Abstract
Objective
The C-type natriuretic peptide (CNP) signaling pathway is a major contributor to postnatal skeletal growth in humans. This study was undertaken to investigate whether CNP signaling could prevent growth delay and cartilage damage in an animal model of inflammatory arthritis.
Methods
We generated transgenic mice that overexpress CNP (B6.SJL-Col2a1-NPPC) in chondrocytes. We introduced the CNP transgene into mice with experimental systemic inflammatory arthritis (K/BxN T cell receptor [TCR]) and determined the effect of CNP overexpression in chondrocytes on the severity of arthritis, cartilage damage, and linear growth. We also examined primary chondrocyte cultures for changes in gene and protein expression resulting from CNP overexpression.
Results
K/BxN TCR mice exhibited linear growth delay (P < 0.01) compared to controls, and this growth delay was correlated with the severity of arthritis. Diminished chondrocyte proliferation and matrix production was also seen in K/BxN TCR mice. Compared to non–CNP-transgenic mice, K/BxN TCR mice with overexpressed CNP had milder arthritis, no growth delay, and less cartilage damage. Primary chondrocytes from mice overexpressing CNP were less sensitive to inflammatory cytokines than wild-type mouse chondrocytes.
Conclusion
CNP overexpression in chondrocytes can prevent endochondral growth delay and protect against cartilage damage in a mouse model of inflammatory arthritis. Pharmacologic or biologic modulation of the CNP signaling pathway may prevent growth retardation and protect cartilage in patients with inflammatory joint diseases, such as juvenile idiopathic arthritis.
Linear growth results from the action of multiple signaling pathways. The pathway that includes C-type natriuretic peptide (CNP) contributes to chondrocyte proliferation, differentiation, and matrix synthesis in skeletal growth plates (1–4). Patients with homozygous loss-of-function mutations in natriuretic peptide receptor B (NPR-B), the CNP receptor, have acromesomelic dysplasia (5), and carriers of the heterozygous mutation frequently have short stature (6). Also, patients with chromosome translocations that cause CNP overexpression develop skeletal overgrowth (7). These conditions indicate a dose-dependent effect of CNP signaling on linear growth. Therefore, linear growth may be affected in patients with acquired diseases that alter the production of CNP or the expression of its receptor, NPR-B.
Children with juvenile inflammatory arthritis (JIA) have impaired linear growth, and 40% develop bone degeneration and deformity adjacent to affected joints (8–11). The mechanism by which chronic systemic inflammation suppresses linear growth in children with JIA is not completely understood (12,13). Alterations in growth hormone and insulin-like growth factor (IGF) signaling pathways have been associated with growth delay in children with chronic arthritis. Although serum growth hormone levels were unchanged in patients with JIA, patients did have reduced levels of IGF-1 and IGF binding protein 3 (IGFBP-3) (14), suggesting that inflammation causes growth hormone resistance. The CNP signaling pathway has not been studied in children with JIA.
Animal models of inflammatory arthritis have been used to delineate pathways that contribute to impaired linear growth and joint deformity (15). Local elevations of tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and IL-1β levels have been observed in affected growth plates. Furthermore, overexpression of IL-6 (16) or TNFα (17) in transgenic mice causes systemic inflammatory arthritis and growth retardation. By 25 days of age, K/BxN T cell receptor (TCR) mice exhibit pronounced joint inflammation that resembles rheumatoid arthritis (17–19). Herein, we report that these mice also develop linear growth delay and cartilage damage that can be lessened by overexpression of CNP in chondrocytes.
MATERIALS AND METHODS
These experiments were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Generation of CNP-transgenic mice
We cloned full-length human CNP (NPPC) complementary DNA (cDNA) into the pKN185 vector, which drives CNP expression under the control of Col2a1 promoters and enhancers (20). Col2a1-NPPC–transgenic mice were produced following pronuclear injection into zygotes on a mixed C57BL/6J-SJL background. One founder animal that had ∼4 copies of the CNP transgene integrated into a single locus, as determined by Southern blotting and Mendelian segregation of the transgene, was then backcrossed for >20 generations onto the C57BL/6J background. C57BL/6J mice were also used as wild-type (WT) controls.
Development of the K/BxN TCR mouse model of arthritis
KRN TCR–transgenic mice, as well as K/BxN TCR mice that develop spontaneous arthritis, have been previously described (21–23). KRN TCR–transgenic mice were a gift from CBDM Lab (Joslin Diabetes Center/Harvard Medical School). K/BxN TCR mice were obtained by crossing KRN TCR–transgenic mice with nonobese diabetic mice (NOD/ShiLtJ; The Jackson Laboratory). Only offspring that inherited the TCR transgene developed inflammatory arthritis on this background; mice that did not inherit the TCR transgene were used as nontransgenic controls.
Breeding the CNP transgene into the mice with experimental arthritis
We bred Col2a1-NPPC–transgenic mice with KRN TCR mice. Offspring that carried both the Col2a1-NPPC and the TCR transgenes were then bred with NOD mice. The phenotypes of offspring that were double heterozygous for the TCR and Col2a1-NPPC transgenes (K/BxN TCR,Col2a1-NPPC) were compared to the phenotypes of offspring that only inherited the TCR transgene (K/BxN TCR). Other offspring resulting from this cross included mice heterozygous for the Col2a1-NPPC transgene only and mice that inherited neither transgene (BxN). Offspring were examined at birth and weekly, from 3 weeks of age until 20 weeks of age.
Small-animal radiography
A Faxitron radiographic inspection unit (model 8050-010; Field Emission) was used to obtain radiographic images of the mice postmortem. Legs were exposed for 1.5 minutes at 35 kVp; the entire body was exposed for 1.5 minutes at 30 kVp.
Clinical scoring of arthritis severity
The severity of clinical arthritis was determined using a previously described scoring system (24,25), with modifications (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html). Higher scores indicate increased severity of clinical arthritis.
Sample collection
Mice were bled under sedation and then euthanized. Samples were collected from mice that were 1, 2, 3, 4, 6, 8, 12, 16, and 24 months old. To evaluate the growth plates, one hind leg (femur and tibia) from each mouse was dissected, fixed with 4% formaldehyde in phosphate buffered saline (PBS) for 24 hours at 4°C, decalcified with 0.5M EDTA for 1 week, and then embedded in paraffin. Coronal sections measuring 4 μm across the femorotibial joint were stained with Safranin O–fast green or hematoxylin and eosin and analyzed by immunohistochemical staining or in situ hybridization. For accurate measurement of articular cartilage and growth plate cartilage, sections were obtained at the point where the anterior cruciate ligament inserts into the tibia.
