Abstract
Eight-carbon (C8) volatiles, such as 1-octen-3-ol, are ubiquitous among fungi. They are the volatiles critical for aroma and flavor of fungi, and assumed to be signals controlling germination of several fungi. In this study, we found that intact Aspergillus flavus conidia scarcely synthesized C8 volatiles but repeated freeze-thaw treatment that made the cell membrane permeable promoted (R)-1-octen-3-ol formation. Loss or down regulation of any one of five fatty acid oxygenases (PpoA, PpoB, PpoC, PpoD or lipoxygenase) hypothesized contribute to 1-octen-3-ol formation had little impact on production of this volatile. This suggested that none of the oxygenases were directly involved in the formation of 1-octen-3-ol or that compensatory pathways exist in the fungus. Germination of the conidia was markedly inhibited at high density (1.0 × 109spores mL−1). It has been postulated that 1-octen-3-ol is an autoinhibitor suppressing conidia germination at high density. 1-Octen-3-ol at concentration of no less than 10 mM was needed to suppress the germination while the concentration of 1-octen-3-ol in the suspension at 1.0 × 109 mL−1 was under the detection limit (<1 µM). Thus, 1-octen-3-ol was not the principal component responsible for inhibition of germination. Instead, it was evident that the other heat-labile factor(s) suppressed conidial germination.
Keywords: Aspergillus flavus, Conidia, Carbon-eight volatiles, 1-Octen-3-ol, Fatty acid oxygenases, Germination, Spore
Introduction
Eight-carbon (C8) volatiles, such as (R)-(-)-1-octen-3-ol, are found almost ubiquitously among fungi, and they are the characteristic of the fungal aroma (Combet et al., 2006). In fungi, they attract flies and mosquitoes, and in some fungi, they repel fungivory (Combet et al., 2006; Brodhun & Feussner, 2011). Interestingly, 1-octen-3-ol reduces dopamine levels and causes dopamine neuron degeneration in Drosophila melanogaster (Inamdar et al., 2013).
C8 volatiles also cause responses in fungi. Conidiation of Trichoderma spp. was induced by C8 volatiles (Nemcovic et al., 2008). On the contrary, it was postulated that 1-octen-3-ol was a volatile autoinhibitor inhibiting unprofitable germination of Penicillium paneum conidia under harsh conditions, such as in highly crowded environments where competition for limited resources would be expected (Chitarra et al., 2004). The ability of C8 volatiles to regulate conidiation and germination of conidia was also reported with Aspergillus nidulans (Herrero-Garcia et al., 2011) and Lecanicillium fungicola (Berendsen et al., 2013). Because of these findings, it has been assumed that C8 volatiles, especially 1-octen-3-ol, perform signaling functions; however, no conclusive evidence supporting this hypothesis has been provided.
(R)-1-Octen-3-ol was derived from linoleic acid through dioxygenation to form linoleic acid (S)-10-hydroperoxide (HPO) and subsequent cleavage reaction to form (R)-1-octen-3-ol and 10-oxo-(E)-8-decenoic acid in mushrooms (Agaricus bisporus) (Wurzenberger & Grosch, 1984). However, the enzyme(s) involved in this pathway has not been identified yet. A group of fatty acid dioxygenases called Ppos [psi (precocious sexual inducer)-producing oxygenases] has been identified in fungi (Brodhun & Feussner, 2011). Aspergillus nidulans has three Ppos (PpoA, PpoB, and PpoC), and studies on the deletion mutants indicated that all the three Ppo enzymes are involved in Psi factor production (Tsitsigiannis et al., 2005). Recombinant A. nidulans PpoC showed an activity to form (R)-10-HPO of linoleic acid, and a portion of the HPO was further converted into C8 volatiles, such as 2-octen-1-ol, 2-octenal, 3-octanone and 1-octen-3-ol probably through non-enzymatic chemical fragmentation (Brodhun et al., 2010). Many fungi also have another type of fatty acid oxygenase, lipoxygenase (LOX) (Brodhun & Feussner, 2011). Ppos and LOXs are candidates for the enzyme involved in C8 volatile formation in fungi.
In this study, we investigated how C8 volatiles, especially, 1-octen-3-ol, are formed in conidia of A. flavus. A. flavus is an opportunistic pathogen of crops causing highly problematic infections because of aflatoxin contamination of seeds. A. flavus has four genes encoding Ppos, namely, ppoA, ppoB, ppoC, and ppoD, and one gene encoding LOX (Brown et al., 2009). We examined the formation of C8 volatiles in mutant lines of these fatty acid oxygenases. Furthermore, we quantified the amount of 1-octen-3-ol produced by conidia, and found that the endogenous amount was too low to be accountable for inhibition of germination of conidia.
