Abstract
Aim
To assess the potential risk of tuberculosis (TB) in patients treated with anti-tumor necrosis factor-alpha (TNF-α) agents in Asia.
Methods
Absolute risk increase (ARI) of TB was estimated for three widely used anti-TNF-α therapies using published standardized incidence ratios (SIR) from the French Research Axed on Tolerance of bIOtherapies registry and incidence (absolute risk [AR]) of TB in Asia. Assuming an association of increased TB risk with anti-TNF-α therapy and country TB AR (incidence), the ARI of TB by country was calculated by multiplying the SIR of the anti-TNF-α therapy by the country's TB AR. The numbers needed to harm (NNH) for each anti-TNF-α agent and numbers needed to treat (NNT) to reduce one TB event using etanercept therapy instead of adalimumab or infliximab were also calculated for each country.
Results
The ARI of TB with anti-TNF-α therapies in Asian countries is substantially higher than Western Europe and North America and the difference between etanercept versus the monoclonal antibodies becomes more evident. The NNH for Asian countries ranged from 8 to 163 for adalimumab, 126 to 2646 for etanercept and 12 to 256 for infliximab. The NNT to reduce one TB event using etanercept instead of adalimumab therapy ranged from 8 to 173, and using etanercept instead of infliximab therapy the NNT ranged from 13 to 283.
Conclusion
Higher numbers of patients are at risk of developing TB with anti-TNF-α therapy in Asia compared with Western Europe and North America. The relative lower risk of TB with etanercept may be particularly relevant for Asia, an endemic area for TB.
Keywords: ankylosing spondylitis, health services and health care economics, psoriatic arthritis, rheumatoid arthritis
Introduction
Therapies directed against tumor necrosis factor-alpha (TNF-α) have significantly advanced the treatment of rheumatoid arthritis (RA), ankylosing spondylitis, Crohn's disease, psoriatic arthritis and psoriasis.1,2 However, increased risks of tuberculosis (TB) and other infections are a major safety concern with anti-TNF-α treatments.3–9 Three widely used anti-TNF-α agents with long-term safety data – etanercept, infliximab and adalimumab – have been shown to increase the risk of developing TB.
Some studies have suggested that the risk for TB reactivation/infection may differ among the anti-TNF-α agents with the monoclonal antibodies (infliximab and adalimumab) having a higher risk of TB than the soluble TNF-α receptor fusion protein (etanercept).7,10–12 Although these differences between the two types of anti-TNF-α agents may not be clinically relevant in Western Europe and North America, this may not be the case in regions such as Asia where TB is endemic.13 The World Health Organization reports the largest number of new TB cases is in Asia, which accounted for 60% of new cases worldwide.14
Data that permit calculations of the risk of TB with anti-TNF-α treatment in Asia and the impact of increased use of anti-TNF-α therapy in this region are limited. Thus this report provides estimates for the potential risk of TB in patients who are candidates for anti-TNF-α therapy in Asia. Estimates were developed for both anti-TNF-α monoclonal antibodies and the soluble TNF-α receptor fusion protein, using data from the Research Axed on Tolerance of bIOtherapies (RATIO) registry in France15 and TB incidence in several Asian countries,13,16 assuming an association of TB incidence with increased risk of TB due to anti-TNF-α therapy. This assumption is based on published reports of several clinical trials in which most cases of TB occurred in endemic regions17–19 and recent data from Japan, Taiwan, the Philippines and Korea related to the risk of TB with anti-TNF-α therapies.8,20–23
Methods
The potential risk of TB with the three most widely used anti-TNF-α agents (adalimumab, etanercept and infliximab) in Asia were estimated using published age and gender standardized incidence ratios (SIR) from the RATIO registry report15 and the absolute risks (AR) or incidence of TB for each country.13,16
The RATIO registry reported the SIRs for individual anti-TNF-α agents as 18.6 (95% confidence interval [CI], 13.4–25.8) for infliximab, 29.3 (95% CI, 20.3–42.4) for adalimumab and 1.8 (95% CI, 0.7–4.3) for etanercept.15 A sensitivity analysis was performed based on the 95% CI of the SIR for each of the anti-TNF-α agents. The deviation of the SIR from 1.00 was tested, assuming a Poisson distribution for the observed cases, given the small number of cases.15
The estimated absolute risk increase (ARI) for each of the anti-TNF-α agents in each Asian country was determined as follows: the SIR of each agent reported in the RATIO registry15 was multiplied by the AR (TB incidence) in that country13,16 Next, the absolute risk increase (ARI) of TB, above that of the general population, due to use of anti-TNF-α agents was calculated by subtracting the AR from the above value for each anti-TNF-α agent in each country (Fig. 1). The number needed to harm (NNH) or produce one TB event was calculated as the reciprocal of the ARI (NNH = 1/ARI).