Histologic scoring system
Histologic evaluation of inflammation in the knee joint and cartilage integrity was performed using two scoring systems. The histologic scoring system described by Pettit et al (26) was used to assess the severity of inflammatory arthritis. The International Cartilage Repair Society scoring system (27) was used to assess the status of the extracellular matrix repair in joint cartilage (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html). An investigator who was not otherwise involved with the study evaluated the mouse knee sections under blinded conditions and assigned a score based on these systems.
Cell proliferation analysis by bromodeoxyuridine (BrdU) labeling
BrdU (300 mg/kg dose; Zymed) was injected intraperitoneally. Mice were euthanized 2 hours after injection. Tissue was processed and embedded in paraffin as described above. BrdU incorporation was detected using a BrdU-staining kit according to the instructions of the manufacturer (Zymed). The percentage of BrdU-positive cells was determined by dividing the number of BrdU-positive chondrocytes by the total number of chondrocytes that were counted in multiple sections of the growth plate.
In situ hybridization
Slides were deparaffinized and fixed in 4% formaldehyde. Sections were digested with proteinase K (1 μg/ml) for 20 minutes at 37°C and acetylated in 0.25% acetic anhydride in 0.1M triethanolamine HCl. After refixation in 4% formaldehyde, sections were hybridized with 35S-labeled riboprobes in hybridization buffer (50% deionized formamide, 300 mM NaCl, 20 mM Tris HCl, pH 8.0, 5 mM EDTA, 0.5 mg/ml yeast transfer RNA, 10% dextran sulfate, and 1× Denhardt's solution) in a humidified chamber at 55°C overnight. After hybridization, slides were washed with 5× saline–sodium citrate (SSC) at 50°C, 50% formamide, 2× SSC at 65°C, and 1× NTE (0.5 M NaCl, 10 mM Tris HCl, pH 8.0, 5 mM EDTA) at 37°C, and then treated with RNase A (20 μg/ml) and RNase T1 (1 unit/μl) in 1× NTE at 37°C for 20 minutes. Slides were further washed in 1× NTE at 37°C, 50% formamide, 2× SSC at 65°C, 2× SSC, 0.1× SSC, and then dehydrated with graded concentrations of ammonium acetate and ethanol. Slides were dipped in NTB emulsion (Kodak) and exposed to the emulsion for 6 weeks, after which they were developed and counterstained with Hoechst 33258 (Sigma). The NPPC probe was made from the pKN185-hCNP vector construct that was originally used to create the transgenic mice. A 5′ 280-bp fragment of the human CNP cDNA sequence plus 118 bp of upstream vector sequence was used for sense and antisense probes (398 bp).
Primary chondrocyte isolation and cytokine treatment
Primary chondrocytes from rib cages of ∼5-day-old mice were isolated by enzymatic digestion using standard methods (28). Briefly, rib cages were collected in sterile PBS. The ribs were rinsed several times before digestion with 10 ml of 3 mg/ml collagenase B (Worthington) in Dulbecco's modified Eagle's medium (DMEM) for ∼1 hour at 37°C. The ribs were then washed several times with PBS and digested overnight at 37°C with collagenase solution (2 ml of 3 mg/ml collagenase B/DMEM plus 10 ml DMEM with 10% fetal bovine serum). The next day, the cell suspension was strained and the cells were pelleted at 1,000 revolutions per minute for 10 minutes and then resuspended in DMEM containing 10% fetal bovine serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml). Viable cell counts were determined by trypan blue staining, and cells were seeded at a density of 1 × 106 cells per 35-mm culture plate in 2.5 ml DMEM (∼1.2 × 105 cells/cm2). Cells were grown in culture medium until ∼90% confluent, with a change of medium every 3 days. Once cells became ∼90% confluent, chondrocyte cultures were serum-starved and treated with cytokines (10 ng/ml TNFα or 10 ng/ml IL-1β; both from R&D Systems) overnight (18 hours) and messenger RNA (mRNA) and proteins were extracted as previously described (29).
Western blotting
Cells were lysed in 500 μl of buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, and Complete protease inhibitor cocktail), and total lysate protein (60 μg/lane) was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Bio-Rad), blocked with nonfat dry milk, resuspended in Tris buffered saline, and probed with primary and secondary antibodies as previously described (29,30). Primary antibodies included anti-phosphorylated and anti-nonphosphorylated p38 MAPK and ERK-1/2 (p38 MAPK phosphorylated #4631, p38 MAPK nonphosphorylated #9212, phospho–ERK-1/2–phospho-MAPK [Thr202/Ty204] [Cell Signaling Technologies]) and anti–NPR-B (sc-25486; Santa Cruz Biotechnology). Immunoreactive proteins were visualized by using 1:1,000 diluted horseradish peroxidase (HRP)–linked secondary antibodies and enhanced chemiluminescence (GE Healthcare) as described (31). HRP-conjugated goat anti-rabbit IgG at a dilution of 1:2,000 (sc-2004; Santa Cruz Biotechnology) was used as the secondary antibody for NPR-B.
RNA extraction and cDNA synthesis
Total cytoplasmic RNA was prepared from primary chondrocytes using an RNeasy kit (Qiagen). RNA (2 μg) was reverse transcribed using a SuperScript II reverse-transcriptase kit (Invitrogen), and the cDNA mixture was diluted 5-fold in nuclease-free water; 5 μl was used for real-time quantitative polymerase chain reaction (qPCR).
Real-time qPCR
Using a StepOnePlus thermocycler (Applied Biosystems), reactions were performed in a 20-μl volume with 0.5 μM of each primer and SYBR Green Master Mix according to the instructions of the manufacturer (Qiagen). The expression level of the housekeeping gene Rlp7 was used to normalize mRNA expression. Information on the primers used to amplify specific cDNA sequences is available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html. All reactions were performed in duplicate.
Statistical analysis
Disease severity and histopathologic scores were compared between groups, using chi-square tests for categorical variables (clinical and histologic scores) and t-tests for continuous variables. Results are expressed as the mean ± SD. To test differences between the groups of mice, data on the nose-to–tail tip length of mice were examined by analysis of variance (ANOVA). ANOVA F test results were reported. P values less than 0.05 were considered significant. We also used Pearson correlation matrices to test for correlations between the clinical and histopathologic data. The data were analyzed using SAS 9.0 and Stata 11.0.