Materials and Methods
Strains and culture conditions
Wild type A. flavus strain, NRRL 3357, was used in this study. The ppo deletion strains, ΔppoA, ΔppoC, and ΔppoD, and the LOX deletion strain, Δlox, prepared by homologous recombination in the pyrG− strain NRRL 3357.5, and the RNAi mutant IRT4, depleted in expression of lox and all 4 ppo genes including ppoB were also used (Brown et al., 2009). A. nidulans (NRRL 1092) was obtained from Japan Collection of Microorganisms at Riken Bioresource Center. All strains were grown at 29 °C on glucose minimal media (GMM) adjusted at pH 6.5 (Shimizu & Keller, 2001) unless otherwise indicated. Conidia suspensions were obtained from surface cultures incubated for 1 week by adding 10 mL of distilled water containing 0.02% (w/v) Tween 20 and by gentle mechanical removal with a sterile glass rod. The suspension was filtered through Miracloth (Calbiochem, La Jolla, CA).
Volatile analysis
For sensitive detection of volatiles a solid phase microextraction (SPME)-GC/MS method was used, while for accurate quantification the volatiles were extracted with organic solvent for GC/MS analysis.
For SPME-GC/MS analysis, fiber coated with 50/30 µm DVB/Carboxen/PDMS Stable Flex (Supelco, Bellefonte, PA) was used. After incubating 300 µL of 1.0 × 109 mL−1 conidial suspension in GMM for 9 h at 29 °C in a glass vial (20 mL), the vial was tightly capped. A portion of vials was kept at −20 °C for 1 h and thawed at 30 °C for 15 min. The fiber was exposed to the headspace of the vial at 22 °C for 30 min. Afterward, the fiber was transferred to an injection port of GC/MS (QP-5050; Shimadzu, Kyoto, Japan) equipped with a 0.25 mm ×30 m Stabiliwax column (Restek, Bellefonte, PA, USA), where compounds were desorbed at 200 °C for 1 min. The column temperature was 40 °C (5 min) to 200 °C (2 min) at 5 °C min−1. The carrier gas (He) was at 1 mL min−1. The mass detector was operated in the electron impact mode with ionization energy of 70 eV. To identify each compound, we used retention indices and MS profiles of corresponding authentic specimens.
For solvent extraction, the conidial suspension (300 µL) was mixed with 2 mL chloroform/methanol (1/2, v/v) containing 5 ng mL−1 nonanyl acetate (as an internal standard). Thereafter, 0.4 mL chloroform and 0.75 mL of 1% (w/v) KCl were added into the mixture, vortexed, and centrifuged at 1000 rpm for 10 min. The organic layer was collected, and directly served to GC-MS analysis under the condition shown above. For resolution of enantiomers of 1-octen-3-ol, Alpha DEX 120 fused silica capillary column (0.25 mm × 30 m, Supelco) was used at a constant column temperature of 75 °C. Racemic 1-octen-3-ol was purchased from Alfa Aesar (Lancashire, UK). Its enantiomers were from Acros Organics (Geel, Belgium). Quantification was done with a calibration curve constructed with pure 1-octen-3-ol in GMM. The detection limit of 1-octen-3-ol was 1 µM with the signal to noise ratio more than 10. Heat inactivation was carried out by immersing the conidial suspension in a tightly sealed tube into boiling water for 10 min.
Germination of conidia
The density of conidia was adjusted to be 1.0 × 106 to 1.0 × 109 mL−1 in GMM containing 0.1% agar. The aqueous solution of 1-octen-3-ol was mixed with the conidial suspension when needed. The suspension (200 µL) was spread on a glass slide (26 × 76 mm), and incubated at 29 °C for 9 h in a humidified closed container. During incubation, the pH of medium did not change substantially. The conidia with the protrusion of a length longer than the diameter of conidia were counted as germinated. The length of hyphae was determined with an ImageJ software (http://rsbweb.nih.gov/ij/). Damaged or dead cells were stained with Evans Blue (Wako Pure Chemicals, Osaka, Japan) (Gaff & Okong’o-Ogola, 1971). The dye [50 mg mL−1 Evans Blue in 10% (w/v) Tween 20, 50 µL] was mixed with the equal volume of conidial suspension, incubated for 30 min, then, diluted four fold with distilled water for observation.