Figure 1.
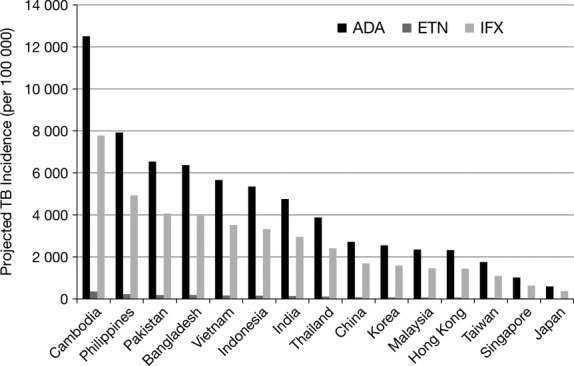
Projected incremental tuberculosis (TB) risks in Asian countries in patients treated with adalimumab, etanercept or infliximab.
A sensitivity analysis was also performed using the 95% CI of SIR for each anti-TNF-α in the RATIO registry report.
The number needed to treat (NNT) to reduce one TB event by using etanercept instead of adalimumab or infliximab was calculated as follows:
The reliability and accuracy of these estimates of TB risk were compared with reported risks for each anti-TNF-α agent in countries where recent data are available (Japan, Taiwan, the Philippines and Korea).8,20–23
Results
Impact of anti-TNF-α therapy on baseline TB risk in Asian countries
Fifteen Asian countries were included in this analysis. According to the World Bank13 and Taiwan TB Control report,16 baseline TB incidence (AR) ranged from a low of 21 per 100 000 in Japan to a high of 442 per 100 000 in Cambodia (Table 1; Fig. 1). Table 1 and Figure 1 show the incremental risk of TB with anti-TNF-α therapy in these 15 Asian countries. The magnitude of increased risk is proportional to the incidence (AR) in each country.
Table 1.
Incidence (AR) and estimated ARI of TB; NNH for adalimumab, etanercept, and infliximab to produce one TB event; and NNT using etanercept instead of adalimumab and infliximab to reduce one TB event
| Country | Incidence/AR (per 100 000) in general population13 | Estimated ARI with use of anti-TNF-α agents (per 100 000) | NNH | NNT using etanercept instead of adalimumab or infliximab | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adalimumab | Etanercept | Infliximab | Adalimumab | Etanercept | Infliximab | Adalimumab | Infliximab | ||
| Cambodia | 442 | 12 951 | 796 | 8221 | 8 | 126 | 12 | 8 | 13 |
| Philippines | 280 | 8204 | 504 | 5208 | 12 | 198 | 19 | 13 | 21 |
| Pakistan | 231 | 6768 | 416 | 4297 | 15 | 241 | 23 | 16 | 26 |
| Bangladesh | 225 | 6593 | 405 | 4185 | 15 | 247 | 24 | 16 | 26 |
| Vietnam | 200 | 5860 | 360 | 3720 | 17 | 278 | 27 | 18 | 30 |
| Indonesia | 189 | 5538 | 340 | 3515 | 18 | 294 | 28 | 19 | 31 |
| India | 168 | 4922 | 302 | 3125 | 20 | 331 | 32 | 22 | 35 |
| Thailand | 137 | 4014 | 247 | 2548 | 25 | 406 | 39 | 27 | 43 |
| China | 96 | 2813 | 173 | 1786 | 36 | 579 | 56 | 38 | 62 |
| Korea | 90 | 2637 | 162 | 1674 | 38 | 617 | 60 | 40 | 66 |
| Malaysia | 83 | 2432 | 149 | 1544 | 41 | 669 | 65 | 44 | 72 |
| Hong Kong | 82 | 2402 | 148 | 1525 | 42 | 678 | 66 | 44 | 73 |
| Taiwan | 62 | 1817 | 112 | 1153 | 55 | 896 | 87 | 59 | 96 |
| Singapore | 36 | 1055 | 65 | 670 | 95 | 1543 | 149 | 101 | 165 |
| Japan | 21 | 615 | 38 | 391 | 163 | 2646 | 256 | 173 | 283 |
AR, absolute risk; ARI, absolute risk increase; TB, tuberculosis; NNH, number needed to harm to produce one TB event; NNT, number needed to treat to reduce one TB event; TNF-α, tumor necrosis factor-alpha.
