Abstract
Focal adhesions are transmembrane protein complexes that attach chondrocytes to the pericellular cartilage matrix and in turn, are linked to intracellular organelles via cytoskeleton. We previously found that excessive compression of articular cartilage leads to cytoskeleton-dependent chondrocyte death. Here we tested the hypothesis that this process also requires integrin activation and signaling via focal adhesion kinase (FAK) and Src family kinase (SFK). Osteochondral explants were treated with FAK and SFK inhibitors (FAKi, SFKi respectively) for 2 hours and then subjected to a death-inducing impact load. Chondrocyte viability was assessed by confocal microscopy immediately and at 24 hours post-impact. With no treatment immediate post-impact viability was 59%. Treatment with 10μM SFKi, 10μM or 100μM FAKi improved viability to 80%, 77%, and 82% respectively (p<0.05). After 24 hours viability declined to 34% in controls, 48% with 10μM SFKi, 45% with 10μM FAKi, and 56% with 100μM FAKi (p<0.01) treatment. These results confirmed that most of the acute chondrocyte mortality was FAK- and SFK-dependent, which implicates integrin-cytoskeleton interactions in the death signaling pathway. Together with previous findings, these data support the hypothesis that the excessive tissue strains accompanying impact loading induce death via a pathway initiated by strain on cell adhesion receptors.
Keywords: Articular cartilage, Chondrocytes, Focal adhesions, Integrins, Posttraumatic osteoarthritis (PTOA)
INTRODUCTION
Osteoarthritis (OA) is a degenerative disease of synovial joints characterized by pain, stiffness and loss of motion. Although the pathogenesis of OA remains poorly understood, the risk factors include aging, joint injury and joint overuse.1–5 Chondrocytes, the specialized resident cells in articular cartilage, maintain the extracellular matrix (ECM) by producing structural molecules including collagens, and glycoproteins.1 Joint use subjects chondrocytes to periodic-mechanical compression transmitted through the ECM. Physiologic compression stimulates chondrocyte anabolic activity that maintains ECM integrity, however, excessive repetitive loading or acute severe mechanical insults result in chondrocyte dysfunction or death, which may contribute to the development of OA.6–12
Current understanding suggests that mechanical insults to articular cartilage result in chondrocyte death through necrosis or by intracellular signaling cascades that lead to apoptosis.13–16 Recently, we demonstrated that mechanical insults to articular cartilage trigger the release of mitochondrial reactive oxygen species (ROS), which causes chondrocyte death. The mortality was significantly decreased by suppression of ROS production with rotenone, an electron transport chain inhibitor, or by N-acetylcysteine, an oxidant scavenger, suggesting that oxidative stress was responsible for chondrocyte death within hours following cartilage mechanical injury.17, 18 In addition, cytoskeletal dissolution by treatment with cytochalasin-B or nocodazole also considerably decreased impact induced chondrocyte death, which suggests that the cytoskeletal linkage between intracellular organelles and cell-surface integrins are required for impact-induced chondrocyte death.19
Focal adhesions which result in cellular attachment are required not only for providing a biochemical signaling hub via tyrosine phosphorylation, but also for carrying out cellular activities such as migration, proliferation and gene expression.20–26 Integrins are a class of transmembrane receptors that cluster in response to mechanical and chemical changes in the ECM to form adhesions which involve multiple intracellular kinases and structural proteins, some of which link integrin complexes to the cytoskeleton.27–31 In articular cartilage, chondrocytes express multiple integrin receptors for type II collagen, fibronectin and other ECM molecules.32 We hypothesized that inhibitors of the adhesion complex-associated protein tyrosine kinases FAK and SFK would reduce impact-induced chondrocyte death.
METHODS
Eleven bovine stifle joints (15–24 months old) were obtained from a local abattoir (Bud’s Custom Meats, Riverside, IA) and 2 × 2 cm2 of osteochondral explants were prepared including the central loaded area from tibial plateau. The explants were rinsed in Hank’s Balanced Salt Solution (HBSS) (Invitrogen™ Life Technologies, Carlsbad, CA, USA) and cultured in 45% Dulbecco’s modified Eagle medium (DMEM) and 45% Ham’s F-12 (F12) supplemented with 10% fetal bovine serum (FBS) (Invitrogen™ Life Technologies), 100U/ml penicillin, 100μg/ml streptomycin, and 2.5μg/ml Amphotericin B at 37°C, 5% CO2 and 5% O2.
