Abstract
Objective
Enterochromaffin cell-derived serotonin (5-HT) promotes intestinal inflammation. We tested hypotheses that peripheral tryptophan hydroxylase (TPH) inhibitors, administered orally, block 5-HT biosynthesis and deplete 5-HT from enterochromaffin cells sufficiently to ameliorate intestinal inflammation; moreover, peripheral TPH inhibitors fail to enter the murine enteric nervous system (ENS) or central nervous systems and thus do not affect constitutive gastrointestinal motility.
Design
Two peripheral TPH inhibitors, LP-920540 and telotristat etiprate (LX1032; LX1606) were given orally to mice. Effects were measured on 5-HT levels in the gut, blood and brain, 5-HT immunoreactivity in the ENS, gastrointestinal motility and severity of trinitrobenzene sulfonic acid (TNBS)-induced colitis. Quantitation of clinical scores, histological damage and intestinal expression of inflammation-associated cytokines and chemokines with focused microarrays and real-time reverse transcriptase PCR were employed to evaluate the severity of intestinal inflammation.
Results
LP-920540 and LX1032 reduced 5-HT significantly in the gut and blood but not in the brain. Neither LP-920540 nor LX1032 decreased 5-HT immunoreactive neurons or fibres in the myenteric plexus and neither altered total gastrointestinal transit time, colonic motility or gastric emptying in mice. In contrast, oral LP-920540 and LX1032 reduced the severity of TNBS-induced colitis; the expression of 24% of 84 genes encoding inflammation-related cytokines and chemokines was lowered at least fourfold and the reduced expression of 17% was statistically significant.
Conclusions
Observations suggest that that peripheral TPH inhibitors uncouple the positive linkage of enterochromaffin cell-derived 5-HT to intestinal inflammation. Because peripheral TPH inhibitors evidently do not enter the murine ENS, they lack deleterious effects on constitutive intestinal motility in mice.
INTRODUCTION
Inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) are inadequately treated medical problems.1–4 Although IBD is not often lethal, it gives rise to substantial morbidity that is difficult to manage. Anti-inflammatory treatment of IBD, moreover, carries risks, such as increased susceptibility to infection and even malignancy. Like IBD, IBS is not inconsequential; IBS is so prevalent that its societal cost is very high and its negative impact on the quality of life can be severe.2,5–7 There is evidence that intestinal inflammation underlies at least a subset of cases of IBS.8 The pathophysiology of neither IBD nor IBS is adequately understood; however, improved comprehension is likely to improve their treatment.
Intestinal inflammation is necessary to defend the bowel against invasion. The luminal microbiome is symbiotic9 but also constitutes a potential threat, requiring of the intestine a barrier to confine microbiota to the lumen and means to combat organisms that penetrate this barrier.10 These means include inflammatory, motor and secretory responses.
Enterochromaffin cells, which are present in the mucosa of all regions of the gut except the oesophagus, contain most of the body’s serotonin (5-HT).11 Enterochromaffin cells express Toll-like receptors and thus may detect microorganisms.12,13 The 5-HT that enterochromaffin cells secrete can evoke peristaltic14,15 and secretory reflexes16; however, enterochromaffin cell 5-HT also appears to contribute to the initiation of intestinal inflammation. Mice that lack the 5-HT transporter (SERT; SERTKO mice), which inactivates 5-HT, are excessively sensitive to experimentally induced colitis and to the spontaneous colitis that arises as a result of interleukin (IL)-10 deletion.17,18 In contrast, mice that lack the rate-limiting enzyme in enterochromaffin cell 5-HT biosynthesis, tryptophan hydroxylase (TPH) 1, are resistant to experimentally induced colitis.19 Enterochromaffin cell-derived 5-HT, therefore, appears to be a multipurpose paracrine factor that can, in addition to stimulating peristaltic and secretory reflexes, initiate host defence mechanisms that include inflammation. When these mechanisms, or the resulting inflammation, become overly active or dysfunctional, therefore, it might be advantageous to deplete enterochromaffin cells of 5-HT to uncouple the serotonergic drive to inflammation.
5-HT is produced in the central nervous system (CNS) and enteric nervous system (ENS) as well as in the gastrointestinal mucosa. Two different genes encode TPH isoforms, TPH1 and TPH2, which are located, respectively, in enterochromaffin cells and neurons.20,21 Currently available TPH inhibitors do not discriminate between TPH1 and TPH2; moreover, studies of mice that lack TPH2 suggest that inhibition of this isoform would be counterproductive, both in the CNS22 and in the ENS.23 The restriction of TPH1 to the intestinal mucosa (enterochromaffin and, in rats and mice, mast cells), however, suggests that a non-selective TPH inhibitor might be physiologically selective for TPH1 if it were to be given orally and if it were unable to cross the blood–brain or blood–myenteric plexus24 barriers. A compound that cannot enter the brain might also fail to enter the myenteric plexus, to which enteric serotonergic neurons are confined.25,26
We tested hypotheses that the oral administration of a peripheral TPH inhibitor can reduce the concentration of 5-HT in the intestinal mucosa sufficiently to protect the gut from the effects of experimentally induced inflammation without lowering brain 5-HT or depleting 5-HT from the ENS. Because, in contrast to TPH2 deletion, selective TPH1 knockout does not affect constitutive gastrointestinal motility,23,27 we also tested the idea that constitutive gastrointestinal motility remains intact following pharmacological depletion of mucosal, but not neuronal, 5-HT. Results suggest that peripheral TPH inhibitors that selectively deplete enteric 5-HT only from the mucosa do not interfere with constitutive gastrointestinal motility and protect the bowel from inflammation. This effect may underlie the utility that peripheral TPH inhibitors have shown in the treatment of non-constipating IBS28 and may also make them useful in therapy of IBD.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (aged 6–8 weeks) were used at Columbia University and male C57 albino mice (aged ~14 weeks) were employed for independent experiments at Lexicon Pharmaceuticals Inc. Institutional Animal Care and Use Committees of Columbia and Lexicon approved experimental protocols carried out at the corresponding institutions.
Compounds
Two different peripheral TPH inhibitors, LP-92054 and LX1032 (telotristat etiprate), were studied to help verify that observed effects are due to their shared ability to inhibit TPH (see discussion of peripheral TPH inhibitors and details of their use in supplementary data, available online only).