Number needed to harm
The NNH for each anti-TNF-α agent per country ranges from 8–163 for adalimumab, 126–2646 for etanercept and 12–256 for infliximab, and is proportional to the incidence (AR) of TB in each country (Table 1). In order to determine how sensitive the analysis is to the uncertainties in the value of the parameters, a one-way sensitivity analysis based on the range of the 95% CI for the SIRs was performed. The NNH calculated based on the upper and lower limits of the 95% CI of SIRs ranged from 5–235 for adalimumab, 53–6803 for etanercept and 9–355 for infliximab, which was consistent with the analysis results.
Number needed to treat
Analysis of the NNT by country is presented in Table 1. The NNT to reduce one TB event for each country with etanercept instead of adalimumab ranged from 8–173. The NNT to reduce one TB event per country with etanercept treatment instead of infliximab therapy ranged from 13–283.
Of the countries included in the analysis, Cambodia had the highest TB incidence, the lowest NNH, and the lowest NNT values, whereas Japan had the lowest TB incidence and the highest NNH and NNT values.
Table 2 shows the ARI of TB in patients with RA treated with anti-TNF-α agents in Japan, Korea, Taiwan and the Philippines based on the model and the published actual ARI of TB in these countries.8,20–23 Actual published data also showed a difference in ARI of TB with etanercept versus adalimumab and infliximab.
Table 2.
Estimated and actual ARI of TB in patients with RA or ankylosing spondylitis treated with anti-TNF-α agents in Japan, Korea, Taiwan and the Philippines
| Anti-TNF-α agent | ARI (incidence of TB per 100 000 population with anti-TNF-α agent use) | |||||||
|---|---|---|---|---|---|---|---|---|
| Japan | Korea | Taiwan | Philippines | |||||
| Estimated | Published20 | Estimated | Published8,21 | Estimated | Published22 | Estimated | Published23 | |
| Etanercept | 38 | NR | 162 | 0, 0 | 112 | NR | 504 | NR |
| Infliximab | 391 | 300 | 1674 | 540, 2558 | 1153 | NR | 5208 | 7813 |
| Adalimumab | 615 | NR | 2637 | 490, NR | 1817 | 9302 | 8204 | NR |
ARI, absolute risk increase; TB, tuberculosis; RA, rheumatoid arthritis; TNF-α, tumor necrosis factor alpha; NR, not reported.
Discussion
The high incidence of TB in many Asian countries presents a major public health problem in this region.14 Compounding this issue is the well-established finding that use of anti-TNF-α agents increases the incidence/risk of TB. Published data from registries suggest that the risk of TB may be less with etanercept than with the monoclonal antibodies infliximab and adalimumab.15,24 This difference between etanercept and the monoclonal antibodies may be more substantial and clinically meaningful in Asia where TB is endemic.