Glucose content
A. flavus conidia were incubated in GMM for 9 h at 29 °C at 1.0 × 109 mL−1, and the medium was cleared with a membrane filter (cellulose acetate 0.5 µm; Advantec, Tokyo, Japan). Glucose content in the medium was determined with a HPLC system equipped with GL-C610H column (10.7 mm i.d. × 300 mm; Hitachi High-Technologies Co., Tokyo, Japan). The mobile phase was water at 0.3 mL min−1, and detection was done with a refractive index detector (L-2490; Hitachi High-Technologies).
Evaluation of an autoinhibiting factor
In order to avoid the effect caused by deprivation of glucose in the medium after incubating conidia in GMM, the glucose content in the cleared medium was adjusted to 1% (w/v) by using the glucose content determined as above. A portion was heat-treated in boiling water for 10 min. Freshly prepared A. flavus conidia were suspended with fresh GMM, with the cleared, spent medium, or with the heat-treated cleared, spent medium at 1.0 × 106 mL−1, and incubated for 9 h at 29 °C as described above to see the germination.
Statistical analysis
Data obtained with at least in triplicate were analyzed using Excel-Toukei 2012 (SSRI, Tokyo, Japan). Germination of conidia was calculated using Kruskal-Wallis one-way ANOVA. Significant differences in their distribution at P < 0.05 are shown with different letters. The amounts of chemicals were calculated using Tukey multiple comparison test. Mean values with different letters are significant at P < 0.05.
Results
Formation of carbon eight volatile compounds from A. flavus conidia
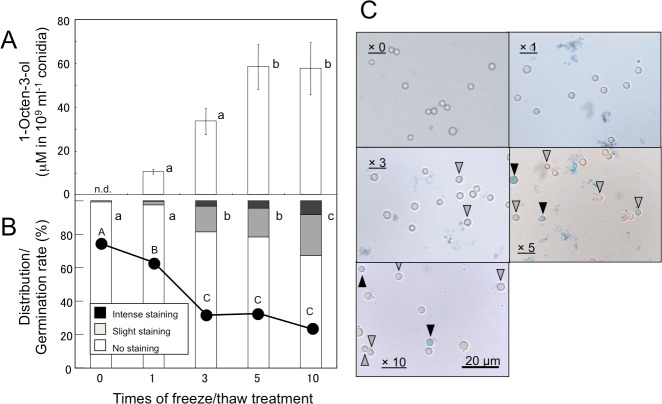
Volatiles were scarcely formed from intact A. flavus (NRRL 3357) conidia suspended in GMM at 1.0 × 109 mL−1 (Fig. 1) and not detected in the headspace of conidiating A. flavus mycelium grown on GMM agar plate. On the contrary, volatiles were released when the suspension of conidia were treated with a freeze-thaw cycle. Among the C8 compounds, 1-octen-3-ol was most abundant, followed by 3-octanone, 2-octen-1-ol, and 1-octen-3-one (Fig. 1). Chiral-phase GC analyses indicated that A. flavus conidia formed (R)-(-)-1-octen-3-ol with an optical purity of >99% enantiomeric excess (Fig. 1, inset). When A. flavus conidia were treated with repeating freeze-thaw cycles, the amount of 1-octen-3-ol positively correlated with increased number of cycles (Fig. 2A). Evans Blue staining of the conidia indicated that the freeze-thaw treatment destroyed the plasma membrane and made them permeable to the pigment (Fig. 2C). The number of Evans Blue-positive cells roughly correlated with numbers of ungerminated cells (Fig. 2B) and with the amount of 1-octen-3-ol (Fig. 2A). Formation of 1-octen-3-ol was completely suppressed when the freeze-thaw treatment was carried out after heat-treatment (in boiling water for 10 min) on conidia.
Figure 1. SPME-GC/MS analysis of volatiles formed from A. flavus conidia.
