Abstract
Diet is highly linked to breast cancer risk, yet little is known about its influence on mammary epithelial populations with distinct regenerative and hence, tumorigenic potential. To investigate this, we evaluated the relative frequency of lineage-negative CD29hiCD24+, CD29loCD24+ and CD29hiThy1+CD24+ epithelial subpopulations in pre-neoplastic mammary tissue of adult virgin MMTV-Wnt1-transgenic mice fed either control (Casein) or soy-based diets. We found that mammary epithelial cells exposed to soy diet exhibited a lower percentage of CD29hiCD24+Lin− population, decreased ability to form mammospheres in culture, lower mammary outgrowth potential when transplanted into cleared fat pads, and reduced appearance of tumor-initiating CD29hiThy1+CD24+ cells, than in those of control diet-fed mice. Diet had no comparable influence on the percentage of the CD29loCD24+Lin− population. Global gene expression profiling of the CD29hiCD24+subpopulation revealed markedly altered expression of genes important to inflammation, cytokine and chemokine signaling, and proliferation. Soy-fed relative to casein-fed mice showed lower mammary tumor incidence, shorter tumor latency, and reduced systemic levels of estradiol 17-β, progesterone and interleukin-6. Our results provide evidence for the functional impact of diet on specific epithelial subpopulations that may relate to breast cancer risk and suggest that diet-regulated cues can be further explored for breast cancer risk assessment and prevention.
Keywords: Mammary tumorigenesis, CD29hiCD24+ epithelial cells, IL6, MMTV-Wnt1 Tg mice, soy
Introduction
Breast cancer is a heterogeneous disease characterized by at least eighteen histopathological and six distinct molecular subtypes, making it extremely difficult to treat (Sørlie et al., 2001). This phenotypic diversity is considered to reflect the epithelial lineage from which a given breast cancer originates (Sørlie et al., 2001; Lim et al., 2009). Although the molecular understanding of the earliest events contributing to breast cancer remains unclear, it is increasingly apparent that a small population of self-renewing epithelial cells in the mammary glands (designated mammary stem cells; MaSC) can undergo oncogenic transformations, thereby, endowing these transformed cells (designated cancer stem cells, CSC) with tumor-initiating properties and the ability to persist in tumors as a distinct population (Al-Hajj et al., 2003; Stingl et al., 2006; Clarke, 2008; Visvader, 2009). CSC and their normal MaSC counterparts are considered to share regulatory programs for their maintenance and propagation. Signaling pathways mediated by Wnt, Notch, Hedgehog, and phosphatidylinositol 3-kinase/Akt have been identified as critical for growth control of normal MaSC and when de-regulated, may underlie aberrant CSC self-renewal and multi-differentiation (Li et al., 2003; Liu et al., 2006; Farnie & Clarke, 2007; Korkaya et al., 2009). Given recent findings of the dynamic plasticity of normal SC and CSC-like cells (Chaffer et al., 2006); that both luminal and myoepithelial (basal) cells can equally contribute to epithelial regeneration (Van Keymeulen et al., 2011); and that luminal and basal epithelial signatures are tightly maintained by autocrine and paracrine-signaling mechanisms (Balko et al., 2012), the identification of novel factors that alter the expansion and biology of epithelial subpopulations has significant implications for breast cancer prevention and treatment.
