Abstract
Clinical studies of patients with chronic myeloid leukemia (CML) revealed that a common pattern of response is a dramatic fall in the circulating population of blast cells, with a minimal or delayed decrease in marrow blasts, suggesting a protective environment. These observations suggest that a greater understanding of the interaction of stromal cells with leukemic cells is essential. Here, we present an in vivo system for monitoring relative tumor accumulation in leukemic mice and residual disease in leukemic mice treated with a tyrosine kinase inhibitor, and an in vitro system for identifying integral factors involved in stromal-mediated cytoprotection. Using the in vivo model, we observed high tumor burden/residual disease in tissues characterized as significant sources of hematopoiesis-promoting stroma, with bone marrow stroma most frequently showing the highest accumulation of leukemia in untreated and nilotinib-treated mice, as well as partial protection of leukemic cells from the inhibitory effects of nilotinib. These studies, which showed a pattern of leukemia distribution consistent with what is observed in imatinib- and nilotinib-treated CML patients, were followed by a more in-depth analysis of stroma-leukemia cell interactions that lead to protection of leukemia cells from nilotinib-induced cytotoxicity. For the latter, we used the human BCR-ABL-positive cell line, KU812F, and the human bone marrow stroma cell line, HS-5, to more closely approximate the bone marrow-associated cytoprotection observed in drug-treated leukemia patients. This in vitro system helped to elucidate stromal-secreted viability factors that may play a role in stromal-mediated cytoprotection of tyrosine kinase inhibitor-treated leukemia cells.
Keywords: leukemias and lymphomas, tumor-stromal cell interactions, protein tyrosine kinases, drug design and optimization, clinical drug resistance
Introduction
There is a growing level of interest in determining the role of the microenvironment, such as stromal cells in the marrow, in regulating growth, self-renewal, and drug resistance of leukemic stem cells. This is likely to be of more than passing interest since it appears possible that small numbers of leukemic CD34+ cells can persist in the marrow microenvironment of patients with CML following years of therapy with imatinib mesylate (Gleevec®, STI571; Novartis Pharma AG). Indeed, the number of leukemic stem cells that exist and that are dependent on stroma to survive is predictive of disease outcome (1).
Hematopoietic and stromal cells, along with other factors including extracellular matrix and vessels, comprise bone marrow; cell-cell interactions and growth factors influence the rate of hematopoiesis (2). The stroma of hematopoietic organs contributes to the development not only of normal hematopoietic cells, but also to that of leukemia cells (3,4). Specifically, bone marrow stroma is the source of signals, such as stem cell factor (SCF), granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF), which either mediate the expansion of, or prevention of the terminal differentiation of hematopoietic stem cells, and that support leukemia cell growth (5-11). Bone marrow stroma and stromal cell-derived soluble factors have been implicated in the long-term survival and growth of various hematologic malignancies, including precursor B-acute lymphoblastic leukemia (ALL) (12, 13). Bone marrow stroma has also been shown to prevent apoptosis of acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL) cells (14-16). In addition, leukemic lymphoblasts that are coupled to bone marrow stroma via gap junction communication are proposed to be held in a quiescent, non-dividing state, which is believed to contribute to resistance to antimitotic agents (17). Splenic stroma and splenocytes also play an important role in the support of the viability and proliferation of both normal and malignant hematopoiesis (18,19).
Here, we track the progression of leukemia growth using an in vivo bioluminescence model of leukemia, which includes monitoring the relative degree and localization of tumor burden in different tissue sources in untreated mice, and the relative degree and localization of residual disease in mice treated with a range of doses of the novel, selective ABL tyrosine kinase inhibitor, nilotinib (AMN107; Tasigna) for varying lengths of time. Using this in vivo model, we demonstrate how leukemia appears to migrate to stroma-associated tissues, with the highest tumor burden accumulating in these regions. This finding mimics the trend that is observed in tyrosine kinase-inhibitor-treated CML and AML patients (residual disease observed in the bone marrow stroma). For the latter half of our studies, we seek to identify the factor(s) mediating the effects of stromal-enhanced viability of leukemia growth.
Materials and Methods
Cell Lines
The human CML cell line, KU812, and the HS-5 bone marrow stromal cell line were purchased from American Type Culture Collection (Rockville, MD). Murine hematopoietic 32D cells were transduced with retrovirus to express p210 BCR-ABL (32D.p210 cells) (20); this cell line is rapidly lethal in syngeneic, non-immunosuppressed C3H mice. KU812F and 32D.p210 cells were transduced with a retrovirus encoding firefly luciferase (MSCV-Luc). Luciferase-expressing cells were selected with G418 at a concentration of 1mg/ml to produce the KU812F- luciferase (luc+) and 32D.p210-luc+ cell lines, respectively.
Cell lines were cultured with 5% CO2 at 37°C, at a concentration of 2×105 to 5×105 in RPMI (Mediatech, Inc., Herndon, VA) with 10% fetal calf serum (FCS) and supplemented with 1% glutamine. Transfected cell lines were cultured in media supplemented with 1mg/ml G418.
Chemical compounds and biologic reagents
Imatinib, nilotinib, and PKC412 were synthesized by Novartis Pharma AG, Basel, Switzerland, and, when used for in vitro experiments, were dissolved in DMSO to make 10 mM stock solutions. Serial dilutions were then made, also in DMSO, to obtain final dilutions for cellular assays.