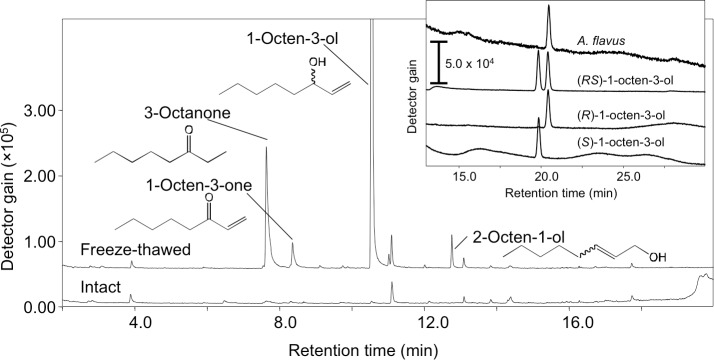
The conidia prepared from 1-week old GMM plates were suspended in GMM liquid medium to be 1.0 × 109 spores mL−1, and immediately (intact) or after one cycle of freeze-thaw treatment (freeze-thawed) the volatiles were collected with SPME fiber at 22 °C, then analyzed with GC-MS. Separation of the enantiomers of 1-octen-3-ol is shown in the inset.
Figure 2. Effect of freeze/thaw treatment on the amount of 1-octen-3-ol and on the integrity of conidia.
(A) Conidia were collected from A. flavus grown for 1-week on GMM plates, and suspended in GMM liquid media to be 1.0 × 109 spores mL−1. They were frozen at −20 °C for 55 min and thawed at 30 °C for 5 min. After given cycles of freeze-thaw treatment, 1-octen-3-ol was extracted from the culture with CHCl3/methanol and analyzed with GC/MS. The values are means of three replicates, and error bars show standard error. Different letters indicate statistically significant differences (P < 0.05) for the cycles determined by ANOVA (Tukey). (B) The numbers of intensely and slightly stained conidia, and not-stained conidia after Evans Blue staining were counted under microscope, and distribution was calculated. Different letters indicate statistically significant differences (P < 0.05, Kruskal-Wallis, n = 300). The freeze-thaw-treated conidia were incubated in GMM for 9 h at 29 °C, and their germination rates were determined under microscope (shown in B with a line chart). Different letters on the symbols indicate statistically significant differences (P < 0.05, Kruskal-Wallis, n = 500). (C) Evans Blue staining was performed with conidia served to freeze-thaw treatment for 0, 1, 3, 5, and 10 cycles. Slightly stained and intensely stained conidia are pointed with open and closed triangles, respectively.
Formation of 1-octen-3-ol from A. flavus mutants
In order to estimate an involvement of each ppo gene and lox gene in 1-octen-3-ol formation, we quantified the volatile formed from conidia of deletion mutants ΔppoA, ΔppoC, ΔppoD, and Δlox (Brown et al., 2009). IRT4 that was deficient in expression of all the five oxygenases (ΔppoD; ppoA, –B, and –C; and lox IRT) (Brown et al., 2009) was also used. As with the wild type spores, substantial amounts of 1-octen-3-ol were only formed after the freeze-thaw treatment of the mutant conidia. The amount of 1-octen-3-ol in each strain showed no significant difference from that in the wild type strain (Table 1). The profiles of the other C8 volatiles detected with SPME-GC/MS were also similar among all strains.
Table 1. The amounts of 1-octen-3-ol formed from the freeze-thaw-treated conidia of ppo and lox mutants.
| Strain | 1-Octen-3-ol (nmol mL−1)a |
|---|---|
| WT | 28.5 ± 6.48 |
| ΔppoA | 39.4 ± 8.74 |
| ΔppoC | 34.1 ± 7.13 |
| ΔppoD | 44.2 ± 13.07 |
| Δlox | 52.1 ± 13.17 |
| IRT4 | 30.7 ± 8.54 |
Notes.
The conidia were suspended with GMM to be 1.0 × 109 ml−1, then, the amount of 1-octen-3-ol was determined after one cycle of freeze-thaw treatment. The mean ± SE is shown (n = 9). There was no statistically significant difference in the amount of 1-octen-3-ol between each strain (ANOVA, Tukey).
Effect of 1-octen-3-ol on germination of conidia
When conidia of A. flavus were suspended in GMM liquid medium at 1.0 × 106 mL−1, more than 90% germinated after 9 h at 29 °C (Fig. 3A). The germination rate decreased dependent on spore density, and at 1.0 × 109 spores mL−1 germination was significantly inhibited. We also examined germination inhibition with A. nidulans under the same experimental condition, and obtained almost the same results (Fig. S1). With both species, vigorous germination of conidia was restored when conidia at 1.0 × 109 mL−1 incubated for 9 h at 29 °C were diluted to 1.0 × 106 mL−1 with fresh GMM.
Figure 3. Germination of conidia and elongation of hyphae were suppressed under high conidial density (A), and in the presence of high concentration of 1-octen-3-ol (B).
