Abstract
The review summarizes the current understanding of the role of hepcidin and ferroportin in normal iron homeostasis and its disorders. It further discusses the various approaches to therapeutic targeting of hepcidin and ferroportin in iron overload diseases (mainly hereditary hemochromatosis and β-thalassemia) and iron-restrictive anemias (anemias associated with infections, inflammatory disorders and certain malignancies, anemia of chronic kidney diseases, iron-refractory iron deficiency anemia).
Hepcidin, ferroportin and iron disorders
Systemic iron homeostasis1
In healthy humans, the concentration of iron in plasma and extracellular fluid is maintained in a relatively narrow range of 10–30 μM, assuring that adequate iron is available for essential cellular functions without incurring iron toxicity. Plasma iron concentration is controlled by the hepatic peptide hormone hepcidin which regulates the major iron flows into plasma: dietary iron absorption in the duodenum (1–2 mg/day), iron recycling from senescent erythrocytes (20 mg/day) and the recovery of iron from storage in hepatocytes and macrophages (a few mg/day depending on iron needs). Transferrin-bound iron exits the plasma compartment destined predominantly for the bone marrow erythrocyte precursors where it is incorporated into heme and hemoglobin. Smaller amounts of iron are taken up by other cells where they are incorporated into myoglobin, redox enzymes and other iron-containing proteins.
Hepcidin and ferroportin
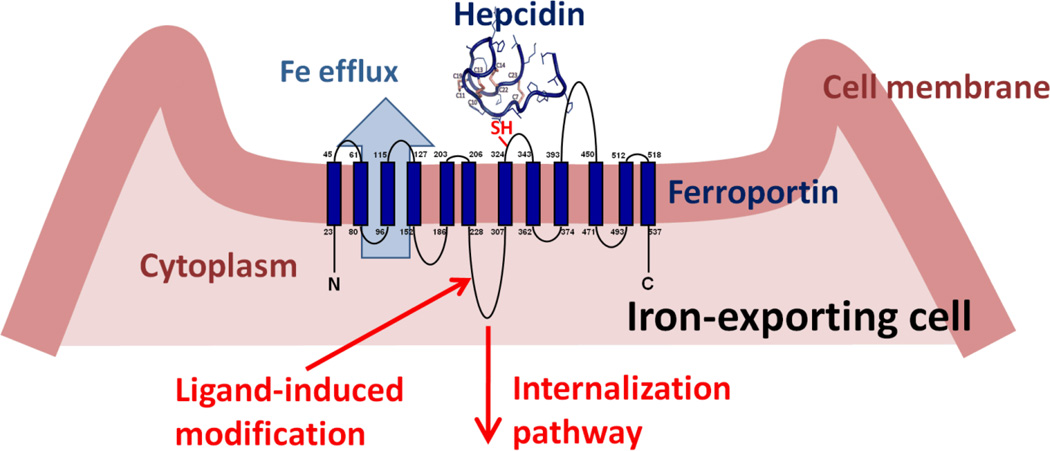
Hepcidin is a 25 amino acid peptide synthesized in hepatocytes as a larger inactive preprohepcidin comprised of a signal peptide and 60 amino acid prohepcidin. Prohepcidin is then cleaved by the prohormone convertase furin to generate mature hepcidin. Hepcidin structure consists of a 4-disulfide crosslinked beta-hairpin whose N-terminal arm is highly conserved and essential for activity. The sole known molecular target of hepcidin is the protein ferroportin2 which functions as a transmembrane conduit for the transfer of cellular iron to plasma. Most cells contain very little ferroportin and do not export iron but use it only for their own metabolic needs. The professional iron exporters, including macrophages, duodenal enterocytes, hepatocytes and placental syncytiotrophoblast, express ferroportin and provide iron for the entire organism. The binding of hepcidin to ferroportin on the membranes of iron-exporting cells induces the endocytosis and proteolysis of ferroportin and thereby decreases the delivery of iron to plasma2 (Figure 1). The specific pathways required for ferroportin internalization and degradation are an evolving area of investigation but there is agreement that ferroportin undergoes ligand-induced ubiquitination. The cellular uptake of iron in its various forms (dietary elemental iron and heme for enterocytes, diferric transferrin, heme-hemopexin, hemoglobin-haptoglobin, and senescent erythrocytes for macrophages) is also subject to regulation but it appears that the regulation of ferroportin expression on the cell membrane is the predominant mode by which iron transport into plasma is controlled.