In vitro proliferation studies
For stromal rescue studies involving the KU812F cell line, we introduced luciferase into the leukemic cells so that we could specifically quantify the viable cell number using light emission. Thus, luciferase expression, which positively correlates with cell viability, was used for proliferation studies involving protection of imatinib- or nilotinib-treated KU812F-luc by stromal-conditioned media (SCM) or cytokines. Luciferase expression in these cell lines was measured by a luminometer. Approximately 10,000 HS-5 stromal cells were plated, and then cultured in the absence of leukemia cells for the reported lengths of time before media was pooled and collected and used in proliferation studies measuring protection of drug (tyrosine kinase inhibitor)-treated cells by SCM.
CML patient cell studies
Frozen vials of bone marrow samples from CML (at least 50% blasts) were thawed and treated with Ficoll-Plaque (Pharmacia, Uppsala, Sweden). The trypan blue exclusion assay has been previously described (21) and was used for proliferation studies involving CML patient samples.
Mouse studies and in vivo imaging
32D.p210 cells were transduced with a retrovirus encoding firefly luciferase (MSCVLuc), and selected with G418 at a concentration of 1mg/ml to produce the 32D.p210-luc+ cell line. 32D.p210-luc+ cells free of Mycoplasma and viral contamination were washed once with Hank's Balanced Salt Solution (HBSS; Mediatech, Inc.,VA), and resuspended in HBSS prior to administration to mice. Nilotinib was formulated by first dissolving powder stock in NMP to give a clear solution (stored for several days at 4°C), and diluting 10-fold in PEG300 just prior to administration to mice. Gavage volumes were fixed according to the individual weights of the mice to achieve 20mg/kg or 100mg/kg nilotinib, depending on the study.
A total of 800,000 32D.p210-luc+ cells were administered via tail vein injection to male NCR-nude mice (5-6 weeks of age; Taconic, NY). Anesthesized mice were imaged 1 day post IV-injection to generate a baseline that was used to establish treatment cohorts with matched tumor burden, and total body luminescence was measured as previously described (22). Cohorts of mice were treated with either oral administration of vehicle (10% NMP-90% PEG300), or oral administration of nilotinib (formulated as above). Mice were monitored for a period of time following the last imaging day and prior to sacrifice. Tissues were preserved in 10% formalin for histopathological analysis and confirmation of tumor burden in vital organ tissues; tissues were also harvested to obtain cells for ex vivo analysis.
The low dose of 20mg/kg nilotinib was pre-determined to be subcurative in vivo (23) and the relatively high dose of 100mg/kg nilotinib was chosen based upon earlier findings that 75mg/kg/day nilotinib administered over a 16-day period was curative in the majority of treated mice over an observation period of approximately 100 days (24).
Results
Leukemia growth in NCR-nude mice treated with vehicle or high dose nilotinib
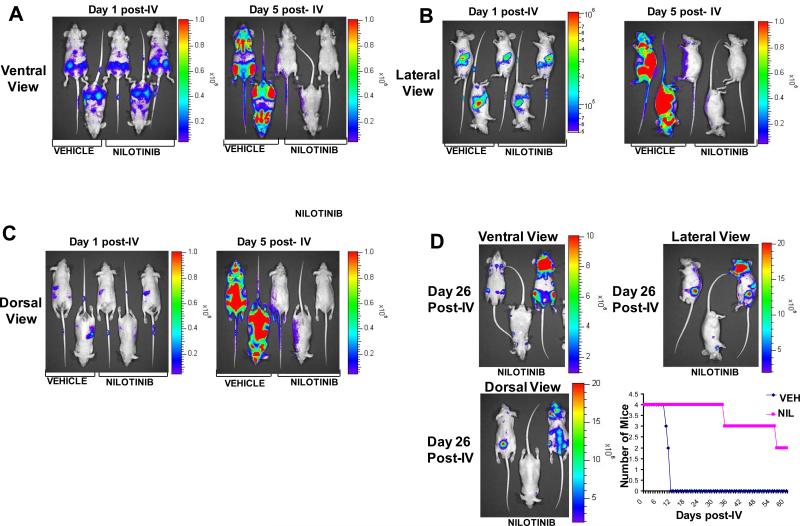
Using a bioluminescent in vivo model of BCR-ABL leukemia, we investigated the growth of BCR-ABL-positive leukemia in vehicle-treated mice, and the pattern of residual disease in nilotinib-treated mice. We observed highest tumor burden in areas of vehicle-treated mice that included the femurs and spleen at baseline, and the femurs, sternum, spine, and spleen of vehicle-treated mice on day 4 post-IV-injection of 32D.p210-luc+ cells (Figure 1A-C). A relatively high dose (100mg/kg) of nilotinib was administered to mice in the drug treatment group for 4 days prior to drug withdrawal. Treatment with this dose caused a dramatic reduction in leukemia burden in mice, although bioluminescence values, while low, were still measurable (Figure 1A-C, Figure 2).
Figure 1. Leukemia growth in NCR-nude mice treated with vehicle or high dose nilotinib.