RESULTS
Col2a1-NPPC–transgenic mice exhibit linear bone overgrowth
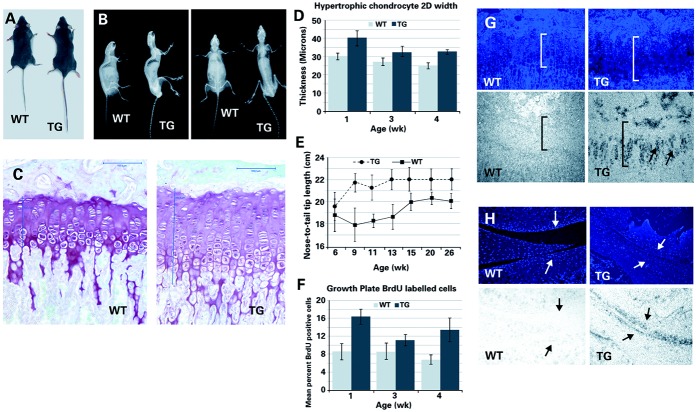
Mice carrying the CNP transgene driven by the Col2a1 promoter/enhancer (Col2a1-NPPC) demonstrated skeletal overgrowth at 4 weeks of age (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html). Overgrowth affected the long bones and the vertebrae (Figures 1A and B) and was associated with increases in growth plate width, the size of hypertrophic chondrocytes (P < 0.01 by F test) (Figures 1C and D), nose-to–tail tip length (Figure 1E), and the number of proliferating chondrocytes (P < 0.01 by F test) (Figure 1F). The mean ± SD width of the growth plate was 148 ±14 μm in Col2a1-NPPC–transgenic mice (n = 12) and 99 ± 5.3 μm in nontransgenic control mice (n = 5) at 4 weeks of age (P < 0.05 by Student's t-test). CNP transgene expression in growth plate and articular chondrocytes was detected by in situ hybridization (Figures 1G and H). Col2a1-NPPC–transgenic mice developed thoracic kyphosis and joint dislocations over time, but no abnormalities outside of the skeletal system were detected.
Figure 1.
Longitudinal overgrowth in Col2a1-NPPC–transgenic (TG) mice. A and B, Photograph (A) and radiographs (B) of 24-week-old nontransgenic wild-type (WT) mice and their female Col2a1-NPPC–transgenic littermates in which C-type natriuretic peptide is overexpressed. C, Photomicrographs of Safranin O–fast green–stained proximal tibia growth plates from 13-week-old female WT and Col2a1-NPPC–transgenic mice. Measurements in the growth plates are shown (vertical lines). Bars = 100 μm. D, Width of hypertrophic chondrocytes in WT and Col2a1-NPPC–transgenic mice at week 1 (n = 3 and n = 4, respectively), week 3 (n = 4 and n = 6, respectively), and week 4 (n = 4 and n = 5, respectively). 2-D = 2-dimensional. E, Linear growth in male and female WT and Col2a1-NPPC–transgenic mice at week 6 (n = 12 and n = 16, respectively), week 9 (n = 6 and n = 10, respectively), week 11 (n = 16 and n = 9, respectively), week 13 (n = 6 and n = 8, respectively), week 15 (n = 8 and n = 12, respectively), week 20 (n = 9 and n = 6, respectively), and week 26 (n = 9 and n = 6, respectively). F, Percent of growth plate chondrocytes that incorporated bromodeoxyuridine (BrdU) in WT and Col2a1-NPPC–transgenic mice at week 1 (n = 4 and n = 5, respectively), week 3 (n = 2 and n = 4, respectively), and week 4 (n = 5 and n = 5, respectively). Values in D–F are the mean ± SD. G and H, Photomicrographs showing growth plate (G) and articular cartilage (H) from 4-week-old WT and Col2a1-NPPC–transgenic mice following Hoechst staining of cell nuclei (top) and in situ hybridization using an antisense NPPC probe (bottom). Original magnification × 20. Note the width of the growth plate (brackets in G). Chondrocytes in G and H are shown (arrows).
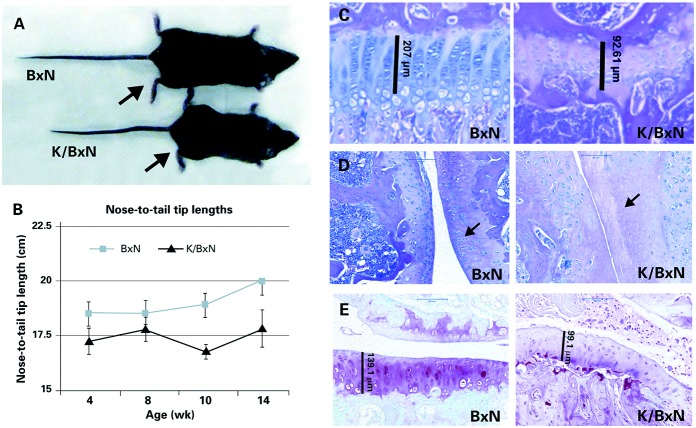
Arthritic K/BxN TCR mice exhibit linear growth delay and articular cartilage damage
K/BxN TCR mice developed arthritis by age 3 weeks and exhibited visible growth delay by age 12 weeks when compared to littermates that did not inherit the TCR transgene (BxN) (Figure 2A). Significant differences in linear growth (as determined by nose-to–tail tip length) between K/BxN TCR mice and BxN littermates were detected by 4 weeks of age (P < 0.05 by F test) (Figure 2B). To prevent length discrepancies, we did not obtain tissue for DNA isolation by clipping tails in order to genotype the mice. In the K/BxN TCR mice, the severity of arthritis was inversely correlated with longitudinal growth; this correlation was strongest at age 14 weeks (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html). The growth plates in the K/BxN TCR mice were narrower and had fewer cells than growth plates in the BxN littermates (Figure 2C). The articular cartilage in K/BxN TCR mice also became less cellular, had less cartilage matrix, and had a more irregular surface than the articular cartilage in BxN mice (Figures 2D and E).
Figure 2.
Growth retardation and cartilage damage in K/BxN T cell receptor (TCR) mice. A, Photograph of 12-week-old female K/BxN mice with the TCR transgene (K/BxN TCR) and without the TCR transgene (BxN). Compared with the BxN mouse, the K/BxN TCR mouse is smaller and has evidence of inflammatory arthritis; the hind paw (arrows) is swollen in the K/BxN TCR mouse. B, Linear growth of male and female BxN and K/BxN TCR mice. Statistical analysis showed significant differences (P < 0.05 by F test) between BxN and K/BxN TCR mice at week 4 (n = 12 and n = 10, respectively), week 8 (n = 15 and n = 8, respectively), week 10 (n = 16 and n = 8, respectively), and week 14 (n = 14 and n = 5, respectively). Values are the mean ± SD. C, Photomicrographs of hematoxylin and eosin (H&E)–stained proximal tibia growth plate cartilage from a 9-week-old female K/BxN TCR mouse and a BxN littermate. The width of the growth plate is shown (vertical lines). Original magnification × 40. D, Photomicrographs of H&E-stained sagittal sections of the femorotibial joint of a 9-week-old female BxN mouse and a K/BxN TCR littermate. Bars = 100 μm. E, Photomicrographs of Safranin O–stained coronal sections of the femorotibial joint of a 12-week-old female BxN mouse and a K/BxN TCR littermate. Bars = 100 μm. K/BxN TCR mice exhibited cartilage thinning (vertical lines in C and D), chondrocyte loss (arrows in D), and reduced proteoglycan content compared to BxN mice.