Figure 1.
After binding hepcidin, ferroportin is covalently modified, internalized and degraded, decreasing cellular iron export
Hepcidin regulation by iron
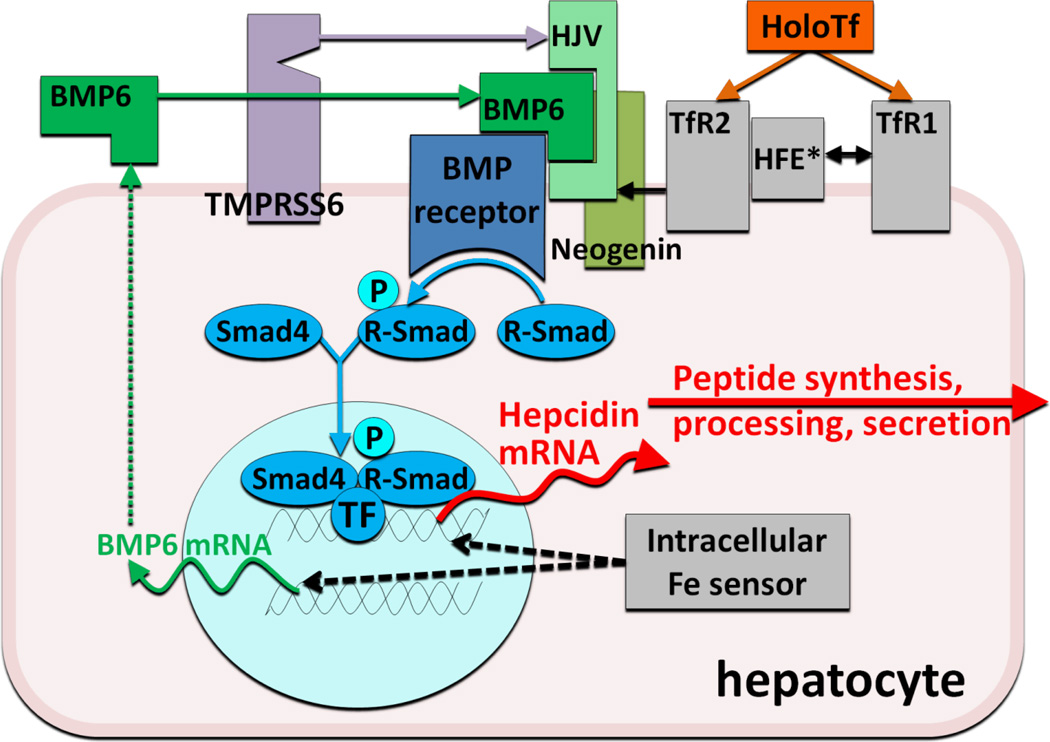
As would be expected of an iron-regulatory hormone, the production of hepcidin is homeostatically regulated by plasma iron concentrations and iron stores3, predominantly through a transcriptional mechanism. Increased hepcidin release in response to increased iron concentrations generates a negative feedback loop that limits iron absorption and retains iron in stores. The regulatory mechanism centers on a bone morphogenetic protein receptor (BMPR) and its SMAD signaling pathway that regulates hepcidin transcription4 (Figure 2). The canonical pathway, which has other important roles in development and tissue remodeling, is adapted for iron regulation by its interaction with proteins specialized in iron sensing or iron-related signaling. BMP6 is an iron-regulated ligand with no other known function but the regulation of hepcidin expression5;6 Similarly, GPI-linked hemojuvelin (HJV)4;7 is the BMPR coreceptor involved solely in hepcidin regulation. HJV membrane expression is modulated by two other proteins. Matriptase 2 (also called TMPRSS6), is a transmembrane serine protease that degrades HJV, possibly in an iron-regulated manner8 and thus is a negative regulator of the BMP pathway. Neogenin, a receptor for netrins, was also found to interact with HJV and BMPRs, although the specific link to iron sensing is still unknown. Finally, BMP pathway signaling is also adjusted by two potential sensors of holo-transferrin concentrations, transferrin receptors 1 and 2, and their interacting partner, transmembrane protein HFE9. Increasing concentrations of holo-transferrin shift the interaction of HFE from TfR1 to TfR2, promote stabilization of TfR2 protein, and enhance SMAD signaling. Although the important role of each of these proteins in hepcidin regulation is supported by the known effects of human and murine mutations on hepcidin regulation, the biochemistry of their interactions is only beginning to be uncovered.