Day 1 post-IV injection of 32D.p210-luc+ cells via tail vein injection are baseline images. NCR-nude mice were treated, starting on this first (baseline) imaging day, for 4 days with either vehicle or nilotinib (100mg/kg). (A) Ventral view images, day 1 and day 5 post-IV injection of 32D.p210-luc+ cells. (B) Lateral view images, day 1 and day 5 post-IV injection of 32D.p210-luc+ cells. (C) Dorsal view images, day 1 and day 5 post-IV injection of 32D.p210-luc+ cells. Representative mice shown. Order of mice in (A), (B), and (C), from left to right: VEH926, VEH929, NIL927, NIL930, NIL928. (D) Day 26 post-IV images of nilotinib (100mg/kg, 4 day) treated NCR-nude mice following drug withdrawal on day 5 post-IV injection of 32D.p210-luc+ cells. Upper left panel: ventral view. Upper right panel: lateral view. Lower left panel: dorsal view. Order of mice in (D), left to right: NIL927, NIL930, NIL928. Lower right panel: survival curve for vehicle- and nilotinib (100mg/kg, 4-days)-treated NCR-nude mice. Two surviving nilotinib-treated mice were sacrificed on day 61 post IV-injection of 32D.p210-luc+ cells. Mice were IV-injected with 800,000 32D.p210-luc+ cells, and were imaged one day later to establish baseline values.
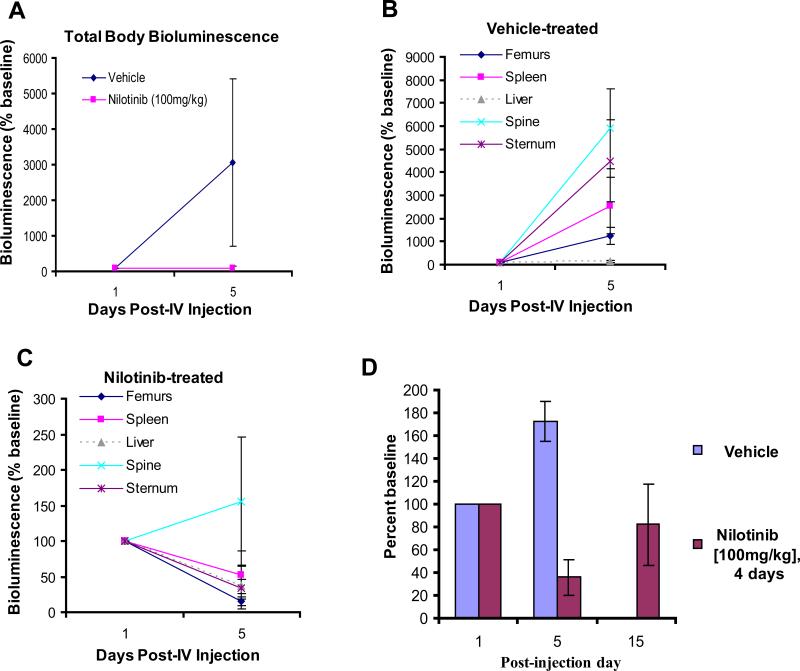
Figure 2. Anti-leukemia drug effects and relative tumor burden in NCR-nude mice treated with high dose nilotinib.
(A) Total body bioluminescence (mean+/-SEM of ventral, dorsal, and lateral views) in vehicle- and nilotinib-treated mice. (B) Relative tumor burden in vehicle-treated NCR-nude mice. (C) Relative tumor burden in nilotinib (100mg/kg, 4-day)-treated NCR-nude mice. Data for (B) and (C) presented as percent of each organ/area's baseline value. (D) White blood cell (WBC) counts (counted by hemacytometer and trypan blue exclusion following red blood cell lysis) in NCR-nude mice treated with vehicle or 100 mg/kg/day of nilotinib for 4 days. Drug was withdrawn on day 5 post-IV, and WBC counts were taken again on Day 15 post-IV. WBC counts are shown as percent of control (baseline WBC counts on day 1 post-IV injection). p=0.003, post IV injection day 5.
Anti-leukemia drug effects and relative tumor burden in NCR-nude mice treated with high dose nilotinib
In addition to high dose nilotinib treatment lowering total body luminescence in mice, it also significantly lowered white blood cell (WBC) counts in mice by day 5 post-IV injection (Figure 2A, D) and led to a significant prolongation of survival as compared to vehicle-treated mice (Figure 1E). However, we observed a visually and measurably detectable recurrence of leukemia burden in the high dose (4-day) nilotinib-treated mice on day 19 post-IV injection of cells (supplementary data) and 26 post-IV (Figure 1D). High tumor burden areas included the femurs, sternum, spleen, and parts of the skull (Figure 1D). Bioluminescence was also observed in the shape, location, and orientation approximately corresponding to the shape, orientation, and anatomical location where superficial/deep cervical lymph nodes have been identified in the mouse (Figure 1D). WBC counts, while still lower in drug-treated mice than vehicle-treated mice on day 15 post-IV injection of leukemia cells, were observed to rise in number in the 4-day nilotinib-treated mice with drug having been withdrawn for 10 days (Figure 2D).
We investigated the relative tumor burden of those tissues in vehicle- and nilotinib-treated mice that either showed highest leukemia burden and are characterized by significant amounts of stroma (i.e. spine, femurs, sternum, spleen) or were known organ targets often used as a marker of leukemia progression (i.e. spleen, liver). Interestingly, in both vehicle- and high dose nilotinib-treated mice, the spine was observed to display the highest relative tumor burden (Figure 2B,C).