After 2 days, the explants were randomly distributed and were treated with fresh culture medium containing 10 or 100μM focal adhesion kinase inhibitor (FAKi) (Santa Cruz Biotechnology, Dallas, TX, USA) to block phosphorylation of FAK at the kinase domain (Try 397) or were treated with fresh culture medium containing 10μM Src family kinase inhibitor (SFKi) (Selleckchem, Houston, TX, USA) to block phosphorylation of SFKs at kinase domain (Tyr 416) for 2 hours. No macroscopic changes in cartilage with 2 hours of inhibition of FAK and SFKs were observed. The explants were securely fixed in customized testing fixtures and were kept submerged in culture medium at all times. Impact energy was controlled by dropping a 2kg mass from a 7cm height, which resulted in an impact energy density of 7 J/cm2 to a cartilage surface through an indenter (flat-faced with 5mm in diameter resting on the explant surface). The cartilage surface was placed parallel to the impact devices to make morphologically repeatable shape of impact injury in cartilage. The explants were then stained with 1μM Calcein-AM, a live cell indicator, and 1μM ethidium-homodimer-2, a dead cell indicator, (Invitrogen™ Life Technologies) for 30 minutes in the same culture condition as previously described.17–19, 33 Confocal laser scanning microscopy (Bio-Rad Laboratories Inc, Hercules, CA, USA) was performed to image impact sites with a depth of 200μm at 20μm intervals. The explants were then placed back into the same culture condition for additional 24 hours and stained again with 1μM Calcein-AM and ethidium-homodimer-2 for confocal microscopy. Percentage of cell viability was calculated as [(live chondrocytes)/(live + dead chondrocytes)] x100 [%] in impact sites using custom automated cell counting program (QCIP™).34 Scanned images were stacked for Z-axis projection using ImageJ (rsb.info.nih.gov/ij).
To confirm if both FAKi and SFKi block phosphorylation of FAK at Tyr 397 and Src at Tyr 416, chondrocytes were isolated from full thickness articular cartilage harvested from a bovine stifle joint using type I collagenase (Sigma-Aldrich, Rochester, NY, USA) dissolved in culture media (0.25 mg/ml) and were cultured in monolayer at 37°C, 5% CO2 and 5% O2 until confluence. Cells were then isolated using 0.0025% trypsin-EDTA (Invitrogen™ Life Technologies) and 1 × 106 cells were cultured in 6-well culture plate with serum containing media for 3 days. Media was switched to serum-free media and cells were cultured for another 24 hours. Cells were treated with 1, 10 or 100μM FAKi for 2 hours and then 100nM N-Formyl-Met-Leu-Phe (fMLF) (R&D Systems, Minneapolis, MN, USA) was added for 30 minutes. For the study of SFKs kinetics, cells were also treated with 0.1, 1 or 10μM SFKi for 2 hours and then 10ng/ml IL1-β and 100ng/ml TNF-α (R&D Systems) were added for 30 minutes. Cells were rinsed with 1X cold PBS and lysed in cold lysis buffer containing protease and phosphatase inhibitor diluted with a 1:100 proteinase inhibitor cocktail III (CalBiochem, San Diego, CA, USA). Total protein concentration was measured with a BCA Protein Assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). The proteins were denatured with 2X sample buffer and reduced with 0.05M Dithiothreitol. 7.5μg proteins from each group were separated in 10% SDS-PAGE gels and blotted onto nitrocellulose membranes. After blocking with 5% non-fat dried milk in tris-buffered saline (20mM Tris-buffer containing 140mM NaCl at pH 7.4) containing 0.1% Tween-20 (TBST) for 1 hour, the blot was incubated at 4°C with total or phosphor-specific FAK (Tyr 397), total or phosphor-specific Src (Tyr 416) and beta-actin antibody (Cell Signaling Technology, Danvers, MA, USA) in a 1:1,000 dilution in 5% BSA in TBST. After overnight incubation, the blots were washed with TBST and incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG in a 1:2,000 dilution in 5% BSA in TBST for 1 hour at room temperature. Then, the blots were reacted with SuperSignal West Dura Chemiluminescent Substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA) and the signals were detected with Kodak Biomax Xar Film from Sigma-Aldrich (Rochester, NY, USA). The integrated density (ID) was measured using ImageJ (rsb.info.nih.gov/ij) and the relative fold changes were calculated.