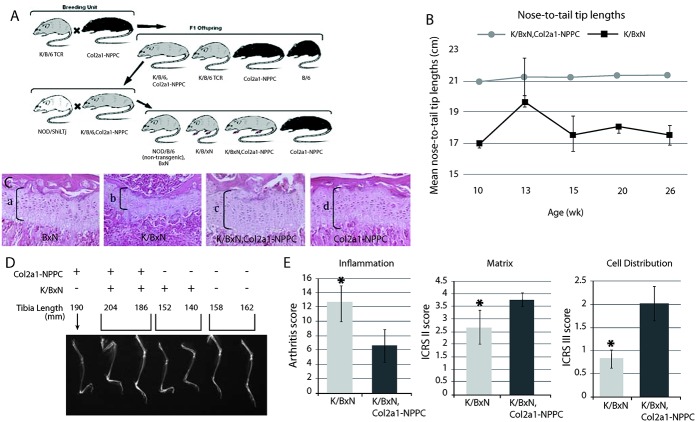
CNP overexpression in chondrocytes improves linear growth and reduces articular cartilage damage in K/BxN TCR mice
Since CNP overexpression enhanced linear growth in Col2a1-NPPC–transgenic mice, we sought to determine whether overexpression could protect against growth impairment in mice with inflammatory arthritis. We first bred Col2a1-NPPC–transgenic mice onto the KRN TCR background and then onto the K/BxN background (Figure 3A).
Figure 3.
C-type natriuretic peptide (CNP) overexpression reduces growth retardation in K/BxN T cell receptor (TCR) mice. A, Breeding strategy used to generate K/BxN TCR mice that overexpress CNP in chondrocytes. Grey coat color indicates the KRN TCR transgene (25). B, Development of arthritis without growth retardation in K/BxN TCR,Col2a1-NPPC–transgenic mice. Nose-to–tail tip lengths of male and female K/BxN TCR,Col2a1-NPPC–transgenic mice and K/BxN TCR mice are shown. Statistical analysis revealed a significant difference (P < 0.05 by F test) between K/BxN and K/BxN TCR,Col2a1-NPPC–transgenic mice at week 10 (n = 16 and n = 6, respectively), week 13 (n = 7 and n = 5, respectively), week 15 (n = 14 and n = 6, respectively), week 20 (n = 7 and n = 5, respectively), and week 26 (n = 7 and n = 5, respectively). Values are the mean ± SD. C, Hematoxylin and eosin–stained coronal sections of proximal tibia growth plates of a 12-week-old nontransgenic mouse (BxN) (a), an arthritic mouse (K/BxN) (b), an arthritic and CNP-overexpressing mouse (K/BxN TCR,Col2a1-NPPC–transgenic) (c), and a CNP-overexpressing mouse (Col2a1-NPPC–transgenic) (d). Original magnification × 20. D, Radiographs of lower extremities of 12-week-old male K/BxN mice with and without the Col2a1-NPPC transgene and/or the TCR transgene. E, Histologic scores from evaluation of inflammation, scores based on International Cartilage Repair Society (ICRS) system II evaluation, and scores based on ICRS system III evaluation in arthritic mice with (K/BxN TCR,Col2a1-NPPC) and without (K/BxN) CNP overexpression. Values are the mean ± SD. ∗ = P < 0.05 versus mice with CNP overexpression.
In K/BxN TCR mice that overexpressed CNP (K/BxN TCR,Col2a1-NPPC–transgenic mice), no growth retardation was seen (P < 0.05 by F test) (Figure 3B), growth plates were increased in width (Figure 3C and online at http://www.case.edu/artsci/biol/skeletal/24114569s.html), and long bones were increased in length (Figure 3D) compared to K/BxN TCR arthritic mice. The increase in growth plate width in mice with the Col2a1-NPPC transgene was associated with an increase in BrdU incorporation (http://www.case.edu/artsci/biol/skeletal/24114569s.html). CNP overexpression reduced the clinical arthritis score in the K/BxN TCR mice, with a mean ± SD arthritis score of 4.37 ± 1.38 in CNP-overexpressing mice (n = 8) compared to 8.66 ± 3.26 in the K/BxN TCR mice (n = 14) (P < 0.05 by t-test).
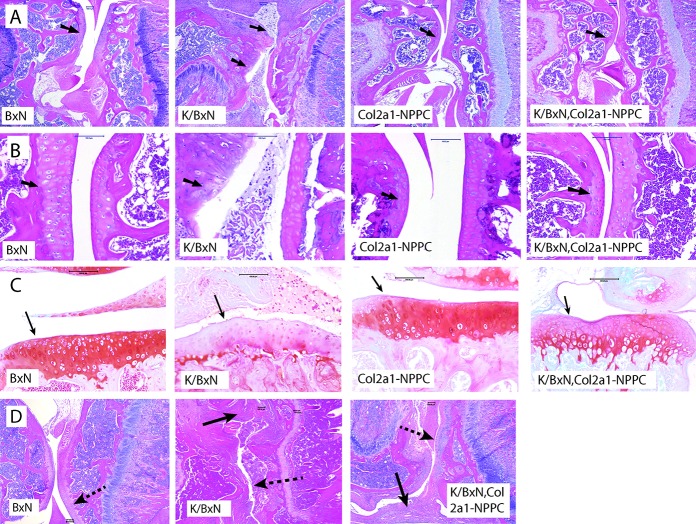
CNP overexpression also reduced the severity of articular cartilage inflammation and damage in the K/BxN TCR mice (Figure 3E). Thirteen-week-old male mice with CNP overexpression had better articular cartilage chondrocyte distribution and organization and cartilage matrix content than the controls. Although CNP overexpression appeared to protect articular cartilage against damage caused by inflammation (Figures 4A–C), overexpression did not reduce inflammation-related changes that occurred in the synovium (Figure 4D).
Figure 4.
C-type natriuretic peptide (CNP) overexpression lessens cartilage damage in K/BxN T cell receptor (TCR) mice. A and B, Photomicrographs of hematoxylin and eosin (H&E)–stained sagittal sections of the femorotibial joint of a 13-week-old male nontransgenic mouse (BxN), an arthritic mouse (K/BxN), a CNP-overexpressing mouse (Col2a1-NPPC–transgenic), and an arthritic and CNP-overexpressing mouse (K/BxN TCR,Col2a1-NPPC–transgenic). Femoral articular cartilage is shown (arrows). The images shown are from mice that had the median score for histologic severity within each genotypic group. Original magnification × 10 in A; × 40 in B. C, Photomicrographs of Safranin O–stained coronal sections of the femorotibial joints of 12-week-old female mice. The cartilage surface of the tibia plateau is shown (arrows). Bars = 100 μm. D, Photomicrographs of H&E-stained sagittal sections of the femorotibial joint of 12-week-old female mice. Although K/BxN TCR,Col2a1-NPPC–transgenic mice have pannus and synovial inflammation (solid arrows), there is less damage to the articular cartilage surface (dashed arrows). Bars = 100 μm.
