Abstract
Aim
The aim of this study was to investigate differences in mortality up to 1 year of age in extremely preterm infants (before 27 weeks) born in seven Swedish healthcare regions.
Methods
National prospective observational study of consecutively born, extremely preterm infants in Sweden 2004–2007. Mortality was compared between regions. Crude and adjusted odds ratios and 95% CI were calculated.
Results
Among 844 foetuses alive at mother's admission for delivery, regional differences were identified in perinatal mortality for the total group (22–26 weeks) and in the stillbirth and perinatal and 365-day mortality rates for the subgroup born at 22–24 weeks. Among 707 infants born alive, regional differences were found both in mortality before 12 h and in the 365-day mortality rate for the subgroup (22–24 weeks) and for the total group (22–26 weeks). The mortality rates were consistently lower in two healthcare regions. There were no differences in the 365-day mortality rate for infants alive at 12 h or for infants born at 25 weeks. Neonatal morbidity rates among survivors were not higher in regions with better survival rates. Perinatal practices varied between regions.
Conclusion
Mortality rates in extremely preterm infants varied considerably between Swedish healthcare regions in the first year after birth, particularly between the most immature infants.
Keywords: Cohort study, Extremely preterm infant, Morbidity, Mortality, Outcome
Key notes.
This study found considerable variations between seven Swedish healthcare regions in stillbirth rates, perinatal mortality rates and 365-day mortality rates for infants born extremely preterm, despite similar healthcare organization and population characteristics.
Improved survival was not associated with any increase in neonatal morbidity.
The regional differences were established within the 12 first hours after birth and may be related to variations in perinatal practices.
Introduction
Perinatal and neonatal mortality due to extremely preterm birth has decreased during recent decades. Mortality rates of 50% or less for infants born at or below 24 weeks of gestation 1–4 are reported as a result of increased centralization and technical advances in perinatal and neonatal care 5–7. Despite overall reductions in perinatal and infant mortality, wide variations exist between countries and between regions within countries 2,4,8–13.
Variations in both practice and outcome are common in all domains of medicine, including the care of mothers and children. However, few population-based studies analyse the variations in survival of infants born extremely preterm 8,11–13. The MOSAIC study evaluated very preterm mortality and morbidity in 10 European regions 11: among foetuses alive at the onset of labour at 24–27 weeks of gestation, the rates of infants born alive varied from 84% to 99% between regions and survival to discharge home ranged from 42% to 81%. In the Canadian NICU network, there were variations in clinical practice and survival rates (72–100%) of infants with birthweights 750–999 g between neonatal intensive care units 13. Despite the highly regionalised and accessible system of neonatal perinatal care, survival differences occurred.
Between 1990 and 1999 in Sweden, Håkansson et al. 8 found variations in survival between healthcare regions in infants born at 22–25 weeks; in regions with better survival, the differences were attributed to a positive attitude to perinatal intervention. Since then, the EXPRESS study in Sweden 3, which includes all infants alive at onset of labour born at <27 weeks of gestation during a 3-year period, has reported higher survival rates than similar national or international studies. Moreover, the national guideline from the 1990s recommending a restrictive attitude to deliveries occurring at 25 weeks or less has been substituted with regional guidelines adopting a more individualized approach to the management of extremely preterm births.
Monitoring of variations in mortality rates is important as they indicate changes in practice that might improve outcome. The aim of the present EXPRESS study was to examine possible regional differences in mortality up to the age of 1 year for infants born extremely preterm (below 27 weeks of gestation) in Sweden, after adjusting for demographic differences. Outcome was considered the result of a continuum of care, starting with the admission for delivery of a mother with a live foetus and ending with outcome at 1 year. The hypothesis was that mortality rates would differ between regions, particularly for the most immature infants.
Study population and methods
The EXPRESS cohort
In the national EXPRESS study (Extremely Preterm Infants in Sweden Study), perinatal and neonatal data were collected prospectively (during a 3-year period, 2004–2007) on all 1011 infants born before 27 completed weeks of gestation 3. Live-born infants (n = 707) and stillbirths (n = 304) with gestational ages (GA) 22 + 0 to 26 + 6 weeks were defined according to the recommendations by the World Health Organization (WHO) 14. The only exclusions were terminations of pregnancy and infants born abroad and transferred to Sweden for neonatal care. The details of the study design, gestational-age-specific survival rates up to 1 year 3 and neonatal morbidity rates 15 are published elsewhere. The mortality rate for the total cohort (22–26 weeks) was 30% 3. For the subcategories used in the present study, the mortality rate was 48% for infants born at 22–24 weeks and 17% for infants born at 25–26 weeks 3.
All Swedish obstetric departments and associated neonatal wards, at all levels of perinatal care, participated in the EXPRESS study. Data collection and quality control were organized by obstetric, paediatric and ophthalmic study coordinators at the seven Swedish healthcare regions. In all seven regions, data were validated through internal and external comparison with patient records in randomly selected samples, and we did not find differences in the quality of data between the regions. The data were electronically transferred to a central EXPRESS database with 220 variables, including demographic characteristics, information on medical and obstetric history, pregnancy course, labour and delivery, and infant data until discharge home or up to 180 postnatal days for infants who were still hospitalized. A list of variables analysed in this study is shown in the supporting information (Table S1).
Data were collected continually throughout the chain of care for all infants who were transferred between departments or hospitals, and information on survival at 1 year was obtained through linkage with the Swedish population register. In 95% of pregnancies, GA was estimated according to the ultrasound performed before 20 postmenstrual weeks: there were no differences between regions regarding the use of ultrasound dating (data not presented). In 664 infants, information was available on GA estimations both by ultrasound and according to the last menstrual period. Median GA estimated by ultrasound was 3 days shorter than GA estimated based on the last menstrual period; however, there were no differences between the healthcare regions (range 2–4 days). Birthweight (BW) standard deviation scores (SDS) were calculated according to the national intrauterine growth standard based on ultrasonically estimated foetal weights 16. Infants with SDS < −2, that is, BW more than 2 SD below the mean, were classified as small for gestational age (SGA).
Preterm prelabour rupture of membranes was defined as spontaneous rupture of the membranes >1 h before the onset of contractions. Pre-eclampsia was defined according to standard criteria. Chorioamnionitis was diagnosed clinically. A mother was considered to have received ‘any’ antenatal corticosteroids if she had been given at least one 12 mg dose of betamethasone, and a ‘complete course’, if two doses of betamethasone were given, and the interval between the first dose and delivery was 24 h or more. Delivery after induced labour or as a prelabour caesarean section on maternal and/or foetal indication was defined as iatrogenic vaginal or caesarean delivery.