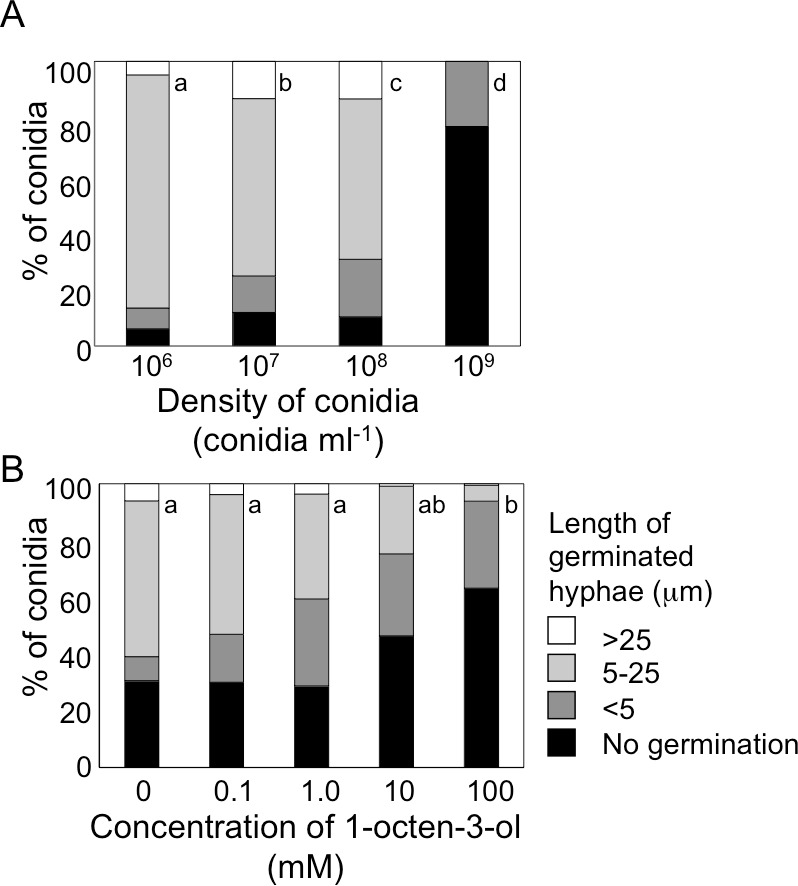
(A) Conidia of A. flavus were prepared from 1-week old GMM plates, then, resuspended in GMM at 1.0 × 106 to 1.0 × 109 spores mL−1. The suspensions were incubated at 29 °C for 9 h, then, the germination rate and the length of hyphae of germinated conidia were examined under microscope. (B) To the conidia set at 1.0 × 106 spores mL−1, 1-octen-3-ol was added. Germination of conidia was examined as above. Different letters indicate statistically significant differences (P < 0.05, Kruskal-Wallis, n = 200).
Next, we added various amounts of 1-octen-3-ol to the conidial suspension set at 1.0 × 106 mL−1. Germination was not affected when the concentration of 1-octen-3-ol was below 1 mM. At 10 mM germination was slightly inhibited, and a marked inhibition was observed at 100 mM (Fig. 3B). The germination of A. nidulans conidia was also inhibited with 1-octen-3-ol at concentration no less than 10 mM (Fig. S1).
Effect of glucose
We noticed that the concentration of glucose in GMM (initially, 1%) decreased to 0.74 and 0.08% after 9 h-incubation of the conidia at the density of 1 × 108 mL−1 and 1 × 109 mL−1, respectively. Therefore, we anticipated that availability of glucose in the medium was accountable for decreased germination. In order to examine this possibility, the conidia were suspended at 2.5 × 105 and 2.5 × 108 mL−1 in GMM containing 0 to 4% glucose, and their germination rate was examined (Fig. 4). Without glucose, the conidia showed poor germination at both the densities, but the germination rate at high density was significantly lower than that at low density. Addition of glucose to the media efficiently promoted germination, and at 2.5 × 105 mL−1 addition of 1% glucose efficiently enhanced the germination rate to more than 95%. At higher density of conidia (2.5 × 108 mL−1), the germination rate increased constantly as increasing the initial concentration of glucose, but the rate was still 40% even with 4% glucose.
Figure 4. Effect of glucose concentration on germination of A. flavus conidia.