Anti-leukemia drug effects and relative tumor burden in NCR-nude mice treated with low dose nilotinib
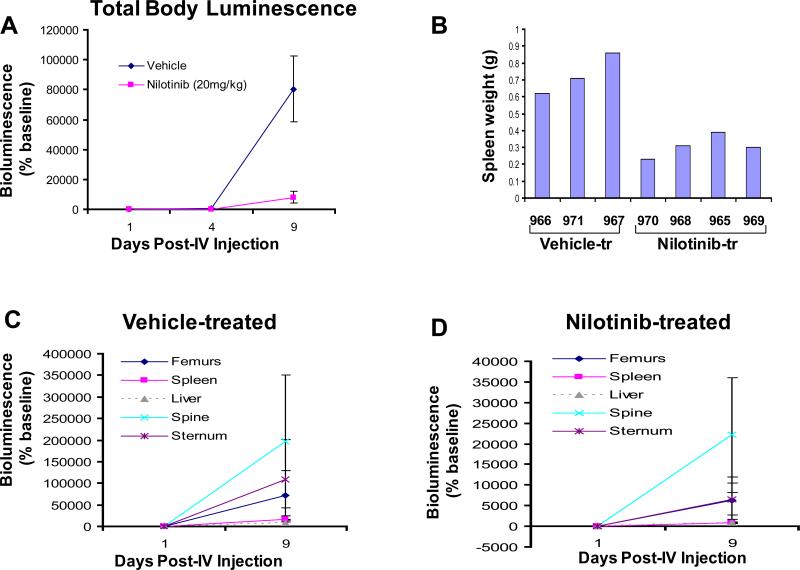
A relatively low dose (20mg/kg) of nilotinib, administered for a total of 8 days, similarly lowered total body luminescence in mice and prevented the increased spleen weights observed in vehicle-treated mice (Figure 3A, B and supplementary data). As with the high dose nilotinib study, the highest tumor burdens as measured by bioluminescence were observed in the femurs, sternum, spine and spleens of vehicle- and low dose nilotinib-treated mice (supplementary data). The relative tumor burden in vehicle- and low dose nilotinib-treated mice was again observed to be highest in the spine on days 4 and 9 post-IV injection of 32D.p210-luc+ cells, followed by femurs (Figure 3C,D and supplementary data).
Figure 3. Anti-leukemia drug effects, relative tumor burden, and stromal-mediated cytoprotection in NCR-nude mice treated with low dose nilotinib.
(A) Total body luminescence: Mean+/-SEM of ventral, dorsal, and lateral views of vehicle-treated and nilotinib (20mg/kg, 8-day)-treated NCR-nude mice. (B) Spleen weights (in grams) for vehicle-treated and nilotinib-treated NCR-nude mice measured on day 16 post-IV injection of 32D.p210-luc+ cells. (C) Relative tumor burden in vehicle-treated NCR-nude mice, days 1 and 9 post-IV injection of 32D.p210-luc+ cells. (D) Relative tumor burden in nilotinib (100mg/kg, 4-day)-treated NCR-nude mice, days 1 and 9 post-IV injection of 32D.p210-luc+ cells. Data for (C) and (D) presented as percent of each organ/area's baseline value.
In another study, we administered 20mg/kg of nilotinib to mice for a total of 3 days. As was observed in the other in vivo imaging studies, the highest tumor burdens as measured by bioluminescence were again observed in the femurs, sternum, spine and spleens of vehicle- and low dose nilotinib-treated mice (supplementary data). The relative tumor burden in low dose nilotinib-treated mice was again observed to be highest in the spine on day 4 post-IV injection of 32D.p210-luc+ cells (supplementary data). However, the spleen showed the highest relative tumor burden in vehicle-treated mice, as compared to spine, liver, and femurs (supplementary data). The overall similarity in the results obtained with high and low dose nilotinib suggests that residual disease in drug-treated mice is associated with tissues bearing high stroma content, regardless of the efficacy of the drug treatment.
Effects of SCM on the proliferation of BCR-ABL-expressing cells
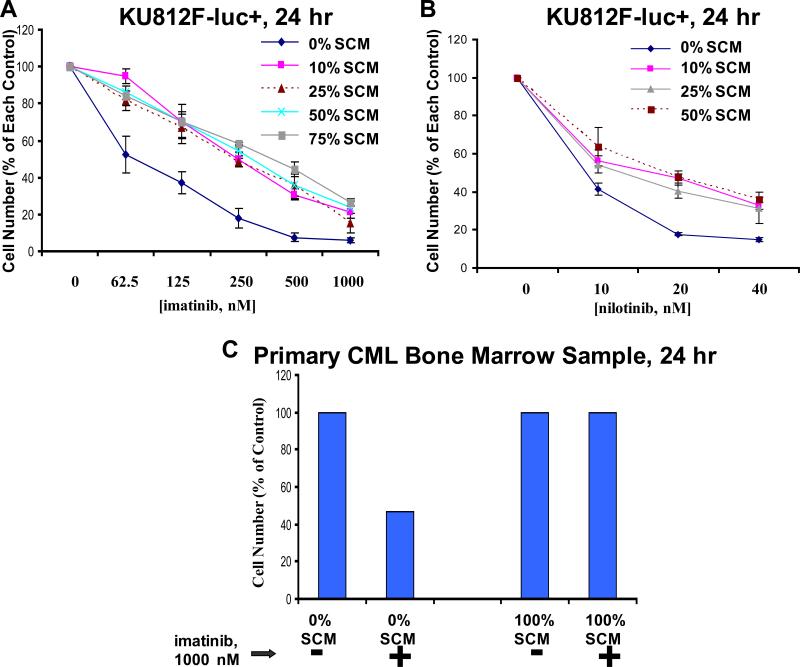
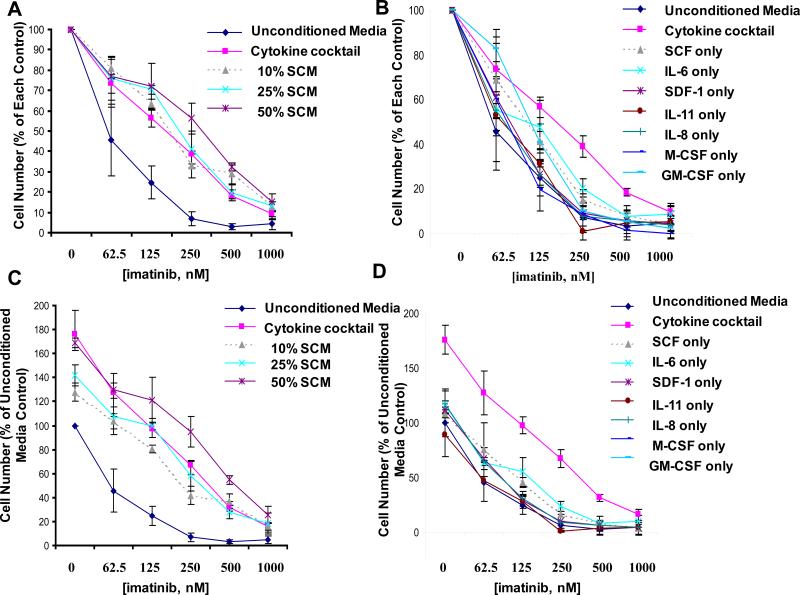
We investigated whether or not stromal-derived, secreted factor(s) could increase BCR-ABL-positive cell proliferation and/or protect cells against the inhibitory effects of selective tyrosine kinase inhibitors. To achieve this, culture media pooled and collected from HS-5 stromal cells cultured for one week was tested with KU812F-luc+ cells, incubated in the absence and presence of imatinib and nilotinib, respectively. We observed that SCM, diluted with FCS-containing RPMI media across a range of concentrations (10-75%), was able to partially protect KU812F-luc+ cells from the inhibitory effects of both imatinib and nilotinib, respectively (Figure 4A, B). In addition, a bone marrow sample derived from a CML-MBC (myeloid blast crisis) patient (not imatinib-naïve) was protected by the inhibitory effects of imatinib when cultured in the presence of SCM (Figure 4C). These results suggest that stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-positive cells appears to involve viability signals in the form of one or more secreted growth factors.