Information on maternal smoking, obtained at the first antenatal visit, was dichotomised as smoking/nonsmoking. Intraventricular haemorrhage (IVH) was graded according to Papile et al. 17, and periventricular leucomalacia (PVL) was defined according to de Vries et al. 18. Severe brain injury was defined either as IVH ≥ grade 3 or as the presence of cystic PVL (cPVL) on ultrasound, with scans read locally by radiologists. Ophthalmological screening for retinopathy of prematurity (ROP) began during the fifth postnatal week and continued weekly or biweekly until the retina was completely vascularized or ROP had regressed. ROP was defined according to the International Classification for ROP 19. Bronchopulmonary dysplasia (BPD) was defined as the need for supplemental oxygen at 36 weeks of GA, and severe BPD was defined as the need for at least 30% oxygen at that time. Major neonatal morbidity was defined as having at least one of severe brain injury, ROP ≥ stage 3 or severe BPD. Neonatal illness severity was estimated with the clinical risk index for babies (CRIB) 20. Congenital anomalies were classified according to the WHO International Classification of Diseases, 10th revision: dislocation of the hip (Q65.0–Q65.5), preauricular tags (Q17.0), undescended testes (Q53.0-Q53.9) and patent ductus arteriosus (Q25.0) were not classified as anomalies.
Study groups and outcome measures
The study cohort was categorized into four subgroups:
Foetuses alive at mother's admission for delivery (n = 844), assessed for GA-specific stillbirth rates, perinatal mortality and 365-day mortality. In this subgroup, the 365-day mortality included stillbirths (median admission to delivery time 2 days; interquartile range 0–5 days) and infants born alive (median admission to delivery time 2 days; interquartile range 0–2 days) who died before the age of 1 year.
Infants born alive (n = 707), assessed for GA-specific mortality before the first 12 h after birth and 365-day mortality.
Infants alive at 12 h (n = 608) and assessed for GA-specific 365-day mortality.
Infants who survived at least 365 days (n = 497), assessed for major neonatal morbidity.
The outcomes relate to the region of birth. Few mothers (<5%) delivered outside their region of domicile, mostly due to temporary stay outside the region or to shortage of neonatal intensive care beds in the region of domicile.
Healthcare regions
Sweden has 9.5 million inhabitants and is divided into seven healthcare regions.
Approximately 100 000 deliveries take place each year at 42 obstetric departments, and home deliveries are extremely rare. Each of the seven regions is served by one level III perinatal centre, to which high-risk pregnancies and very preterm births are recommended to be transferred. There is no national guideline regarding the management of extremely preterm infants. However, regional guidelines, based on professional consensus, do exist. The characteristics of the healthcare regions (Table S2) reveal a wide variation in area (range 9.7 × 103–236.4 × 103 km2), population size (range 0.56–2.1 million) and population density (range 3.7–216 per km2). The Stockholm region also differed in population education and income. The map of Sweden including the seven healthcare regions is shown in Figure 1.
Figure 1.
Map of Sweden and the seven healthcare regions.
Statistics
Categorical variables were analysed with either Fischer's exact test or chi-square test, as appropriate. Where specified, adjustments were made for GA (entered as a second-grade polynoma in analyses based on three or more GA groups, otherwise entered as a continuous, linear variable), multiple birth (yes/no), infant gender (male/female) and birthweight SD score (entered as a continuous linear variable). Maternal characteristics were not included in the multiple logistic regression models as we found no association between maternal characteristics and survival (data not shown), and previously 15, we did not find associations between maternal characteristics and neonatal morbidity among survivors. ‘The R2 statistics’ was used to show to what extent regional differences contributed to the overall variance in mortality or morbidity. Statistical analyses were performed with Gauss (Gauss™; Aptech Systems Inc., Maple Valley, WA, USA).
The Regional Research Ethics Board at Lund University approved the study. Parents were informed and consented to the data acquisition and study purposes.
Results
Regional group characteristics
Maternal characteristics for the 844 foetuses alive at mother's admission for delivery are presented in Table 1. Except for minor variations in the distribution of maternal age and foetal gender, there were no differences between the regions.
Table 1.
Maternal and foetal characteristics of pregnancies with foetuses alive (n = 844) at mother's admission for delivery
| Region | |||||||
|---|---|---|---|---|---|---|---|
| Stockholm | Uppsala | Linköping | Lund | Göteborg | Örebro | Umeå | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Maternal characteristics | |||||||
| Total number of pregnancies | 187 | 128 | 62 | 121 | 147 | 23 | 78 |
| Non-Nordic origin | 27 (14) | 15 (12) | 4 (6.5) | 11 (9.1) | 15 (10) | 1 (4.3) | 8 (10) |
| Age* (years), median [range] | 32 [14–45] | 32 [17–45] | 32 [19–43] | 29 [16–43] | 31 [17–43] | 28 [15–38] | 31 [18–46] |
| Age <20 years | 3 (1.6) | 7 (5.5) | 3 (4.8) | 6 (5.0) | 3 (2.0) | 3 (13) | 1 (1.3) |
| Age ≥35 years | 68 (36) | 39 (30) | 14 (23) | 33 (27) | 37 (25) | 1 (4.3) | 24 (31) |
| Smoking† | 18 (9.6) | 21 (16) | 9 (14) | 17 (14) | 17 (12) | 0 (0.0) | 9 (12) |
| Parity 0 | 119 (64) | 79 (62) | 38 (61) | 65 (54) | 86 (58) | 17 (74) | 42 (54) |
| Parity 3+ | 8 (4.3) | 12 (9.4) | 4 (6.5) | 3 (2.5) | 10 (6.8) | 0 (0.0) | 7 (9.0) |
| Any pregnancy complication‡ | 105 (56) | 75 (59) | 34 (55) | 88 (73) | 92 (63) | 12 (52) | 48 (62) |
| Foetal characteristics | |||||||
| Total number of foetuses | 213 | 147 | 69 | 142 | 161 | 26 | 86 |
| Male gender* | 115 (54) | 87 (59) | 29 (42) | 79 (54) | 81 (50) | 22 (85) | 49 (57) |
| Multiple pregnancy | 50 (24) | 38 (26) | 14 (20) | 38 (27) | 28 (17) | 5 (19) | 16 (19) |
p < 0.05 among regions; other variables not significant (p > 0.05).