Figure 2.
Hepcidin regulation by iron
Hepcidin regulation by erythroid factors
Low hepcidin concentrations were observed in iron-deficiency anemia, in hereditary anemias with ineffective erythropoiesis, and in mouse models of anemia due to bleeding or hemolysis. Substantial evidence points to the existence of a hepcidin-regulating signal originating in erythroid precursors in the bone marrow1. This “erythroid” factor whose biochemical nature is not yet known, physiologically suppresses hepcidin in proportion to the erythropoietic activity of the marrow. Suppression of hepcidin in turn allows greater availability of iron for erythropoiesis.
Hepcidin regulation by inflammation
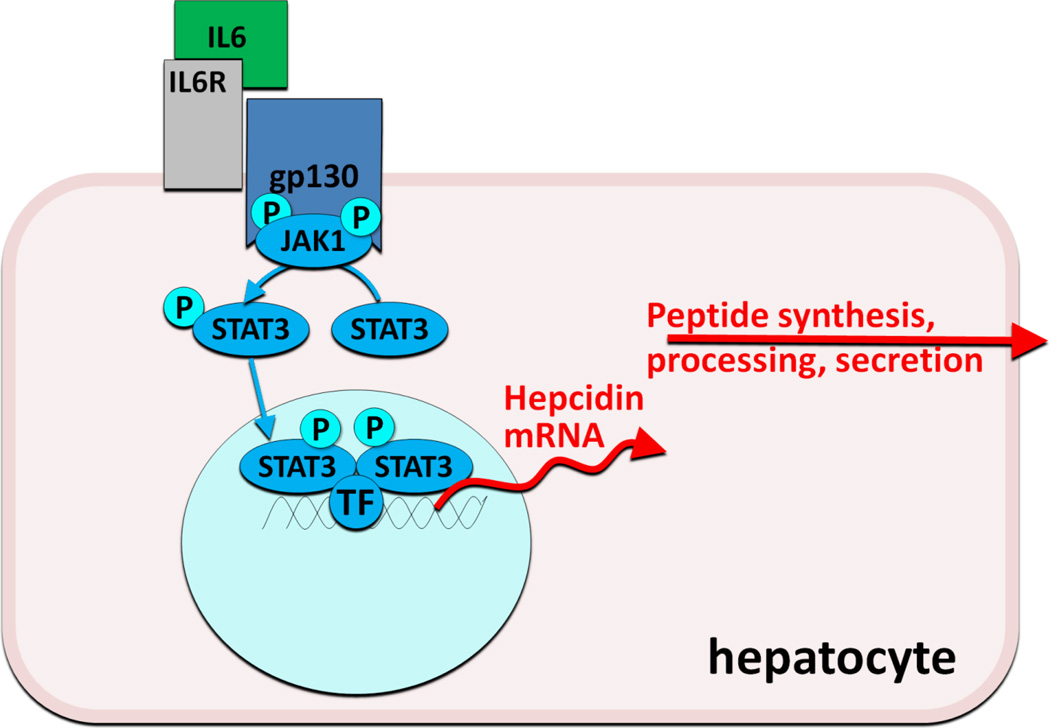
Infection and inflammation generate signals that dramatically increase hepcidin synthesis and release, resulting in the characteristic hypoferremia, restriction of iron flow to erythropoiesis, and anemia of inflammation (anemia of chronic diseases). Hepcidin transcription is particularly responsive to stimulation by interleukin-6, an inflammatory cytokine that activates the JAK-STAT pathway (Figure 3). After phosphorylation by JAK2, the STAT3 transcription factor binds to cognate motifs in the hepcidin promoter and increases hepcidin transcription10. Other inflammatory cytokines and microbial products may also activate hepcidin transcription.
