Abstract
Melanoma is the most dangerous form of skin cancer due to its metastatic potential and is an important public health concern. Melanoma incidence has been increasing worldwide. While potentially curable when diagnosed early, metastatic melanoma carries a poor prognosis. Until recently, systemic therapy for metastatic melanoma was ineffective, but the recent successes in the development of new therapies for metastatic melanoma, such as mitogen-activated protein kinase (MAPK) pathway inhibitors, anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) and Programmed cell death protein 1 (PD-1)/ Programmed cell death 1 ligand 1 (PD-L1) pathway blocking antibodies, as well as combinatorial strategies of cytotoxic chemotherapy and inhibitors of angiogenesis, have all yielded promising results, changing the continually evolving landscape of therapeutic options for patients with this disease. The aim of this review is to summarize the evolution of and recent advances in the treatment of metastatic melanoma. The present review is based on a comprehensive PubMed search between the dates of January 1, 1960, to November 15, 2013, using the search term melanoma or metastatic melanoma combined with terms, such as chemotherapy, immunotherapy, CTLA-4, PD-1, PDL-1, adoptive T cell, targeted therapy, MAPK, molecular biology and survival.
Introduction
The incidence rates of melanoma have been rising for the past 30 years and the malignancy ranks currently as the fifth most common cancer in men and sixth in women in the United States.1 In 2013, approximately 76,690 new cases of melanoma (45,060 in men and 31,630 in women) will be diagnosed, and an estimated 9,480 deaths will occur from this disease.1 As such, the lifetime probability of developing melanoma in the US is now estimated at 1 in 37 for men and 1 in 56 for women, considerably higher than just a few decades ago (1 in 600 for both sexes combined in 1960)1 Given the high incidence rates among young adults and the large number of deaths, melanoma results in significant years of potential life lost (YPLL) and lost productivity.2 Although a minority of patients present with distant metastases at diagnosis, approximately one quarter to one third of all melanoma patients will eventually experience recurrence and development of more advanced stage disease. It is currently estimated that there are nearly 1 million melanoma survivors living in the US.3–5 Although melanoma accounts for only 4% of all dermatologic cancers, it is responsible for 80% of deaths from skin cancer.6 While potentially curable when diagnosed early, historically the prognosis of patients with metastatic disease has been poor, with a median survival time of under 1 year and an overall 5-year mortality rate close to 90%.7 For almost 40 years before the approval of ipilimumab in 2011, no single drug or combination of drugs has demonstrated a significant impact on the overall survival of patients with metastatic melanoma.8 However, recent research in the fields of tumor biology and immunology has led to the development of new targeted agents promising immunotherapeutic agents which prolong progression-free survival and overall survival of patients with advanced melanoma. This review provides an overview of the latest advances in understanding the biology of melanoma and the treatment options for patients with advanced/metastatic disease. The present review is based on a comprehensive PubMed search between the dates of January 1, 1960, to November 15, 2013, using the search term melanoma or metastatic melanoma combined with terms, such as chemotherapy, immunotherapy, CTLA-4, PD-1, PDL-1, adoptive T cell, targeted therapy, MAPK, molecular biology and survival.
Role of Immunotherapy
The interplay between tumors and their immunologic microenvironments is complex, dynamic, and difficult to decipher; it is however, of pivotal importance for understanding the functionality and efficacy of immunotherapeutic drugs. The recent molecular characterization of various mechanisms mediated by cancer cells to evade immune detection has sharpened the focus of cancer immunotherapy to develop targeted molecules capable of manipulating the tumor microenvironment in favor of an antitumor immune response. The increased immune specificity of such agents has resulted in better tolerability and a more favorable side-effect profile than that of high dose interleukin 2 (IL-2), the first non-specific immunomodulator approved for the treatment of advanced/metastatic melanoma.
Individual human tumors harbor a multitude of gene mutations, the products of which are potentially recognizable as foreign antigens.9 Many, however, are mistakenly identified as “self” by the host immune system, thus aiding cancer cell escape from immune detection and allowing tumors to survive and grow. Indeed, escape from immune control is now viewed as one of the hallmarks of cancer.10
There is increasing evidence indicating that some patients with cancer mount an adaptive immune response specifically directed against antigenic proteins expressed in their tumors. T cells secrete cytokines, which in turn, generate acute inflammation that results in expansion of cytotoxic T cells (CTLs), tissue destruction, and the potential control or even elimination of malignancy.11 Unfortunately, T-cell functionality is impeded within cancers because the tumor milieu contains suppressive elements, including regulatory T cells and myeloid-derived suppressor cells, soluble factors such as IL-6, IL-10, vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), and ligands for co-inhibitory receptors that downregulate CTLs activity.12 Signaling via co-inhibitory receptors, or “checkpoint molecules” such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed death 1 (PD-1), contribute to the down-modulation of CD8+ and CD4+ effector T-cell function, making these receptors logical targets for drugs such as ipilimumab (anti-CTLA-4 monoclonal antibody), nivolumab and lambrolizumab (anti-PD-1 monoclonal antibodies). Melanoma cells have been shown to express high levels of PD-L1 (B7-H1) protein, a ligand for PD-1 receptor.13
Role of Targeted Therapy
It is becoming increasingly clear that melanoma, like other cancers, arises from complex molecular aberrations in genes that alter critical signaling pathways that control cell proliferation, differentiation, and cell death. Approximately 50% of melanomas contain mutations that activate the RAS/RAF/MEK/ERK (MAPK) pathway, making it a prime therapeutic target.14–16 The most common BRAF somatic mutation in cutaneous melanoma, particularly those without chronic sun damage, results from substitution of glutamic acid for valine at amino acid 600 in the gene encoding the serine-threonine protein kinase BRAF V600E.14 The second most common BRAF mutation is BRAF V600K substituting lysine for valine, that represents 5-6 %.17 Another reported mutation in melanoma of the skin that has not been chronically exposed to sun involves the NRAS gene, although such alterations seem to be less common (10-20%) than and mutually exclusive from BRAF.17,18 Genetic aberrations affecting the upstream tyrosine kinase-like KIT are less common and occur mainly in melanomas arising on the mucosa, acral skin, and skin with sun-induced damage, but generally do not occur in melanomas of the skin without chronic sun exposure .19 Mutations in PI3K/PTEN/AKT (AKT) signaling pathway are also observed, although at lower frequencies.20
Evolution of the Treatment of Metastatic Melanoma
Prior to 2011, Dacarbazine (DTIC) and IL-2 were the only FDA approved agents for the treatment of metastatic melanoma. The first milestone in the treatment of advanced melanoma occurred in 1975, with the FDA's approval of DTIC; yet, the agent has not been demonstrated in phase III trials to improve overall survival compared to placebo in metastatic melanoma, and is associated with modest response rates of 12%.21 Nevertheless, DTIC has been the reference chemotherapeutic treatment against which many of the newly developed therapies were tested. Temozolomide (TMZ, Temodar) is an orally available alkylating agent, analog of DTIC, with comparable efficacy, and a more favorable toxicity profile than DTIC21 and although not currently FDA approved for this indication, TMZ is routinely used for therapy of melanoma in lieu of intravenous DTIC. Multiple other chemotherapeutic agents have shown some activity in melanoma including nitrosureas (carmustine, lomustine, fotemustine), platinum analogues (cisplatin, carboplatin), vinca alkaloids (vinblastine, vindesine), and taxanes (paclitaxel, docetaxel).