Unknown in mothers of 61 of 844 foetuses alive at mother's admission (7.2%).
Pregnancy complication includes any of following: preterm prelabour rupture of membranes, abnormal antenatal cardiotocogram, suspected intrauterine growth retardation, iatrogenic preterm birth indicated by maternal disease, pre-eclampsia, eclampsia, essential hypertension, chorioamnionitis, placenta praevia, placental abruption, polyhydramnion, oligohydramnion, diabetes mellitus or gestational diabetes.
In the total cohort of infants born alive (n = 707), mean (SD) GA was 25 (1.3) weeks and mean (SD) BW was 709 (196) g. Infant characteristics according to the healthcare region are presented in Table 2. There were differences in the rate of SGA and low Apgar scores (<4) at 1 and 5 min. However, there were no differences in mean GA, mean BW or rate of multiple births.
Table 2.
Characteristics of infants born alive (n = 707) in seven Swedish healthcare regions
| Region | |||||||
|---|---|---|---|---|---|---|---|
| Stockholm | Uppsala | Linköping | Lund | Göteborg | Örebro | Umeå | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 177 | 135 | 59 | 117 | 126 | 21 | 72 |
| Male gender | 95 (54) | 81 (60) | 25 (42) | 63 (54) | 64 (51) | 18 (86) | 42 (58) |
| Gestational age, weeks | |||||||
| Mean ± SD | 25.0 ± 1.4 | 25.1 ± 1.3 | 25.0 ± 1.4 | 25.1 ± 1.1 | 25.1 ± 1.2 | 24.8 ± 1.4 | 25.1 ± 1.1 |
| Birthweight, g | |||||||
| Mean ± SD | 707 ± 203 | 711 ± 192 | 720 ± 206 | 691 ± 190 | 709 ± 182 | 732 ± 237 | 723 ± 202 |
| Range | 266–1500 | 375–1218 | 280–1125 | 348–1235 | 425–1160 | 400–955 | 320–1161 |
| SGA*, † | 21 (12) | 31 (23) | 11 (19) | 24 (21) | 14 (11) | 2 (9.5) | 11 (15) |
| Apgar 1 min <4** | 77 (44) | 27 (20) | 23 (39) | 37 (32) | 52 (41) | 8 (38) | 20 (28) |
| Apgar 5 min <4* | 45 (25) | 14 (10) | 15 (25) | 19 (16) | 23 (18) | 4 (19) | 9 (13) |
| Multiple birth infants | 41 (23) | 37 (27) | 13 (22) | 30 (26) | 20 (16) | 2 (9.5) | 15 (21) |
| Birth defects‡ | 19 (11) | 16 (12) | 5 (8.5) | 9 (7.7) | 15 (12) | 1 (4.8) | 12 (17) |
| CRIB>10§ | 13 (9.5) | 13 (10) | 6 (13) | 18 (17) | 17 (16) | 0 (0.0) | 6 (9.2) |
CRIB = clinical risk index for babies (20).
p < 0.05;
p < 0.01 among regions; other variables not significant (p > 0.05).
SGA: small for gestational age, birthweight < mean −2 SD of the intrauterine growth standard 16.
See Materials and Methods.
For infants alive at 12 h (n = 608).
Perinatal practices
The variation in the rates of obstetric intervention between regions was wide and statistically significant (p < 0.05), except for the use of tocolysis (p = 0.06; Table 3). The proportion of infants delivered at level III hospitals varied between 68% and 86%. After exclusion of the Örebro region (too few cases), the proportion of infants receiving a full course of antenatal steroids ranged from 40% to 72%. There were considerable variations in the rate of neonatal interventions (p < 0.05). After exclusion of the Örebro region (too few cases), the proportion of infants receiving surfactant varied from 35% to 73% and the rate of intubation at birth ranged from 36% to 75%.
Table 3.
Frequency of obstetric and neonatal interventions between healthcare regions
| Region | |||||||
|---|---|---|---|---|---|---|---|
| Stockholm | Uppsala | Linköping | Lund | Göteborg | Örebro | Umeå | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Obstetric interventions | |||||||
| Foetuses alive at admission* | 213 | 147 | 69 | 142 | 161 | 26 | 86 |
| Birth at level III hospital | 164 (77) | 103 (70) | 47 (68) | 109 (77) | 138 (86) | 18 (69) | 64 (74) |
| Antenatal steroids, 2 doses | 85 (40) | 91 (62) | 36 (52) | 87 (61) | 98 (61) | 9 (35) | 62 (72) |
| Caesarean section | 61 (29) | 79 (54) | 27 (39) | 85 (60) | 48 (30) | 12 (46) | 49 (57) |
| Tocolytic treatment† | 108 (68) | 70 (70) | 47 (89) | 81 (79) | 92 (75) | 18 (81) | 45 (70) |
| Neonatal interventions | |||||||
| Infants born alive | 177 | 135 | 59 | 117 | 126 | 21 | 72 |
| Surfactant within 2 h after birth | 65 (37) | 99 (73) | 21 (36) | 78 (67) | 44 (35) | 10 (24) | 50 (69) |
| Neonatologist present at birth | 130 (73) | 108 (80) | 48 (81) | 106 (91) | 109 (87) | 20 (90) | 66 (92) |
| Intubation at birth | 53 (48) | 101 (75) | 21 (36) | 79 (68) | 53 (42) | 8 (38) | 54 (75) |
| Admitted to NICU§ | 150 (92) | 132 (99) | 50 (89) | 111 (98) | 124 (98) | 18 (90) | 69 (100) |
Foetus alive at mother's admission for delivery.
For the subgroup with spontaneous preterm labour only (denominator N values: 158, 101, 53, 103, 122, 22 and 64 for the seven regions, respectively).
For infants alive 30 min after birth (denominator N values: 163, 133, 56, 113, 126, 20 and 69 for the seven regions, respectively).
Tocolysis p = 0.06; all other obstetric and neonatal interventions p < 0.05.
Outcomes
Crude mortality rates for the total cohorts (22–26 weeks) and for the subgroups (22–24 weeks and 25–26 weeks, respectively) are displayed according to the region in Table 4.
Table 4.