Statistical analysis was performed by One-way ANOVA with the Tukey test for pair-wise comparison using SPSS software (IBM Corporation, Armonk, New York, USA). The level of significance was set at p<0.05.
RESULTS
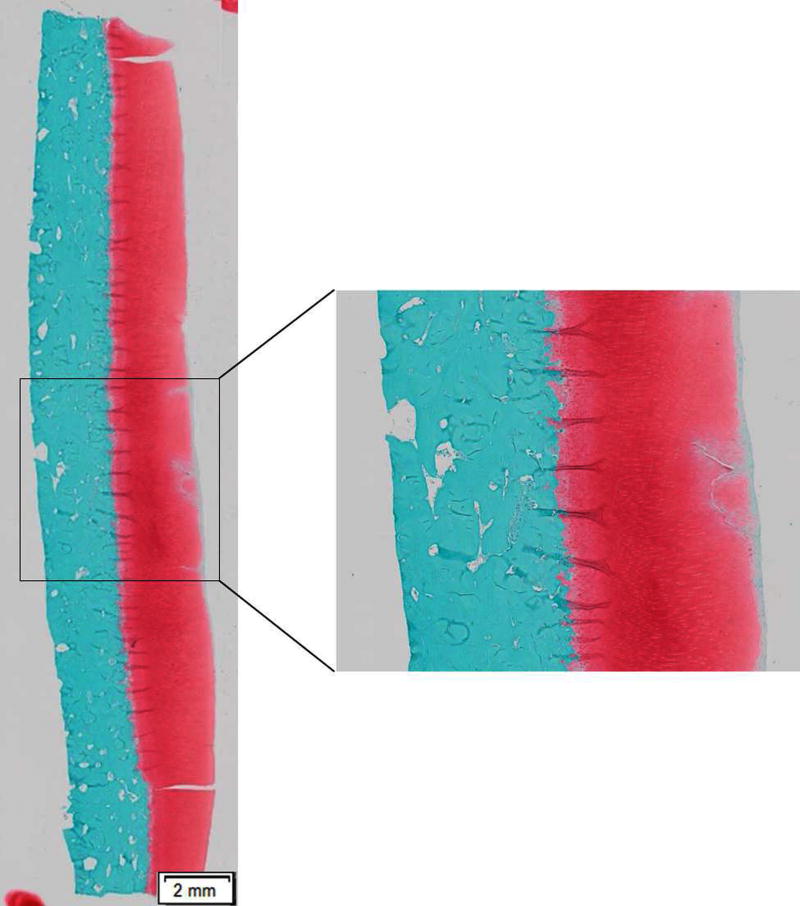
Confocal microscopy immediately after impact showed that 7 J/cm2 impact to articular cartilage created approximately 5-mm diameter impact site in the ECM (Fig 2). Dead chondrocytes were located primarily in and near the impact sites, which was consistent with previous studies of impact injury.17
Figure 2. Structural injury caused by 7 J/cm2 impact in an osteochondral explant.
A safranin-O and fast green-stained sagittal section of the explant shows that a 7 J/cm2 mechanical insult resulted in structural damage in ECM.