CNP overexpression enhances signal transduction via NPR-B and reduces the sensitivity of this signaling pathway to the proinflammatory cytokines TNFα and IL-1β
We performed cGMP enzyme-linked immunosorbent assays, real time qPCR, and Western blotting using cultures of rib cartilage primary chondrocytes harvested from Col2a1-NPPC–transgenic and WT control mice. The mean ± SD intracellular cGMP level in the Col2a1-NPPC–transgenic mouse chondrocytes was ∼8 ± 0.7–fold higher than in the WT mouse chondrocytes, confirming enhanced CNP signaling. In Col2a1-NPPC–transgenic mouse chondrocytes, levels of protein and mRNA for NPR-B (Npr2) were increased (Figures 5, 6A, and online at http://www.case.edu/artsci/biol/skeletal/24114569s.html); Col2a1-NPPC–transgenic mouse chondrocytes also had increased expression of mRNA for the pro-chondrogenic growth factors Vegf and Tgfb1 and increased expression of Col2a1 compared to WT mouse chondrocytes (Figure 5).
Figure 5.
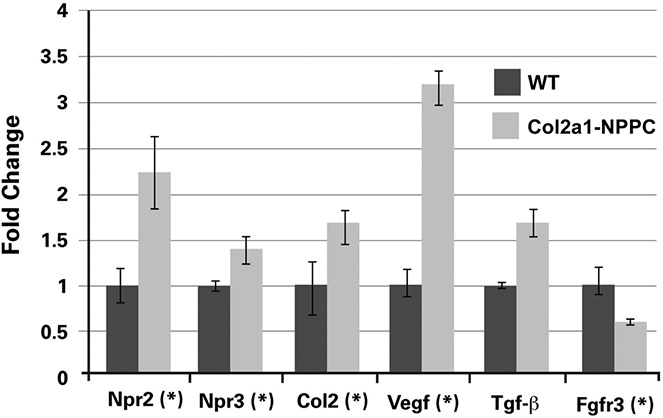
C-type natriuretic peptide overexpression in chondrocytes increases the expression of genes associated with cartilage anabolism. Results of quantitative real-time polymerase chain reaction analysis of primary chondrocyte cultures recovered from Col2a1-NPPC–transgenic mice and wild-type (WT) mice for several genes associated with cartilage growth are shown. Values are the mean ± SD. ∗ = P < 0.05.
Figure 6.
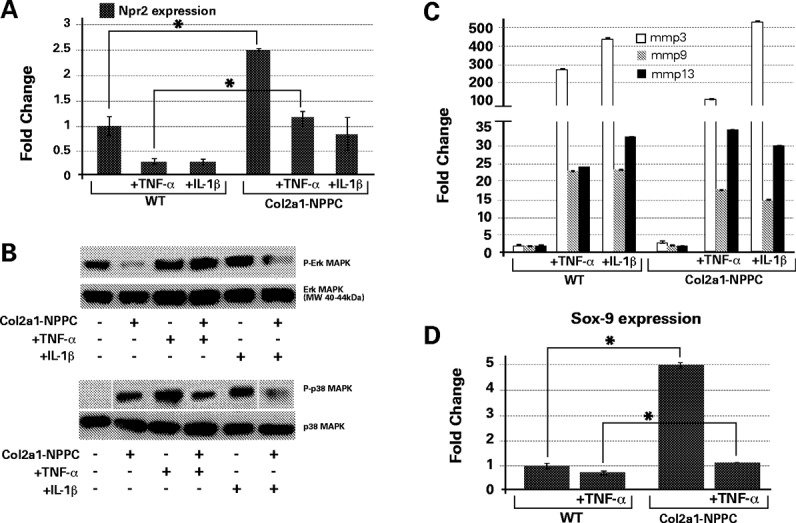
C-type natriuretic peptide (CNP) overexpression in chondrocytes dampens the response to inflammatory cytokines. A, Relative levels of mRNA for Npr2 in primary cultures obtained from nontransgenic and Col2a1-NPPC–transgenic mice that had or had not been exposed to tumor necrosis factor α (TNFα) (10 ng/ml) and interleukin-1β (IL-1β) (10 ng/ml). B, Western blot analysis of p44 ERK-1 and p38 MAPK phosphorylation. ERK-1 phosphorylation was inhibited by the CNP transgene. TNFα treatment increased p44 ERK-1 phosphorylation in mouse chondrocytes (even in those from CNP-transgenic mice), but IL-1β treatment did not change suppression of p44 ERK-1 phosphorylation in Col2a1-NPPC–transgenic mouse chondrocytes. Phosphorylation of p38 MAPK was increased in Col2a1-NPPC–transgenic mouse cartilage as compared to cartilage from nontransgenic littermates. Overnight treatment with TNFα or IL-1β significantly increased the p38 phosphorylation in nontransgenic mouse chondrocytes, while this effect was only modest in the Col2a1-NPPC–transgenic mice. C, Real-time quantitative polymerase chain reaction (qPCR) analysis of matrix metalloproteinase 3 (MMP-3), MMP-9, and MMP-13 in primary chondrocytes isolated from the rib cages of wild-type (WT) and Col2a1-NPPC–transgenic mice. D, Real-time qPCR analysis of SOX9 transcription factor in primary chondrocytes isolated from the rib cages of WT and Col2a1-NPPC–transgenic mice. Values in A, C, and D are the mean ± SD, normalized to levels in WT mice. ∗ = P < 0.05.
Immunostaining revealed that vascular endothelial growth factor (VEGF) was expressed in a wider region of the growth plate of Col2a1-NPPC–transgenic mice (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html). VEGF staining was found to encompass an area that surrounds not only the hypertrophic and proliferating chondrocytes, but also the trabecular bone beneath the hypertrophic chondrocytes where the blood vessels reside (http://www.case.edu/artsci/biol/skeletal/24114569s.html). In addition, as has been previously reported (32), we confirmed that p38 MAPK phosphorylation is increased and MAPK ERK-1 phosphorylation is decreased in CNP-overexpressing chondrocytes compared to WT mouse chondrocytes (Figure 6B). We also observed that expression of mRNA for matrix metalloproteinase 3 (MMP-3), MMP-9, and MMP-13 was mildly increased in Col2a1-NPPC–transgenic mouse chondrocytes as compared to WT mouse chondrocytes (Figure 6C).