Mortality and morbidity by healthcare region and gestational age groups
| Region | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Stockholm | Uppsala | Linköping | Lund | Göteborg | Örebro | Umeå | ||
| n/N (%) | n/N (%) | n/N (%) | n/N (%) | n/N (%) | n/N (%) | n/N (%) | ||
| Foetus alive at mother's admission for delivery (n = 844) | ||||||||
| Stillbirth | ||||||||
| 22–24 weeks | 30/110 (27) | 4 /57 (7.0) | 9/33 (27) | 22/72 (30) | 29/78 (37) | 4/15 (27) | 10/39 (26) | <0.001 |
| 25–26 weeks | 6/103 (5.8) | 8/90 (8.8) | 1/36 (2.7) | 3/70 (4.3) | 6/83 (3.6) | 1/11 (9) | 4/47 (8.5) | 0.81 |
| 22–26 weeks | 36/213 (17) | 12/147 (8.2) | 10/69 (15) | 25/142 (18) | 35/161 (22) | 5/26 (19) | 14/86 (16) | 0.06 |
| Perinatal death | ||||||||
| 22–24 weeks | 70/110 (64) | 12/57 (21) | 23/33 (70) | 38/72 (53) | 49/78 (63) | 10/15 (67) | 16/39 (41) | <0.001 |
| 25–26 weeks | 14/103 (14) | 20/90 (22) | 6/36 (17) | 9/70 (13) | 13/83 (16) | 2/11 (18) | 7/47 (15) | 0.74 |
| 22–26 weeks | 84/213 (39) | 32/147 (22) | 29/69 (42) | 47/142 (33) | 62/161 (39) | 12/26 (46) | 23/86 (27) | <0.01 |
| Died before 365 days | ||||||||
| 22–24 weeks | 78/110 (71) | 22/57 (43) | 25/33 (75) | 41/72 (60) | 54/78 (69) | 11/15 (73) | 19/39 (49) | <0.001 |
| 25–26 weeks | 21/103 (20) | 24/90 (27) | 9/36 (25) | 16/70 (23) | 16/83 (19) | 3/11 (27) | 8/47 (17) | 0.84 |
| 22–26 weeks | 99/213 (46) | 46/147 (31) | 34/69 (49) | 57/142 (40) | 70/161 (43) | 14/26 (54) | 27/86 (31) | 0.12 |
| Infant born alive (n = 707) | ||||||||
| Died before 12 h | ||||||||
| 22–24 weeks | 37/80 (46) | 6/53 (11) | 11/24 (46) | 11/50 (22) | 16/49 (33) | 4/11 (36) | 5/29 (17) | <0.001 |
| 25–26 weeks | 3/97 (3.1) | 3/82 (3.7) | 2/35 (5.7) | 2/67 (3.0) | 3/77 (3.9) | 1/10 (10) | 2/43 (4.7) | 0.60 |
| 22–26 weeks | 40/177 (23) | 9/135 (6.7) | 13/59 (22) | 13/117 (11) | 19/126 (15) | 5/21 (24) | 7/72 (9.7) | <0.01 |
| Died before 365 days | ||||||||
| 22–24 weeks | 48/80 (60) | 18/53 (34) | 16/24 (67) | 19/50 (38) | 25/49 (51) | 7/11 (64) | 9/29 (31) | <0.001 |
| 25–26 weeks | 15/97 (15) | 16/82 (20) | 8/35 (23) | 13/67 (19) | 10/77 (13) | 2/10 (20) | 4/43 (9.3) | 0.60 |
| 22–26 weeks | 63/177 (36) | 34/135 (25) | 24/59 (41) | 32/117 (27) | 35/126 (28) | 9/21 (43) | 13/72 (18) | 0.02 |
| Infant alive at 12 h (n = 608) | ||||||||
| Died before 365 days | ||||||||
| 22–24 weeks | 11/43 (26) | 14/49 (29) | 5/13 (38) | 11/42 (26) | 9/33 (27) | 3/7 (43) | 5/25 (20) | 0.88 |
| 25–26 weeks | 12/94 (13) | 13/79 (16) | 7/34 (21) | 11/65 (17) | 7/74 (9.4) | 1/9 (11) | 2/41 (4.8) | 0.32 |
| 22–26 weeks | 23/137 (17) | 27/128 (21) | 12/47 (26) | 22/107 (21) | 16/107 (15) | 4/16 (25) | 7/66 (11) | 0.32 |
| Infant alive at 365 days (n = 497) | ||||||||
| Major neonatal morbidity* | ||||||||
| 22–24 weeks | 26/32 (81) | 27/35 (77) | 7/8 (88) | 22/31 (71) | 19/24 (79) | 2/4 (50) | 11/20 (55) | 0.33 |
| 25–26 weeks | 38/82 (46) | 27/66 (41) | 13/27 (48) | 26/54 (48) | 32/67 (48) | 3/8 (38) | 18/39 (46) | 0.98 |
| 22–26 weeks | 64/114 (56) | 54/101 (50) | 20/35 (57) | 48/85 (56) | 51/91 (56) | 5/12 (42) | 29/59 (49) | 0.92 |
In foetuses alive at mother's admission for delivery and delivered at 22–26 weeks, the rate of stillbirth ranged from 8.2% to 22% (p = 0.06), perinatal mortality rate ranged from 22% to 46% (p < 0.01), and 365-day mortality ranged from 31% to 54% (p = 0.12). For the 22- to 24-week group, regional variations in mortality were wide and all outcomes differed (p < 0.001). There were no regional mortality differences in the 25- to 26-week group.
In infants born alive at 22–26 weeks (Table 4), the 365-day mortality rate ranged from 18% to 43% (p = 0.02). In the 22- to 24-week group, the 365-day mortality difference between regions was wider and significant (p < 0.001). There were large regional differences in the rate of death before 12 h after birth in both the total cohort (p < 0.01) and the 22- to 24-week group (p < 0.001), but only trivial and nonsignificant differences in 365-day mortality between infants alive at 12 h. The rate of major neonatal morbidities between survivors at 1 year did not differ between the regions.
Adjusted odds ratios (ORs) for death at various times for foetuses that were alive on the mother's admission for delivery in seven healthcare regions are presented in Figure 2, with Stockholm as the reference region. Significant ORs are shown in Table 5. The risk for stillbirth was lower in the Uppsala region, and the risk for perinatal death and death before 365 days was lower in the Uppsala and Umeå regions compared with the reference region. The stillbirth risk was increased in the Göteborg region. There were no significant differences between infants born at 25–26 weeks. Complete crude and adjusted ORs are presented in the supplementary information (Table S3). The adjusted proportion of the variation that could be explained by regional differences varied from 2% to 10% (Table S3).