Figure 4. Effects of SCM on the proliferation of BCR-ABL-expressing cells.
Treatment of KU812F-luc+ cells for 24 hr with imatinib (A) or nilotinib (B) in the absence and presence of SCM at varying concentrations (collected and pooled from HS-5 stroma cultured for a total of 7 days). Cell number for drug-treated KU812F-luc+ cells cultured in the absence of SCM is shown as the percent of the no SCM control. Cell number for drug-treated cells cultured in the presence of each respective percentage of SCM is shown as the percent of each respective percentage of SCM control. Samples for this experiment were set up in triplicate, and error bars represent the standard error of the mean. (C) Treatment of a primary bone marrow CML-MBC patient sample (progressed s/p gleevec, HHT/ara-C) for 24 hr with imatinib in the absence and presence of SCM (media conditioned for 7 days in the presence of stromal cells).
Effects of human stroma-derived cytokines on the proliferation of BCR-ABL-expressing cells
To explore this possibility further, we randomly screened the effects of stromal derived factor-1 (SDF-1; 10ng/mL), which is constitutively produced by bone marrow stromal cells and behaves as a chemoattractant supporting the homing of stem cells, as well as a mixture of cytokines previously shown to be secreted at high concentrations in SCM derived from HS-5 stromal cells (including SCF, interleukin (IL)-6, IL-8, IL-11, M-CSF and GM-CSF; each at a concentration of 10ng/mL) (25), to determine if a cocktail of these cytokines would be able to protect imatinib-treated, BCR-ABL-positive cells from the inhibitory effects of the drug. We found the cytokine cocktail was able to potentiate the growth of untreated KU812F-luc+ cells and to partially protect imatinib-treated KU812F-luc+ cells to a similar extent as SCM across a range of concentrations (10-50%), which was collected and pooled from stromal cells cultured for 22 days (Figure 5A,C and supplementary data). When each cytokine (at 10ng/mL) was compared to the mixture of cytokines, we observed varying potentiation of KU812F-luc+ cell growth and protective capacities of the individual cytokines, all of which were generally less than that of the cytokine cocktail (Figure 5B,D). These results suggest that two or more of the tested cytokines are likely required in combination to achieve the maximum protection observed when drug-treated KU812F-luc+ cells are co-cultured with SCM or cytokine cocktail.
Figure 5. Effects of human stroma-derived cytokines on the proliferation of BCR-ABL-expressing cells.
(A) 24-hour treatment of KU812F-luc+ cells with imatinib in presence and absence of cytokine cocktail or SCM (conditioned for 22 days). Cell number for drug-treated KU812F-luc+ cells cultured in the absence of conditioned media is shown as the percent of the unconditioned media control, whereas cell number for drug-treated cells cultured in the presence of conditioned media is shown as the percent of the conditioned media control. Samples for this experiment were set up in triplicate, and error bars represent the standard error of the mean. (B) 24-hr treatment of KU812F-luc+ cells with imatinib in presence and absence of cytokine cocktail (10ng/mL of each cytokine pooled together) vs individual cytokines (10 ng/mL each). Values are presented as percent of each respective control. Samples for this experiment were set up in triplicate, and error bars represent the standard error of the mean. (C, D) Same as data shown in (A, B, respectively), except cell number for drug-treated cells cultured in the absence and presence of conditioned media, cytokine cocktail, or individual cytokines is shown as the percent of the unconditioned media control.