The nitrosoureas include fotemustine, carmustine (BCNU), lomustine (CCNU), and semustine (methyl CCNU). Fotemustine has shown RR of 12-27% in metastatic melanoma, 22,23 and was the first drug to show significant efficacy in brain metastases.24 A phase III randomized study on 229 patients comparing DTIC versus fotemustine showed higher RR in the intent-to-treat population (n = 229; 15.2% v 6.8%; P = 0.043) for fotemustine; however, there were no differences in the median time to progression (1.9 and 1.8 months, respectively) or median survival (7.3 and 5.6 months, respectively) between the two arms. 25 Moreover, grade 3 to 4 hematological toxicities (neutropenia, thrombocytopenia) were more frequent in the fotemustine group. The agent is currently being studied for intrahepatic artery chemoembolization in patients with isolated liver metastases from primary uveal melanoma (NCT00110123). Although not available in the United States, fotemustine is still used as 1st line therapy in some European countries.
Cisplatin and carboplatin have shown modest activity as single agents with responses ranging from 15% to 20%.26,27 Paclitaxel, used as a single agent or in combination with other agents, has antitumor activity (RR = 16%–26%) in patients with metastatic melanoma, including patients whose disease has progressed on prior chemotherapies.28–30 Three phase II trials employing paclitaxel as a single agent for patients with advanced melanoma showed a mean response rate of approximately 17%, mainly partial responses.31–33 Other formulations of paclitaxel, such as nab-Paclitaxel (ABI-007, Abraxane), a 130-nM, Cremophor-free, albumin-bound (nab) paclitaxel particle, has been shown to be relatively well tolerated and to have single-agent activity in metastatic melanoma seen in a phase II trial.34, Based on this, a phase III trial was conducted which randomized patients to nab-paclitaxel versus dacarbazine. Progression-free survival was significantly increased with nab-paclitaxel (median 4.8 versus 2.5 months, HR 0.79, 95% CI 0.63-0.99) and there was a statistically nonsignificant trend toward increased overall survival (median 12.8 versus 10.7 months; HR 0.83, p = 0.09).35 Nab-Paclitaxel has several advantages over paclitaxel, including a lower rate of allergic reactions.36
The modest single agent activities led to investigation of combinations of these agents to improve outcomes. The combination of carboplatin and paclitaxel (CP) has been shown to be active in a wide range of malignancies including modest activity in advanced melanoma37–41and is currently a “community standard” (NCCN cited) for off-protocol treatment of patients with metastatic disease. In a more recent study, the combination of carboplatin and nab-paclitaxel was found to have an ORR of 25.6% and a median PFS of 4.5 months in previously untreated patients (chemotherapy naïve cohort) and 4.1 months in the cohort of patients who had previously received treatment for metastatic melanoma. Median OS was 11.1 months in the chemo-naive group and 10.9 months in the previously treated group.36
There were efforts to combine more than 2 cytotoxic agents as well. The Dartmouth regimen, combination of cisplatin, dacarbazine, carmustine (BCNU), and tamoxifen (CDBT), was initially reported having a high overall RR of 54% in 1980-90s.42,43 However, a phase III multicenter trial involving 240 patients failed to show any statistically significant benefit in favor of the regimen vs dacarbazine monotherapy in terms of RR (16.8% versus 9.9%, respectively; P = 0.13) or OS (7.7 versus 6.3 months; P = 0.52).44 A prospective evaluation of another triple-drug regimen containing cisplatin, vinblastine, and dacarbazine (CVD) for metastatic melanoma showed an ORR of 40% in a phase II study.45 The treatment was associated with significant gastrointestinal and hematological toxicity. The CVD regimen was later used along with IL-2 and interferon-alpha (IFNa) to develop biochemotherapy regimens.46 Based upon these results, higher responses can be seen with combinations of cytotoxic agents, compared to dacarbazine monotherapy, but these regimens fail to extend survival significantly and are associated with higher toxicity.
The role of angiogenesis inhibitors is also actively being explored in metastatic melanoma. New blood vessel formation (angiogenesis) is a fundamental event in the process of tumor growth and metastatic dissemination and the vascular endothelial growth factor (VEGF) pathway is well established as one of the key regulators of this process.47 In melanoma, there is substantial evidence that VEGF is important in growth and metastases. Increased serum concentrations of VEGF correlate with tumor progression and survival, and the expression of VEGF in sites of melanoma metastasis was found to be much higher than in primary tumors.48 Moreover, transcriptional up regulation of VEGF has been demonstrated as an important escape mechanism of melanoma cells when exposed the conventional chemotherapeutic agents (DTIC).49 Despite this, a phase III, randomized, placebo-controlled study of the multikinase and VEGF-receptor inhibitor sorafenib in combination with CP as second-line treatment for patients with unresectable stage III or IV disease showed no benefit in PFS or OS upon addition of sorafenib.37 Bevacizumab is a recombinant humanized murine anti-VEGF monoclonal antibody.50 A phase II trial evaluated the antitumor activity of the angiogenesis inhibitor bevacizumab when combined with temozolamide or nab-paclitaxel and carboplatin in patients with stage IV melanoma. Median PFS and OS with nab-paclitaxel, carboplatin and bevacizumab combined were 6.7 months and 13.9 months, respectively, with an overall RR of 33.3% (95% CI: 20.8%-47.9%), suggestive of a promising activity with the addition of an angiogenesis inhibitor.51 However, a larger phase II study which randomized 214 patients in a 2:1 ratio to CP versus CP and bevacizumab did not meet the primary objective of statistically significant improvement in PFS with the addition of bevacizumab to carboplatin plus paclitaxel (4.2 months for the CP arm and 5.6 months for the CPB arm; HR= 0.78; P = .1414). A larger phase III trial will be necessary to determine whether there is benefit of adding bevacizumab to cytotoxic chemotherapy in this disease setting.52
Biochemotherapy
The term biochemotherapy refers to regimens that combine cytotoxic agents with IL-2 and/or IFNa. IL-2 was FDA approved for treatment of malignant melanoma based on the analysis of 270 metastatic melanoma patients treated in 8 clinical trials between 1985 and 1993.53 The overall RR was 16%, with complete responses seen in 6% and partial responses in 10% of patients. However, high-dose IL-2 has been associated with significant dose-dependent toxicities which limit its clinical utility.54 IFNa, approved for use as adjuvant therapy for early stage melanoma, has also demonstrated only modest benefits55 Based upon this activity , multiple clinical trials have evaluated biochemotherapy regimens using drugs such as DTIC, tamoxifen, cisplatin, vinblastine, carmustine, or temozolomide along with various combinations and doses of IL-2 and IFNa.56–64 Although there were some initial promising results with higher response rates and improved progression-free survival, they failed to translate into overall survival benefit for the biochemotherapy arms.56,61 Biochemotherapy may require a reduction in the IL-2 dose to combine it safely with chemotherapeutic agents. Durable responses may be compromised due to reduced IL-2 dose, as suggested by one study, in which a CR rate of 15.4% was achieved for patients who failed biochemotherapy and were subsequently treated with high dose IL-2.65 Biochemotherapy also produced substantially more constitutional, hemodynamic, and hematologic side effects. One study66 evaluated the quality of life in the patients included in a randomized control trial of biochemotherapy versus chemotherapy and found that the overall quality of life significantly declined through the 5th treatment cycle with biochemotherapy (p = 0.03).
Furthermore, a meta-analysis that evaluated 18 trials including 2621 patients found higher response rates in patients treated with biochemotherapy compared with those receiving chemotherapy alone (odds ratio = 0.59; 95% CI = 0.49–0.72; P < 0.00001), but no significant improvement in survival (odds ratio = 0.99; 95% CI = 0.91–1.08; P = 0.9)67. In addition, the increased response rate was associated with an increase in toxicity. Based upon these results, biochemotherapy cannot be considered a standard of care for treatment of metastatic melanoma.