Diet and lifestyle are highly modifiable determinants of breast cancer risk. In support of this, clinical and epidemiological investigations have demonstrated an inverse association between high soy food intake and breast cancer susceptibility (Fink et al., 2007; Wu et al., 2008), with the strongest and most consistent association demonstrated when dietary intake occurred during early childhood than at any other life stage (Korde et al., 2009). Nevertheless, the molecular mechanisms underlying the linkage between diet and breast cancer risk remain poorly understood, in part because translating epidemiological data to clinical (human) studies and equating these with experimental evidence from animal models of breast cancer has not been completely straightforward. Previously, we showed that dietary intake of soy-rich foods reduced chemically-induced mammary tumor formation in rodent models, partly through inhibition of Wnt-signaling and upregulation of PTEN expression (Simmen et al., 2005; Dave et al., 2005; Su et al., 2007; Su and Simmen, 2009; Rahal and Simmen, 2010). Using the human breast cancer cell lines MCF-7 and MDA-MB-231 that display a subpopulation of cells with SC-like properties, we further showed that the soy isoflavone genistein can limit the expansion and differentiation potential of this subpopulation, as measured by their decreased ability to form mammospheres when cultured under non-adherent conditions (Montales et al., 2012). Since PTEN and Wnt signaling pathways are functionally linked (Li et al., 2003; Korkaya et al, 2009) and constitute key regulators of mammary SC and CSC biology (Liu et al., 2004; Farnie et al., 2007; Zhou et al., 2007; Korkaya et al., 2009), our findings raise the intriguing possibility that epithelial cells with mammosphere-forming activities, suggestive of regenerative potential, are early in vivo targets of dietary factors for anti-tumor protection.
Little is known about the influence of diet on specific mammary epithelial populations in vivo. To investigate this, we evaluated the relative frequency and functional behavior of distinct epithelial subpopulations in pre-neoplastic (hyperplastic) mammary tissues of MMTV-Wnt1-transgenic mice (Tsukamoto et al., 1988) by using well-characterized cell-surface antigens for fluorescence-activated cell sorting (FACS) and soy-based foods as dietary paradigm. Wnt1-Tg female mice are prone to spontaneous mammary tumors due to SC expansion (Cho et al., 2008; Vaillant et al., 2008). Hyperplastic mammary glands from these mice display increased repopulating activity while resultant mammary tumors show enhanced accumulation of CSC with tumor-initiating ability. We show here that the frequency of CD29hiCD24+Lin− epithelial cells, unlike that of the CD29loCD24+Lin− is reduced in hyperplastic mammary glands of Wnt1-Tg mice exposed to dietary soy protein isolate (SPI) beginning at weaning and throughout lifetime when compared to those of mice fed the control diet. We also show that the reduction of this epithelial subpopulation with dietary SPI exposure is associated with decreased self-renewal capacity and lower outgrowth potential of mammary epithelial cells and reduced mammary tumor incidence. Gene expression profiling of this mammary epithelial subpopulation further revealed a suppressed transcriptional network important to inflammation, cytokine and chemokine signaling, and proliferation. Our results provide evidence for the functional impact of diet on specific epithelial subpopulations that may relate to breast cancer risk and suggest that diet-regulated cues can be further explored for breast cancer risk assessment and prevention.
Materials and methods
Animals and diets
Animal studies were carried out in accordance with approved protocols from the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. Mice were housed in polycarbonate cages under conditions of 24 °C, 40% humidity and a 12-h light, 12-h dark cycle. Wnt1 transgenic (Wnt1-Tg) male mice [B6SJL-Tg(Wnt1)1Hev/J] were obtained from Jackson Laboratories (Bar Harbor, Maine, USA) and mated with wildtype (WT) females of the same strain to generate both WT and Wnt1-Tg offspring. During mating and throughout pregnancy and lactation, dams were fed the American Institute of Nutrition (AIN-93G)-based diet containing casein (CAS) as the sole protein source (Simmen et al., 2005). At weaning [post-natal day (PND) 21], female Wnt1-Tg mice were assigned to one of two semi-purified isocaloric AIN-93 based diets with either CAS or soy protein isolate (SPI; containing 430 mg of total isoflavones/kg diet) as sole protein source. Food and water were provided ad libitum. Spontaneous mammary tumor formation was monitored in Wnt1-Tg mice (n=30 per diet group) by weekly palpation from four weeks of age until 8 months (Fig. 1a). Tumors were measured using a caliper at initial detection, and 2 weeks later, were excised, weighed, and re-measured. Tumor volume was calculated as previously described (Rahal et al., 2013). Mice which did not manifest tumors at age 8 months were euthanized.