Immunocytochemistry
Tissues were fixed with 4% formaldehyde (from paraformaldehyde) in 0.2 M phosphate buffer (pH 7.4). Laminar preparations containing the longitudinal muscle with attached myenteric plexus (LMMP) were dissected from the intestines of 6–8-week-old mice and examined as whole mounts. Biotinylated mouse monoclonal antibodies to HuC/D (diluted 1/100; Molecular probes, Carlsbad, California, USA) were visualised with streptavidin coupled to Alexa 488 (diluted 1/ 500; Molecular probes, Carlsbad, California, USA). Rabbit polyclonal antibodies to 5-HT (diluted 1/250; Immunostar, Hudson, Wisconsin, USA) were visualised with donkey antibodies coupled to Alexa 594 (diluted 1/500; Molecular probes, Carlsbad, California, USA). Coverslips were mounted with 50% glycerol in 0.5 M bicarbonate buffer (pH 8.6).
Determination of whole gastrointestinal transit time
A 6% solution of carmine (natural red 4; Sigma Aldrich, St Louis, Missouri, USA) was prepared in 0.5% methylcellulose and 0.3 mL/mice were administered intragastrically.29 The time that elapsed between the gavage of carmine and the appearance of the first red fecal pellet was recorded as total gastrointestinal transit time.
Measure of colonic motility
The time required to eject a glass bead (diameter, 3 mm) inserted into the rectum a distance of 2 cm from the anal verge was used to estimate colonic motility.30
Gastric emptying and small intestinal propulsion
A solution containing rhodamine B dextran (100 μL; 10 mg/mL in 2% methylcellulose) was administered by gavage. Animals were killed 15 min after gavage; the stomach, small intestine, caecum and colon were collected in 0.9% NaCl. The small intestine was divided into 10 segments of equal length, and the colon (used to obtain total recovered rhodamine B fluorescence) was divided in half. Each piece of tissue was then homogenised in 4 mL of 0.9% NaCl, centrifuged (2000×g), and the fluorescence of 1 mL aliquots of the supernatant was measured. The proportion of the rhodamine B dextran that emptied from the stomach was calculated; small intestinal transit was estimated from the position of the geometric centre4 of the rhodamine B dextran in the small bowel.
Experimental colitis
Colitis was induced with trinitrobenzene sulfonic acid (100 mg/kg) as previously described31 (see details in supplementary data, available online only). During treatment, a clinical disease activity index was computed daily based on changes in body weight (five-point scale), stool consistency (four-point scale) and blood in stools (three-point scale).
Histological scoring of inflammation
Transverse sections (5 μM) of paraffin-embedded distal colon (3 cm) were stained with haematoxylin and eosin. An expert pathologist, blinded to each animal’s treatment, scored the tissue on an 11-point scale (see supplementary data, available online only, for details of scoring).
Reverse transcriptase PCR
RNA was extracted with Trizol (Invitrogen) and treated with DNase I (1 U/mL). PCR, utilising primers for β-actin, confirmed the absence of DNA contamination. Real-time PCR was employed to quantify transcript abundance. Reverse transcriptase (High Capacity cDNA Archive Kit; Applied Biosystems) was used to convert 1 μg of sample to complementary DNA. Transcript abundance was normalised to that of glyceraldehyde 3-phosphate dehydrogenase (see supplementary data, available online only, for further details).
PCR microarray
Focused PCR microarrays (84 genes encoding key inflammatory cytokines and chemokines; PAMM-011Z SABiosciences; Frederick, Maryland, USA) were used. cDNA samples were mixed with sufficient master mix to be loaded into the wells of 96-well PCR array plates. Microarrays were carried out and plates were read using a TaqMan 7500 PCR machine. A web-based integrated PCR array expression analysis suite provided by SaBiosciences was employed for image analysis and data acquisition. The intensity of signals was normalised to those of glyceraldehyde 3-phosphate dehydrogenase and β-actin.
5-HT in tissue
5-HT was extracted from tissues by homogenising in a buffer containing 300 mM trichloroacetic acid, 100 mM sodium acetate, pH 3.5, 0.01 mM EDTA and 20 mM sodium bisulfate. The lysates were centrifuged and 5-HT in the supernatants was analysed by using reverse-phase high-pressure liquid chromatography, employing a C18 column (mobile phase: 97% of 100 mM sodium acetate, pH 3.5 and 3% acetonitrile) with an in-line fluorescence detector (excitation wavelength=280 nM, emission wavelength=330 nM).
Statistical analyses
Student’s t test and one-way analysis of variance (ANOVA) were used, respectively, to compare single and multiple means. Two-way ANOVA was used to analyse the significance of the contributions to observed variation of time (days) and treatment (peripheral TPH inhibitor-treated versus vehicle-treated) to the clinical course of TNBS-induced colitis.
RESULTS
Peripheral TPH inhibitors selectively decrease gastrointestinal and blood 5-HT concentrations
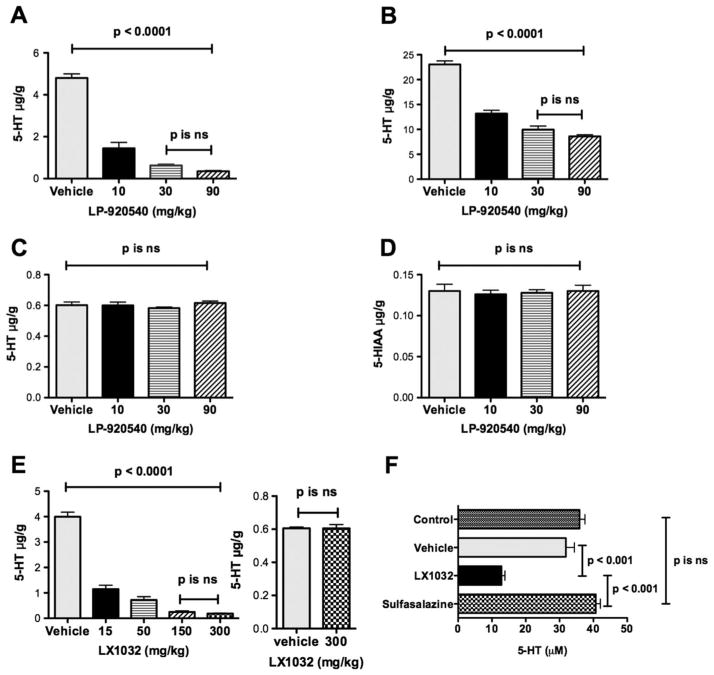
We first verified that orally administered LP-920540 and LX1032 selectively deplete gastrointestinal 5-HT. After the gavage of LP-920540 (five mice at each dose) or vehicle (n=5 mice), 5-HT levels were measured in the jejunum, colon and brain. The 5-hydroxyindoleacetic acid (5-HIAA) level was also quantified in the brain, where, in contrast to the gut,32 5-HIAA is the major 5-HT metabolite. In both the jejunum (figure 1A) and colon (figure 1B), LP-920540 dose-dependently depleted 5-HT; however, 5-HT was more thoroughly depleted in the jejunum than in the colon. In contrast, neither brain 5-HT (figure 1C) nor 5-HIAA (figure 1D) levels were affected significantly by oral LP-920540. The effects of LX1032 were similar to those of LP-920540. Again, after gavage of LX1032 (n=10) small intestinal 5-HT, but not brain 5-HT, was significantly less than that in mice (n=6) receiving vehicle (figure 1E). Both LP-920540 (n=10) and LX1032 (n=10), at their highest dose, achieved a greater than 90% reduction in small intestinal 5-HT. LX1032 and LP-920540, but not sulfasalazine (a non-5-HT-related anti-inflammatory compound; n=10), also reduced blood 5-HT significantly (figure 1F and data not shown).