Figure 3.
Hepcidin regulation by IL-6 during inflammation
Hepcidin clearance
As a small peptide that does not appear to be strongly bound to plasma proteins, hepcidin is cleared from plasma by the kidneys and by ferroportin-mediated endocytosis and proteolysis. Accordingly, plasma hepcidin concentrations can also be increased by disease processes that diminish the clearance of hepcidin by the kidneys11.
Hepcidin deficiency in iron overload disorders
Dysregulation of the hepcidin-ferroportin axis gives rise to two phenotypically opposite groups of disorders. In iron overload disorders, ferroportin is hyperactive, stimulating intestinal iron absorption and the release of iron from macrophages, and causing an increase in plasma iron concentrations, transferrin saturation and iron deposition in the liver and other organs. In hereditary hemochromatoses, increased ferroportin activity is caused by deficient hepcidin production due to genetic lesions that dysregulate the signals controlling hepcidin transcription, disrupt the hepcidin gene itself, or in rare cases, make ferroportin resistant to internalization by hepcidin. In iron-loading anemias associated with ineffective erythropoiesis (β-thalassemias and congenital dyserythropoietic anemias), hepcidin production is suppressed12 by a pathological signal13 from an expanded population of erythrocyte precursors that fail to mature to fully differentiated erythrocytes.
Hepcidin excess in iron-restrictive disorders
In iron-restrictive disorders plasma hepcidin concentrations are excessive because of increased production of hepcidin, driven by systemic inflammation14;15, autonomous production by tumors16, or genetic lesions in TMPRSS6/MT-217, the negative regulator of hepcidin transcription. Alternatively, hepcidin concentrations may increase because of decreased hepcidin clearance by diseased kidneys11;18. Regardless of the specific cause, increased hepcidin concentrations inhibit iron absorption in the duodenum and restrict iron release from macrophages, limiting the flow of iron to hemoglobin synthesis, and resulting in iron-restricted anemias. Other mechanisms, including shortened erythrocyte lifespan, direct suppression of erythropoiesis by cytokines and diminished erythropoietin production may also contribute to anemia in these settings, depending on the underlying disease.
Hepcidin agonists
The rationale for hepcidin agonists
In hereditary hemochromatoses, phlebotomy is an inexpensive and effective treatment for iron overload that is acceptable to most but not all patients. Iron-loading anemias, on the other hand, cannot be treated in this manner and require iron chelation therapy, which is not well tolerated by many patients both because of toxic side effects (all current chelators) and the burden of parenteral administration (desferoxamine). Alternatives to existing treatments are needed. In both of these disorders, hepcidin agonists, i.e. agents that replace hepcidin activity or stimulate its endogenous production, would be expected to prevent iron accumulation. Although this strategy would not reverse established iron overload, it could diminish iron-mediated tissue injury by redistributing iron from parenchymal tissues to macrophages19, where iron is less toxic. Recent studies in a mouse model of β-thalassemia suggest that hepcidin agonists could not only prevent iron overload but also improve erythropoiesis20, perhaps by decreasing excessive α-globin synthesis or diminishing oxidative stress in erythrocyte precursors.