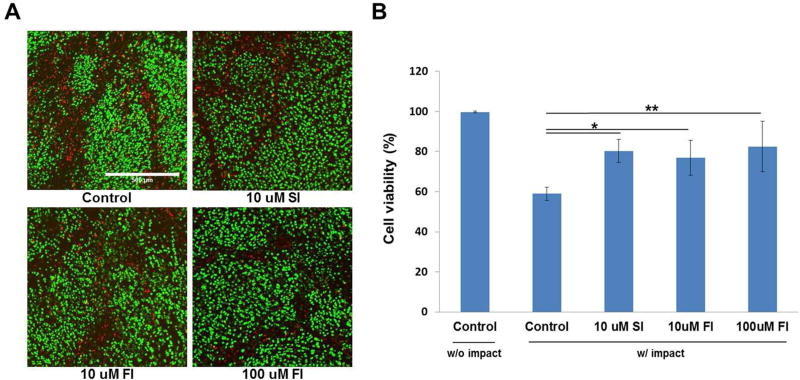
Focal adhesion protein tyrosine kinase inhibitors significantly reduced cell death (Fig 3A). Fifty-nine percent of the chondrocytes were viable in the untreated articular cartilage, compared with, 80% (p<0.05) in 10μM SFKi treated samples, 77% (p<0.05) in 10μM FAKi treated samples and 82% (p<0.001) in 100μM FAKi treated group. Between the two tested concentrations, no difference of chondrocyte viability was observed (Fig. 3B).
Figure 3. Confocal microscopy and cell viability immediately after impact injury.
(A) Confocal micrographs show live (green) and dead (red) chondrocytes in an impact site in an untreated control explant, and in explants treated with 10μM SFKi and either 10 or 100μM FAKi. Compared to control, fewer dead chondrocytes were observed in SFKs or FAKi treated groups. (B) Statistical analysis revealed that chondrocyte viability was significantly higher in SFKs or FAKi treated explants compared to control. Between two tested concentrations, 100μM FAKi was more effective than 10μM. Asterisk represents statistically significant (*p<0.05, **p<0.01). Bars = 500 μm.
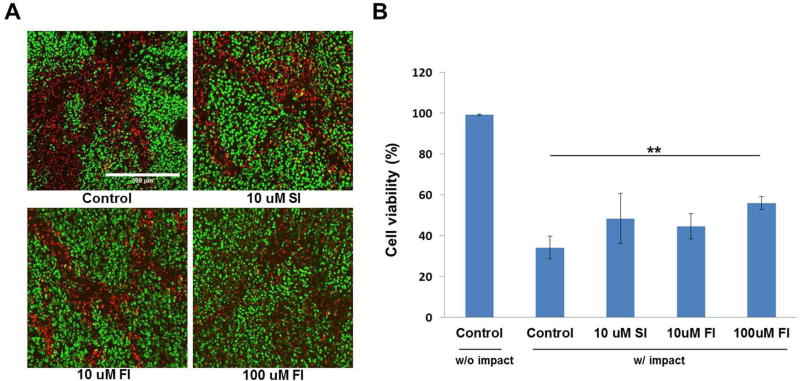
After confocal microscopy, the explants were placed back in culture. Confocal microscopy performed 24 hours after impact revealed that viability in all experimental groups had declined relative to measurements taken immediately post-impact (Fig. 4A). Viability was reduced to 34% in controls, 48% in 10μM SFKi treated samples, 45% in 10μM FAKi treated samples and 56% in the 100μM FAKi treated group. All inhibitor-treated groups showed no statistical significance in chondrocyte viability compared to controls except 100uM FAKi treated group (p<0.01) (Fig. 4B). Chondrocyte viability in non-impact cartilage at the beginning and post 24 hours showed no difference which implies that the viability was not affected by the experimental procedure.
Figure 4. Confocal microscopy and cell viability at post 24 hours after impact injury.
(A) Confocal micrographs taken 24 hours post-impact show live (green) and dead (red) chondrocytes in an un-treated control explant, and in explants treated with 10μM SFKi and either 10 or 100μM FAKi. The numbers of dead chondrocytes increased relative to immediate post-impact in all groups. (B) Statistical analysis revealed that 100μM FAKi was the only treatment that had a statistically significant effect. Asterisks indicate statistically significance versus control and other treatment (*p<0.05, **p<0.01). Bars = 500μm.