In recent years, a new, safer T-cell activating agent has gained significant attention as therapy for advanced melanoma. Ipilimumab is a monoclonal antibody against CTLA-4, which prevents downregulation of T-cell activation, thus allowing sustained immune responses to tumor antigens on malignant melanocytes. When exposed to the antigen presenting cells (APCs), T-cell activation requires co-stimulatory signals.68 First, tumor-associated antigens presented by major histocompatibility complex I or II on specialized APCs bind to T-cell receptors. A 2nd signal required for T-cell activation occurs when B7 molecules on the APC bind to CD28 receptors on the T-cell. In the absence of a second signal, a T cell becomes anergic and fails to mount a full immune response.69 T cells that do receive both signals become activated, able to proliferate and target the tumor. Shortly after T-cell activation, CTLA-4 is upregulated, competitively inhibiting the binding of B7 molecules to CD28 and halting T-cell activation and proliferation.
Ipilimumab has received FDA approval in March of 2011 on the basis of a randomized phase III clinical trial in which 676 HLA-A*0201 positive patients with unresectable metastatic melanoma were randomly assigned in a 3:1:1 ratio to ipilimumab plus a glycoprotein 100 (gp100) vaccine, ipilimumab alone, or gp100 alone.70 Ipilimumab at 3 mg/kg and the gp100 vaccine were given every three weeks for four doses. Patients with confirmed responses or with stable disease for three months or more were allowed to receive reinduction with their original treatment if they subsequently had disease progression. Overall survival was significantly increased in patients receiving ipilimumab (ipilimumab versus gp100 alone 10.1 versus 6.4 months, HR 0.66). Benefits were seen across all subsets and were independent of sex, age, stage at presentation (M0, M1a, and M1b versus M1c), baseline LDH, or prior use of IL-2. Of note, a retrospective analysis of four phase II trials confirmed that Ipilimumab has similar efficacy regardless of HLA status.71 Currently, the recommended ipilimumab regimen for clinical use as specified by the FDA consists of 4 cycles of 3 mg/kg/dose given 3 weeks apart at an approximate cost of $30,000 per dose.70
A significant consequence of the T-cell activation and proliferation resulting from treatment with ipilimumab is the development of immune-mediated adverse reactions. These may involve any organ system. In clinical studies approximately 10%-26% of patients have experienced severe (≥ grade 3) autoimmune-related events including diarrhea/colitis, rash, hypothyroidism, hypopituitarism, hypophysitis, and adrenal insufficiency.72–74 Prompt evaluation, institution of supportive measures, interruption of ipilimumab dosing, and/or early intervention with corticosteroids (1-2 mg/kg/day prednisone or equivalent), depending upon the severity of patient symptoms, is essential in order to prevent progression to more serious and potentially irreversible toxicity. To this effect, the FDA has created a Risk Elimination and Management System (REMS) which provides additional information to both healthcare providers and patients, and which is widely available.75 The majority of these immune-mediated reactions typically do not occur until several weeks after the initiation of ipilimumab; however, some have been observed weeks to months after discontinuation of therapy.
A recent phase II study presented at the 2013 American Society of Clinical Oncology (ASCO) meeting evaluated the combination of ipilimumab and granulocyte macrophage-colony stimulating factor (GM-CSF) versus ipilimumab alone in patients with metastatic melanoma. Ipilimumab was administered at 10 mg/kg every 3 weeks for 4 doses followed by maintenance therapy with 10 mg/kg every 3 months, which is different from the FDA-approved regimen. GMCSF was administered as a 250 mcg subcutaneous daily injection on days 1 through 14 of a 21-day cycle. An interim analysis showed that the survival rate of patients after one year of treatment with the ipilimumab/GM-CSF combination was 68.9% compared with 52.9% in those receiving ipilimumab monotherapy, with a hazard ratio (HR) of mortality of 0.64 (stratified one-sided log rank p = .014). The median overall survival (OS) of patients in the combination arm was 17.5 months compared with 12.7 months in the group of patients receiving ipilimumab alone. The overall safety profile was favorable, and actually the combination therapy resulted in a decreased incidence of high-grade AEs (grades 3-5 toxicities occurred in 44.9% versus 58.3% of patients) and a lower incidence of pulmonary and gastrointestinal high-grade immune-related toxicity.76 The proposed explanation for the lower incidence of GM-CSF-related toxicity is thought to be due to the effect of GM-CSF on the microenvironment of gastrointestinal and pulmonary mucosa, which is different from other organs, and may possess healing properties in these enviornments.77–79
Long-term follow-up on ipilimumab responders was recently reported on 177 patients with advanced melanoma who were treated on three of the earliest ipilimumab trials. The median duration of objective responses was up to 88 months, suggesting potentially curative durable tumor regressions in a small group of patients.80
Tremelimumab is another anti-CTLA4 antibody which demonstrated durable responses in about 10% of the patients in phase I and II trials,81 similar to ipilimumab, but the phase III trial failed to demonstrate survival advantage over chemotherapy (12.6 months versus 10.7, p = 0.127) and therefore has not been FDA approved. It is speculated that subtle differences in the design of phase III trials of ipilimumab and tremelimumab may have played a role in those insignificant results.82
PD-1 & PDL-1 Inhibitors
PD-1 is an immunoinhibitory receptor in the CD28 family that plays a vital role in tumor immune escape.83 The interaction of PD-1 with the ligand Programmed death ligand 1 (PD-L1, also known as B7-H1) inhibits T-cell proliferation, survival, and effector functions (cytotoxicity, cytokine release),84 induces apoptosis of tumor-specific T cells, promotes the differentiation of CD4+ T cells into immunosuppressive regulatory T cells (Tregs),85 and increases the resistance of tumor cells to CTL attack.86,87 In recent years, the PD-1/PD-L1 pathway has been found to play an important role in tumor-induced immunosuppression in melanoma and is an increasingly exploited therapeutic target in this disease and other advanced malignancies.88–90 Blockade of PD-1 or PD-L1 can reinvigorate exhausted T-cells (such as TILs), enhancing their expansion, cytokine production, and cytolytic functions. Improved understanding of PD-1 pathway biology and preclinical demonstration of its pivotal role in immunosuppression have driven the clinical development of PD-1 pathway blockade for cancer therapy, and both anti-PD-1 and anti-PD-L1 agents are currently being tested in the clinic.91
Nivolumab (BMS-936558, MDX-1106) is a fully human IgG4 antibody that blocks the PD-1 receptor. This agent was evaluated in patients with treatment-refractory metastatic solid tumors90, and clinical activity was observed in patients with melanoma, renal-cell cancer (RCC), and non–small-cell lung cancer (NSCLC). Expression of PD-L1 on the surface of tumor cells seemed to correlate with the potential to achieve an objective response89, suggesting a role for PD-L1 as a potential predictive marker of response to anti-PD-1 blockade. However, the utility of tumor PD-L1 expression in patient selection for such treatment will need to be confirmed in larger prospective trials.
A recent phase I trial reported on the long-term follow-up of safety and response in patients with advanced melanoma treated with single-agent nivolumab. The response rate (RR) across all dose cohorts was 31%, and there was no clear dose-response curve.92 Responses were durable, with a median duration of 104 weeks (2 years), suggesting that maintenance therapy may not be required. In addition, the responses were fairly rapid, with 45% of patients showing an objective response at the time of the first tumor assessment (8 weeks). The median OS in this study was 16.8 months for the entire population, and the 1- and 2-year OS rates were 62% and 43%, respectively.