Figure 1.
Peripheral tryptophan hydroxylase inhibitors deplete serotonin (5-HT) from the gut and blood but not from the brain. (A) Oral LP-920540 depletes 5-HT from the jejunum; the effect is significant and maximal at 30 mg/kg. (B) Oral LP-920540 depletes 5-HT from the colon. (C) Oral LP-920540, in doses equal to those that deplete 5-HT from the small and large intestines (A, B), does not deplete 5-HT from the brain. (D) Oral LP-920540 does not deplete the brain of 5-hydroxyindoleacetic acid (5-HIAA). (E) Oral LX1032 depletes 5-HT from the jejunum but not the brain. The effect of LX1032 on the jejunum is maximal at 150 mg/kg and even 300 mg/kg fails to affect brain 5-HT levels. (F) Oral LX1032 depletes 5-HT from the blood. Neither vehicle nor sulfasalazine affect blood 5-HT.
Peripheral TPH inhibitors do not deplete enteric neuronal 5-HT
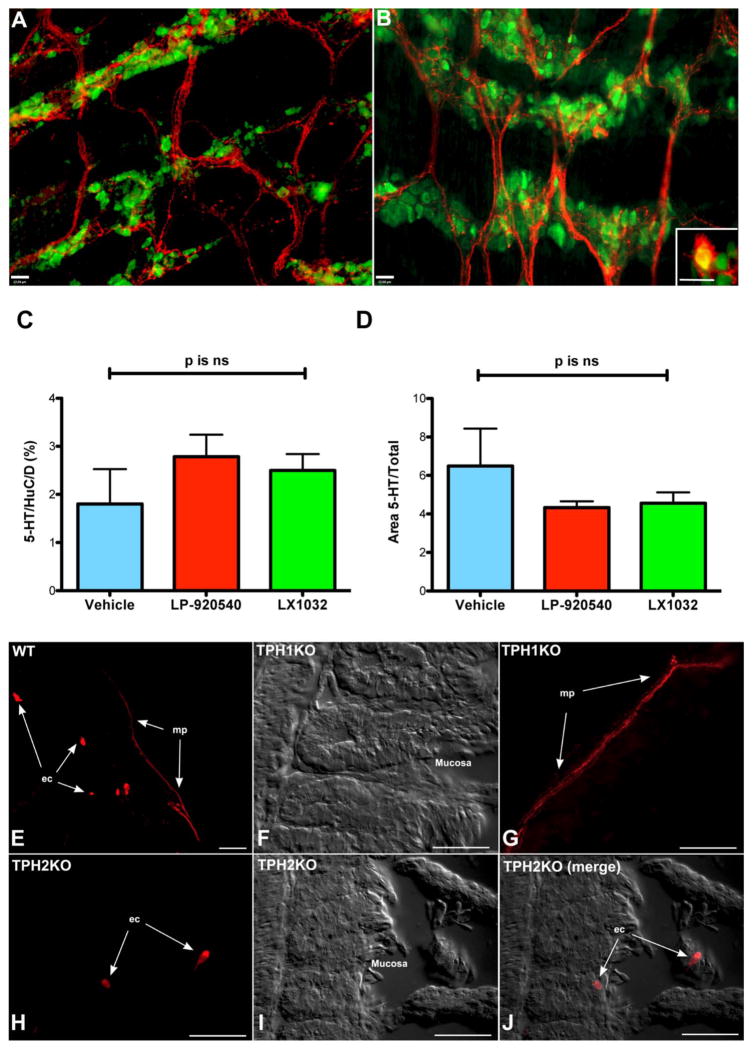
Measurements of 5-HT levels in whole bowel predominantly reflect mucosal 5-HT. Immunocytochemistry was therefore used to examine the 5-HT content of the ENS after gavage of the peripheral TPH inhibitors, LP920540 (n=6), LX1032 (n=6) and LX1033 (the parent compound of LX1032; n=6) or vehicle (n=6). The underlying logic was that 5-HT immunoreactivity in myenteric nerve cell bodies and neurites would decrease and fewer would be at the threshold needed for demonstration if these inhibitors gained access to the TPH2 of enteric serotonergic neurons. Sections from wild-type (WT), TPH1KO, and TPH2KO mice were examined as antibody controls. Because enteric serotonergic neurons express SERT, the uptake of mucosally secreted 5-HT might, in control mice, account for some of their 5-HT content. Peripheral TPH inhibitors, however, deplete mucosal and blood 5-HT (see figure 1). The maintenance of enteric neuronal 5-HT immunoreactivity in treated animals, therefore, would imply that peripheral TPH inhibitors fail to penetrate the blood–myenteric plexus.
