal of Clinical Endocrinology and Metabolism. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Journal of the American Medical Association. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Fischl B, Salat D, Quinn BT, Makris N, Dale AM. Cortical volume and speed-of-processing are complementary in prediction of performance intelligence. Neuropsychologia. 2005;43:704–713. doi: 10.1016/j.neuropsychologia.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiology of Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]