The conidia were suspended with GMM liquid medium containing 0–4% glucose. After incubating at 29 °C for 8.5 h, the germination of conidia was examined under microscope. Different letters indicate statistically significant differences among respective density (P < 0.05, Kruskal-Wallis, n = 300).
Autoinhibitor
When A. flavus conidia were suspended with fresh GMM at 1.0 × 106 mL−1, germination of 90% of them was observed after 9 h at 29 °C (Fig. 5). We recovered GMM that had been used to incubate the conidia at 1.0 × 109 mL−1 for 9 h, adjusted its glucose concentration to 1%, and suspended the conidia with the spent medium at 1.0 × 106 mL−1. The germination of conidia was significantly suppressed, and only 40% of them germinated (Fig. 5). When the spent medium was heated with boiling water for 10 min, the germination of conidia recovered, therefore, the factor that suppressed germination was heat-labile.
Figure 5. Effect of a factor secreted from the conidia at high density.
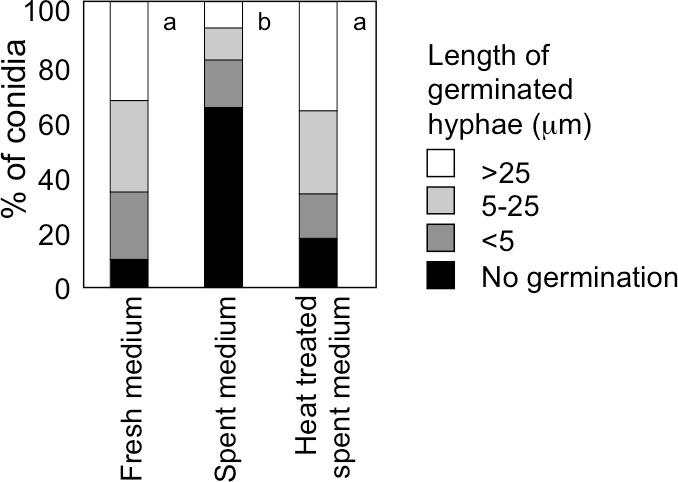
The medium recovered from the conidial suspension incubated for 9 h at 29 °C at the density of 1.0 × 109 mL−1 (spent medium), the used medium after heat treatment at 100 °C for 10 min (heat treated spent medium), or fresh GMM medium was used to suspend conidia at 1.0 × 106 mL−1. Germination of conidia was examined after incubation at 29 °C for 9 h as above. Different letters indicate statistically significant differences (P < 0.05, Kruskal-Wallis, n = 200).
Discussion
We found that disintegration of cellular architecture caused by freeze-thaw treatment elicited formation of 1-octen-3-ol in A. flavus conidia. When sporophores of mushrooms (Agaricus bisporus) were sliced or homogenized, the amount of C8 volatiles increased (Combet et al., 2009). Penicillium roqueforti also formed significant amount of 1-octen-3-ol after homogenization (Kermasha et al., 2002). This suggests that the enzymes are not active in intact tissues/cells of fungi or are lacking accessibility to the substrates (linoleic acid and oxygen), perhaps due to localization of enzyme and substrates in different intracellular compartments. Compartmentalization would be destroyed during loss of cell wall/membrane integrity thus allowing for mixing of enzymes with their substrates or with the factors that might activate the enzymes.
Volatile analysis of oxygenase mutants of A. flavus suggested that none of the five oxygenases found in its genome were directly involved in 1-octen-3-ol formation. A. fumigatus PpoC forms (R)-10-HPO from linoleic acid (Garscha et al., 2007) while (R)-1-octen-3-ol is formed stereospecifically from (S)-10-HPO in mushrooms (Agaricus bisporus) (Wurzenberger & Grosch, 1984). Even though product specificity of A. flavus PpoC has not been identified yet, the discrepancy in stereochemistry further reduces the possibility that PpoC is involved in (R)-1-octen-3-ol formation in A. flavus. This is unexpected because these are the only genes showing substantial homology to the fatty acid oxygenases involved in oxylipin pathways in animal and plant cells. Because the ability to form 1-octen-3-ol after disruption of conidia was abolished by heat-treatment, there must be another enzyme system(s) that is responsible for 1-octen-3-ol formation. It is known that deletion or overexpression of a ppo gene can result in abberant regulation of other ppo genes through feedback regulation. For example, overexpression of ppoA in A. nidulans led to reduced expression of ppoC, and deletion of ppoB increased expression of ppoC (Brown et al., 2009; Tsitsigiannis et al., 2004). Possibly some unknown feedback mechanism had impact on the volatile production as assessed in this study.