Due to the limited amount of real-world published data, this study estimated the increased risk of TB with anti-TNF-α therapy in Asia. Data from clinical trials of multiple anti-TNF-α monoclonal antibodies indicate an association between the risk of developing TB on anti-TNF-α therapy and the incidence of TB in the region. Most cases of TB in these clinical trials occurred in regions with a higher incidence of TB, especially Asia.17–19 Results from the RATIO registry showed an increase in the risk of TB among patients treated with various anti-TNF-α agents compared with the general population, as well as a difference among the various anti-TNF-α agents in this respect. Assuming that the increase in TB incidence/risk (ARI) with use of anti-TNF-α therapy depends on the TB incidence (AR), the ARI for each anti-TNF-α agent for each country can be estimated using the SIRs for each agent reported by Tubach et al. (RATIO registry in France)15 and the known TB incidence (AR) for each country.13,16 The data were then used to determine the NNH and NNT for each anti-TNF-α agent in each Asian country. The NNH, used in conjunction with the NNT, assessed potential adverse effects of an intervention by providing the number of treatments needed to harm a patient.25 The NNT for using etanercept instead of adalimumab or infliximab provided the number of patients that need to be treated in order to prevent one event of TB. Together, NNH and NNT allow physicians to compare different treatment options and outcomes for the same disease, enabling them to translate the results from clinical trials and systemic reviews for use in routine clinical practice.25–27 The NNH due to anti-TNF-α therapy in our analysis was substantially lower in Asian countries (ranging from 8–2646) than previously published values in Western Europe (ranging from 232–6410).15,24 Our study also indicates that the monoclonal antibody treatments adalimumab and infliximab had substantially lower NNH than the soluble TNF-α receptor fusion protein etanercept.
The major differences between the actions of the two classes of drugs in vivo appear to be related to effects on granulomas and infections.28 While the soluble receptor may have high affinity for the TNF-α molecule, the monoclonal antibodies have a higher avidity for transmembrane TNF-α and thus bind TNF-α more tightly.29–32
Our analysis has a number of limitations. Due to the lack of published data in Asia, we estimated the increased risk of TB with anti-TNF-α therapy using data from the French RATIO registry. These data may not be applicable to Asian countries, given the differences in health care standards, co-morbidities and the overall socioeconomic conditions. However, given the higher standards of health care, lower incidence of TB and generally better socioeconomic conditions in France, these analyses may actually underestimate the risk of TB in most regions of Asia. The analyses will increase the awareness of a higher risk of TB in Asia and will encourage more research in this field. Because the RATIO study was conducted in a population with different socioeconomic backgrounds and with different levels of health care, it is possible the ARI for Asian populations may be either under- or overestimated. However, given the much worse socioeconomic conditions and standards of health care, one would expect the actual ARI based on local data would be worse. This is essentially confirmed by comparisons of the limited published data with the estimated ARI. Some actual (but limited) data have recently been reported from Japan, Taiwan, the Philippines and Korea regarding the TB risk associated with infliximab. For example, Japanese post-marketing data showed a < 1% incidence of TB among 5000 RA patients taking infliximab (most of whom had received isoniazid prophylaxis).33 In a 14-week study of 87 Chinese RA patients receiving infliximab, only one developed TB. However, patients with positive tuberculin tests or radiographic evidence of active or occult TB at screening were excluded from this study.34 A literature review on anti-TNF therapy in India revealed a TB reactivation rate of 10.6% in patients with spondyloarthropathy receiving standard doses of infliximab, which was 56 times greater than the incidence in the general Indian population. By contrast, etanercept was reported to cause reactivation tuberculosis in only 5% of RA patients after 1 year of treatment.35 In a Philippine study, TB developed in 5/64 (7.8%) patients with rheumatic disease receiving infliximab (at study enrolment, one of these patients had active TB that was being treated, and four were receiving isoniazid for latent TB).23 The probability of new infection with anti-TNF-α therapy increases where TB is endemic; under- or overestimated data may be influenced by the prophylaxis or surveillance strategies of the specific country. However, the available data are very limited and sporadic and could very well be a function of inadequate sample size. Our estimates do not take into account the administration of only a few, intermittent doses of TNF inhibitors in some Asian countries (since this was not recommended by product labels), or the widespread use of corticosteroids. These factors could confound extrapolation of the French data to Asian countries and might affect the calculation of NNT and NNH.