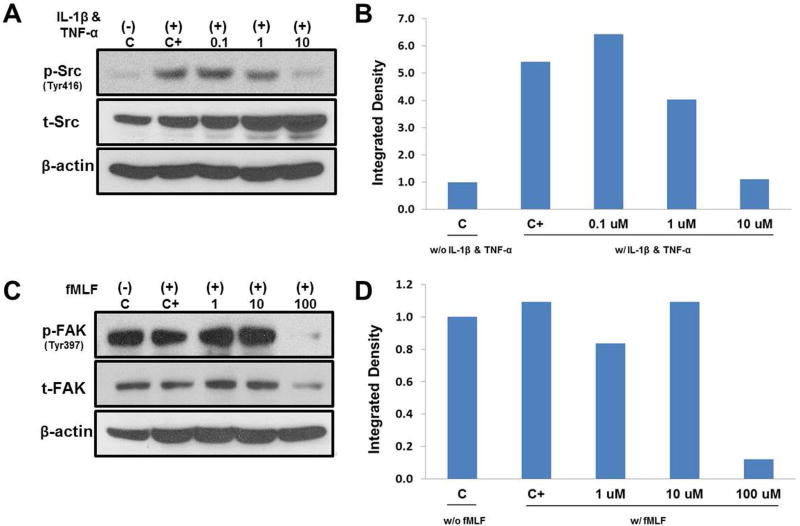
Phospho-specific immunoblot analysis confirmed that SFKi significantly reduced SFKs autophosphorylation at Tyr 416. Significantly enhanced SFKs phosphorylation was observed with 30 minute treatments with 10 ng/ml IL-1β and 100 ng/ml TNF-α and SFKi blocked this response in a dose-dependent manner (Fig. 5A). IL-1β and TNF-α was to ensure to induce inflammatory stimulus for chondrocytes. Analysis of the integrated densities of the bands revealed that the phosphor- to total SFKs ratio decreased with increasing SFKi (Fig. 5B). The inhibitory effect of FAKi on FAK autophosphorylation at Tyr 397 was also tested. Although 100nM fMLF did not stimulate Tyr 397 phosphorylation, FAKi at a concentration of 100μM significantly reduced basal phosphorylation (Fig 5C & D).
Figure 5. Kinetics of FAK and SFKi by western blot analysis.
(A) Immunoblot analysis showed that treatment with 10 ng/ml IL-1β and 100 ng/ml TNF-α for 30 minutes significantly increased SFKs phosphorylation at Tyr 416. 0.1, 1 and 10μM SFKi diminished this response dose dependently. (B) Analysis of the integrated densities of the bands with the phosphor- to total SFKs ratio. C represents untreated control and C+ represents 10 ng/ml IL-1β and 100 ng/ml TNF-α treated only. (C) Immunoblots show that treatment with 100nM fMLF for 30 min did not enhance FAK phosphorylation at Tyr 397; however 100μM FAKi significantly reduced FAK phosphorylation among tested concentrations of 1, 10 and 100μM. (D) Analysis of the integrated densities of the bands with the phosphor- to total FAK ratio. C represents untreated control and C+ represents 100nM fMLF treated only.
DISCUSSION
Since focal adhesion complexes associated with integrins process information on ECM strain by triggering tyrosine phosphorylation of FAK and SFK,35–37 we hypothesized that treatment with FAK and SFK inhibitors would significantly reduce chondrocyte death induced by impact injury, which causes excessive strain.38, 39 We chose FAKi at concentrations of 10μM and 100μM and SFKi at a concentration of 10μM since they were previously shown to diminish the activation of FAK and SFKs by blocking tyrosine phosphorylation.40–43 Phosphoprotein analysis confirmed that tyrosine phosphorylation was diminished by pre-treatment with FAKi or SFKi, supporting the conclusion that inhibiting the formation of focal adhesions by blocking phosphorylation of FAK or SFKs enhanced viability after impact-induced cartilage injury. Although we did not observe that FAKi blocked FAK activation in a clear dose-dependent manner, FAK activation was significantly reduced at a concentration of 100 μM. This suggests that the autophosphorylation of FAK at Tyr 397 was activated by some other unknown factors and 100nM fMLF failed to stably stimulate its phosphorylation. However, among the tested doses only 100μM FAKi significantly blocked its activation.