Figure 2.
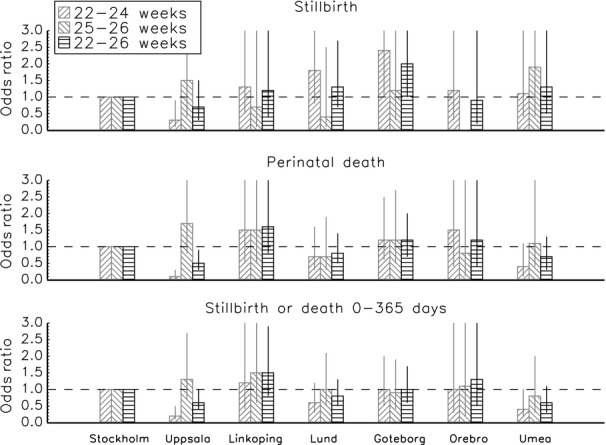
Adjusted odds ratios for death among foetuses alive at mother's admission for delivery among the Swedish healthcare regions (reference = Stockholm region), according to the time of death. Odds ratios are adjusted for gestational age (weeks) and (weeks2), multiple births (yes/no), gender and birthweight standard deviation score 16. Adjusted proportion of variation that can be explained by the regional difference (R2): stillbirth: 22–24 weeks, 0.05; 22–26 weeks, 0.01. Perinatal death: 22–24 weeks, 0.10; 22–26 weeks 0.02. Death before 365 days: 22–24 weeks, 0.06; 22–26 weeks, 0.01.
Table 5.
Risk for death among foetuses alive at maternal admission for delivery, among infants born alive, and risk for neonatal morbidity in six Swedish healthcare regions compared with the reference region (Stockholm). Only regions and outcomes with significantly different risks compared with the reference are shown
| Region | Gestational age, weeks | OR 95% CI | ||
|---|---|---|---|---|
| Crude | Adjusted† | |||
| Alive at maternal admission | ||||
| Stillbirth | Uppsala | 22–24 | 0.2, 0.1–0.6 | 0.3, 0.1–0.9 |
| Uppsala | 22–26 | 0.4, 0.2–0.8 | ||
| Göteborg | 22–24 | 2.4, 1.0–5.2 | ||
| Perinatal death | Uppsala | 22–24 | 0.2, 0.1–0.3 | 0.1, 0.0–0.3 |
| Uppsala | 22–26 | 0.4, 0.3–0.7 | 0.5, 0.3–0.9 | |
| Umeå | 22–24 | 0.4, 0.2–0.8 | ||
| Umeå | 22–26 | 0.6, 0.3–1.0 | ||
| Death before 365 days | Uppsala | 22–24 | 0.3, 0.1–0.5 | 0.2, 0.1–0.5 |
| Uppsala | 22–26 | 0.5, 0.3–0.8 | ||
| Umeå | 22–24 | 0.4, 0.2–0.8 | 0.4, 0.2–1.0* | |
| Umeå | 22–26 | 0.5, 0.3–0.9 | ||
| Born alive | ||||
| Death before 365 days | Uppsala | 22–24 | 0.3, 0.2–0.7 | 0.3, 0.1–0.7 |
| Uppsala | 22–26 | 0.6, 0.4–1.0 | ||
| Umeå | 22–24 | 0.3, 0.1–0.7 | 0.4, 0.1–1.0** | |
| Umeå | 22–26 | 0.4, 0.2–0.8 | 0.4, 0.2–0.8 | |
| Death before 12 h | Uppsala | 22–24 | 0.1, 0.1–0.3 | 0.0, 0.0–0.1 |
| Uppsala | 22–26 | 0.2, 0.1–0.4 | 0.1, 0.0–0.4 | |
| Lund | 22–24 | 0.2, 0.1–0.5 | 0.3, 0.1–0.9 | |
| Lund | 22–26 | 0.3, 0.2–0.7 | ||
| Umeå | 22–24 | 0.2, 0.1–0.5 | 0.2, 0.1–0.5 | |
| Umeå | 22–26 | 0.3, 0.1–0.8 | ||
| Major neonatal morbidity | Umeå | 22–24 | 0.3, 0.1–1.0 | 0.3, 0.1–1.0 |
Among infants born alive, the risk for death before 365 days was lower in the Uppsala and Umeå regions and the risk for death before 12 h was lower in the Uppsala, Umeå and Lund regions compared with the reference region (Table 5, Figure 3). Most differences were confined to the 22- to 24-week group; there were no significant differences for the 25- to 26-week group for any of the outcomes. Complete crude and adjusted ORs are presented in the supplementary information (Table S4). The adjusted proportion of the variation that could be explained by regional differences varied from 5% to 13% (Table S4).
Figure 3.
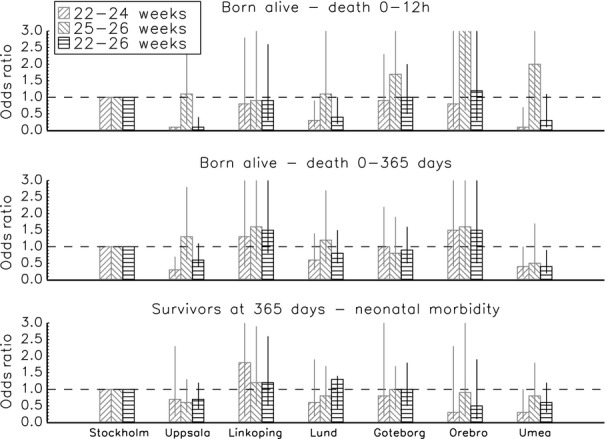
Adjusted Odds ratios for death among infants born alive and neonatal morbidity among survivors to 365 days between the Swedish healthcare regions (reference = Stockholm region), according to the time of death. Odds ratios are adjusted for gestational age (weeks) and (weeks2), multiple births (yes/no), gender and birthweight standard deviation score 21. Adjusted proportion of variation that can be explained by the regional difference (R2): death before 12 h: 22–24 weeks, 0.13; 22–26 weeks, 0.05. Death 0–365 days: 22–24 weeks, 0.06; 22–26 weeks 0.02. Neonatal morbidity: 22–24 weeks, 0.06; 22–26 weeks, 0.01. Neonatal morbidity includes any of retinopathy of prematurity stage ≥3 19, intraventricular haemorrhage grade ≥3 17, cystic periventricular leucomalacia 18 or severe bronchopulmonary dysplasia (need for at least 30% oxygen at 36 weeks of corrected age).