Doubly labelled preparations were used to determine the proportion of total nerve cell bodies (HuC/D-immunoreactive) co-stained with antibodies to 5-HT in vehicle and LP-920540-treated mice (figure 2A, B). Because serotonergic neurons (figure 2B inset, C) are a relatively small proportion of the total that project extensively within the ENS, computer-assisted imaging was employed to estimate the proportion of the total area of the myenteric plexus occupied by 5-HT-immunoreactive neurites (figure 2D). The proportion of nerve cell bodies that were 5-HT immunoreactive was not significantly different from control in mice receiving either LP-920540 or LX1032 (figure 2C). The proportion of myenteric area occupied by serotonergic neurites was not significantly different from that of vehicle-treated animals in LP920540 or LX1032-treated mice (figure 2D). Similar results were obtained with mice receiving LX1033 (not illustrated). Antibodies to 5-HT appeared to be specific in that both enterochromaffin cells and myenteric nerve fibres were 5-HT immunoreactive in WT mice (figure 2E); no enterochromaffin cells (figure 2F) but myenteric nerve fibres (figure 2G) were 5-HT immunoreactive in TPH1KO mice; only enterochromaffin cells were 5-HT immunoreactive in TPH2KO mice (figure 2H–J). These observations suggest that peripheral TPH inhibitors, in doses that deplete 5-HT from enterochromaffin cells (see figure 1), fail to affect 5-HT significantly in the myenteric plexus.
Figure 2.
Peripheral tryptophan hydroxylase inhibitors do not deplete enteric neuronal serotonin (5-HT). Neurons and 5-HT were identified immunocytochemically, respectively, with antibodies to HuC/D (green) and 5-HT (red). Vehicle, LP-920540 (100 mg/kg), LX1032 (200 mg/kg) and LX1033 (200 mg/kg) were administered orally. (A) Dissected longitudinal muscle with adherent myenteric plexus (LMMP); vehicle administration. 5-HT-immunoreactive neurites (red) are prominent both within ganglia and in interganglionic connectives. (B) LMMP; LP-920540 administration. The appearance cannot be distinguished from that of the animal given only vehicle (A). Inset: 5-HT-immunoreactive nerve cell body. The neuron (Dogiel type 1 morphology) is doubly immunostained with antibodies to 5-HT and HuC/D (yellow). (C) The 5-HT-immunoreactive proportion of total nerve cell bodies in animals given LP-920540 or LX1032 was not significantly different from that in animals treated with vehicle. (D) The proportion of myenteric plexus area occupied by 5-HT-immunoreactive neurites is not significantly different in mice receiving vehicle, LP-920540 or LX1032. (E) 5-HT immunoreactivity—wild-type (WT) mouse. (F) 5-HT immunoreactivity/interference contrast—TPH1KO mouse (because there is no immunoreactivity, the interference image is shown to identify the field examined). (G) 5-HT immunoreactivity—TPH1KO mouse (serotonergic neurites are immunoreactive. (H–J) 5-HT immunoreactivity—TPH2KO mouse (enterochromaffin cells are labelled but there are no 5-HT immunoreactive nerve fibres. Interference microscopy shows the field examined. (E–J) The bars=50 μm.
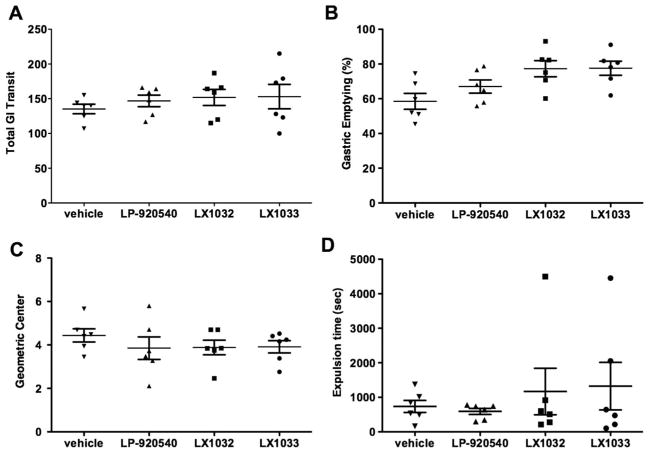
Peripheral TPH inhibitors do not alter constitutive gastrointestinal motility in mice
Total gastrointestinal transit time (figure 3A), gastric emptying (figure 3B), small intestinal transit (figure 3C) and colonic motility (figure 3D) did not differ significantly from vehicle in LP-920540 or LX1032, or LX1033 (the parent compound of LX1032)-treated mice (each group contained six mice). Peripheral TPH inhibitors, therefore, fail to alter constitutive gastrointestinal motility significantly, despite their ability to reduce mucosal levels of 5-HT profoundly. These data are consistent with the previous observation that all of the above measured parameters of gastrointestinal motility in TPH1KO do not differ significantly from those in WT mice.23,27 In TPH2KO mice,23 transit is abnormally slow in the small and large intestines, while gastric emptying is accelerated (although it is not yet clear whether the abnormal motility of TPH2KO mice is due to impaired serotonergic neuro-transmission or ENS abnormalities due to the absence of 5-HT-promoted neurogenesis/survival). The inability of peripheral TPH inhibitors to alter constitutive motility in either the intestine or the stomach is thus consistent with the idea that these compounds do not significantly affect TPH2 in enteric neurons.
Figure 3.
Peripheral tryptophan hydroxylase inhibitors fail to affect measures of constitutive gastrointestinal (GI) motility. Effects of oral LP-920540 (100 mg/kg), LX1032 (200 mg/kg), LX1033 (200 mg/kg) and vehicle. (A) Total gastrointestinal transit time. (B) Gastric emptying (% of administered rhodamine B dextran emptied from the stomach in 15 min). (C) Small intestinal transit (the position of the geometric centre of rhodamine B dextran in the small intestine after 15 min). (D) Colonic motility (time to expel an intrarectal glass bead inserted 2 cm from the anal verge). The errors are broad for LX1032 and LX1033 because of an outlier in each group.