It was noted that CTLA-4 and PD-1 appear to play complementary roles in regulating anti-tumor immunity. A phase I trial combining93 CTLA-4 and PD-1 blockade via administration of ipilimumab and nivolumab, respectively, was recently reported on 86 patients with unresectable, metastatic melanoma. The trial included four concurrent and two sequenced cohorts. In the concurrent cohorts, patients received four doses of nivolumab (0.3-3 mg/kg) and ipilimumab (1-3 mg/kg) every 3 weeks, followed by nivolumab alone every 3 weeks for four doses. In the sequenced cohorts, patients previously treated with ipilimumab (outside of the study) received nivolumab alone (1 or 3 mg/kg) every 2 weeks. The objective response rate (ORR) was 40 across all dose levels % (95% confidence interval [CI], 27 to 55) for patients in the concurrent-regimen cohort. At a median follow-up of 13 months, 90% of responses were maintained in the concurrent therapy arm. The estimated 1-year survival rate with the concurrent regimen was 82%. Among patients who received maximum doses (concurrent arm cohort 2, with nivolumab at a dose of 1 mg per kilogram and ipilimumab at a dose of 3 mg per kilogram), 9 of 17 patients (53%; 95% CI, 28 to 77) showed objective response, including 3 who had a complete response. Of note, all 9 patients who had a response had tumor reduction of 80% or more at the first scheduled tumor assessment. Response was seen in all subgroups of patients, including those with elevated pretreatment LDH level, M1c disease, and bulky, multifocal tumor burden. It was also noted that the sequential regimen was effective in patients with previous radiologic progression on ipilimumab. The concurrent treatment resulted in grade 3-4 treatment-related AEs in 53% of patients, and serious AEs in 49% of patients. However, the only grade 3-4 AEs occurring in > 10% of patients were asymptomatic laboratory abnormalities (mainly elevated levels of lipase, aspartate and alanine aminotransferase) which resolved with conservative measures in most patients. Grade 3-4 diarrhea and immune-related gastrointestinal toxicity was also more common in the patients receiving concurrent, compared with sequential therapy (6% versus 0%, and 9% versus 0%, respectively). Based on these results, a phase III trial comparing concurrent nivolumab plus ipilimumab versus either agent alone has been activated and is currently recruiting patients with advanced melanoma (NCT01844505). The dosing scheme designated to move forward in this phase III trial included nivolumab to be administered at 1 or 3 mg/kg and ipilimumab at 3 mg/kg.94 The combination is also being evaluated in patients with NSCLC and RCC.95
Lambrolizumab (MK-3475) is a highly selective, humanized IgG4-kappa isotype monoclonal antibody against PD-1. A previous phase I escalation trial in patients with treatment-resistant advanced malignancies of different histologies reported clinical responses at 1mg/kg, 3 mg/kg, and 10 mg/kg doses without reaching a maximum-tolerated dose.96 In April 2013, lambrolizumab received “breakthrough therapy” designation from the FDA based on early interim results from a single-arm, open-label phase Ib study in 85 patients with surgically unresectable metastatic melanoma, in which an impressive overall 51% ORR was observed.97 The most recent update of this phase I trial reported data on 135 patients with advanced melanoma who had or had not received prior therapy with ipilimumab; these patients were treated at different doses and dose frequencies in non-randomized concurrent cohorts.98 The confirmed ORR was 38% (44/117 patients) for the entire cohort, and responses seemed to be independent of previous ipilimumab therapy. The highest response rate was an impressive 52% in the patient cohort receiving the highest dose in the study (10 mg/kg every 2 weeks).98 Overall, 77% of patients had a reduction in tumor burden. Similarly to patients treated with nivolumab, the responses were rapid and durable: the majority of patients demonstrated an objective response by the first tumor assessment at 12 weeks, and 81% of patients who had a response were continuing therapy at a median follow-up of 11 months. The treatment was well-tolerated, and the majority of therapy-related AEs were grade 1-2. Grade 3-4 AEs were noted in 12.6% of patients and were generally managed by dose interruptions or medical management.
MPDL3280A is a human monoclonal anti-PD-L1 antibody that blocks the binding of PD-L1 to PD-1 and B7-1. A recent study reported on 45 metastatic melanoma patients who received treatment at doses ranging from 1 to 20 mg/kg.99 An ORR of 26% was observed .The 24-week progression-free survival (PFS) rate was 35%. The incidence of all grade 3-4 AEs, regardless of attribution, was 33%, including hyperglycemia (7%), elevated ALT (7%), elevated AST. No cases of pneumonitis or colitis were reported. Stable disease was achieved in 87% of patients with PD-L1 positive tumors compared to 20% of patients with PD-L1 negative tumors. Overall, MPDL3280A as monotherapy was well tolerated and associated with durable responses.
BMS-936559 is also a fully human IgG4 PD-L1 antibody, which was evaluated in phase I trial which included 52 patients with melanoma. Multiple dose levels from 0.3 g/kg to 10 mg/kg were tested. The response rate ranged from 6% to 29% across dose levels. Responders (9/52) demonstrated durable responses with manageable toxicities, 5/9 patients had ongoing response for over a year. 14/52 (27%) melanoma patients had stable disease for over 24 weeks.100
In conclusion, PD-1 receptor blockers have shown overall higher response rates than ipilimumab, with higher tolerability and fewer grade 3 and 4 side effects. A striking feature of immune checkpoint inhibitors is the impressive duration of response in select patients, noted initially with ipilimumab, and now with the newest PD-1/PDL-1 blocking agents. The ability of PD-1 blockade to induce rapid and sustained responses regardless of previous anti-CTLA-4 therapy makes this approach an extremely promising therapeutic option.