Small peptides that mimic the action of hepcidin
The extracellular loop of ferroportin surrounding the thiol cysteine C326 appears to be essential for hepcidin binding (Figure 1), as indicated by the lack of hepcidin binding to otherwise fully functional ferroportin containing the isosteric mutation C326S21. Structural and mutagenesis analysis of the interface between hepcidin and ferroportin showed that the segment comprised of 9 N-terminal aminoacid segment of hepcidin is sufficient for binding and internalization of ferroportin22. Further refinement by the introduction of unnatural aminoacids and lipid/bile acid modification of this minihepcidin scaffold has generated peptides whose molar activity exceeds that of hepcidin. These small modified minihepcidin peptides show bioactivity in vivo as determined by their ability to induce hypoferremia in mice and prevent iron accumulation in hepcidin-deficient mice. When engineered for resistance to proteolysis and uptake by intestinal transporters, some of these peptides retain activity even when administered by gavage (Preza et al., manuscript in revision).
Stimulants of hepcidin synthesis
In murine models, proof of principle has been demonstrated for two approaches to stimulate hepcidin production in patients with hepcidin deficiency. In the first, pharmacologic doses of BMP6, the natural ligand of the BMP receptor involved in hepcidin regulation, were shown to activate the pathway (Figure 2) that regulates hepcidin transcription23 (Figure 2) and to correct the high iron saturation and iron maldistribution in the HFE model of hereditary hemochromatosis23. Relatively high doses of BMP6 were required for this effect, and the chronic administration caused peritoneal calcifications23. In the second approach, transgenic inactivation of the membrane protease TMPRSS6 in HFE mice increased hepcidin expression and reversed their iron overload phenotype24, suggesting that the administration of a specific inhibitor of the enzymatic activity of TMPRSS6 could be used to treat hereditary hemochromatosis. Titration of the inhibition may be necessary as the HFE mice developed iron-restricted anemia when TMPRSS6 was completely inactivated.
Hepcidin antagonists
The rationale for hepcidin antagonists
Iron-restrictive anemias caused or exacerbated by hepcidin excess (due to inflammation, renal failure or genetic lesions) are currently treated by a combination of erythropoiesis stimulating agents and parenteral iron25. Although these modalities are often effective, concerns about their side effects have spurred the search for alternatives. Hepcidin antagonists, i.e. agents that decrease hepcidin production or interfere with its effect on ferroportin, would be expected to relieve hepcidin-mediated iron-restriction and release more iron for erythropoiesis. Because iron-restrictive anemias are not life-threatening, the safety profile of effective hepcidin antagonists will be the key determinant of their therapeutic use. Mouse models have provided a proof of concept for the potential benefit of hepcidin antagonists, and several have reached preclinical stage with a few reaching human trials for iron-restrictive anemia indications. In principle, hepcidin antagonists can target cytokines that stimulate hepcidin production (BMPs and IL-6), interfere with the cytokine signaling pathways (STAT3, BMPR-SMAD), stimulate erythropoiesis, bind and neutralize the hepcidin peptide (antibodies, other binding molecules), prevent hepcidin binding to ferroportin or interfere with ferroportin internalization pathways.
Antagonists of BMPs or their pathways
The BMP pathway is at the core of transcriptional regulation of hepcidin synthesis by iron. Various sulfated proteoglycans are known to bind BMPs, and the glycosaminoglycan heparin was recently shown to decrease hepcidin concentrations in mice and in humans with treated for venous thrombosis26. Other natural and modified natural BMP antagonists, including the BMP family protein noggin27, the soluble form of the BMP coreceptor hemojuvelin28;29, and the soluble ALK3 component of the type I BMP receptor30 manifested hepcidin-lowering activity in vitro and/or in mouse models. Dorsomorphin and its congeners, synthetic small molecule antagonists of the kinase activity of the BMP receptor, also lowered hepcidin in mouse models30;31. BMPs and their pathways are involved in bone formation, wound healing, hematopoiesis, morphogenesis and many other essential processes. It remains to be seen whether further development of anti-BMP agents can achieve sufficient activity and specificity for iron-related signaling to make them safe and useful for the treatment of anemia.