Peripheral TPH inhibitors ameliorate TNBS-induced colitis
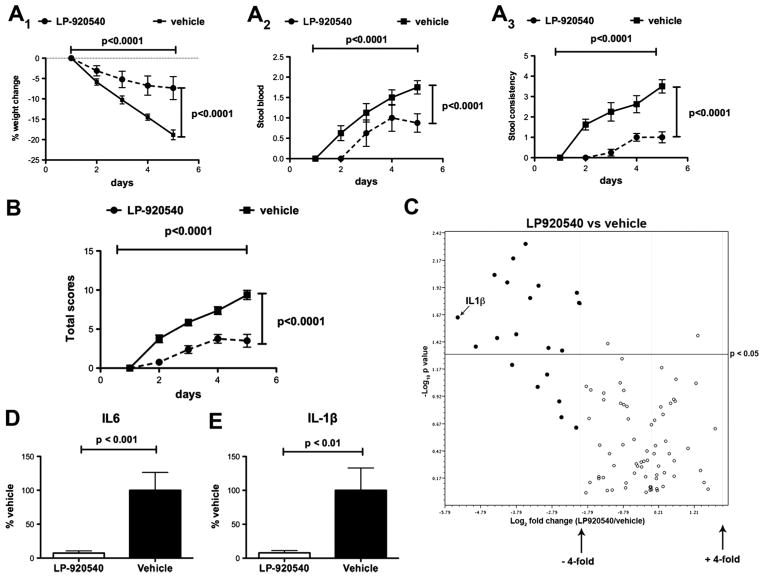
We tested the hypothesis that mucosal 5-HT depletion with LP-920540 or LX1032 confers protection from intestinal inflammation (doses were chosen to be the smallest able to deplete mucosal 5-HT, see figure 1). For LP-920540, a total clinical score was determined consisting of weight loss, stool consistency and the presence or absence of blood in the stool. The clinical condition of the mice was scored daily for 5 days following the administration of TNBS and two-way ANOVA was employed to analyse the effects of time and LP-929540 treatment. The total clinical scores and those of each of its components in six mice treated with LP-920540 was significantly less than in six vehicle-treated control animals (figure 4A1–3, B). The scores of each parameter increased significantly as a function of time after TNBS, but did so to a significantly smaller degree in mice given LP-920540.
Figure 4.
Oral administration of the peripheral tryptophan hydroxylase inhibitor, LP-920540, ameliorates the severity of trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice. The severity of inflammation was estimated from the clinical course and intestinal expression of cytokines. Two-way analysis of variance was used to compare clinical measurements in vehicle and LP-920540-treated mice as a function of time after TNBS administration. Weight loss (A1), stool blood (A2), stool consistency (A3) and the aggregated total clinical scores (B) all increase significantly with time after TNBS in both LP-920540 and vehicle-treated mice; however, the increments in each parameter are significantly greater in mice treated only with vehicle. (C) Expression of transcripts encoding 84 inflammation-associated cytokines and chemokines was quantified with a focused microarray in animals subjected to TNBS-induced colitis. A volcano plot is illustrated displaying statistical significance as the negative log10 of the p value on the y-axis against the log2 of the fold-change of expression on the x-axis. The combination of the p value statistical test with the fold-change in regulation enables genes with either large or small expression changes that are statistically significant to be identified. Values for transcripts corresponding to each of the inflammation-associated transcripts in eight animals treated with LP-920540 (filled circles) were compared to those for the corresponding transcripts from six animals receiving only vehicle (open circles). The boundaries of the fourfold upregulation or downregulation cut-offs are shown as is the cut-off of statistical significance (p<0.05). Transcripts encoding 14 (17%) of the assayed inflammation-associated molecules are both fourfold less abundant in the colon in LP-920540 (filled circles) than in vehicle-treated mice and significantly different. An additional six transcripts in the colons of LP-920540-treated mice were fourfold less abundant than in vehicle-treated colon, but the difference did not reach statistical significance. No transcripts were either fourfold or significantly more abundant in vehicle than in LP-920540-treated mice. The position of interleukin (IL)-1β is indicated (see also E). (D) Abundance of transcripts encoding IL-6 during TNBS-induced colitis (vehicle >LP-920540; not included in array). (E) Abundance of transcripts encoding IL-1β during TNBS-induced colitis (vehicle >LP-920540).
The expression of genes encoding molecules involved in mediating or regulating colonic inflammation was investigated to validate the clinical suggestion that TNBS-induced colitis is less severe in LP-920540 than in vehicle-treated mice (figure 4B). Focused microarrays were used to facilitate the simultaneous study of the expression of 84 inflammatory pathway genes in six vehicle and eight LP-920540-treated mice. After TNBS-induced colitis, transcripts encoding 24% of such transcripts were at least fourfold more abundant in vehicle-treated than in LP-920540-treated animals (figure 4C). The lower abundance of 14 (17%) of these transcripts in LP-920540-treated animals was statistically significant (p<0.05). No transcripts were expressed to a fourfold greater extent in LP-920540 than in vehicle-treated mice. Real-time PCR confirmed these data in six additional vehicle and LP-9205420-treated animals for transcripts encoding IL-6 (figure 4D; not included in the array) and IL-1β (figure 4E; included in the array; see position in figure 4C). Transcripts encoding IL-6 and IL-1β were each significantly more abundant during TNBS-induced colitis in vehicle-treated than in LP-920540-treated mice. These observations support the idea that LP-920540 reduces the severity of TNBS-induced colitis.
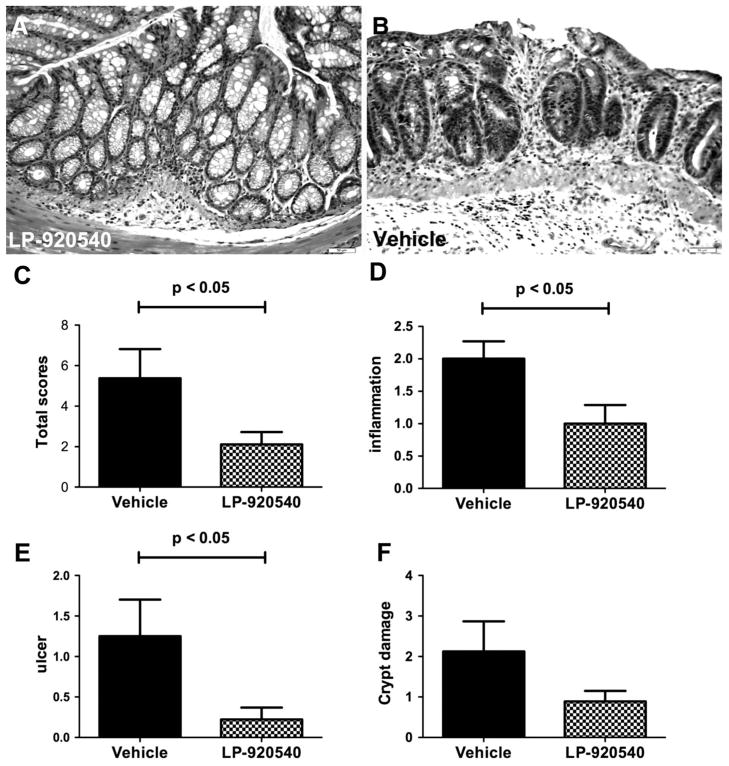
Histological examination of tissue from six vehicle and six LP-920540-treated mice confirmed the protective effect of LP-920540 (figure 5A, B). A total score was compiled (figure 5C), consisting of inflammatory infiltrates (figure 5D), ulceration (figure 5E) and crypt damage (figure 5F). A veterinary pathologist, who was blinded to the identity of the material, scored the preparations. The total histological score (figure 5C) for vehicle-treated mice was 5.4±1.4 while that for animals receiving LP-920540 was 2.1±0.6 (p<0.05). The total histological score for naive mice was 1±0 (n=5), all of which was derived from inflammatory infiltrates.