When deaths before 12 h were excluded, no differences in the 365-day mortality risk remained between the 608 infants still alive for the total cohort or the subgroups born at 22–24 weeks and 25–26 weeks. In a separate analysis, the risk for death was adjusted for CRIB scores. The ORs were similar to our previous findings, and there were no regional differences in the 365-day mortality risk for infants alive at 12 h.
The risk for major neonatal morbidity among infants alive at 365 days was lower in the Umeå region among infants born at 22–24 weeks (Table 5, Figure 3); no other differences were seen.
Discussion
In a national cohort of extremely preterm births in Sweden, wide regional variations in the risk for stillbirth, perinatal death and 365-day mortality existed, despite the mortality rate in the whole population being lower in the EXPRESS study 3 than in comparable population-based studies 1,2,11,21–23. However, there were no indications that regions with lower mortality rates had an increased rate of major neonatal morbidity, measured as a composite morbidity outcome of either severe brain injury, ROP stage 3 or more, or severe BPD.
Variations in perinatal and neonatal outcome are reported between centres 4,9,10,13, regions 2,8,12 and regions within various countries 11. For infants with birthweights 401–1000 g admitted to the Neonatal Research Network of the National Institute of Child Health and Human Development (NICHD) centres, Vohr et al. 10 found striking differences in survival (range 51–72%) between centres. In the Canadian NICU network, Lee et al. 13 observed variations in the practices and survival (72–100%) of infants with birthweights 750–999 g between neonatal intensive care units, and the Swiss Neonatal Network reported considerable centre-to-centre variation between infants born at <26 weeks (the standardized survival ratios ranged from 0.8 to 1.4) 9. The MOSAIC study evaluated very preterm mortality and morbidity in 10 European regions 24 and noted large differences between European regions in obstetric practices that were related to outcome, especially at 24–25 weeks. In Sweden, Håkansson et al. 8 found an increased survival (OR 2.5; 95% CI 1.7–3.6) in infants born at 22–25 weeks during 1995–1999 in the two northern healthcare regions than in the remaining five regions.
In the present study, the total cohort was divided into two GA groups, 22–24 weeks and 25–26 weeks, and most differences in mortality risks occurred within the 22- to 24-week group. There were no differences in any outcome for the 25- to 26-week group. There were wide variations between regions for the risk of stillbirth, perinatal death and 365-day mortality in the cohort of foetuses alive at mother's admission for delivery at 22–24 weeks. For example, in the 22- to 24-week group, the adjusted OR for stillbirth ranged from 0.3 (95% CI 0.1–0.9) to 2.4 (95% CI 1.0–5.2) between regions, compared with the reference region. Similarly, wide variations between infants born alive at 22–24 weeks were found: the adjusted OR for the region with the lowest risk for 365-day mortality was 0.3 (95% Cl 0.1–0.7), compared with the reference region. The magnitudes of the regional variations in mortality were similar to, or frequently much larger than, the reported effect sizes of many routinely used interventions, such as surfactant administration, antenatal corticosteroids or centralization of perinatal care 5–7.
In agreement with the study by Håkansson et al. 8, the differences in mortality risks between regions were nullified when early deaths (<12 h) were excluded. This suggested that different mortality rates were predominantly accounted for by different practices regarding delivery, resuscitation, initial stabilization at birth and during the first hours after admission to the NICU. As we assumed that variations in perinatal and neonatal practices would be a major determinant of outcome, mortality risk was only adjusted for biological factors. However, when the cohort of infants alive at 12 h was adjusted for CRIB score, there were no differences in 365-day mortality risk, compared with the unadjusted risk.
Under the Swedish healthcare system, all people living or working in the country have equal access to heavily subsidized health care, which is taxpayer-funded, and all inhabitants are covered by a general health insurance. Antenatal care is standardized, free of charge and utilized by almost 100% of pregnant women. The organization and availability of perinatal care, including technical equipment and expertise at regional level, is similar in all seven healthcare regions in Sweden. Therefore, the prerequisites for providing advanced neonatal care to extremely preterm infants are uniform throughout the country, contrary to the situation in some other countries, for example, the United States 25.
Many studies reporting variations in outcome comprise populations that are more heterogeneous 4,10–12 than the Swedish population. With the exception of income in the Stockholm region, the region-specific differences were small with respect to educational achievement and income, and maternal background, demographic and pregnancy data, and infant characteristics were similar. As Sweden is a homogenous country without extreme poverty or large regional differences in ethnic composition, variations in regional outcomes are unlikely to be attributed to differences in healthcare organization or population characteristics. In the total EXPRESS cohort 3, intrapartum death rate (6.5%) and 365-day mortality in infants born alive (30%) were lower than in similar population-based studies 1,2,11,21–23. With the homogeneity of the background data and the overall favourable outcome, the wide variations in mortality are conspicuous.
The EXPRESS study has previously reported considerable neonatal morbidity among surviving infants 15. However, in the present study, and in agreement with some studies 8,9,26, but not all 27,28, no evidence was found to support the notion that regions with lower mortality risk would have an increased risk for survival with major neonatal morbidities measured by the composite outcome of neonatal morbidity used.
As in other studies 8,13,24,27, a wide variation in the use of obstetric and neonatal interventions around the time of birth was found. This finding might reflect that most perinatal interventions involving extremely preterm births are not standardized or evidence-based and that perinatal management is influenced by the perception of the long-term neurodevelopmental outcome, which is a matter of both concern and controversy 2,29.
To be able to compare preterm birth outcome data in different regions, it is crucial that the estimation of GA is comparable between regions. In the EXPRESS study, ultrasound dating was performed for almost all pregnancies (95%), without differences in prevalence between regions. Median GA estimated by ultrasound was shorter than LMP-based estimations, but the regional difference between the two methods were similar. Thus, it is reasonable to assume there were no systematic regional differences in the estimation of gestational length.