Since CNP overexpression in Col2a1-NPPC–transgenic mice appeared to protect articular cartilage in vivo against the damaging effect of inflammation, we treated primary chondrocytes from Col2a1-NPPC–transgenic mice and control mice with the proinflammatory cytokines TNFα and IL-1β. TNFα and IL-1β each significantly reduced Npr2 mRNA expression in both Col2a1-NPPC–transgenic and WT mouse chondrocytes. However, Npr2 mRNA expression in Col2a1-NPPC–transgenic mouse chondrocytes after TNFα or IL-1β treatment was comparable to Npr2 expression in untreated WT mouse chondrocytes (Figure 6A).
In Col2a1-NPPC–transgenic mouse chondrocytes, TNFα exposure had no effect on p38 MAPK phosphorylation, whereas IL-1β reduced p38 MAPK phosphorylation (Figure 6B). In contrast, both treatment with TNFα and treatment with IL-1β increased p38 MAPK phosphorylation in WT mouse chondrocytes. The expression of mRNA for the MMPs was either the same or less elevated in Col2a1-NPPC–transgenic mouse chondrocytes compared to WT mouse chondrocytes following exposure to these cytokines (P > 0.05) (Figure 6C).
It has been reported that IGF-1, IGF receptor I, and IGFBP-3 levels are diminished in serum of children with JIA, accounting for the delay in longitudinal growth. To understand whether CNP overexpression affects Igf1, Igf1r, and Igfbp3 mRNA expression, we performed real-time qPCR using primary chondrocyte cultures of rib cartilage harvested from Col2a1-NPPC–transgenic and WT mice. Col2a1-NPPC–transgenic mouse primary chondrocytes did not have increased levels of mRNA for Igf1 or Igf1r, but did have ∼6–7-fold increased levels of mRNA for Igfbp3 compared to WT mouse chondrocytes. When exposed to TNFα (10 ng/ml) and IL-1β (10 ng/ml) overnight, Igf1 and Igf1r expression levels in chondrocytes were suppressed significantly in both Col2a1-NPPC–transgenic and WT mouse chondrocytes. Interestingly, Igfbp3 expression levels did not differ between Col2a1-NPPC–transgenic and WT mouse chondrocytes exposed to proinflammatory cytokines; in addition, Igfbp3 expression remained up-regulated in the Col2a1-NPPC–transgenic mouse chondrocytes (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html).
Earlier reports indicate that TNFα and IL-1β inhibit chondrocyte differentiation and DNA synthesis in growth plate and costal chondrocytes by suppressing Sox9 expression (33). It is known that CNP up-regulates Sox9 expression in chondrocytes (34,35). Thus, we sought to determine whether CNP overexpression in primary chondrocytes from Col2a1-NPPC–transgenic mice also up-regulates Sox9 mRNA expression. Results showed that in primary chondrocytes from Col2a1-NPPC–transgenic mice, Sox9 expression is up-regulated 5-fold as compared to WT mouse chondrocytes. As expected, when chondrocytes were exposed to TNFα overnight, Sox9 expression was reduced in both WT and Col2a1-NPPC–transgenic mouse chondrocytes, but in Col2a1-NPPC–transgenic mouse chondrocytes that reduction brought Sox9 expression to the level seen in untreated WT mice (Figure 6D). We suggest that CNP overexpression in Col2a1-NPPC–transgenic mice protected against down-regulation of Sox9 expression levels during TNFα exposure.
DISCUSSION
It has been posited that in children with JIA the inhibitory effects of proinflammatory cytokines (IL-6, TNFα, and IL-1 β) on growth plate chondrocytes contribute to growth suppression (36,37). Elevation of proinflammatory cytokines (e.g., TNFα and IL-1β) in synovial fluid has also been associated with destruction of growth plates adjacent to an arthritic joint. Proinflammatory cytokines decrease the width of the proliferating zone in growth plate cartilage, which leads to a decrease in endochondral bone growth. IL-1β has been shown to reduce expression of cartilage collagens and proteoglycans (13,38).
In this study, we tested whether the growth-promoting effects of the CNP/NPR-B signaling pathway would lessen the severity of growth failure and cartilage degeneration in a mouse model of inflammatory arthritis. We generated mice in which CNP is overexpressed in chondrocytes resulting in increased chondrocyte proliferation, matrix production, and hypertrophic chondrocyte size, consistent with previous reports (2). We then bred the transgene for CNP overexpression into the K/BxN TCR mouse model of chronic inflammatory arthritis and observed that CNP overexpression reduced the damaging effects of chronic inflammatory arthritis on linear growth and articular cartilage.
There are several possible mechanisms by which CNP overexpression protects growth plate and articular cartilage against inflammation-induced damage. One possibility is that CNP overexpression increases chondrocyte functioning, thereby blunting the consequences of the negative effects of inflammatory cytokines. Interestingly, activation of MAPKs, particularly p38 MAPK, is a critical event that leads to the production of several mediators of cartilage damage, including the MMPs, in an arthritic joint (39). Therefore, it was surprising that overexpression of CNP protected articular cartilage while also significantly increasing p38 MAPK phosphorylation. CNP signaling may favor the activation of anabolic pathways downstream of p38 MAPK, whereas inflammatory cytokines favor the activation of catabolic pathways.
It has been suggested that growth delay in children with JIA is due, at least in part, to reduced levels of IGF-1 and IGFBP-3. Therefore, we studied the effect of CNP overexpression on Igf1 mRNA expression in the primary chondrocytes isolated from rib cage cartilage of Col2a1-NPPC–transgenic mice and WT mice, using real-time qPCR. Results showed that while there was no significant suppressive effect of CNP overexpression on Igf1 and Igf1r, there was a significant increase in Igfbp3 expression levels. In a previous human study, the investigators showed that a patient with acromesomelic dysplasia, Maroteaux type lacking NPR2 function had low serum levels of IGF-1. It may be possible that lack of CNP signaling negatively regulates IGF-1 levels. However, patients with acromesomelic dysplasia, Maroteaux type have skeletal dysplasia that is distinct from that seen in IGF-1 deficiency. Furthermore, patients with this disease are able to produce IGF-1 when an IGF-1 generation test is performed (40). Our data on the Col2a1-NPPC–transgenic mice do not suggest any direct regulatory effects of excess CNP on IGF-1 signaling pathway.
Igf1 expression and Igf1r expression were similarly suppressed in both Col2a1-NPPC–transgenic mouse primary chondrocytes and WT mouse chondrocytes when cultures were exposed to TNFα and IL-1β overnight, while Igfbp3 expression remained up-regulated. However, in the absence of a significant change in Igf1 and Igf1r expression in Col2a1-NPPC–transgenic mouse chondrocytes, we do not believe the protective effects of CNP seen in cartilage are due to its regulatory effects on Igf1 signaling.