RAS/RAF/MEK/ERK Pathway-Targeted Therapies
Vemurafenib
Several new molecules targeting mutated genes in the RAS/RAF/MEK/ERK pathway are being tested in the clinic. BRAF is the most frequently mutated protein kinase in human cancers,101 and vemurafenib was developed as a specific, potent BRAF inhibitor.102 Somatic mutations in BRAF, a serine-threonine kinase, are present in 40-60% of metastatic melanomas. The vast majority of mutations involve a substitution for valine (typically glutamine) at the 600th amino acid position (V600E). Unlike the nonselective BRAF inhibitor sorafenib, which demonstrated a 5% response rate, selective BRAF inhibitors have shown impressive results in melanoma.103 A phase III trial (BRIM-3) randomized 675 patients with unresectable, previously untreated stage IIIC or IV melanoma in a 1:1 fashion to receive either oral vemurafenib 960 mg twice daily or dacarbazine 1000 mg/m2 IV every 3 weeks. However, after reviewing interim analysis data, crossover from dacarbazine to vemurafenib was recommended for patients who progressed on chemotherapy. In the vemurafenib group, most patients had a detectable decrease in tumor size, and 48% experienced an OR. Median PFS was improved compared to dacarbazine (5.3 versus 1.6 months in the vemurafenib versus dacarbazine groups, respectively) as was the OS rate at 6 months (84% versus 64%).104 The most commonly observed AEs with vemurafenib were fatigue, arthralgia, rash, photosensitivity, edema, nausea, alopecia and pruritus. The most common (≥5%) grade 3 AEs were cutaneous squamous cell carcinomas (cuSCC) and rash. Secondary cuSCC and keratoacanthomas, often containing RAS mutations, occur in approximately 20% of patients, usually in the first 2–3 months of therapy, and are treated by simple excision.105 Unfortunately all patients, including those who experience initial profound tumor regression, ultimately develop resistance to vemurafenib through reactivation of the MAPK or alternative pathways.106 Thus, combination treatment with agents blocking downstream and alternate pathways is desired107 and it is currently thought that simultaneous targeting of RAF and MEK by individual inhibitors may be more effective in cancer therapy than targeting either kinase alone. This is based in part on the existence of intricate feedback loops involved in ERK signaling that can inhibit RAF and MEK.108
Dabrafenib
Dabrafenib is another agent active against BRAF. On May 29, 2013, the FDA approved dabrafenib for the treatment of patients with BRAF V600E mutation-positive unresectable or metastatic melanoma based on the results of a phase III randomized controlled trial comparing dabrafenib with dacarbazine.109 In this trial, 250 patients with unresectable, previously untreated BRAF V600E melanoma were randomized 3:1 to receive either oral dabrafenib 150 mg daily versus dacarbazine 1000 mg/m2 intravenously every 3 weeks. Median progression-free survival was 5.1 months for dabrafenib and 2.7 months for dacarbazine, with a hazard ratio (HR) of 0·30 (95% CI 0·18-0·51; p<0·0001). While vemurafenib and dabrafenib seem to have similar efficacy in terms of ORR in the two phase III trials , the rates of cuSCC/keratoacanthoma which is an important AE with vemurafenib, were different (18-25% in vemurafenib trials versus 6-11% in dabrafenib trials).110
Dabrafenib in Brain Metastasis
Interestingly, dabrafenib seems to be quite effective in the treatment of intracranial metastases in patients with advanced melanoma, although the agent was synthesized specifically with minimal blood-brain barrier penetrative capability as a way of limiting toxic neurologic AEs. BREAK-MB was an open label international phase II study in which patients with BRAF V600E/K metastatic melanoma with previously treated or untreated asymptomatic brain metastases (>= then 0.5 cm but <= 4cm) were treated with dabrafenib 150 mg BID.111 In patients whose tumors harbored the V600E mutation, the overall intracranial response rate (OIRR; primary endpoint) was 39.2% (95% CI 28.0–51.2%) for the treatment-naive patients (n = 74) and 30.8% (95% CI 19.9–43.4%) in patients who had received prior local therapy (n = 65). ORRs were lower in V600K patients (15%); however, the number of patients was significantly lower than those with the V600E mutation. The overall intracranial disease control rate (CR+PR+SD) in V600E patients was 81%, which was similar to the overall extracranial disease control rate (83% for previously treated versus 80% for brain treatment-naive patients). Preliminary reports evaluating vemurafenib in the same clinical setting remain controversial.112,113
Trametinib
In preclinical models, mutations of BRAF were associated with enhanced sensitivity to MEK inhibition; pharmacological MEK blockade was shown to completely abrogate tumor growth in BRAF mutant-positive xenografts.114 Trametinib, a potent and highly selective MEK1/2 inhibitor, received FDA approval on May 29, 2013 for the 1st line treatment of patients with unresectable or metastatic melanoma harboring a BRAF V600E/K mutation (currently, trametinib is not indicated for patients who have received prior BRAF inhibitor therapy). Approval was based upon the results of the phase III METRIC trial in which 322 patients with advanced melanoma were randomly assigned in a 2:1 ratio to receive either trametinib (2 mg/day orally) or chemotherapy (dacarbazine or paclitaxel). Compared with patients receiving chemotherapy, patients treated with trametinib demonstrated significant improvement in median PFS (1.5 months versus 4.8 months) and 6-month OS (67% versus 81%; HR for death 0.54, 95% CI 0.32- 0.92) in all subgroups, including patients with brain metastases and M1c disease, despite permitted crossover to trametinib. Although cutaneous toxicity—mainly papulopustular rash— was noted in 87% of patients, notably, no cuSCC was observed during trametinib treatment.115 Other less common, but potentially significant, toxicities observed with MEK inhibition by trametinib included a decrease in cardiac ejection fraction or ventricular dysfunction, visual problems (i.e. retinal pigment epithelial detachment or retinal vein occlusion), and interstitial lung disease.116
Dabrafenib/Trametinib Combinatorial Therapy
Resistance to therapy with BRAF kinase inhibition develops quickly, typically within 6 to 7 months of therapy initiation.109 To address this issue and potentially delay the development of resistance to treatment, as well as to minimize toxicity associated with BRAF inhibition, combination therapy with dabrafenib and trametinib has been investigated. The combination has received accelerated FDA approval in January 2014 for use in the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation. Approval was based on the results of phase I/II study,117 in which, 162 patients were randomly assigned in a 1:1:1 fashion to receive 150 mg of dabrafenib twice daily, or 150 mg of dabrafenib twice daily plus either 1 or 2 mg trametinib daily (150/2 mg or 150/1 mg). The response rates and response duration were 76% (95% CI: 62, 87) and 10.5 months (95% CI: 7, 15), respectively, in the trametinib 2 mg plus dabrafenib combination arm and 54% (95% CI: 40, 67) and 5.6 months (95% CI: 5, 7), respectively, in the single-agent dabrafenib arm. The incidence of acneiform dermatitis, the most common and dose-limiting toxic effect of trametinib, was reduced in the combination group. Proliferative skin lesions, including cuSCC, papillomas, and hyperkeratosis, which are commonly seen with BRAF inhibition monotherapy, were less frequently observed. (cuSCC incidence was 19% with dabrafenib monotherapy versus 7% with combination therapy). Phase III randomized control trials comparing dabrafenib plus trametinib to dabrafenib (NCT01584648) or to vemurafenib (NCT01597908) monotherapy are ongoing and FDA approval of the combination treatment is contingent upon results of these trials.
KIT Inhibitors
Somatic mutations in KIT occur infrequently in patients with melanoma, with a 2-3% incidence.118KIT mutations are most frequent in acral and mucosal melanoma subtypes and are rarely reported in cutaneous melanomas, particularly those associated with intermittent ultraviolet exposure. Mutations of c-kit in melanomas are variable, and only select KIT alterations are truly oncogenic and indicative of an effective therapeutic target.119 In one trial, 28 patients with KIT mutations or amplifications were treated with imatinib, demonstrating a durable response rate of 16% and median PFS of 12 weeks. The majority of patients (72%) experienced temporary disease stabilization, at minimum.120 This study suggested that, melanoma patients who harbor mutations in exons 11 or 13 in c-KIT or with an amplification of c-KIT may benefit more from imatinib treatment, which may serve as a predictive biomarker for c-KIT inhibition trials.
Emerging Immunotherapies
Various immunotherapeutic approaches are currently being studied in many cancer types. Thus far, however, studies have been most extensively conducted in melanoma, providing valuable experience potentially applicable to other cancers and making melanoma a model system for identifying therapeutic biomarkers.121 Immunotherapy using autologous T cells has the potential to induce durable responses, especially in patients who have failed multiple prior treatments.122 A number of small nonrandomized clinical trials with autologous t-cell therapy have reported high response rates of around 40% and durable progression-free survival, in patients with metastatic melanoma.122,123 This investigational approach is increasingly being explored at centers around the world. Monoclonal antibodies under investigation in the treatment of melanoma include those targeting the receptors CD40, CD137 (4-1BB), CD134 (OX40), glucocorticoid-induced TNF receptor (GITR), KIR, and TGF-β. CD40 is an important mediator of the immune response and has been shown to be expressed on different types of APCs and on various tumor tissues.124 The interaction of this molecule with its natural ligand, CD40L, is critical in the communication between T cells and DCs and between T-helper cells and B cells during humoral immune responses.125 A trial of the anti-CD40 monoclonal antibody CP-870,893 in combination with tremelimumab in patients with metastatic melanoma is ongoing (NCT01103635). Assessments of oncolytic virotherapy126 are ongoing and include a recently reported phase III trial of an oncolytic herpes virus (Talimogene Laherparepvec, T-VEC).127
Conclusion
The future has never been brighter for metastatic melanoma patients and for academicians and clinicians working tirelessly to improve patient survival. Immunotherapeutic approaches with checkpoint inhibitors and targeted therapies have substantially increased the treatment armamentarium and revolutionized the clinical approach to melanoma in the last few years. Along with approved therapies and a long pipeline of new molecules and treatment modalities in development, increased collaboration between cancer geneticists and immunologists will be of vital importance to determine the best treatment for each patient.