The strengths of this study were the prospective enrolment of all births before 27 gestational weeks in Sweden, including intrapartum stillbirths, the cohort was stratified by GA estimated by ultrasound in practically all pregnancies, and mortality was determined at several times up to 1 year of age. The study was conducted in a uniform society, which minimized differences in outcomes related to nonmedical factors. In addition, the regional mortality rates were adjusted for a large number of possible confounders. One limitation was that stratification of the births by region rendered smaller numbers with subsequently larger confidence intervals for several estimates. Despite this limitation, differences between regions were detected. Indeed, due to lack of power, the degree of variation might have been underestimated. On the other hand, we performed multiple testing, and some associations considered significant might have occurred by chance. However, the associations were very consistent; most differences occurred in the 22- to 24-week gestational age group, and the same two regions had lower mortality risks in most of the comparisons. Thus, we believe that the regional differences in mortality found in our study represent true findings.
In summary, there were wide regional variations in mortality rates up to 1 year between extremely preterm infants. These differences, both in the population comprising foetuses alive at mother's admission for delivery and in the population of infants born alive, were present in the most immature group of infants, born at 22–24 weeks. The regional mortality differences arose early in life (<12 h) and persisted to 1 year of age. The motives for restrictive treatment policies relate to concerns about the burdens of poor long-term outcomes. We have recently shown, however, that the outcome at 2.5 years of the EXPRESS cohort was favourable compared with studies with lower survival rates, despite perinatal care in the EXPRESS study being more proactive 30. The present study shows that the rates of obstetric and neonatal interventions around birth vary between the Swedish healthcare regions; these differences may account for the regional variations in survival. Further analyses are underway to explore this relationship and to ascertain that the favourable short-term outcomes achieved in regions with high intervention rates translate into lasting long-term benefits.
Acknowledgments
Technical assistance with data collection by Ms. Grozda Pajic, Lund University, is gratefully acknowledged. The authors thank Marius Kublickas, MD, PhD (MedSciNet AB, Stockholm, Sweden), for the design and maintenance of the study database, and Associate Professor Stellan Håkansson, MD, PhD, main coordinator of National Perinatal Quality Registry PNQ, and Petra Otterblad Olausson, PhD, Epidemiological Centre, The National Board of Health and Welfare, Stockholm, for valuable support. The English language of the manuscript was revised by Sue Pajuluoma.
Glossary
- BPD
Bronchopulmonary dysplasia
- BW
Birthweight
- CI
Confidence interval
- CRIB
Clinical risk index for babies
- CTG
Cardiotocogram
- EXPRESS
Extremely Preterm Infant Study in Sweden
- GA
Gestational age
- IVH
Intraventricular haemorrhage
- LMP
Last menstrual period
- NICU
Neonatal intensive care unit
- OR
Odds ratio
- PMA
Postmenstrual age
- PPROM
Preterm premature rupture of membranes
- PVL
Periventricular leucomalacia
- cPVL
Cystic periventricular leucomalacia
- ROP
Retinopathy of prematurity
- SD
Standard deviation
- SGA
Small for gestational age
- WHO
World Health Organization
Appendix
Members of the EXPRESS Group
Professor Mats Blennow, MD, PhD, Dept. of Pediatrics, Karolinska University Hospital Huddinge, Stockholm.
Professor Uwe Ewald, MD, PhD, Dept. of Pediatrics, Uppsala University, Uppsala.
Professor Vineta Fellman, MD, PhD, Dept. of Pediatrics, Lund University, Uppsala.
Thomas Fritz, MD, Dept. of Obstetrics and Gynecology, Sahlgrenska University Hospital, Göteborg.
Professor Lena Hellström-Westas, MD, PhD, Dept. of Pediatrics, Uppsala University, Uppsala.
Associate Professor Per Åke Holmgren, MD, PhD, Dept. of Pediatrics, Umeå University, Umeå.
Professor Gerd Holmström, MD, PhD, Dept. of Ophthalmology, Uppsala University, Uppsala. Expert Group member.
Annika Jeppsson, MD, Dept. of Obstetrics and Gynecology, Linköping University, Linköping.
Associate Professor Karin Källén, PhD, Centre for Reproductive Epidemiology, Lund University, Lund. Steering Group member.
Late Professor Ricardo Laurini, MD, PhD, Dept. of Pathology, Bodö Central Hospital, Bodö, Norway. Expert Group member.
Eva Lindberg, MD, PhD, Dept. of Pediatrics, Örebro University, Örebro.
Anita Lundqvist, PhD, Dept. of Health Sciences, Lund University, Lund.
Professor Karel Maršál, MD, PhD, Dept. of Obstetrics and Gynecology, Lund University, Lund. (Principal investigator), Steering Group member.
Professor Tore Nilstun, PhD, Dept. of Medical Ethics, Lund University, Lund. Steering Group member.
Associate Professor Solveig Nordén-Lindeberg, MD, PhD, Dept. of Obstetrics and Gynecology, Uppsala University, Uppsala.
Professor Mikael Norman, MD, PhD, Dept. Dept. of Pediatrics, Karolinska University, Stockholm, Steering Group member.
Elisabeth Olhager, MD, PhD, Dept. of Pediatrics, Linköping University, Linköping.
Ingrid Östlund, MD, PhD, Dept. of Obstetrics and Gynecology, Örebro University, Örebro.
Professor Fredrik Serenius, MD, PhD, Dept. of Pediatrics, Norrland University, Umeå. Steering Group member.
Marija Simic, MD, Dept. of Obstetrics and Gynecology, Karolinska University Hospital Solna, Stockholm.
Gunnar Sjörs, MD, PhD, Dept. of Pediatrics, Uppsala University, Uppsala.
Lennart Stigson, MD, Dept. of Pediatrics, Sahlgrenska University Hospital, Göteborg.
Professor Karin Stjernqvist, Dept of Psychology, Lund University, Lund. Expert Group member.
Associate Professor Bo Strömberg, Dept. of Pediatrics, Uppsala University, Uppsala. Steering Group member.
Late Associate Professor Margareta Wennergren, MD, PhD, Dept. of Obstetrics and Gynecology, Sahlgrenska University Hospital, Göteborg. Steering Group member.
Professor Magnus Westgren, MD, PhD, Dept. of Obstetrics and Gynecology, Karolinska University Hospital Huddinge, Stockholm.
Financial disclosures
This study was supported by the Swedish Research Council (Grant 2006–3858 and 2009-4250), the Swedish Neonatal Society and the Evy and Gunnar Sandberg Foundation.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Table S1 List of variables.
Table S2 Characteristics of the seven Swedish healthcare regions.