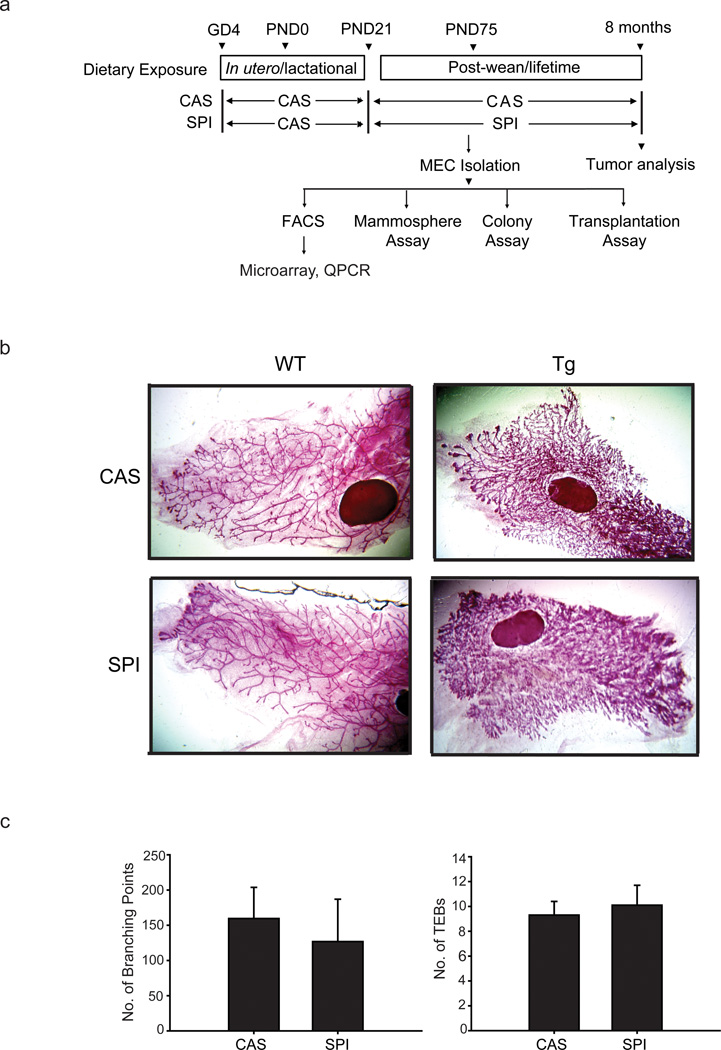
Figure 1.
Description of the animal model and experimental design. (a) Overview of the dietary regimen and subsequent analyses. Wildtype female mice were mated with Wnt1-Transgenic (Tg) males to generate wildtype and Wnt-1 Tg offspring. Female Wnt1-Tg mice at weaning [postnatal day (PND) 21] were randomly assigned to either casein (CAS) or soy protein isolate (SPI) diets and were fed their respective diets thereafter. Mice (n=30 mice per diet group) were followed for mammary tumor appearance (by palpation), and those with tumors were sacrificed two weeks after initial tumor appearance. Mice that did not develop tumors were sacrificed at 8 months of age. A similar set of mice were sacrificed at postnatal day (PND) 75 for the conduct of the various assays shown. Abbreviations used: GD4, gestation day 4; MEC, mammary epithelial cells; FACS, fluorescence-activated cell sorting; QPCR, quantitative real-time polymerase chain reaction. (b) Representative carmine-stained whole mounts of mammary glands (left mammary gland #4) from Wnt1-Tg females and their wildtype (WT) siblings of the two diet groups. Wnt1-Tg and WT mice were fed either CAS or SPI diet beginning at PND21 (weaning) and mammary tissues at PND50 were collected for the analyses, following previously described procedures (Rahal et al., 2013). (c) Mammary ductal branching and terminal end buds (TEBs) were quantified in PND50 Wnt1-Tg mice of the two diet groups. Branching density for each gland was quantified by counting the number of branched points within a box of fixed dimensions. The TEBs located at the leading edge of the fat pad were counted from whole mounts of the left mammary gland #4. Results are mean ± SEM (n=5 and 4 individual mice, respectively for CAS and SPI diets).