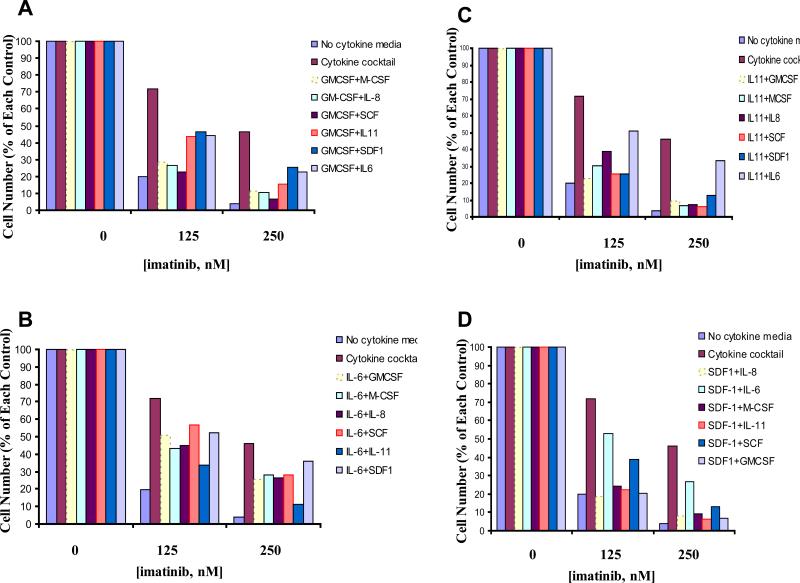
To further investigate this, we tested the combination of paired cytokines to assess the extent of the protective effects of each pair against imatinib-treated KU812F-luc+ cells as compared to the cytokine cocktail. We did observe varying degrees of protection with the different cytokine pairings, however, as was observed with individual cytokines, none of the paired cytokines were able to protect drug-treated cells to the full extent of the cytokine cocktail (Figure 6).
Figure 6. Effects of paired human stroma-derived cytokines on the proliferation of BCR-ABL-expressing cells.
KU812F-luc+ cells treated for 24 hr with imatinib in the absence and presence of paired cytokines. Cell number for drug-treated KU812F-luc+ cells cultured in the absence of cytokines is shown as the percent of the no cytokine control, whereas cell number for drug-treated cells cultured in the presence of cytokines is shown as the percent of the respective cytokine control. (A) Imatinib treatment of KU812F-luc+ cells in absence and presence of individual cytokines paired with GMCSF (10ng/mL). (B) Imatinib treatment of KU812F-luc+ cells in absence and presence of individual cytokines paired with IL-6 (10ng/mL). (C) Imatinib treatment of KU812F-luc+ cells in absence and presence of individual cytokines paired with IL-11 (10ng/mL). (D) Imatinib treatment of KU812F-luc+ cells in absence and presence of individual cytokines paired with SDF-1 (10ng/mL).
Discussion
Investigators have been actively exploring the relationship between malignant cells and the bone marrow microenvironment to better understand the source and nature of viability-promoting factors that can aid in disease progression. A recent study utilized a stromal cell system, derived via bone biopsy, to investigate the interactions between CLL B-cells, stroma cells, and stroma-secreted factors that protect the leukemic cells from naturally-occurring and drug-induced apoptosis (26).
In the present study, we monitored leukemia progression in vivo and noted that a variety of tissues showed high tumor accumulation, namely those characterized by high levels of bone marrow stroma (i.e. spine, sternum, skull) and those characterized by hematopoiesis-supporting stroma (i.e. spleen). These same tissues showed a high degree of residual disease in drug-treated mice, both acutely, as in the case of relatively low dose (20mg/kg) nilotinib-treated mice observed shortly after drug withdrawal, and chronically, as in the case of relatively high dose (100mg/kg) nilotinib treated mice observed several weeks following drug withdrawal. These data are consistent with the findings of others that show movement of leukemia cells, including ALL and CLL, into the stroma of bone marrow (27-29).
While the spine of nilotinib-treated mice was found to consistently show the highest relative tumor burden when compared to other high tumor burden tissues, such as spleen, femurs and liver, the highest relative tumor burden in vehicle-treated mice was observed to be in both the spleen as well as the spine. This finding may be in part explained by the fact that soluble growth factors and cytokines produced by splenic stroma have been indicated in hematopoiesis from bone marrow (19), and viability signals provided by diseased splenocytes contribute to the survival and expansion of leukemic cells (18). These reports suggest that the spleen, like bone marrow, may be a significant source of viability signals that have the ability to promote leukemic cell growth.
The stromal compartment of the spleen influences the proliferation and viability of both normal and diseased hematopoietic cells. Studies have been performed that support the idea that splenic stroma supports the development of white blood cells, such as dendritic cells. Dendritic cell hematopoiesis is supported by long-term splenic stroma cultures (30), and dendritic cell development is supported by splenic stroma and stroma-derived growth factors (31). The differentiation of dendritic cells can be influenced by endothelial-like splenic stromal cells (32).