Article Highlights.
The incidence of melanoma has been rising for the past 3 decades.
Historically, metastatic melanoma has had a dismal long-term survival due to an absence of effective treatment.
Substantial recent research in the melanoma field has changed the treatment landscape with 4 new drugs being FDA approved for advanced melanoma in the past 2 years.
Immunotherapy is shown to induce durable responses in select patients with metastatic melanoma. A striking feature of immune checkpoint inhibitors is the impressive duration of response in select patients, noted initially with ipilimumab, and now with the newest PD-1-blocking agents.
Targeted agents are effective in inducing responses in select patients with metastatic melanoma carrying specific genetic mutations; unfortunately resistance develops in virtually all patients.
Combinatorial treatment strategies including chemotherapy, targeted agents, and immunotherapy, will undoubtedly transform the clinical approach to this disease and promise to improve patient survival in the coming years.
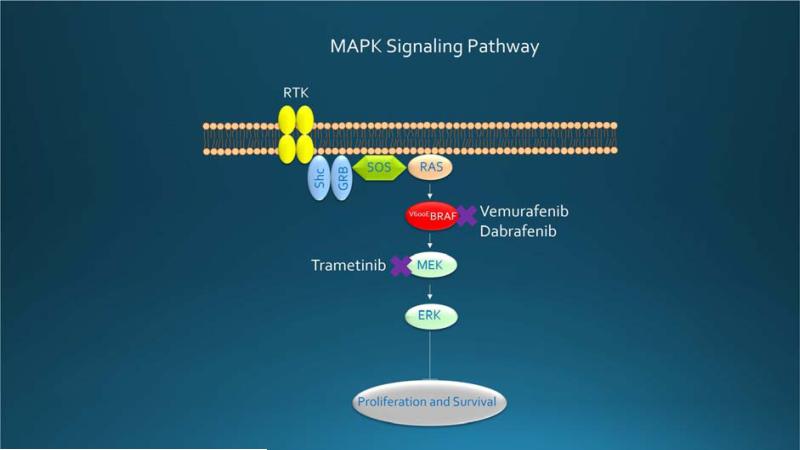
Figure 1.
MAPK pathway signaling in malignant melanoma leads to phosphorylation and activation of RAS, RAF, MEK, and ERK, a process that ultimately regulates the cell cycle, differentiation, and apoptosis. Mutated BRAF, which is 500-fold activated, stimulates constitutive signaling, proliferation, and survival, making it an excellent target for vemurafenib and dabrafenib. Trametinib inhibits MEK, situated downstream. MAPK = mitogen-activated protein kinase
Figure 2.
Timeline depicting FDA approvals and landmark clinical trials in metastatic melanoma. HR = hazard ratio; N = number randomized; OS = overall survival; RR = response rate; PFS = progression-free survival
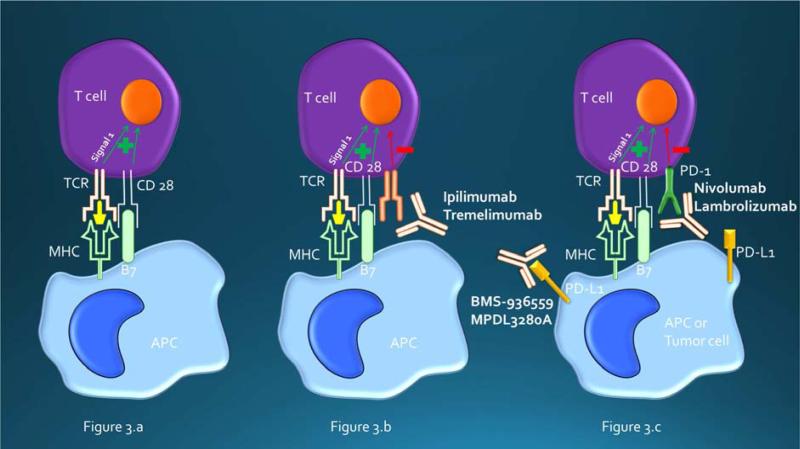
Figure 3.
Figure 3.a. T-cell activation with positive co-stimulation.
Figure 3.b. CTLA-4 is a negative regulator of T-cell activation. CTLA-4 inhibitors binds to CTLA-4 and blocks the interaction of CTLA-4 with its ligand, B7. Blockade of CTLA-4 augments T-cell activation and proliferation.
Figure 3.c. Engagement of PD-1 expressed on T cells with PDL-1 expressed on APC or tumor cells results in T-cell suppression and tumor protection. Blockade of this interaction with either PD-1 or PDL-1 blocking antibodies can “wake up” exhausted T cells, resulting in a T-cell response against tumor. APC = antigen-presenting cell; CTLA-4 = cytotoxic T-lymphocyte antigen-4; MHC = major histocompatibility complex; PD-1 = programmed death 1; PDL-1 = programmed death ligand-1; TCR = T-cell receptor
Acknowledgements
This publication was in part made possible by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We thank Dr Atman Shah and Dr Ramesh Kumar for their constructive recommendations on this project. We thank Catalyst Prose for grammar editing assistance in some parts of the manuscript.
Financial support and disclosure: The authors have nothing to disclose. All the authors stated that they had no interests which might be perceived as posing a conflict or bias.