Antagonists of the IL-6 pathway
Reflecting the important role of IL-6 in inflammatory hepcidin regulation, anti-IL-6 strategies, originally developed for other indications, appear to have robust hepcidin-lowering effects32;33. Although published reports utilized anti-IL-6-receptor antibody, approaches targeting IL-6 itself34 or its signaling pathway35 would also be expected to suppress hepcidin when it is increased due to infection or inflammation. The beneficial effects of these agents on other manifestations of inflammatory diseases will facilitate studies of their effects on anemia in these settings. As these agents are introduced for human use in various inflammatory disorders, we will learn more about their safety profile and effects on host defense, and whether they are suitable as first line agents for the treatment of anemia of inflammation.
Nucleic acid-based inhibitors of hepcidin synthesis
Anti-sense oligonucleotides and siRNAs directed against hepcidin or its regulators (Figures 2 and 3) may be well suited as inhibitors of hepcidin synthesis because of their preferential effect on hepatocytes. Other components of the hepcidin-regulatory network in hepatocytes could also be targeted by these approaches. Development programs in this area have been announced by Alnylam and the ISIS/Xenon consortium.
Erythropoiesis-stimulating agents
Erythropoietin and erythropoiesis-stimulating agents (ESAs) have been reported to suppress hepcidin production in animal models and in humans36. Generation of the putative hepcidin-suppressing "erythroid factor" by developing erythrocytes is a likely mechanism mediating this effect. In addition, agents such as prolyl hydroxylase inhibitors37 may also reduce hepcidin expression by increasing the activity of hypoxia-inducible factor (HIF), a pathway proposed to be directly involved in the regulation of hepcidin transcription 38.
Agents that bind and neutralize hepcidin
Human or humanized monoclonal antibodies (MAb) against hepcidin have been developed by scientists at Lilly (US Patent 7820163) and Amgen (US Patent Application 12/022515). Amgen’s anti-hepcidin Mab reversed iron restriction and erythropoietin resistance in a humanized mouse model of anemia of inflammation39 in which mouse hepcidin genes were replaced by a human hepcidin gene. Lilly’s Mab (LY2787106) is already undergoing phase I human trials in cancer-related anemia. Besides Mabs, other protein and nonprotein scaffolds could be used to developed hepcidin-trapping agents. Considering the high rate of hepcidin synthesis during inflammation, the main hurdle is whether it is technically and economically feasible to deliver effective amounts of these neutralizing drugs into the bloodstream. This concern could be addressed by developing agents that recycle after delivering their hepcidin cargo for degradation by macrophages.
Antagonists of hepcidin effect on ferroportin
Agents that block the hepcidin-binding site on ferroportin without inducing its internalization are also under development. Researchers at Lilly developed a Mab that targets the extracellular ferroportin loop adjacent to the hepcidin-binding site (WIPO Patent Application WO/2010/065496 and Figure 1). As LY2928057, the antiferroportin Mab is undergoing studies in healthy volunteers. Our group developed a high-content screen using cells expressing ferroportin-GFP fusion protein to identify small molecules acting as hepcidin antagonists40. We identified compounds which allow continuous iron export from cells in the presence of hepcidin. The small molecule hepcidin antagonists include thiol modifiers that inhibit hepcidin binding to ferroportin. It remains to be seen whether any of these activities have sufficient selectivity to form a basis for drug development.
Conclusions
Considering the central role of the hepcidin-ferroportin axis in iron regulation and in the pathogenesis of common iron disorders, it is not surprising that this system has been targeted for drug development. In the coming years, these efforts are likely to yield new medications for the treatment of anemia and iron overload diseases.