It should be noted that none of the 69 cases in the RATIO study had been treated with correct chemoprophylaxis against TB before anti-TNF-α therapy was initiated. Over the past few years, there has been greater awareness of this problem, and many countries have developed better screening programs that may eventually reduce the number of TB cases by decreasing the number of reactivations of latent TB. For example, a screening program consisting of a tuberculin skin test, QuantiFERON-TB Gold (QTG) test, standard chest radiograph, and contrast enhanced-computerized tomography of the chest has been recommended in India due to the high prevalence of TB.36 However, such screening procedures will not impact de novo TB infection. The risk of de novo TB is higher in areas with a greater incidence of the disease.19 Thus, patients in Asia treated with TNF inhibitors will remain at a higher risk of contracting a new infection with TB despite the use of screening procedures, and vigilance is needed while patients are being treated with such therapies. Early detection and treatment of TB while on TNF-inhibitor therapy may result in better outcomes for the patient.17,19
The findings of this study provide additional insights about the use of anti-TNF-α therapies in Asian countries where patients are at high risk for TB. The lower risk of developing TB with etanercept relative to adalimumab and infliximab may be more pronounced and more clinically relevant in Asia given the higher risk of TB which is endemic in many of these countries. This issue warrants further evaluation using results from registries and real-world practice data from Asia. The use of anti-TNF-α agents in this area will require health care providers to become familiar with the warnings and precautions on the labels of all such therapies. Guidelines have been developed that emphasize the importance of screening for TB before initiating treatment with biologic agents.37–39
Acknowledgments
Editorial/medical writing support was provided by WC Hatch at ACUMED and funded by Pfizer Inc.
Funding
This research received no specific grant from any funding agency from the public, commercial or not-for-profit sectors.
Author contribution
Authors contributed equally to this work. All authors fulfilled the ICMJE guidelines for authorship criteria.
Competing interests
Sandra Navarra is on the speakers' bureau of Pfizer, Inc., Roche, GlaxoSmithKline/Human Genome Sciences, and Merck Sharp & Dohme. Boxiong Tang and Mahboob Rahman are employees of Pfizer, Inc., and have employment benefits and stock options. Chi Chiu Mok is on the speakers' bureau and has served as an advisor/consultant for Pfizer Inc. Dr. Mok has also attended an advisory board for GlaxoSmithKline. All other authors declare no competing interests.
References
- Wiedmann MW, Mössner J, Baerwald C, Pierer M. TNF alpha inhibition as treatment modality for certain rheumatologic and gastrointestinal diseases. Endocr Metab Immune Disord Drug Targets. 2009;9:295–314. doi: 10.2174/187153009789044347. [DOI] [PubMed] [Google Scholar]
- Silva LC, Ortigosa LC, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2:817–33. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
- Askling J, Fored CM, Brandt L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005;52:1986–92. doi: 10.1002/art.21137. [DOI] [PubMed] [Google Scholar]
- Dixon WG, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–76. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor-α therapy. Arthritis Rheum. 2003;48:3013–22. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Seong SS, Choi CB, Woo JH, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007;34:706–11. [PubMed] [Google Scholar]
- Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50:372–9. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- Mohan AK, Coté TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004;39:295–9. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39:1254–5. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- Incidence of tuberculosis (per 100,000 people). The World Bank Group Web site. [Accessed 23 March 2012.] Available from URL: http://data.worldbank.org/indicator/SH.TBS.INCD.
- Tuberculosis factsheet (No. 104). World Health Organization Web site. [Accessed 23 March 2012.] Available from URL: http://www.who.int/mediacentre/factsheets/fs104/en/
- Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60:1884–94. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwan Tuberculosis Control Report 2009. Center for Disease Control Taiwan Web site. [Accessed 30 April 2012.] Available from URL: http://www.cdc.gov.tw/uploads/files/cd38b0db-50f5–40ff-abfe-fd11902c1f0b.pdf.