When examining what factors could be involved in germination inhibition at high spore densities, we found that availability of glucose was an important factor as addition of glucose could partially remediate the germination defect. We also found that a heat-labile component in the media recovered from A. flavus conidia suspension incubated for 9 h at high density (1.0 × 109 mL−1) was partly accountable for inhibition of germination. However, in our set up, it did not appear that 1-octen-3-ol was contributing to inhibition of germination. The maximum concentration of 1-octen-3-ol–57.6 µM after 10 cycles of freeze-thaw treatment of 1.0 × 109 spores/mL−1–was well under the 10 mM of exogenous 1-octen-3-ol found to inhibit germination. This is also the case with A. nidulans (Fig. S1). From these results, it appears that 1-octen-3-ol is not the principal endogenous autoinhibitor responsible for inhibiting germination at high spore density for either A. flavus or A. nidulans. In previous studies showing 1-octen-3-ol suppression of germination of conidia, its amount formed from the conidia had not been determined (Chitarra et al., 2004; Herrero-Garcia et al., 2011). In order to confirm the role of 1-octen-3-ol in controlling germination of fungal conidia, the amount of 1-octen-3-ol formed by the conidia should be precisely quantified, then, the effect of 1-octen-3-ol at the physiological concentration should be re-evaluated.
In this study, we found that A. flavus conidia synthesized C8 volatiles after disruption of their cell integrity. (R)-1-Octen-3-ol was most abundantly formed volatile. Analyses on mutant strains revealed that fatty acid oxygenases identified in A. flavus so far, namely, ppoA, ppoB, ppoC, ppoD, and lox were not essential to form 1-octen-3-ol. It is suggested that another, heat-labile enzyme, is involved in 1-octen-3-ol biosynthesis. Even though increasing spore density was correlated with germination inhibition for A. flavus, the amount of 1-octen-3-ol formed from the conidia was much lower than that found to inhibit germination of conidia, thus, 1-octen-3-ol is not directly involved in inhibition of germination and the physiological significance of 1-octen-3-ol formation in conidia remains an open question.
Abbreviations
- C8
carbon-eight
- SPME
solid phase microextraction
- Ppo
Psi-factor producing oxygenase
- LOX
lipoxygenase
Supplemental Information
(A) Conidia of A. nidulans were prepared from 1-week old GMM plates, then, resuspended in GMM at 1.0 × 106 to 1.0 × 109 spores mL−1. The suspensions were incubated at 29 °C for 9 h, then, the germination rate and the length of hyphae of germinated conidia were examined under microscope. (B) To the conidia set at 1.0 × 106 spores mL−1, 1-octen-3-ol was added. Germination of conidia was examined as above. Different letters indicate statistically significant differences (P < 0.05, Kruskal-Wallis, n = 200).
Funding Statement
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) [KAKENHI (No. 23580151)] and by the Yamaguchi University (Yobimizu Project) to MK, and in part by NSF IOS-0965649 funds to NPK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Nancy P. Keller is an Academic Editor for PeerJ. The other authors declare there are no competing interests.
Author Contributions
Kana Miyamoto, Tomoko Murakami and Pattana Kakumyan performed the experiments, analyzed the data, prepared figures and/or tables.