Reference List
- 1.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu.Rev.Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53:1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat.Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 5.Meynard D, Kautz L, Darnaud V, et al. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat.Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 6.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat.Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat.Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri L, Pagani A, Nai A, et al. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goswami T, Andrews NC. Hereditary Hemochromatosis Protein, HFE, Interaction with Transferrin Receptor 2 Suggests a Molecular Mechanism for Mammalian Iron Sensing. J.Biol.Chem. 2006;281:28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 10.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaritsky J, Young B, Wang HJ, et al. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin.J Am Soc Nephrol. 2009;4:1051–1056. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardenghi S, Grady RW, Rivella S. Anemia, ineffective erythropoiesis, and hepcidin: interacting factors in abnormal iron metabolism leading to iron overload in beta-thalassemia. Hematol.Oncol.Clin.North Am. 2010;24:1089–1107. doi: 10.1016/j.hoc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat.Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 14.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin.Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein DA, Roy CN, Fleming MD, et al. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 17.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat.Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomosugi N, Kawabata H, Wakatabe R, et al. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108:1381–1387. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 19.Viatte L, Nicolas G, Lou DQ, et al. Chronic hepcidin induction causes hyposideremia and alters the pattern of cellular iron accumulation in hemochromatotic mice. Blood. 2006;107:2952–2958. doi: 10.1182/blood-2005-10-4071. [DOI] [PubMed] [Google Scholar]
- 20.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin.Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes A, Preza GC, Phung Y, et al. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114:437–443. doi: 10.1182/blood-2008-03-146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preza GC, Ruchala P, Nemeth E, Ganz T. Minihepcidins: Small Peptides Involved in Disulfide Exchange with Ferroportin Act as Agonists. FASEB J. 2011;24 (Meeting Abstract Supplement): Abstract 1011.1. [Google Scholar]
- 23.Corradini E, Schmidt PJ, Meynard D, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139:1721–1729. doi: 10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117:4590–4599. doi: 10.1182/blood-2010-10-315507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 26.Poli M, Girelli D, Campostrini N, et al. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117:997–1004. doi: 10.1182/blood-2010-06-289082. [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Valore EV, Nemeth E, et al. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106:2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 29.Babitt JL, Huang FW, Xia Y, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin.Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinbicker AU, Sachidanandan C, Vonner AJ, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. doi: 10.1182/blood-2010-10-313064. Prepublished on March 10, 2011, as blood-2010-10-313064 [pii];10.1182/blood-2010-10-313064 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat.Chem.Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song SN, Tomosugi N, Kawabata H, et al. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010;116:3627–3634. doi: 10.1182/blood-2010-03-271791. [DOI] [PubMed] [Google Scholar]
- 33.Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol.Int. 2010;30:917–923. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- 34.Xu Z, Bouman-Thio E, Comisar C, et al. Pharmacokinetics, Pharmacodynamics, and Safety of a Human Anti-IL-6 Monoclonal Antibody (Sirukumab) in Healthy Subjects in a First-in-Human Study. British Journal of Clinical Pharmacology. doi: 10.1111/j.1365-2125.2011.03964.x. Prepublished on March 11, 2011, as 10.1111/j.1365-2125.2011.03964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fatih N, Camberlein E, Island M, et al. Natural and synthetic STAT3 inhibitors reduce hepcidin expression in differentiated mouse hepatocytes expressing the active phosphorylated STAT3 form. Journal of Molecular Medicine. 2010;88:477–486. doi: 10.1007/s00109-009-0588-3. [DOI] [PubMed] [Google Scholar]
- 36.Ashby DR, Gale DP, Busbridge M, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95:505–508. doi: 10.3324/haematol.2009.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klaus S, Langsetmo I, Spong S, et al. Induction of Erythropoiesis and Iron Utilization by the HIF Prolyl Hydroxylase Inhibitor FG-4592. J Am Soc Nephrol. 2005;16:49A. Abstract F-FC050. [Google Scholar]
- 38.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin.Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 40.Fung E, Hsu J, Damoiseaux R, Ganz T, Nemeth E. Identification of hepcidin antagonists through a high throughput screening approach. FASEB J. 2011;24 (Meeting Abstract Supplement): Abstract 1011.6. [Google Scholar]