Abbreviations
- CTLA-4
cytotoxic T-lymphocyte-associated antigen-4
- PD-1
programmed death 1
- FDA
Food and Drug Administration
- AEs
adverse events
- TILs
tumor-infiltrating lymphocytes
- MAPK
mitogen-activated protein kinase
- ASCO
American Society of Clinical Oncology
- HR
hazard ratio
- OS
overall survival
- PDL1
programmed death ligand 1
- RR
response rate
- ORR
objective response rate
- OIRR
the overall intracranial response rate
- PFS
progression-free survival
- CR
complete response
- PR
partial response
- SD
stable disease
- IL-2
interleukin-2
- cuSCC
cutaneous squamous cell carcinomas
- YPLL
years of potential life lost
- VEGF
vascular endothelial growth factor
- TGF-β
transforming growth factor beta
- IFNa
Interferon alfa
- APCs
antigen presenting cells
- Tregs
regulatory T cells
- CP
carboplatin and paclitaxel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharmacoeconomics. 2011;29(10):863–74. doi: 10.2165/11589300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Soong SJ, Harrison RA, McCarthy WH, Urist MM, Balch CM. Factors affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol. 1998;67(4):228–33. doi: 10.1002/(sici)1096-9098(199804)67:4<228::aid-jso4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society . Cancer Facts & Figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 6.Miller AJ, Mihm MC. Melanoma. N Engl J Med. 2006;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AMM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2011;47(14):2150–7. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68(3):889–92. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28(29):4531–8. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 15.Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell. 2002;2(1):5–7. doi: 10.1016/s1535-6108(02)00089-2. [DOI] [PubMed] [Google Scholar]
- 16.Polsky D, Cordon-Cardo C. Oncogenes in melanoma. Oncogene. 2003;22(20):3087–91. doi: 10.1038/sj.onc.1206449. [DOI] [PubMed] [Google Scholar]
- 17.Ascierto PA, Kirkwood JM, Grob J-J, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sensi M, Nicolini G, Petti C, et al. Mutually exclusive NRASQ61R and BRAFV600E mutations at the single-cell level in the same human melanoma. Oncogene. 2006;25(24):3357–64. doi: 10.1038/sj.onc.1209379. [DOI] [PubMed] [Google Scholar]
- 19.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 20.Omholt K, Kröckel D, Ringborg U, Hansson J. Mutations of PIK3CA are rare in cutaneous melanoma. Melanoma Res. 2006;16(2):197–200. doi: 10.1097/01.cmr.0000200488.77970.e3. [DOI] [PubMed] [Google Scholar]
- 21.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 22.Jacquillat C, Khayat D, Banzet P, et al. Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer. 1990;66(9):1873–8. doi: 10.1002/1097-0142(19901101)66:9<1873::aid-cncr2820660904>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Kleeberg UR, Engel E, Israels P, et al. Palliative therapy of melanoma patients with fotemustine. Inverse relationship between tumour load and treatment effectiveness. A multicentre phase II trial of the EORTC-Melanoma Cooperative Group (MCG). Melanoma Res. 1995;5(3):195–200. doi: 10.1097/00008390-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Khayat D, Giroux B, Berille J, et al. Fotemustine in the treatment of brain primary tumors and metastases. Cancer Invest. 1994;12(4):414–20. doi: 10.3109/07357909409038234. [DOI] [PubMed] [Google Scholar]
- 25.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22(6):1118–25. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 26.Glover D, Glick JH, Weiler C, Fox K, Guerry D. WR-2721 and high-dose cisplatin: an active combination in the treatment of metastatic melanoma. J Clin Oncol. 1987;5(4):574–8. doi: 10.1200/JCO.1987.5.4.574. [DOI] [PubMed] [Google Scholar]
- 27.Evans LM, Casper ES, Rosenbluth R. Phase II trial of carboplatin in advanced malignant melanoma. Cancer Treat Rep. 1987;71(2):171–2. [PubMed] [Google Scholar]
- 28.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106(2):375–82. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 29.Wiernik PH, Einzig AI. Taxol in malignant melanoma. J Natl Cancer Inst Monogr. 1993;(15):185–7. [PubMed] [Google Scholar]
- 30.Nathan FE, Berd D, Sato T, Mastrangelo MJ. Paclitaxel and tamoxifen: An active regimen for patients with metastatic melanoma. Cancer. 2000;88(1):79–87. doi: 10.1002/(sici)1097-0142(20000101)88:1<79::aid-cncr12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Legha SS, Ring S, Papadopoulos N, Raber M, Benjamin RS. A phase II trial of taxol in metastatic melanoma. Cancer. 1990;65(11):2478–81. doi: 10.1002/1097-0142(19900601)65:11<2478::aid-cncr2820651114>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Bedikian AY, Weiss GR, Legha SS, et al. Phase II trial of docetaxel in patients with advanced cutaneous malignant melanoma previously untreated with chemotherapy. J Clin Oncol. 1995;13(12):2895–9. doi: 10.1200/JCO.1995.13.12.2895. [DOI] [PubMed] [Google Scholar]
- 33.Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs. 1991;9(1):59–64. doi: 10.1007/BF00194546. [DOI] [PubMed] [Google Scholar]
- 34.Hersh EM, O'Day SJ, Ribas A, et al. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer. 2010;116(1):155–63. doi: 10.1002/cncr.24720. [DOI] [PubMed] [Google Scholar]
- 35.Hersh E, Del Vecchio M, Brown M, et al. Phase 3, randomized, open-label, multicenter trial of nab-paclitaxel (nab-P) versus dacarbazine (DTIC) in previously untreated patients with metastatic malignant melanoma (MMM). Pigment Cell Melanoma Res. 2012;25(6):863. [Google Scholar]
- 36.Kottschade LA, Suman VJ, Amatruda T, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma : a North Central Cancer Treatment Group Study, N057E(1). Cancer. 2011;117(8):1704–10. doi: 10.1002/cncr.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27(17):2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 38.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30(1):34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimpfer-Rechner C, Hofmann U, Figl R, et al. Randomized phase II study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: a multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG). Melanoma Res. 2003;13(5):531–6. doi: 10.1097/00008390-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106(2):375–82. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 41.Hodi FS, Soiffer RJ, Clark J, Finkelstein DM, Haluska FG. Phase II study of paclitaxel and carboplatin for malignant melanoma. Am J Clin Oncol. 2002;25(3):283–6. doi: 10.1097/00000421-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Lattanzi SC, Tosteson T, Chertoff J, et al. Dacarbazine, cisplatin and carmustine, with or without tamoxifen, for metastatic melanoma: 5-year follow-up. Melanoma Res. 1995;5(5):365–9. doi: 10.1097/00008390-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Del Prete SA, Maurer LH, O'Donnell J, Forcier RJ, LeMarbre P. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep. 1984;68(11):1403–5. [PubMed] [Google Scholar]
- 44.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745–51. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 45.Legha SS, Ring S, Papadopoulos N, Plager C, Chawla S, Benjamin R. A prospective evaluation of a triple-drug regimen containing cisplatin, vinblastine, and dacarbazine (CVD) for metastatic melanoma. Cancer. 1989;64(10):2024–9. doi: 10.1002/1097-0142(19891115)64:10<2024::aid-cncr2820641010>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 46.Legha SS, Ring S, Bedikian A, et al. Treatment of metastatic melanoma with combined chemotherapy containing cisplatin, vinblastine and dacarbazine (CVD) and biotherapy using interleukin-2 and interferon-alpha. Ann Oncol. 1996;7(8):827–35. doi: 10.1093/oxfordjournals.annonc.a010762. [DOI] [PubMed] [Google Scholar]
- 47.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 48.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19(2):577–83. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 49.Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003;2(8):753–63. [PubMed] [Google Scholar]
- 50.Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 51.Kottschade L a Suman VJ, Perez DG, et al. A randomized phase 2 study of temozolomide and bevacizumab or nab-paclitaxel, carboplatin, and bevacizumab in patients with unresectable stage IV melanoma : a North Central Cancer Treatment Group study, N0775. Cancer. 2013;119(3):586–92. doi: 10.1002/cncr.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30(1):34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16(11 Suppl 13):11–20. [PubMed] [Google Scholar]
- 55.Petrella T, Verma S, Spithoff K, et al. Adjuvant interferon therapy for patients at high risk for recurrent melanoma: an updated systematic review and practice guideline. Clin Oncol (R Coll Radiol) 2012;24(6):413–23. doi: 10.1016/j.clon.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordin. J Clin Oncol. 2008;26(35):5748–54. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson DB, Adena M, McLeod GR, et al. Interferon-alpha 2a does not improve response or survival when combined with dacarbazine in metastatic malignant melanoma: results of a multi-institutional Australian randomized trial. Melanoma Res. 1993;3(2):133–8. [PubMed] [Google Scholar]
- 58.Bajetta E, Di Leo A, Zampino MG, et al. Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. J Clin Oncol. 1994;12(4):806–11. doi: 10.1200/JCO.1994.12.4.806. [DOI] [PubMed] [Google Scholar]
- 59.Falkson CI, Ibrahim J, Kirkwood JM, Coates AS, Atkins MB, Blum RH. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: an Eastern Cooperative Oncology Group st. J Clin Oncol. 1998;16(5):1743–51. doi: 10.1200/JCO.1998.16.5.1743. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol. 1999;17(3):968–75. doi: 10.1200/JCO.1999.17.3.968. [DOI] [PubMed] [Google Scholar]
- 61.Eton O, Legha SS, Bedikian AY, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol. 2002;20(8):2045–52. doi: 10.1200/JCO.2002.07.044. [DOI] [PubMed] [Google Scholar]
- 62.Ridolfi R, Chiarion-Sileni V, Guida M, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol. 2002;20(6):1600–7. doi: 10.1200/JCO.2002.20.6.1600. [DOI] [PubMed] [Google Scholar]
- 63.Kaufmann R, Spieth K, Leiter U, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. J Clin Oncol. 2005;23(35):9001–7. doi: 10.1200/JCO.2005.01.1551. [DOI] [PubMed] [Google Scholar]
- 64.Bajetta E, Del Vecchio M, Nova P, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol. 2006;17(4):571–7. doi: 10.1093/annonc/mdl007. [DOI] [PubMed] [Google Scholar]
- 65.Tarhini AA, Kirkwood JM, Gooding WE, Cai C, Agarwala SS. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J Clin Oncol. 2007;25(25):3802–7. doi: 10.1200/JCO.2006.10.2822. [DOI] [PubMed] [Google Scholar]
- 66.Chiarion-Sileni V, Del Bianco P, De Salvo GL, et al. Quality of life evaluation in a randomised trial of chemotherapy versus bio-chemotherapy in advanced melanoma patients. Eur J Cancer. 2003;39(11):1577–85. doi: 10.1016/s0959-8049(03)00372-1. [DOI] [PubMed] [Google Scholar]
- 67.Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol. 2007;25(34):5426–34. doi: 10.1200/JCO.2007.12.0253. [DOI] [PubMed] [Google Scholar]
- 68.Boasberg P, Hamid O, O'Day S. Ipilimumab: Unleashing the Power of the Immune System Through CTLA-4 Blockade. Semin Oncol. 2010;37(5):440–449. doi: 10.1053/j.seminoncol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192(1):161–80. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 70.Hodi F, O'Day S. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010;10:9. [PMC free article] [PubMed] [Google Scholar]
- 72.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 73.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21(8):1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 74.Hersh EM, O'Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29(3):489–98. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 75.Yervoy (ipilimumab): Risk Evaluation and Mitigation Strategy (REMS) - Severe Immune-Mediated Adverse Reactions. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm249770.htm. Accessed November 14, 2013.