Figure 5.
Histological examination of tissue from mice subjected to trinitrobenzene sulfonic acid (TNBS)-induced colitis confirms that inflammation is less severe in LP-920540 than in vehicle-treated animals. (A) Colon of a mouse subjected to TNBS-induced colitis and treated with LP-920540. The appearance of the mucosa is relatively normal. (B) Colon of a mouse subjected to TNBS-induced colitis and treated with vehicle. The mucosa is ulcerated and an inflammatory infiltrate can be seen in the lamina propria that extends through the muscularis mucosa into the underlying submucosa. (C) Total histological score (vehicle >LP-920540). (D) Inflammatory infiltrates (vehicle >LP-920540). (E) Degree of ulceration (vehicle >LP-920540). (F) Appearance of crypt damage.
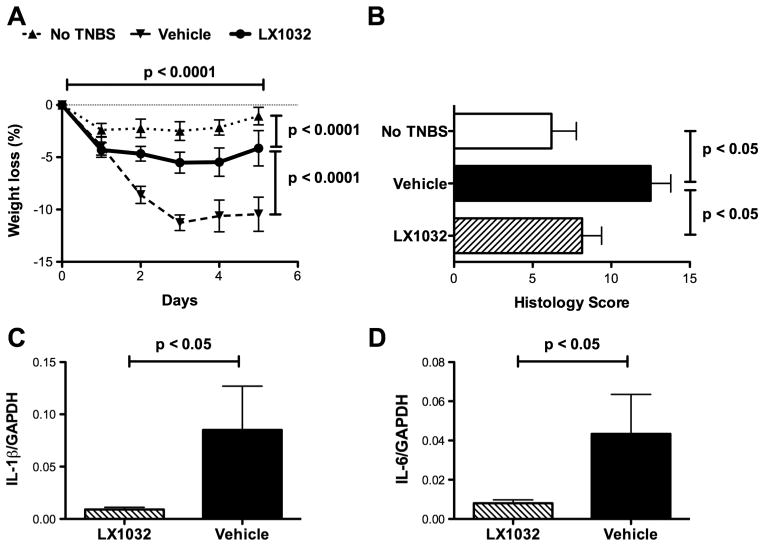
A second peripheral TPH inhibitor, LX1032 (200 mg/kg; a dose that significantly reduced intestinal 5-HT; see figure 1E), was evaluated to determine whether it shared the ability of LP-920540 to inhibit TNBS-induced colitis. Weight loss after the induction of colitis with TNBS was significantly greater in 10 mice treated orally with vehicle than in 10 animals receiving LX1032 (figure 6A). Histological damage in the colons of mice receiving TNBS was scored, summated and normalised to that in five control mice, which received no TNBS. Histological damage scores in mice receiving vehicle (n=5) were significantly higher than in animals receiving LX1032 (figure 6B; n=10). The abundance of transcripts encoding IL-1β (figure 6C) and IL-6 (figure 6D) was lower in 10 mice treated with LX1032 than in five mice given vehicle. Blood neutrophils were elevated in mice subjected to TNBS-induced colitis. The neutrophil count was determined in blood samples drawn 1 day before and 5 days following TNBS administration. No difference in mean counts was found in the blood of control mice (n=5), which did not receive TNBS, but a significant elevation was detected after TNBS in 10 mice treated with vehicle (data not illustrated). In contrast, no significant increase in blood neutrophils was observed in 10 mice treated with LX1032 after the induction of colitis with TNBS; thus, as a result, the blood neutrophil count after TNBS-induced colitis was significantly lower in animals treated with LX1032 than with vehicle (data not illustrated). These observations indicate that LX1032, like LP-920540, provides significant protection to mice from the effects of TNBS-induced colitis.
Figure 6.
LX1032 (200 mg/kg) shares the ability of LP-920540 to alleviate the severity of trinitrobenzene sulfonic acid (TNBS)-induced colitis. (A) Two-way analysis of variance was used to compare the degree of weight loss in naive, vehicle, and LX1032-treated mice as a function of time after the administration of TNBS. The horizontal lines in the legend above the graph depict statistical comparisons between treatments. Weight loss after TNBS in vehicle and LX1032-treated mice is greater than that in control animals given enemas containing vehicle but no TNBS (p<0.0001); however, the time-dependent increment in loss of weight is significantly greater in mice treated with vehicle than with LX1032 (p<0.0001). (B) TNBS-induced histological damage scores are significantly greater in vehicle-treated mice than in control animals not given TNBS. Histological damage following TNBS-induced colitis, however, is greater in vehicle than in LX1032-treated mice. (C) Abundance of transcripts encoding IL-1β during TNBS-induced colitis (vehicle >LX1032). (E) Abundance of transcripts encoding IL-6 during TNBS-induced colitis (vehicle >LX1032). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
DISCUSSION
We tested the hypothesis that oral peripheral TPH inhibitors, which do not cross the blood–brain barrier, protect mice from TNBS-induced colitis. This hypothesis relies on the accessibility of enterochromaffin cells, which contain TPH1, to the compounds. Inhibition of 5-HT biosynthesis in enterochromaffin cells was expected to deplete mucosal 5-HT and thus mimic the protective effect of TPH1KO.19 Peripheral TPH inhibitors do not distinguish TPH1 from TPH2.28,33,34 To avoid adverse effects, therefore, it is necessary that they not gain access to TPH2, which is located in central35,36 and enteric serotonergic neurons.23,37,38 Deletion of TPH2 in the brain is associated with abnormal behaviour,22 while deletion of TPH2 in the ENS is accompanied by deficient gastrointestinal motility and abnormal enteric neuronal development or survival.23 The inability of peripheral TPH inhibitors to cross the blood–brain barrier protects central 5-HT stores; however, the possibility that the blood–myenteric plexus24 might similarly sequester enteric TPH2 and prevent the depletion of enteric neuronal 5-HT had to be verified.