Considerable clinical and experimental evidence shows that acute severe joint injuries and excessive repetitive mechanical loading due to post-injury joint incongruity and instability cause post-traumatic osteoarthritis, and that excessive repetitive loading in uninjured joints causes osteoarthritis.44, 45 Since chondrocytes are responsible for maintaining the ECM by producing collagens, proteoglycans and glycoproteins, and in adults the cells have limited ability to replace themselves, significant chondrocyte death from cartilage mechanical injury is likely to initiate cartilage degradation which could lead to osteoarthritis. Moreover, recent work suggests that chondrolytic factors such as matrix metalloproteinases (MMPs), tumor necrosis factor (TNF) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) are highly up-regulated in cartilage injury sites via activating mitogen-activated protein kinases (MAP kinases) such as p-38 and ERK 1/2 and those signals are closely associated with propagation of chondrocyte death.46 Other studies have shown that rapid production of reactive oxygen species (ROS) immediately after cartilage mechanical injury directly led to massive chondrocyte death. Immediate or delayed treatment with N-acetylcysteine, a free radical scavenger, and rotenone, an electron transport chain inhibitor, significantly reduced the level of ROS, which resulted in increased chondrocyte survival. Those findings demonstrated that mitochondria were responsible for the injury induced ROS production that immediate chondrocyte death following mechanical injury results from oxidative stress.17, 18 In addition, pre-impact treatment with cytochalasin-B or nocodazole, which block formation of contractile microfilaments and microtubules, significantly decreased chondrocyte death suggesting that relief of cytoskeletal tension enhanced the survivability of chondrocytes in impact-injured cartilage.19 Taken together, this previous work shows that cartilage injury releases mediators that cause chondrocyte death and ECM degradation and suggest that immediate intervention to stop progressive chondrocyte death after cartilage injury may prevent articular cartilage from progressive degradation that can lead to osteoarthritis.
The organization of focal adhesions and actin in chondrocytes in cartilage differs substantially from the organization in monolayer culture.47 In cartilage, chondrocytes show small adhesion complexes and diffuse cortical actin distribution, which is in contrast to the much larger complexes and stress fibers found in chondrocytes cultured in monolayers.48 As the adhesion sites of chondrocytes in situ are difficult to image and quantify we focused on studying downstream effects of the inhibitors on viability.
The decrease in cell viability over the 24 hours following impact is striking: from 59% to 34% in untreated control samples (p<0.001), from 80% to 48% in 10μM SFKi treated samples (p<0.001), from 77% to 45% in 10μM FAKi treated samples (p<0.001), and from 82% to 56% in 100μM treated samples (p<0.001). The decrease in viability 24 hours after impact was also found in previous studies and suggesting that mechanical insults to cartilage cause acute chondrocyte necrosis and subacute apoptosis.18 Although the data suggest that acute inhibition of focal adhesions might decrease progressive cell death, only the 100μM FAKi saved significantly more chondrocytes 24 hours after impact. To test whether the inhibitors had a protective effect on chondrocyte viability downstream of their effect on adhesion (e.g anti-oxidant activity), we challenged explants with 75mM hydrogen peroxide (H2O2) for 30 minutes in the presence and absence of FAK or SFKs inhibition. The result revealed no protective effect of either inhibitor.
In conclusion, the present data are consistent with the previous observation that cytoskeletal dissolution reduced impact-associated cell death. Together the findings support the hypothesis that joint surface impact causes chondrocyte death by inducing the activation of a mechanotransduction pathway that transmits extracellular strains to intracellular organelles via integrins and the cytoskeleton. Further advances in understanding the mechanisms by which mechanical forces cause cartilage loss have the potential to lead to new treatments of injured joints that could decrease the risk of OA following joint injuries.