- Westhovens R, Yocum D, Han J, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54:1075–86. doi: 10.1002/art.21734. [DOI] [PubMed] [Google Scholar]
- Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:889–94. doi: 10.1136/ard.2005.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia EC, Cush JJ, Matteson EL, et al. Comprehensive tuberculosis screening program in patients with inflammatory arthritides treated with golimumab, a human anti-tumor necrosis factor antibody, in phase III clinical trials. Arthritis Care Res. 2013;65:309–13. doi: 10.1002/acr.21788. [DOI] [PubMed] [Google Scholar]
- Dabbous O, Gilmer K, Tatsuki Y, et al. Tuberculosis in Japanese patients with rheumatoid arthritis treated with infliximab: findings from the post marketing surveillance trial. Ann Rheum Dis. 2007;66(Suppl 2):167. [Google Scholar]
- Kim E, Uhm W, Bae S, Yoo DH, Kim TH. Incidence of tuberculosis among Korean patients with ankylosing spondylitis who are taking tumor necrosis factor blockers. J Rheumatol. 2011;38:2218–23. doi: 10.3899/jrheum.110373. [DOI] [PubMed] [Google Scholar]
- Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 2008;59:800–6. doi: 10.1002/art.23705. [DOI] [PubMed] [Google Scholar]
- Navarra SV, Raso A, Lichauco JJ, Tan PP. Clinical experience with infliximab among Filipino patients with rheumatic diseases. APLAR J Rheumatol. 2006;9:150–6. [Google Scholar]
- Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:522–8. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiri M, Suarez-Almazor M, Wells G, Robinson V, Tugwell P. Number needed to treat (NNT): implication in rheumatology clinical practice. Ann Rheum Dis. 2003;62:316–21. doi: 10.1136/ard.62.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L. Show me the evidence: using number needed to treat. South Med J. 2007;100:881–4. doi: 10.1097/SMJ.0b013e3180f63246. [DOI] [PubMed] [Google Scholar]
- Straus SE. Individualizing treatment decisions. The likelihood of being helped or harmed. Eval Health Prof. 2002;25:210–24. doi: 10.1177/016327870202500206. [DOI] [PubMed] [Google Scholar]
- Kohno T, Tam LT, Stevens S, Louie JS. Binding characteristics of tumor necrosis factor receptor-Fc fusion proteins vs anti-tumor necrosis factor mAbs. J Investig Dermatol Symp Proc. 2007;12:5–8. doi: 10.1038/sj.jidsymp.5650034. [DOI] [PubMed] [Google Scholar]
- Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-α monoclonal antibody cA2 binds recombinant transmembrane TNF-α and activates immune effector functions. Cytokine. 1995;7:251–9. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–26. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- Santora LC, Kaymakcalan Z, Sakorafas P, Krull IS, Grant K. Characterization of noncovalent complexes of recombinant human monoclonal antibody and antigen using cation exchange, size exclusion chromatography, and BIAcore. Anal Biochem. 2001;299:119–29. doi: 10.1006/abio.2001.5380. [DOI] [PubMed] [Google Scholar]
- Siegel SA, Shealy DJ, Nakada MT, et al. The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine. 1995;7:15–25. doi: 10.1006/cyto.1995.1003. [DOI] [PubMed] [Google Scholar]
- Oka H, Nishioka K, Togo M, Ochi T. The efficacy of infliximab for patients with rheumatoid arthritis in Japan: results of 5000 cases by post-marketing surveillance data. APLAR J Rheumatol. 2006;9:142–5. [Google Scholar]
- Zhang FC, Hou Y, Huang F, et al. Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a preliminary study from China. APLAR J Rheumatol. 2006;9:127–30. [Google Scholar]
- Kumar A. Experience with anti-tumor necrosis factor-α therapy in India. APLAR J Rheumatol. 2006;9:136–41. [Google Scholar]
- Malaviya AN, Kapoor S, Garg S, et al. Preventing tuberculosis flare in patients with inflammatory rheumatic diseases receiving tumor necrosis factor-alpha inhibitors in India – An audit report. J Rheumatol. 2009;36:1414–20. doi: 10.3899/jrheum.081042. [DOI] [PubMed] [Google Scholar]
- Lichauco JJ, Tankeh-Torres SA, Navarra SV, Dans LF for the Task Force for TB Screening Prior to Use of Biologic Agents. Philippine guidelines on the screening for tuberculosis prior to the use of biologic agents. APLAR J Rheumatol. 2006;9:184–92. [Google Scholar]
- Mok CC, Tam LS, Chan TH, et al. Management of rheumatoid arthritis: consensus recommendations from the Hong Kong Society of Rheumatology. Clin Rheumatol. 2011;30:303–12. doi: 10.1007/s10067-010-1596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]