The splenic microenvironment has also been implicated in promotion of the viability and expansion of transformed cells. Indeed, enlargement of the spleen, or splenomegaly, characterizes many different hematological malignancies, such as CML, especially during the later stages of the disease (33). In vivo, spleen removal from erythroleukemic mice was observed to prolong survival (18). Furthermore, the in vitro proliferation of primary erythroleukemic blast cells co-cultered in the presence of either leukemic-derived spleen cells or the conditioned media derived from such cells was enhanced compared to the proliferation of primary erythroleukemic cells co-cultured with normal splenocytes, with elevated secretion by the diseased splenocytes of viability factors including IL-6, IL-12p70, IL-2, macrophage chemoattractant protein-5 (MCP-5), vascular endothelial growth factor-A (VEGF-A), soluble tumor necrosis factor receptor-1 (sTNFR1), and tumor necrosis factor-α (TNF-α) (18).
Both media conditioned by human stromal cells and a cocktail of cytokines secreted in high concentrations by stroma are able to partially protect BCR-ABL-expressing cells from the inhibitory effects of tyrosine kinase inhibitors, such as imatinib and nilotinib, on cellular proliferation. These findings are consistent with those of others, as cytokines such as IL-6 and GM-CSF have been found to protect myeloid leukemia cells from chemotherapy-induced apoptosis (34).
Unlike the present findings with BCR-ABL-positive leukemia, not all hematologic malignancies are able to be rescued from apoptosis by secreted factors in the absence of direct contact with stromal cells. For example, a need for direct bone marrow fibroblast cell-leukemic cell interaction was observed for protection of AML cells from apoptosis (35,36), and for protection of B-lineage ALL cells from chemotherapy-induced apoptosis (37). Similarly, protection of myeloma cells from drug-induced apoptosis is dependent upon both adhesion between bone marrow stromal cells and myeloma cells, and soluble factors induced by the cell-cell interaction (38). Finally, the adherence of CLL cells to bone marrow stromal cell layers was necessary for their protection from apoptosis (39).
Our results suggest that significant reservoirs for tumor expansion and accumulation appear to be tissues characterized by stroma having the ability to support normal hematopoietic and malignant stem cell development. In addition, we found that stromal cells secrete cytokines having the ability to partially rescue BCR-ABL-expressing cells from the cytotoxic effects of protein tyrosine kinase inhibitors such as imatinib and nilotinib.
In summary, we present a novel, qualitative and quantitative in vivo approach to tracking tumor progression. We also identify factors that may play a role in stromal-mediated cytoprotection from anti-leukemia agents. The panel of cytokines that we identified as collectively mimicking the cytoprotective effects of stroma offers significant insight into putative factors that could serve as molecular targets to improve the efficacy of existing therapies and minimize residual disease in patients. It is therefore anticipated that this information may be used in the development of new strategies to override stromal-mediated chemoresistance.
Supplementary Material
Acknowledgments
Grant Support: J.D.G is supported by NIH grant CA66996, and a Specialized Center of Research Award from the Leukemia and Lymphoma Society. J.D.G. is also supported by NIH grants CA36167 and DK50654. A.L.K. and J.D.G. have a financial interest with Novartis Pharma AG.
Abbreviations list
- CML
Chronic myeloid leukemia
- SCF
Stem Cell Factor
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte macrophage colony-stimulating factor
- ALL
Acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- CLL
chronic lymphocytic leukemia
- luc+
luciferase
- FCS
fetal calf serum
- SCM
stromal-conditioned media
- HBSS
Hank's Balanced Salt Solution
- WBC
white blood cell
- SDF-1
stromal derived factor-1
- IL
interleukin
- MCP-5
macrophage chemoattractant protein-5
- VEGF-A
vascular endothelial growth factor-A
- sTNFR1
soluble tumor necrosis factor receptor-1
- TNF-α
tumor necrosis factor-α
References
- 1.Kumagai M, Manabe A, Pui CH, Behm FG, Raimondi SC, Hancock ML, et al. Stroma-supported culture in childhood B-lineage acute lymphoblastic leukemia cells predicts treatment outcome. J Clin Invest. 1996;97:755–760. doi: 10.1172/JCI118474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996;87:4109–4119. [PubMed] [Google Scholar]
- 3.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27:375–386. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 4.Litwin C, Leong KG, Zapf R, Sutherland H, Naiman SC, Karsan A. Role of the microenvironment in promoting angiogenesis in acute myeloid leukemia. Am J Hematol. 2002;70:22–30. doi: 10.1002/ajh.10092. [DOI] [PubMed] [Google Scholar]
- 5.Verfaillie CM. Soluble factor(s) produced by human bone marrow stroma increase cytokine-induced proliferation and maturation of primitive hematopoietic progenitors while preventing their terminal differentiation. Blood. 1993;82:2045–53. [PubMed] [Google Scholar]
- 6.Liesveld JL, harbol AW, Abboud CN. Stem cell factor and stromal cell co-culture prevent apoptosis in a subculture of the megakaryoblastic cell line, UT-7. Leuk Res. 1996;20:591–600. doi: 10.1016/0145-2126(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 7.Harrison PR, Nibbs RJ, Bartholomew C, O'Prey J, Qiu J, Walker M, et al. Molecular mechanisms involved in long-term maintenance of erythroleukaemia cells by stromal cells. Leukemia. 1997;11(suppl 3):474–477. [PubMed] [Google Scholar]