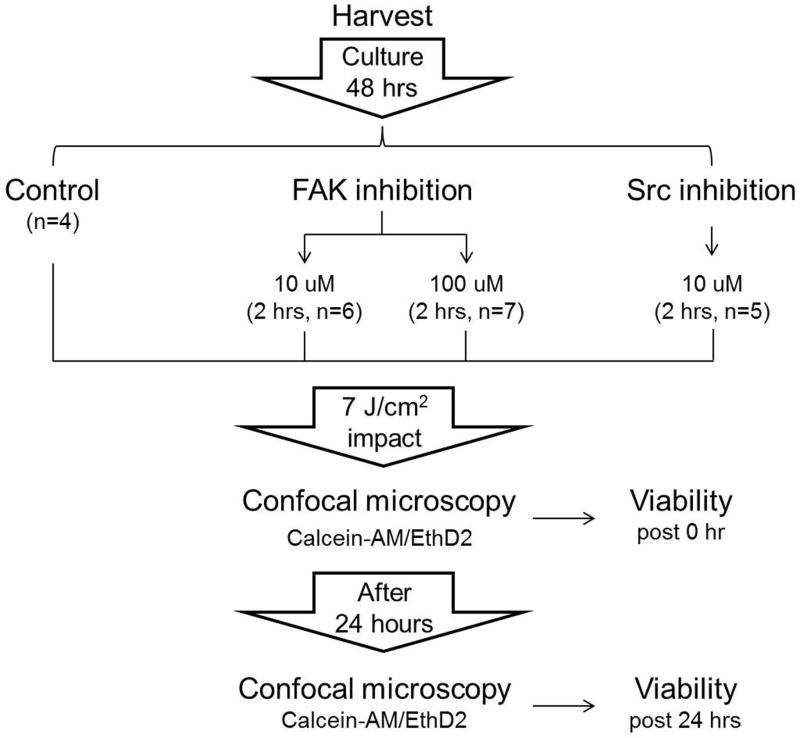
Figure 1. Schematic diagram of experimental design.
Osteochondral explants from bovine stifle joints were cultured in 5% O2/CO2 at 37°C for 48 hours. The explants were then divided into 4 experimental groups: un-treated control group (n=4), 10μM (n=6) or 100μM (n=7) FAKi treated group and 10μM SFKi treated group (n=5). Explants were subjected to a 7 J/cm2 impact injury and cartilage was stained with 1μM Calcein-AM/EthD-2. Confocal laser scanning microcopy was performed immediately after and post 24 hours of post-impact injury. Cell viability was calculated by counting live and dead chondrocytes.
Acknowledgments
The authors thank Dr. Lei Ding for providing valuable advice in experimental design, Nick Stroud for developing a drop tower device, Brice Journot for developing custom automated cell counting program, Drs. Dongrim Seol, Prem S. Ramakrishnan and Mitchell C. Coleman for experimental support, and Abigail D. Smith, Barbara J. Laughlin and John F. Bierman for harvesting osteochondral explants and ordering reagents.
References
- 1.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect; 2005;54:465–80. [PubMed] [Google Scholar]
- 2.Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology; 2002;3:257–64. doi: 10.1023/a:1020185404126. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J; 2001;21:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect; 1998;47:487–504. [PubMed] [Google Scholar]
- 5.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev; 2006;58:150–67. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Ikenoue T, Trindade MC, Lee MS, et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res; 2003;21:110–6. doi: 10.1016/S0736-0266(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 7.Leong DJ, Hardin JA, Cobelli NJ, et al. Mechanotransduction and cartilage integrity. Ann N Y Acad Sci; 2011;1240:32–7. doi: 10.1111/j.1749-6632.2011.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis; 1999;58:15–9. doi: 10.1136/ard.58.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimuta JF, Levenston ME. Response of cartilage and meniscus tissue explants to in vitro compressive overload. Osteoarthritis Cartilage; 2012;20:422–9. doi: 10.1016/j.joca.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004:7–16. [PubMed] [Google Scholar]
- 11.Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma; 2006;20:739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 12.Borrelli J, Jr, Silva MJ, Zaegel MA, et al. Single high-energy impact load causes posttraumatic OA in young rabbits via a decrease in cellular metabolism. J Orthop Res; 2009;27:347–52. doi: 10.1002/jor.20760. [DOI] [PubMed] [Google Scholar]
- 13.Kurz B, Lemke AK, Fay J, et al. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat; 2005;187:473–85. doi: 10.1016/j.aanat.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Chen CT, Burton-Wurster N, Borden C, et al. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res; 2001;19:703–11. doi: 10.1016/S0736-0266(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen CT, Burton-Wurster N, Lust G, et al. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J Orthop Res; 1999;17:870–9. doi: 10.1002/jor.1100170612. [DOI] [PubMed] [Google Scholar]
- 16.Borrelli J, Jr, Tinsley K, Ricci WM, et al. Induction of chondrocyte apoptosis following impact load. J Orthop Trauma; 2003;17:635–41. doi: 10.1097/00005131-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin W, McCabe D, Sauter E, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res; 2010;28:1057–63. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin JA, McCabe D, Walter M, et al. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am; 2009;91:1890–7. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauter E, Buckwalter JA, McKinley TO, et al. Cytoskeletal dissolution blocks oxidant release and cell death in injured cartilage. J Orthop Res; 2012;30:593–8. doi: 10.1002/jor.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci; 2010;123:1007–13. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 21.Humphries JD, Wang P, Streuli C, et al. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol; 2007;179:1043–57. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mofrad MR, Golji J, Abdul Rahim NA, et al. Force-induced unfolding of the focal adhesion targeting domain and the influence of paxillin binding. Mech Chem Biosyst; 2004;1:253–65. [PubMed] [Google Scholar]