Table S3 Risk of death among fetuses alive at maternal admission for delivery (n = 844) in six healthcare regions compared with the reference region (Stockholm) by gestational age groups. Bold letters indicate statistically significant OR.
Table S4 Risk of death among infants born alive and morbidity among survivors to 365 days in six healthcare regions compared with the reference region (Stockholm) by gestational age. Bold letters indicate statistically significant OR.
References
- Markestad T, Kaaresen PI, Ronnestad A, Reigstad H, Lossius K, Medbø S, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005;115:1289–98. doi: 10.1542/peds.2004-1482. [DOI] [PubMed] [Google Scholar]
- Tommiska V, Heinonen K, Lehtonen L, Renlund M, Saarela T, Tammela O, et al. No improvement in outcome of nationwide extremely low birth weight infant populations between 1996–1997 and 1999–2000. Pediatrics. 2007;119:29–36. doi: 10.1542/peds.2006-1472. [DOI] [PubMed] [Google Scholar]
- The EXPRESS Group. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301:2225–33. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks' gestation. JAMA. 2011;306:2348–58. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle WA American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008;121:419–32. doi: 10.1542/peds.2007-3283. [DOI] [PubMed] [Google Scholar]
- Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants: a meta-analysis. JAMA. 2010;304:992–1000. doi: 10.1001/jama.2010.1226. [DOI] [PubMed] [Google Scholar]
- Håkansson S, Farooqi A, Holmgren PA, Serenius F, Högberg U. Proactive management promotes outcome in extremely preterm infants: a population-based comparison of two perinatal management strategies. Pediatrics. 2004;114:58–64. doi: 10.1542/peds.114.1.58. [DOI] [PubMed] [Google Scholar]
- Fischer N, Steurer MA, Adams M, Berger TM. Swiss Neonatal Network Survival rates of extremely preterm infants (gestational age <26 weeks) in Switzerland: impact of the Swiss guidelines for the care of infants born at the limit of viability. Arch Dis Child Fetal Neonatal Ed. 2009;94:F407–13. doi: 10.1136/adc.2008.154567. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–9. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- Draper ES, Zeitlin J, Fenton AC, Weber T, Gerrits J, Martens G, et al. Investigating the variations in survival rates for very preterm infants in 10 European regions: the MOSAIC birth cohort. Arch Dis Child Fetal Neonatal Ed. 2009;94:F158–63. doi: 10.1136/adc.2008.141531. [DOI] [PubMed] [Google Scholar]
- Tromp M, Eskes M, Reitsma JB, Erwich JJ, Brouwers HA, Rijninks-van Driel GC, et al. Regional perinatal mortality differences in the Netherlands; care is the question. BMC Public Health. 2009;9:102. doi: 10.1186/1471-2458-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU network: 1996-1997. Pediatrics. 2000;106:1070–9. doi: 10.1542/peds.106.5.1070. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Report 2005: Make every mother and child count. . Available at: http://www.who.int/whr/2005/whr2005_en.pdf (accessed no March 23, 2013) [DOI] [PubMed] [Google Scholar]
- EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) Acta Paediatr. 2010;99:978–92. doi: 10.1111/j.1651-2227.2010.01846.x. [DOI] [PubMed] [Google Scholar]
- Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1–6. doi: 10.1016/s0166-4328(05)80189-5. [DOI] [PubMed] [Google Scholar]
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The International Neonatal Network. Lancet. 1993;342:193–8. [PubMed] [Google Scholar]
- Vanhaesebrouck P, Allegaert K, Bottu J, Debauche C, Devlieger H, Docx M, et al. The EPIBEL study: outcomes to discharge from hospital for extremely preterm infants in Belgium. Pediatrics. 2004;114:663–75. doi: 10.1542/peds.2003-0903-L. [DOI] [PubMed] [Google Scholar]
- Field DJ, Dorling JS, Manktelow BN, Draper ES. Survival of extremely premature babies in a geographically defined population: prospective cohort study of 1994–1999 compared with 2000–2005. BMJ. 2008;336:1221–3. doi: 10.1136/bmj.39555.670718.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollée LA, Cuttini M, Delmas D, Papiernik E, den Ouden AL, Agostino R, et al. MOSAIC Research group. Obstetric interventions for babies born before 28 weeks of gestation in Europe: results of the MOSAIC study. BJOG. 2009;116:1481–91. doi: 10.1111/j.1471-0528.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- Lorch SA, Maheshwari P, Even-Shoshan O. The impact of certificate of need programs on neonatal intensive care units. J Perinatol. 2012;32:39–44. doi: 10.1038/jp.2011.47. [DOI] [PubMed] [Google Scholar]
- Serenius F, Ewald U, Farooqi A, Holmgren PA, Håkansson S, Sedin G. Short-term outcome after active perinatal management at 23–25 weeks of gestation. A study from two Swedish perinatal centres. Part 3: neonatal morbidity. Acta Paediatr. 2004;93:1090–7. [PubMed] [Google Scholar]
- Bottoms SF, Paul RH, Iams JD, Mercer BM, Thom EA, Roberts JM, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960–6. doi: 10.1016/s0002-9378(97)70386-7. [DOI] [PubMed] [Google Scholar]
- de Kleine MJ, den Ouden AL, Kollée LA, Ilsen A, van Wassenaer AG, Brand R, et al. Lower mortality but higher neonatal morbidity over a decade in very preterm infants. Paediatr Perinat Epidemiol. 2007;21:15–25. doi: 10.1111/j.1365-3016.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- Rijken M, Stoelhorst GM, Martens SE, van Zwieten PH, Brand R, Wit JM, et al. Mortality and neurologic, mental, and psychomotor development at 2 years in infants born less than 27 weeks' gestation: the Leiden follow-up project on prematurity. Pediatrics. 2003;112:351–8. doi: 10.1542/peds.112.2.351. [DOI] [PubMed] [Google Scholar]
- Serenius F, Källén K, Blennow M, Ewald U, Fellman V, Holmström G, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–20. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of variables.
Table S2 Characteristics of the seven Swedish healthcare regions.
Table S3 Risk of death among fetuses alive at maternal admission for delivery (n = 844) in six healthcare regions compared with the reference region (Stockholm) by gestational age groups. Bold letters indicate statistically significant OR.
Table S4 Risk of death among infants born alive and morbidity among survivors to 365 days in six healthcare regions compared with the reference region (Stockholm) by gestational age. Bold letters indicate statistically significant OR.