- 8.Breems DA, Blokland EA, Ploemacher RE. Stroma-conditioned media improve expansion of human primitive hematopoietic stem cells and progenitor cells. Leukemia. 1997;11:142–50. doi: 10.1038/sj.leu.2400530. [DOI] [PubMed] [Google Scholar]
- 9.O'Prey J, Leslie N, Itoh K, Ostertag W, Bartholomew C, Harrison PR. Both stroma and stem cell factor maintain long-term growth of ELM erythroleukemia cells, but only stroma prevents erythroid differentiation in response to erythropoietin and interleukin-3. Blood. 1998;91:1548–1555. [PubMed] [Google Scholar]
- 10.Leslie NR, O'Prey J, Bartholomew C, Harrison PR. An activating mutation in the kit receptor abolishes the stroma requirement for growth of ELM erythroleukemia cells, but does not prevent their differentiation in response to erythropoietin. Blood. 1998;92:4798–4807. [PubMed] [Google Scholar]
- 11.Shih CC, Hu MC, Hu J, Medeiros J, Forman SJ. Long-term ex vivo maintenance and expansion of transplantable human hematopoietic stem cells. Blood. 1999;94:1623–36. [PubMed] [Google Scholar]
- 12.Bradstock K, Bianchi A, Makrynikola V, Filshie R, Gottlieb D. Long-term survival and proliferation of precursor-B acute lymphoblastic leukemia cells on human bone marrow stroma. Leukemia. 1996;10:813–820. [PubMed] [Google Scholar]
- 13.Ashley DM, Bol SJ, Kannourakis G. Human bone marrow stromal cell contact and suluble factors have different effects on the survival and proliferation of paediatric B-lineage acute lymphoblastic leukaemic blasts. Leuk Res. 1994;18:337–46. doi: 10.1016/0145-2126(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 14.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 15.Lagneaux L, Delforge A, De Bruyn C, Bernier M, Bron D. Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leukemia Lymphoma. 1999;35:445–453. doi: 10.1080/10428199909169609. [DOI] [PubMed] [Google Scholar]
- 16.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 17.Paraguassú-Braga FH, Borojevic R, Bouzas LF, Barcinski MA, Bonomo A. Bone marrow stroma inhibits proliferation and apoptosis in leukemic cells through gap junction-mediated cell communication. Cell Death and Differentiation. 2003;10:1101–1108. doi: 10.1038/sj.cdd.4401279. [DOI] [PubMed] [Google Scholar]
- 18.Shaked Y, Cervi D, Neuman M, Chen L, Klement G, Michaud CR, et al. The splenic microenvironment is a source of proangiogenesis/inflammatory mediators accelerating the expansion of murine erythroleukemic cells. Blood. 2005;105:4500–4507. doi: 10.1182/blood-2004-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Despars G, O'Neill HC. Splenic endothelial cell lines support development of dendritic cells from bone marrow. Stem Cells. 2006;24:1496–1504. doi: 10.1634/stemcells.2005-0530. [DOI] [PubMed] [Google Scholar]
- 20.Matulonis U, Salgia R, Okuda K, Druker B, Griffin JD. IL-3 and p210 BCR-ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line. Exp Hematol. 1993;21:1460–1466. [PubMed] [Google Scholar]
- 21.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitors PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, Den Boer ML, et al. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–83. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg E, Catley L, Wright RD, Moreno D, Banerji L, Ray A, et al. Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood. 2007;109:2112–20. doi: 10.1182/blood-2006-06-026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human Papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- 26.Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch.”. Leuk Res. 2007;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43:461–6. doi: 10.1080/10428190290011921. [DOI] [PubMed] [Google Scholar]
- 28.Miyake K, Hasunuma Y, Yagita H, Kimoto M. Requirement for VLA-4 and VLA-5 integrins in lymphoma cells binding to and migration beneath stromal cells in culture. J Cell Biol. 1992;119:653–662. doi: 10.1083/jcb.119.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makrynikola V, Bianchi A, Bradstock K, Gottlieb D, Hewson J. Migration of acute lymphoblastic leukemia into human bone marrow stroma. Leukemia. 1994;8:1734–43. [PubMed] [Google Scholar]
- 30.Ni K, O'Neill HC. Long-term stromal cultures produce dendritic-like cells. Brit J Haematol. 1997;97:710–725. doi: 10.1046/j.1365-2141.1997.00135.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson HL, Ni K, O'Neill HC. Proliferation of dendritic cell progenitors in long term culture is not dependent on granulocyte macrophage-colony stimulating factor. Exp Hematol. 2000;28:193–202. doi: 10.1016/s0301-472x(99)00146-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nature Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal DS. In: Hodgkin's disease and non-Hodgkin's lymphomas. 2nd ed. Murphy GP, Lenard RE, editors. American Cancer Society; Atlanta, GA: 1995. pp. 451–485. [Google Scholar]
- 34.Lotem J, Sachs L. Hematopoietic cytokines inhibit apoptosis induced by transforming growth factor beta 1 and cancer chemotherapy compounds in myeloid leukemic cells. Blood. 1992;80:1750–7. [PubMed] [Google Scholar]
- 35.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5). Exp Hematol. 2001;29:448–57. doi: 10.1016/s0301-472x(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 36.Bendall LJ, Daniel A, Kortlepel K, Gottlieb DJ. Bone marrow adherent layers inhibit apoptosis of acute myeloid leukemia cells. Exp Hematol. 1994;22:1252–60. [PubMed] [Google Scholar]
- 37.Mudry RE, Fortney JE, York T, Hall BM, Gibston LF. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96:1926–32. [PubMed] [Google Scholar]
- 38.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–82. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 39.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br. J. Haematol. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.