Oral administration of two peripheral TPH inhibitors, LP-920540 and LX1032, were each found to deplete intestinal 5-HT; moreover, neither compound affected brain concentrations of 5-HT or its chief CNS metabolite, 5-HIAA. As would be expected, following the depletion of 5-HT from enterochromaffin cells, which are the primary source of circulating 5-HT,30,39–41 LX1032 also induced a significant fall in blood 5-HT. This effect of LX1032 was not shared with oral sulfasalazine, which alleviates intestinal inflammation. Because mucosal 5-HT dwarfs that in the ENS, mucosal contamination makes enteric neuronal 5-HT difficult to measure chemically; neuronal 5-HT was therefore evaluated immunocytochemically. Neither LP-920540 nor LX1032 reduced the proportion of myenteric 5-HT-immunoreactive nerve cell bodies or the area of myenteric plexus occupied by 5-HT-immunoreactive nerve fibres. Enteric neuronal 5-HT stores are thus maintained even when peripheral TPH inhibitors deplete mucosal 5-HT; therefore, peripheral TPH inhibitors evidently fail to enter the myenteric plexus or inhibit enteric neuronal TPH2. Biosynthesis mediated by the sequestered TPH2 is thus probably more important than the uptake of mucosal 5-HT to maintain 5-HT in enteric serotonergic neurons.
Although 5-HT secretion from enterochromaffin cells stimulates peristaltic reflexes,14,15 this action in mice does not appear to be necessary for constitutive gastrointestinal propulsion. Constitutive motility is not impaired in TPH1KO mice, which lack mucosal 5-HT;23 moreover, colonic migrating motor complexes have been observed even after removal of the mucosa.42 The current observations that neither LP-920540 nor LX1032 significantly affect total gastrointestinal transit time, gastric emptying, small intestinal or colonic motility are thus consistent with the idea that mucosal 5-HT can be depleted in mice without adversely affecting gastrointestinal motility. Because gastrointestinal transit time and gastric emptying are abnormal in TPH2KO mice,23 the observations are also consistent with the idea that peripheral TPH inhibitors fail to enter the murine myenteric plexus, block TPH2, or affect neuronal stores of 5-HT. LX1032, however, relieves the excessive motility seen in patients with carcinoid syndrome,43 implying that LX1032 reaches the systemic circulation and inhibits 5-HT biosynthesis by metastatic symptom-inducing tumour cells.
Both LP-920540 and LX1032 ameliorated the severity of TNBS-induced colitis. Total clinical scores, as well as its components of weight loss, stool blood and stool consistency were all significantly lower in LP-920540-treated mice than in animals treated with vehicle. Focused microarrays also revealed that 24% of a panel of transcripts encoding molecules related to inflammation were at least fourfold more highly expressed in vehicle than in LP-920540-treated mice; moreover, the LP920540-associated reduction in the expression of 17% of these genes was statistically significant. The LP-920540-induced reduction in proinflammatory gene expression was verified in the expression of transcripts encoding IL-6 (not included in the array) and IL Histological examination of the colons of mice treated either with vehicle or LP-920540 verified that LP-920540 protected the colon.
The current observations demonstrate the ability of selective pharmacological depletion of mucosal 5-HT to reduce the severity of TNBS-induced colitis. This action of peripheral TPH inhibitors thus extends results previously obtained with TPH1KO19 and SERTKO17 mice and shows that deletion of mucosal 5-HT reduces the severity of intestinal inflammation, no matter whether it is achieved chronically by the knockout of TPH1, or acutely by spatially restricted pharmacological TPH inhibition. Because of the acute nature of 5-HT depletion following treatment with peripheral TPH inhibitors, the effect is unlikely to be due to an adaptive change, which might potentially occur in a knockout animal in which the deficit in mucosal 5-HT begins in fetal life. The concordance of pharmacological and knockout actions thus provides compelling evidence that enterochromaffin cell-derived 5-HT is not only a proinflammatory mediator, but also a necessary one. The observations further support the concept that enterochromaffin cells function as defensive sensors triggering inflammation when needed. Observations are consistent with the small number of publications that are relevant to mucosal 5-HT in IBD. Particularly in ulcerative colitis, mucosal levels of 5-HT have been found to be reduced,44 transcripts encoding TPH1 and SERT are less abundant,45 and in severe disease the 24-h urinary excretion of 5-HIAA is elevated.46 Secretion of 5-HT in response to IL-1β and lipopolysaccharide is greater in enterochromaffin cells derived from inflamed bowel,12 suggesting that 5-HT could contribute to abnormalities of bowel function in Crohn’s disease. As a result, the inhibition of 5-HT secretion from enterochromaffin cells has been proposed as an alternative therapeutic strategy to ameliorate symptoms in IBD.
These observations suggest that IBD affects enterochromaffin cells, although they cannot distinguish whether the inflammatory process releases 5-HT and interferes both with its biosynthesis and reuptake, or whether 5-HT release contributes to inflammation. In any case, when inflammation is abnormal, as in IBS or IBD, the uncoupling of the proinflammatory drive of mucosal 5-HT from the inflammatory process by means of therapy with peripheral TPH inhibitors is likely to be beneficial. It is also likely to be safe because of the sequestration of TPH2 within the confines of the CNS and ENS, which peripheral TPH inhibitors do not reach.
Supplementary Material
Significance of this study.
What is already known on this subject?
Treatment of disorders of the gut that involve inflammation, such as IBD and IBS, is not adequate.
Mucosal 5-HT is positively coupled to intestinal inflammation, the severity of which is ameliorated in mice that lack TPH1, the rate-limiting enzyme in mucosal 5-HT biosynthesis.
Constitutive gastrointestinal motility is normal in mice that lack TPH1, but abnormal in animals that lack TPH2, the rate-limiting enzyme in neuronal 5-HT biosynthesis.
Peripheral TPH inhibitors have been developed, which reduce 5-HT levels in the gut but not the brain because they do not cross the blood–brain barrier.
What are the new findings?
Oral administration of the peripheral TPH inhibitors, LP-920540 or LX1032, deplete 5-HT in the gut and blood but not the brain.
Oral administration of LP-920540 does not affect enteric neuronal 5-HT and thus LP-920540 probably does not cross the blood–myenteric plexus barrier.
Despite the depletion of mucosal 5-HT, oral administration of neither LP-920540 nor LX1032, affect total gastrointestinal transit time, gastric emptying, small bowel transit, or colonic motility.
Oral administration of the peripheral TPH inhibitors, LP-920540 or LX1032, protects mice against haptene-induced colitis.
How might it impact on clinical practice in the foreseeable future?