Earlier reports have indicated that TNFα and IL-1β inhibit chondrocyte differentiation by suppressing DNA synthesis in the growth plate and costal chondrocytes (33). TNFα suppresses DNA synthesis, chondrocyte differentiation, and matrix synthesis by unknown mechanisms (41). It has been suggested that TNFα suppresses expression of Sox9, a key chondrocyte transcription factor, in inflamed cartilage. Our data suggest that CNP might improve chondrocyte proliferation and differentiation during inflammation by its regulatory effect on Sox9 expression. Sox9 mRNA expression was up-regulated 5-fold in chondrocytes of Col2a1-NPPC–transgenic mice. SOX9 and CNP are both known to increase matrix synthesis (42). Although Sox9 expression was significantly reduced following TNFα exposure in Col2a1-NPPC–transgenic mouse chondrocytes, expression was comparable to the level found in untreated WT mouse chondrocytes. Thus, it can be speculated that CNP expression overcomes the effect of proinflammatory cytokines on cartilage at the level of transcription factor Sox9 expression. Sox9 regulates chondrocyte differentiation, which is needed not only for longitudinal growth, but also for the matrix production that maintains the cartilage integrity.
We also cannot rule out the possibility that CNP overexpression directly modulates the inflammatory response. It has been reported that CNP inhibits cytokine-induced leukocyte rolling and has an antiplatelet/antithrombotic effect (43). Reports suggest that CNP secretion by vascular endothelial cells is increased in response to inflammatory stimuli such as IL-1β, TNFα, and lipopolysaccharide (44,45). Additionally, CNP suppresses lipopolysaccharide-activated murine macrophage secretion of prostaglandin E2 (46) and inhibits vascular inflammation and intimal hyperplasia in experimental vein grafts (47). Therefore, it is possible that CNP overexpression protects growth and prevents joint damage by diminishing chronic inflammation. Consequently, studies investigating the antiinflammatory effects of CNP in models of arthritis are warranted. While our data demonstrate that linear growth retardation in K/BxN TCR mice can be prevented by CNP overexpression, it remains to be determined whether this protective effect will be observed in other animal models of inflammation and whether pharmacologic manipulation of the CNP signaling pathway will have similar effects.
Reduction in linear growth is one complication of joint inflammation. Another common complication is widening of the metaphysis, which has been attributed to neovascularization and VEGF overexpression in the growth plate. CNP may play a role in this neovascularization, since chondrocytes that overexpress CNP were shown to produce increased Vegf mRNA levels (Figure 5), and VEGF immunostaining revealed an area that encompasses hypertrophic and proliferating chondrocytes, as well as the trabecular bone where the blood vessels reside (available online at http://www.case.edu/artsci/biol/skeletal/24114569s.html). Consequently, Col2a1-NPPC–transgenic mice developed widening at the ends of their long bones. We did not observe worsening periarticular overgrowth in the K/BxN TCR,Col2a1-NPPC–transgenic mice, perhaps because of the protective effect of CNP overexpression on the chondrocyte response to inflammatory cytokines. However, further studies are needed. An analog of CNP has been shown to improve skeletal growth in mice harboring a knockin Fgfr3 allele that causes thanatophoric dysplasia in humans (48). It will be interesting to determine whether this analog will be able to improve the cartilage phenotype in K/BxN TCR mice.
CNP overexpression by mouse chondrocytes prevented endochondral growth delay and reduced articular cartilage damage in a mouse model of systemic inflammatory arthritis. The likely mechanism for this effect is a cell-autonomous increase in chondrocyte differentiation, proliferation, hypertrophy, and matrix production, and a cell-autonomous resistance to the growth-suppressive effects of proinflammatory cytokines. These data suggest that the CNP/NPR-B pathway may represent a novel therapeutic target to preserve growth plate and joint cartilage integrity during systemic inflammatory diseases. Although our findings are of particular relevance to JIA, their impact may extend to other forms of inflammatory arthritis and other inflammatory diseases of childhood that stunt longitudinal growth, such as inflammatory bowel disease.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Bükülmez had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Bükülmez, Khan, Haqqi, Warman.
Acquisition of data. Bükülmez, Khan, Bartels, Ortiz-Lopez.
Analysis and interpretation of data. Bükülmez, Khan, Murakami, Sattar, Haqqi, Warman.
Acknowledgments
We thank Diana Mathis, PhD, and Christophe Benoist, PhD (Joslin Diabetes Center/Harvard Medical School) for the generous gift of K/BxN T cell receptor mice. We also thank Yoshihiko Yamada, PhD (National Institute of Dental and Craniofacial Research) for the Col2a1 promoter and enhancer construct pKN185. We gratefully acknowledge Arnold Caplan, PhD for use of the laboratories at the Skeletal Research Center and for discussing study results, providing help with data interpretation, and for critical review of the manuscript. We are also thankful for the generous assistance of Jean F. Welter, MD, PhD for scoring mouse joint cartilage, reviewing data, and helping with the manuscript.