Whole mount analyses
Whole mounts of mammary fat pads were prepared and analyzed for branching density and numbers of terminal end buds (TEBs) following previously described procedures (Rahal et al., 2013). Four to five mice were used for each diet group.
Isolation of primary mammary epithelial cells
Primary mammary epithelial cell (MEC) isolation followed previously described procedures (LaMarca et al., 2010). Briefly, MECs were isolated from hyperplastic mammary glands (#3–5; without lymph nodes) of PND75 Wnt1-Tg mice fed either CAS or SPI beginning at weaning (PND21) (Fig. 1a). The glands were mechanically minced until pasty and digested with collagenase A (2mg/ml/0.1g tissue) (Roche Applied Science, Indianapolis, IN) in DMEM/F12 containing 1X antibiotic-antimycotic (ABAM; Invitrogen, Carlsbad, California, USA) at 37 °C for 1 hr in a rotary shaker at 200 rpm. Samples were centrifuged at 600×g for 10 minutes, washed three times in wash buffer [DMEM/F12, 5% fetal bovine serum (FBS), and 1% ABAM (all from Invitrogen)] at 425×g for 2 s and then once in PBS before trypsinization in 0.05% Trypsin/EDTA (Invitrogen) for 20 minutes. Trypsin was diluted in HBSS+ [HBSS (Invitrogen) containing 2% FBS and 10 mmol/L HEPES (Invitrogen)]. Cells were filtered through a 40 µm strainer, centrifuged at 450×g for 5 min, and washed once in HBSS+ prior to use for further analyses (Fig. 1a).
Fluorescence activated cell sorting (FACS)
Freshly isolated MECs were resuspended in HBSS+ (1×107cells/ml) and labeled with selected antibodies or isotype controls (Supplementary Table S1) for 30 min on ice. Cells were then washed in HBSS+ and incubated with streptavidin-APC. After incubation for 20 min on ice, cells were washed once with HBSS+ and then subjected to FACS on a FACS Aria Cell Sorting Flow Cytometer (BD Bioscience, San Jose, California, USA) (Supplementary Figure S2). Dead cells were excluded using 4’,6-diamidino-2-phenylindole (DAPI; 1 µg/ml; Sigma-Aldrich, St. Louis, MO). Cell purity was routinely greater than 95%. Data analysis was performed using FACS Diva software (BD Bioscience).
Mammosphere and colony assays
Freshly isolated MECs from hyperplastic mammary glands of PND75 Wnt1-Tg mice fed either CAS or SPI or FACS-sorted epithelial subpopulations were used for both mammosphere and colony forming assays. Mammosphere culture was performed as previously described (Montales et al., 2012). Cells were grown in non-adherent culture dishes and allowed to form primary mammospheres (P1). After 5 days, primary mammospheres were collected, dissociated into single cells and plated for formation of secondary mammospheres (P2). Mammosphere-forming units (MFUs) were manually counted at day 5 and day 7 of primary and secondary passages, respectively. Matrigel colony forming assay followed previously described protocols (Stingl et al., 2006). Colonies (per 5000 seeded cells) were counted on day 14 of culture. Results shown for isolated MECs are from seven (mammosphere assay) and five (colony assay) independent experiments, with each experiment representing mammary epithelial cells isolated from a different mouse and performed in 8–16 replicates. Mammosphere-formation and clonogenic assays of FACS-sorted cells are from three independent experiments, with each experiment representing an individual mouse and carried out in triplicates.
MEC transplantation assays
MECs isolated from hyperplastic mammary glands of PND75 Wnt1-Tg mice (donor; n=8 each for CAS and SPI groups) were suspended at a concentration of 10,000 cells/10 µl in a 1:1 solution of PBS and Matrigel (BD Bioscience) (LaMarca et al., 2010). For each donor mouse, ten microliters of suspended MECs were injected into cleared fat pads of an individual recipient (PND21) wildtype mouse (CAS-MECs on right side; SPI-MECs on left side) using a 26G needle attached to a 50 µl Hamilton glass syringe (Hamilton Company, Reno, Nevada, USA). Recipient mice (total of 8) were lifetime fed the control CAS diet and sacrificed 8 weeks after transplantation. Left and right mammary glands (#4) for each injected mouse were collected for whole mount analysis as previously described (Rahal et al., 2013).