When intestinal inflammation contributes to disease, as in IBS or IBD, therapy with a peripheral TPH inhibitor may beneficially remove the proinflammatory drive that mucosal 5-HT provides to the inflammatory process.
Such treatment is likely to lack adverse effects on gastrointestinal motility or mood because peripheral TPH inhibitors do not reach TPH2, which is sequestered within the confines of the ENS and CNS.
Acknowledgments
The authors would like to thank the following groups at Lexicon Pharmacueticals: Medicinal Chemistry for providing the compounds; Metabolism and Cardiology and Pharmaceutical Discovery for compound dose–response studies, DMPK for formulation, DMPK, Molecular Biology and Pathology for help in the analysis of LX1032 IBD biomarkers.
Funding: This study was supported by grants NS12969 and NS15547 from the National Institutes of Health and Lexicon Pharmaceutical Inc and The Irving Institute for Clinical and Translational Research at Columbia University.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/gutjnl-2013-304901).
Contributors: KGM, MDG, QMY and BZ contributed to all phases of the work. KS and ZL contributed to data acquisition. KGJ and TO studied LX1032 effects on inflammation.
Competing interests: None.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement There are no additional unpublished data. The authors commit to assisting any investigators seeking to replicate or follow up these studies with advice and, if needed, reagents.
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Monnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011;45(Suppl):S98–101. doi: 10.1097/MCG.0b013e31821fbf44. [DOI] [PubMed] [Google Scholar]
- 3.Quigley EM, Abdel-Hamid H, Barbara G, et al. A global perspective on irritable bowel syndrome: a consensus statement of the World Gastroenterology Organisation Summit Task Force on Irritable Bowel Syndrome. J Clin Gastroenterol. 2012;46:356–66. doi: 10.1097/MCG.0b013e318247157c. [DOI] [PubMed] [Google Scholar]
- 4.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–17. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 5.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Katzka DA. Genetic epidemiology and pharmacogenetics in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1075–84. doi: 10.1152/ajpgi.00537.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–59. doi: 10.1038/ajg.2011.201. quiz 60. [DOI] [PubMed] [Google Scholar]
- 8.Schmulson M, Chey WD. Abnormal immune regulation and low-grade inflammation in IBS: does one size fit all? Am J Gastroenterol. 2012;107:273–5. doi: 10.1038/ajg.2011.427. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Wang B, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–22. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motility J European Gastrointest Motil Soc. 2012;24:503–12. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erspamer V. Occurrence of indolealkylamines in nature. In: Erspamer V, editor. Handbook of experimental pharmacology: 5-hydroxytryptamine and related indolealkylamines. New York: Springer-Verlag; 1966. pp. 132–81. [Google Scholar]
- 12.Kidd M, Gustafsson BI, Drozdov I, et al. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil. 2009;21:439–50. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogunovic M, Dave SH, Tilstra JS, et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–83. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol (Lond) 1959;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bülbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol. 1958;13:444–57. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke HJ, Sidhu M, Wang Y-Z. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol Mot. 1997;9:181–6. doi: 10.1046/j.1365-2982.1997.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 17.Haub S, Ritze Y, Bergheim I, et al. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil J European Gastrointest Motil Soc. 2010;22:826–34. e229. doi: 10.1111/j.1365-2982.2010.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–95. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 19.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–60. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal TPH1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–30. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 22.Savelieva KV, Zhao S, Pogorelov VM, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershon MD, Bursztajn S. Properties of the enteric nervous system: limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J Comp Neurol. 1978;180:467–88. doi: 10.1002/cne.901800305. [DOI] [PubMed] [Google Scholar]
- 25.Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea pig small intestine. Neuroscience. 1982;7:341–50. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- 26.Costa M, Furness JB, Cuello AC, et al. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience. 1982;7:351–63. doi: 10.1016/0306-4522(82)90272-x. [DOI] [PubMed] [Google Scholar]
- 27.Yadav VK, Balaji S, Suresh PS, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–12. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown PM, Drossman DA, Wood AJ, et al. The tryptophan hydroxylase inhibitor LX1031 is effective for patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–16. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimball ES, Palmer JM, D’Andrea MR, et al. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–73. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- 30.Chen JJ, Zhishan L, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high affinity serotonin transporter (SERT): abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–61. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis KG, Stevanovic K, Karamooz N, et al. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology. 2011;141:588–98. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershon MD, Ross LL. Radioisotopic studies of the binding, exchange, and distribution of 5-hydroxytryptamine synthesized from its radioactive precursor. J Physiol. 1966;186:451–76. doi: 10.1113/jphysiol.1966.sp008046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Yang Q, Sun W, et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 34.Jin H, Cianchetta G, Devasagayaraj A, et al. Substituted 3-(4-(1,3,5-triazin-2-yl)-phenyl)-2-aminopropanoic acids as novel tryptophan hydroxylase inhibitors. Bioorg Med Chem Lett. 2009;19:5229–32. doi: 10.1016/j.bmcl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Gutknecht L, Waider J, Kraft S, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–32. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 36.Gutknecht L, Kriegebaum C, Waider J, et al. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol. 2009;19:266–82. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–86. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross ER, Gershon MD, Margolis KG, et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–17. e2. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humphrey JH, Toh CC. Absorption of serotonin (5-hydroxytryptamine) and histamine by dog platelets. J Physiol. 1954;124:300–4. doi: 10.1113/jphysiol.1954.sp005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrissey JJ, Walker MN, Lovenberg W. The absence of tryptophan hydroxylase activity in blood platelets. Proc Soc Exp Biol Med. 1977;154:496–9. doi: 10.3181/00379727-154-39702. [DOI] [PubMed] [Google Scholar]
- 41.Matondo RB, Punt C, Homberg J, et al. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2009;296:G963–8. doi: 10.1152/ajpgi.90709.2008. [DOI] [PubMed] [Google Scholar]
- 42.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138:659–70. 70 e1–2. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Pappas C, Turnage A, Frazier KS, et al. LX1032: a potential new therapy for carcinoid syndrome (CS) J Clin Oncol. 2009;27(Suppl 1):abstract e14555. [Google Scholar]
- 44.Magro F, Vieira-Coelho MA, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216–24. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- 45.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and IBS. Gastroentrology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Wisniewska-Jarosinska M, Boznanska-Swietaszek P, Harasiuk A, et al. 24-hour urinary 5-hydroxyindole acetic acid excretion in patients with ulcerative colitis. Pol Merkur Lekarski. 2009;26:452–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.