Nancy P. Keller analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Kenji Matsui conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
References
- Berendsen et al. (2013).Berendsen RL, Kalkhove SIC, Lugones LG, Baars JJP, Wösten HAB, Bakker PAHM. Effect of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Applied Microbiology and Biotechnology. 2013;97:5535–5543. doi: 10.1007/s00253-013-4793-1. [DOI] [PubMed] [Google Scholar]
- Brodhun & Feussner (2011).Brodhun F, Feussner I. Oxylipins in fungi. FEBS Letters. 2011;278:1047–1063. doi: 10.1111/j.1742-4658.2011.08027.x. [DOI] [PubMed] [Google Scholar]
- Brodhun et al. (2010).Brodhun F, Schneider S, Göbel C, Hornung E, Feussner I. PpoC from Aspergillus nidulans is a fusion protein with only one active haem. Biochemical Journal. 2010;425:553–565. doi: 10.1042/BJ20091096. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2009).Brown SH, Scott JB, Bhaheetharan J, Sharpee WC, Milde L, Wilson RA, Keller NP. Oxygenase coordination is required for morphological transition and the host-fungus interaction of Aspergillus flavus. Molecular Plant-Microbe Interactions. 2009;22:882–894. doi: 10.1094/MPMI-22-7-0882. [DOI] [PubMed] [Google Scholar]
- Chitarra et al. (2004).Chitarra GS, Abee T, Rombouts FM, Posthumus MA, Dijksterhuis J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Applied and Environmental Microbiology. 2004;70:2823–2829. doi: 10.1128/AEM.70.5.2823-2829.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combet et al. (2006).Combet E, Henderson J, Eastwood DC, Burton KS. Eight-carbon volatiles in mushrooms and fungi: properties, analysis, and biosynthesis. Mycoscience. 2006;47:317–326. doi: 10.1007/S10267-006-0318-4. [DOI] [Google Scholar]
- Combet et al. (2009).Combet E, Henderson J, Eastwood DC, Burton KS. Influence of sporophore development, damage, storage, and tissue specificity on the enzymic formation of volatiles in mushrooms (Agaricus bisporus) Journal of Agricultural and Food Chemistry. 2009;57:3709–3717. doi: 10.1021/jf8036209. [DOI] [PubMed] [Google Scholar]
- Gaff & Okong’o-Ogola (1971).Gaff DF, Okong’o-Ogola O. The use of non-permeating pigments for testing the survival of cells. Journal of Experimental Botany. 1971;22:756–758. doi: 10.1093/jxb/22.3.756. [DOI] [Google Scholar]
- Garscha et al. (2007).Garscha U, Jernerén F, Chung D, Keller NP, Hamberg M, Oliw EH. Identification of dioxygenases required for Apergillus development. Studies of products, stereochemistry, and the reaction mechanism. Journal of Biological Chemistry. 2007;282:34707–34718. doi: 10.1074/jbc.M705366200. [DOI] [PubMed] [Google Scholar]
- Herrero-Garcia et al. (2011).Herrero-Garcia E, Garzia A, Cordobés S, Espeso EA, Ugalde U. 8-Carbon oxylipins inhibit germination and growth, and stimulate aerial conidiation in Aspergillus nidulans. Fungal Biology. 2011;115:393–400. doi: 10.1016/j.funbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Inamdar et al. (2013).Inamdar AA, Hossain MM, Bernstein AI, Miller GE, Richardson JR, Bennett JW. Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proceedings of National Academy of Sciences of the United States of America. 2013;110:19561–19566. doi: 10.1073/pnas.1318830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermasha et al. (2002).Kermasha S, Perraud X, Bisakowski B, Husson F. Production of flavor compounds by hydroperoxide lyase from enzymatic extract of Penicillium sp. Journal of Molecular Catalysis B: Enzymatic. 2002;19–20:479–487. doi: 10.1016/S1381-1177(02)00202-3. [DOI] [Google Scholar]
- Nemcovic et al. (2008).Nemcovic M, Jakubikova L, Viden I, Farkas V. Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiology Letters. 2008;284:231–236. doi: 10.1111/j.1574-6968.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- Shimizu & Keller (2001).Shimizu K, Keller NP. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillu nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsigiannis et al. (2004).Tsitsigiannis DI, Kowieski TM, Zarnowski R, Keller NP. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryotic Cell. 2004;3:1398–1411. doi: 10.1128/EC.3.6.1398-1411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsigiannis et al. (2005).Tsitsigiannis DI, Kowieski TM, Zarnowski R, Keller NP. Three putative oxylipin biosynthetic genes integrate sexual and asexual development in Aspergillus nidulans. Microbiology. 2005;151:1809–1821. doi: 10.1099/mic.0.27880-0. [DOI] [PubMed] [Google Scholar]
- Wurzenberger & Grosch (1984).Wurzenberger M, Grosch W. The formation of 1-octen-3-ol from the 10-hydroperoxide isomer of linoleic acid by a hydroperoxide lyase in mushrooms (Psalliota bispora) Biochimica et Biophysica Acta. 1984;794:25–30. doi: 10.1016/0005-2760(84)90293-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Conidia of A. nidulans were prepared from 1-week old GMM plates, then, resuspended in GMM at 1.0 × 106 to 1.0 × 109 spores mL−1. The suspensions were incubated at 29 °C for 9 h, then, the germination rate and the length of hyphae of germinated conidia were examined under microscope. (B) To the conidia set at 1.0 × 106 spores mL−1, 1-octen-3-ol was added. Germination of conidia was examined as above. Different letters indicate statistically significant differences (P < 0.05, Kruskal-Wallis, n = 200).