- 76.Hodi FS, Lee SJ, McDermott DF, et al. Multicenter, randomized phase II trial of GM-CSF (GM) plus ipilimumab (Ipi) versus ipi alone in metastatic melanoma: E1608. J Clin Oncol. 2013;31(suppl) abstr CRA9007. [Google Scholar]
- 77.GM-CSF/Ipilimumab combination extends melanoma survival. Cancer Discov. 2013;3(7):OF6. doi: 10.1158/2159-8290.CD-NB2013-083. [DOI] [PubMed] [Google Scholar]
- 78.Sturrock A, Seedahmed E, Mir-Kasimov M, Boltax J, McManus ML, Paine R. GM-CSF provides autocrine protection for murine alveolar epithelial cells from oxidant-induced mitochondrial injury. Am J Physiol Lung Cell Mol Physiol. 2012;302(3):L343–51. doi: 10.1152/ajplung.00276.2011. [DOI] [PubMed] [Google Scholar]
- 79.Egea L, Hirata Y, Kagnoff MF. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol. 2010;4(6):723–31. doi: 10.1586/egh.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27(7):1075–81. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 82.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–22. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193(7):839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(27):9331–6. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1(8):1223–1225. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taube JM, Anders R a, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taneja SS. Re: Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. J Urol. 2012;188(6):2148–9. doi: 10.1016/j.juro.2012.08.169. [DOI] [PubMed] [Google Scholar]
- 91.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sznol M, Kluger H, Hodi S, et al. Survival and long-term follow-up of safety and response in patients (pts) with advanced melanoma (MEL) in a phase I trial of nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol. 2013;31(suppl) abstr CRA9006^. [Google Scholar]
- 93.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phase 3 Study of Nivolumab or Nivolumab Plus Ipilimumab Versus Ipilimumab Alone in Previously Untreated Advanced Melanoma (CheckMate-067). Bristol-Myers Squibb 2013. ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT01844505. Accessed September 5, 2013.
- 95.Bailey A, McDermott DF. Immune checkpoint inhibitors as novel targets for renal cell carcinoma therapeutics. Cancer J. 2013;19(4):348–52. doi: 10.1097/PPO.0b013e31829e3153. [DOI] [PubMed] [Google Scholar]
- 96.Patnaik A, Kang SP, Tolcher AW, et al. Phase I study of MK-3475 (anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. J Clin Oncol. 2012;30(suppl) doi: 10.1158/1078-0432.CCR-14-2607. abstr 2512. Available at: http://meetinglibrary.asco.org/content/100724-114. Accessed August 5, 2013. [DOI] [PubMed] [Google Scholar]
- 97.Hamid O. Efficacy and Safety of MK-3475 in Patients with Advanced Melanoma.. Paper presented at The Society for Melanoma Research 2012 Congress; Hollywood, CA. Nov 8–11, 2012. [Google Scholar]
- 98.Hamid O, Robert C, Daud A, et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(suppl) abstr 9010. [Google Scholar]
- 100.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Greenman C, Stephens P, Smith R, Al. E. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim P. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(3):596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng W, Gopal YNV, Scott A, Chen G, Woodman SE, Davies M a. Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell Melanoma Res. 2012;25(2):248–58. doi: 10.1111/j.1755-148X.2011.00950.x. [DOI] [PubMed] [Google Scholar]
- 104.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su F, Viros A, Milagre C. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013;49(6):1297–304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Nijenhuis CM, Haanen JB a G, Schellens JHM, Beijnen JH. Is combination therapy the next step to overcome resistance and reduce toxicities in melanoma? Cancer Treat Rev. 2013;39(4):305–12. doi: 10.1016/j.ctrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 108.McCubrey JA, Steelman LS, Chappell WH, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3(10):1068–111. doi: 10.18632/oncotarget.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hauschild A, Grob J-J, Demidov L V, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 110.Menzies AM, Kefford RF, Long G V. Paradoxical oncogenesis: are all BRAF inhibitors equal? Pigment Cell Melanoma Res. 2013;26(5):611–5. doi: 10.1111/pcmr.12132. [DOI] [PubMed] [Google Scholar]
- 111.Long G V, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 112.Rochet NM, Dronca RS, Kottschade L a, et al. Melanoma brain metastases and vemurafenib: need for further investigation. Mayo Clin Proc. 2012;87(10):976–81. doi: 10.1016/j.mayocp.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rochet NM, Kottschade L a, Markovic SN. Vemurafenib for melanoma metastases to the brain. N Engl J Med. 2011;365(25):2439–41. doi: 10.1056/NEJMc1111672. [DOI] [PubMed] [Google Scholar]
- 114.Solit DB, Garraway L a, Pratilas C a, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439(7074):358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 116.Mekinist (package insert). GlaxoSmithKline, Res Triangle Park NC 27709. May, 2013.
- 117.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Handolias D, Salemi R, Murray W, et al. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res. 2010;23(2):210–5. doi: 10.1111/j.1755-148X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- 119.Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904–9. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 120.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–34. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ascierto P a, Kalos M, Schaer D a, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19(5):1009–20. doi: 10.1158/1078-0432.CCR-12-2982. [DOI] [PubMed] [Google Scholar]
- 122.Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17(7):1664–73. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- 123.Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19(17):4792–800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 124.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19(5):1035–43. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto P a. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19(5):997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 126.Chan WM, Rahman MM, McFadden G. Oncolytic myxoma virus: The path to clinic. Vaccine. 2013;31(39):4252–8. doi: 10.1016/j.vaccine.2013.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andtbacka RHI, Collichio FAAT, et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2013;31(suppl) abstr LBA9008. [Google Scholar]