- 23.Hall JE, Fu W, Schaller MD. Focal adhesion kinase: exploring Fak structure to gain insight into function. Int Rev Cell Mol Biol; 2011;288:185–225. doi: 10.1016/B978-0-12-386041-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 24.Parsons JT, Martin KH, Slack JK, et al. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene; 2000;19:5606–13. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 25.Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science; 1997;275:1308–11. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 26.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell; 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 27.Huveneers S, Danen EHJ. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci; 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 28.Giancotti FG, Ruoslahti E. Integrin signaling. Science; 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 29.Aplin AE, Howe A, Alahari SK, et al. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev; 1998;50:197–263. [PubMed] [Google Scholar]
- 30.Howe A, Aplin AE, Alahari SK, et al. Integrin signaling and cell growth control. Curr Opin Cell Biol; 1998;10:220–31. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 31.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science; 1995;268:233–9. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 32.Vinall RL, Lo SH, Reddi AH. Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp Cell Res; 2002;272:32–44. doi: 10.1006/excr.2001.5395. [DOI] [PubMed] [Google Scholar]
- 33.Seol D, McCabe DJ, Choe H, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum; 2012;64:3626–37. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouillette MJ, Ramakrishnan PS, Wagner VM, Sauter EE, Journot BJ, McKinley TO, et al. Strain-dependent oxidant release in articular cartilage originates from mitochondria; Biomech Model Mechanobiol; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang DD, Gunst SJ. Depletion of focal adhesion kinase by antisense depresses contractile activation of smooth muscle. Am J Physiol Cell Physiol. 2001;280:C874–83. doi: 10.1152/ajpcell.2001.280.4.C874. [DOI] [PubMed] [Google Scholar]
- 36.Seufferlein T, Rozengurt E. Sphingosine induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in Swiss 3T3 cells. J Biol Chem; 1994;269:27610–7. [PubMed] [Google Scholar]
- 37.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. Journal of Cell Biology; 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn TM, Allen RG, Schalet BJ, et al. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. Journal of Orthopaedic Research; 2001;19:242–49. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 39.Burgin LV, Aspden RM. A drop tower for controlled impact testing of biological tissues. Med Eng Phys; 2007;29:525–30. doi: 10.1016/j.medengphy.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Hochwald SN, Nyberg C, Zheng M, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle; 2009;8:2435–43. doi: 10.4161/cc.8.15.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golubovskaya VM, Nyberg C, Zheng M, et al. A small molecule inhibitor 1, 2,,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–16. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z, Chang PC, Yang JC, et al. Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors. Genes Cancer; 2010;1:40–9. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong M, Rice L, Lepler S, et al. Impact of the Src inhibitor saracatinib on the metastatic phenotype of a fibrosarcoma (KHT) tumor model. Anticancer Res; 2010;30:4405–13. [PubMed] [Google Scholar]
- 44.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res; 2011;29:802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramakrishnan P, Hecht BA, Pedersen DR, et al. Oxidant conditioning protects cartilage from mechanically induced damage. J Orthop Res; 2010;28:914–20. doi: 10.1002/jor.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding L, Heying E, Nicholson N, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage; 2010;18:1509–17. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamin M, Archer CW, Ralphs JR. Cytoskeleton of cartilage cells. Microsc Res Tech; 1994;28:372–7. doi: 10.1002/jemt.1070280503. [DOI] [PubMed] [Google Scholar]
- 48.Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports; 2009;19:457–69. doi: 10.1111/j.1600-0838.2009.00912.x. [DOI] [PubMed] [Google Scholar]