Abstract
The use of adult-tissue stem cells to treat gastrointestinal diseases holds much promise. A method for in vitro growth of gut stem cells and their use in repairing damaged intestines in mice has been described.
Adult-tissue stem cells have a property that makes them attractive to researchers in the emerging field of regenerative medicine. In contrast to most other cells from adult tissues, they can self-renew and generate the specific cell types that comprise the organs from which they are derived. Under the correct conditions, adult stem cells should be capable of generating fully functional, organ-specific tissues that would retain regenerative capacity and could restore the normal organ function of patients. In particular, stem-cell therapies might be beneficial for gastrointestinal disorders in which normal gut is lacking or damaged, as in short-bowel syndrome or inflammatory bowel disease. Now Yui and colleagues1 describe in Nature Medicine how single stem cells from the mouse colon can be grown in vitro to produce mini-organs of epithelial tissue that can engraft onto a damaged intestine in mice.
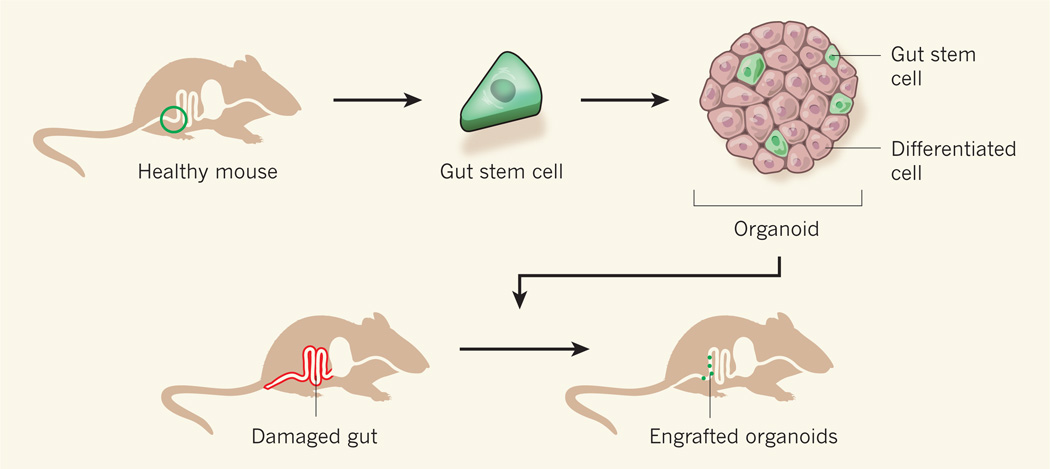
The authors’ work builds on the previous identification2 of the Lgr5 protein as a marker for gut stem cells and on the establishment3,4 of culture conditions that induce such cells to give rise to gut organoids — mini-organs that contain stem cells and other cell types that are typical of the intestinal epithelium. To test the possibility that gut stem cells could be used to regenerate intestinal tissue in live animals, Yui et al. isolated individual Lgr5-expressing stem cells from the colons of healthy mice (Fig. 1). These mice had been genetically engineered so that cells expressing Lgr5 also expressed a fluorescent protein, which facilitated their isolation. By culturing the individual stem cells, the researchers generated organoids and expanded them in vitro.
Figure 1. Fixing a damaged intestine.
Yui et al.1 isolated individual stem cells from the gut of healthy mice and grew them in vitro using a medium that induced the cells to produce mini-organs (organoids) that contained stem cells and other types of differentiated cells commonly found in the colon. The authors expanded the organoids in vitro and then introduced them into mice whose intestines had been superficially damaged. Some of the organoids successfully attached to the damaged areas and survived for the six-month duration of the experiment.
Yui et al. used the organoids to treat mice whose gut epithelial lining had been damaged in a way that resembles the effects of human ulcerative colitis — a form of inflammatory bowel disease. The authors introduced the organoids into the animals’ intestine using an enema, which is a drug-delivery method often used in humans. Remarkably, the transplanted organoids were found to attach to the damaged areas and generated an epithelium that was reminiscent of that of normal colon and contained all the relevant cell types. Furthermore, because the donor Lgr5-expressing cells were fluorescent, the researchers were able to trace the engrafted tissue and determine that it was still self-renewing 25 weeks after treatment. The treated mice gained more weight than untreated animals during the experiment, suggesting possible disease amelioration.
The authors’ findings1 have exciting implications. It has been shown5,6 that organoids isolated directly from normal intestine contain stem cells, can engraft onto damaged gut epithelium, and can support long-term epithelial regeneration. However, the current study goes one step further by showing that donor stem cells can be expanded in vitro to grow large numbers of organoids, which can then be transplanted and successfully engrafted into a host with a damaged gut. Therefore, using these techniques, stem cells from a patient’s gut could be cultured in the laboratory to generate organoids that could then be used to provide functional intestinal tissue to the original donor. The stem cells could be isolated from samples taken during routine diagnostic procedures such as gastrointestinal endoscopy. What’s more, the authors’ study suggests that an enema-based system might be a viable mode of delivery.
However, much work remains to be done before a similar treatment in humans could be considered. In addition to weight gain, other parameters that are indicative of accelerated tissue healing should be monitored in future studies. Such parameters include the lack of diarrhoea, of blood in stools and of microscopic tissue damage. It will also be crucial to show that the transplanted epithelium not only is apparently intact but also functions normally. In Yui and colleagues’ work, the organoids were engrafted only on areas of damaged tissue, which could be a limitation for the application of this approach in patients with short-bowel syndrome, a disorder in which areas of the small intestine are missing because of surgery or a birth defect. For these patients, engraftment onto the residual, normal intestine — in addition to generation of muscle, nerves and connective tissue — would be required.
Furthermore, the authors report a low rate of engraftment — this would need to be optimized to achieve reasonable therapeutic efficacy. Although it is encouraging that Yui et al. did not detect precancerous changes or polyps in the mice up to 25 weeks after transplantation, longer observation periods will be required to fully address the safety of the therapy. Despite these caveats, the study represents an important step towards realizing the promise of stem-cell-based therapies for gastrointestinal disorders.
References
- 1.Yui S, et al. Nature Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, et al. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, et al. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, et al. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Booth C, O’Shea JA, Potten CS. Exp. Cell. Res. 1999;249:359–366. doi: 10.1006/excr.1999.4483. [DOI] [PubMed] [Google Scholar]
- 6.Tait IS, Evans GS, Flint N, Campbell FC. Am. J. Surg. 1994;167:67–72. doi: 10.1016/0002-9610(94)90055-8. [DOI] [PubMed] [Google Scholar]