REFERENCES
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–21. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–6. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Ogawa Y, Chusho H, Yasoda A, Tamura N, Komatsu Y, et al. Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology. 2002;143:3604–10. doi: 10.1210/en.2002-220307. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem. 2005;280:14288–92. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, et al. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab. 2006;91:1229–32. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, et al. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat. 2007;28:724–31. doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- Ortoft G, Oxlund H, Jorgensen PH, Andreassen TT. Glucocorticoid treatment or food deprivation counteract the stimulating effect of growth hormone on rat cortical bone strength. Acta Paediatr. 1992;81:912–7. doi: 10.1111/j.1651-2227.1992.tb12134.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S, Jones KL, Wehrenberg WB, Culler FL. Inhibition by prednisone of growth hormone (GH) response to GH-releasing hormone in normal men. J Clin Endocrinol Metab. 1988;67:1258–61. doi: 10.1210/jcem-67-6-1258. [DOI] [PubMed] [Google Scholar]
- White PH. Growth abnormalities in children with juvenile rheumatoid arthritis. Clin Orthop Relat Res. 1990;259:46–50. [PubMed] [Google Scholar]
- Bacon MC, White PH, Raiten DJ, Craft N, Margolis S, Levander OA, et al. Nutritional status and growth in juvenile rheumatoid arthritis. Semin Arthritis Rheum. 1990;20:97–106. doi: 10.1016/0049-0172(90)90022-8. [DOI] [PubMed] [Google Scholar]
- Liem JJ, Rosenberg AM. Growth patterns in juvenile rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:663–8. [PubMed] [Google Scholar]
- MacRae VE, Farquharson C, Ahmed SF. The pathophysiology of the growth plate in juvenile idiopathic arthritis. Rheumatology (Oxford) 2006;45:11–9. doi: 10.1093/rheumatology/kei091. [DOI] [PubMed] [Google Scholar]
- Simon D, Lucidarme N, Prieur AM, Ruiz JC, Czernichow P. Treatment of growth failure in juvenile chronic arthritis. Horm Res. 2002;58(Suppl 1):28–32. doi: 10.1159/000064770. [DOI] [PubMed] [Google Scholar]
- Takahi K, Hashimoto J, Hayashida K, Shi K, Takano H, Tsuboi H, et al. Early closure of growth plate causes poor growth of long bones in collagen-induced arthritis rats. J Musculoskelet Neuronal Interact. 2002;2:344–51. [PubMed] [Google Scholar]
- De Benedetti F, Meazza C, Martini A. Role of interleukin-6 in growth failure: an animal model. Horm Res. 2002;58(Suppl 1):24–7. doi: 10.1159/000064757. [DOI] [PubMed] [Google Scholar]
- Mangialaio S, Ji H, Korganow AS, Kouskoff V, Benoist C, Mathis D. The arthritogenic T cell receptor and its ligand in a model of spontaneous arthritis. Arthritis Rheum. 1999;42:2517–23. doi: 10.1002/1529-0131(199912)42:12<2517::AID-ANR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. A new mouse model of rheumatoid arthritis: organ-specific disease provoked by systemic autoimmunity. Ryumachi. 1997;37:147. [PubMed] [Google Scholar]
- Yamada Y, Miyashita T, Savagner P, Horton W, Brown KS, Abramczuk J, et al. Regulation of the collagen II gene in vitro and in transgenic mice. Ann N Y Acad Sci. 1990;580:81–7. doi: 10.1111/j.1749-6632.1990.tb17920.x. [DOI] [PubMed] [Google Scholar]
- Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, et al. Genetic influences on the end-stage effector phase of arthritis. J Exp Med. 2001;194:321–30. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, et al. Critical roles for interleukin 1 and tumor necrosis factor α in antibody-induced arthritis. J Exp Med. 2002;196:77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach P, Hattori K, Huang H, Hyatt E, Morse J, Nguyen L, et al. The K/BxN mouse model of inflammatory arthritis: theory and practice. Methods Mol Med. 2007;136:269–82. doi: 10.1007/978-1-59745-402-5_20. [DOI] [PubMed] [Google Scholar]
- Monach PA, Mathis D, Benoist C. The K/BxN arthritis model. Curr Protoc Immunol. 2008;15:15.22. doi: 10.1002/0471142735.im1522s81. [DOI] [PubMed] [Google Scholar]
- Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A(Suppl 2):45–57. [PubMed] [Google Scholar]
- Gartland A, Mechler J, Mason-Savas A, MacKay CA, Mailhot G, Marks SC, Jr, et al. In vitro chondrocyte differentiation using costochondral chondrocytes as a source of primary rat chondrocyte cultures: an improved isolation and cryopreservation method. Bone. 2005;37:530–44. doi: 10.1016/j.bone.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Rasheed Z, Akhtar N, Haqqi TM. Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther. 2010;12:R195. doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther. 2004;308:767–73. doi: 10.1124/jpet.103.059220. [DOI] [PubMed] [Google Scholar]
- Singh R, Ahmed S, Malemud CJ, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate selectively inhibits interleukin-1β-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J Orthop Res. 2003;21:102–9. doi: 10.1016/S0736-0266(02)00089-X. [DOI] [PubMed] [Google Scholar]
- Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, et al. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev Biol. 2007;7:18. doi: 10.1186/1471-213X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-α. J Biol Chem. 2000;275:3687–92. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102:14665–70. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti F, Massa M, Pignatti P, Albani S, Novick D, Martini A. Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest. 1994;93:2114–9. doi: 10.1172/JCI117206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangge H, Kenzian H, Gallisti S, Neuwirth G, Liebmann P, Kaulfersch W, et al. Serum cytokines in juvenile rheumatoid arthritis: correlation with conventional inflammation parameters and clinical subtypes. Arthritis Rheum. 1995;38:211–20. doi: 10.1002/art.1780380209. [DOI] [PubMed] [Google Scholar]
- MacRae VE, Farquharson C, Ahmed SF. The restricted potential for recovery of growth plate chondrogenesis and longitudinal bone growth following exposure to pro-inflammatory cytokines. J Endocrinol. 2006;189:319–28. doi: 10.1677/joe.1.06609. [DOI] [PubMed] [Google Scholar]
- Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS, et al. Activation of p38 MAPK is a key step in tumor necrosis factor–mediated inflammatory bone destruction. Arthritis Rheum. 2006;54:463–72. doi: 10.1002/art.21626. [DOI] [PubMed] [Google Scholar]
- Olney RC. C-type natriuretic peptide in growth: a new paradigm. Growth Horm IGF Res. 2006;16(Suppl A):S6–14. doi: 10.1016/j.ghir.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Klooster AR, Bernier SM. Tumor necrosis factor α and epidermal growth factor act additively to inhibit matrix gene expression by chondrocyte. Arthritis Res Ther. 2005;7:R127–38. doi: 10.1186/ar1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchalova K, Krejci P, Wilcox WR. C-natriuretic peptide: an important regulator of cartilage. Mol Genet Metab. 2007;92:210–5. doi: 10.1016/j.ymgme.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A, et al. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci U S A. 2005;102:14452–7. doi: 10.1073/pnas.0504961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S, Itoh H, Komatsu Y, Ogawa Y, Hama N, Yoshimasa T, et al. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells: evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology. 1993;133:3038–41. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Ahluwalia A, Hobbs AJ. C-type natriuretic peptide in vascular physiology and disease. Pharmacol Ther. 2005;105:85–93. doi: 10.1016/j.pharmthera.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kiemer AK, Lehner MD, Hartung T, Vollmar AM. Inhibition of cyclooxygenase-2 by natriuretic peptides. Endocrinology. 2002;143:846–52. doi: 10.1210/endo.143.3.8680. [DOI] [PubMed] [Google Scholar]
- Schachner T, Zou Y, Oberhuber A, Mairinger T, Tzankov A, Laufer G, et al. Perivascular application of C-type natriuretic peptide attenuates neointimal hyperplasia in experimental vein grafts. Eur J Cardiothorac Surg. 2004;25:585–90. doi: 10.1016/j.ejcts.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Lorget F, Kaci N, Peng J, Benoist-Lasselin C, Mugniery E, Oppeneer T, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet. 2012;91:1108–14. doi: 10.1016/j.ajhg.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]