Microarray analysis
Total RNA was isolated from freshly sorted CD29hiCD24+Lin− epithelial subpopulation of hyperplastic mammary glands (#3–5) of PND75 Wnt1-Tg mice fed either CAS or SPI, using the Arcturus PicoPure RNA isolation kit (Life Technologies Corporation, Carlsbad, California, USA). RNA (100 ng) was amplified using the 3’ IVT Express kit (Affymetrix, Santa Clara, California, USA) prior to hybridization onto Affymetrix 430 2.0 array chips, which contain over 45,000 probes interrogating 34,000 well-characterized mouse genes. Isolated RNAs pooled from 3–4 mice per diet group (CAS or SPI) constituted one biological replicate; two independent biological replicates (representing a total of 6–8 independent RNA samples per diet group) were used for the array analyses. Expression data was analyzed using the GeneSpring GX version 11 software (Agilent Technologies, Santa Clara, California, USA). Processing of the .CEL files was performed using the GeneChip robust multiarray analysis (RMA) with quantile normalization as previously described (Pabona et al., 2012). Additional gene expression analysis was performed using genome set enrichment analysis (GSEA) (Subramanian et al., 2005). Ontology and biological function analyses used the GeneSpring and Affymetrix NetAffx programs; genes with at least 1.3-fold change (P ≤ 0.05) were analyzed using Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Redwood City, California, USA). Data were deposited in Gene Expression Omnibus (GSE37849).
Quantitative real-time PCR (QPCR)
Preparation of cDNAs and QPCR analyses were performed as described (Rahal et al., 2010). Primers (Supplementary Table S3) were synthesized by Integrated DNA Technologies, Inc. (Coralville, Iowa, USA). mRNA expression was calibrated to a standard curve generated using pooled cDNA stocks and normalized to that of TATA-box binding protein (Tbp) or 18s rRNA.
Serum analysis for steroid hormones and IL6
Analyses were carried out using sera collected from PND75 Wnt1-Tg mice assigned for FACS, mammosphere, colony-forming, and transplantation assays (Fig. 1a). Estradiol levels (n=15 mice per diet group) were quantified using an Estradiol Ultrasensitive RIA Kit (Beckman Coulter Inc., Brea, California, USA). IL6 levels (n=13 mice per diet group) were measured using a Mouse IL6 Quantikine ELISA Kit (R&D Systems, Inc., Minneapolis, Minnesota, USA). Progesterone levels (n=8 mouse per diet group) were determined using a Progesterone EIA Kit (Cayman Chemical Company, Ann Arbor, Michigan, USA).
Statistical analysis
Data are presented as mean ± SEM and were compared by t-test or one way ANOVA, using the SigmaStat 3.5 software (SPSS, Chicago, Illinois, USA). Differences in tumor incidence between diet groups were determined by Fisher’s Exact test. P ≤ 0.05 was considered to be statistically significant.
Results
Dietary SPI lowers the relative frequency of the CD29hiCD24+Lin− epithelial subpopulation in hyperplastic mammary glands of Wnt1-Tg mice
Whole mounts of mammary glands from Wnt1-Tg virgin mice (PND50) displayed extensive ductal side-branching and highly developed lobuloalveoli relative to same-aged wildtype (WT) mice and did not visibly differ with CAS and SPI dietary exposure (Fig. 1b). This was supported by the comparable numbers of ductal branching points and terminal end buds in Tg mammary glands of both diet groups (Fig. 1c). Diet did not also alter the MEC yield from these tissues (8.29 ± 1.12×106 vs. 8.03 ± 1.38×106 cells/gram of mammary tissue; CAS vs. SPI, respectively).
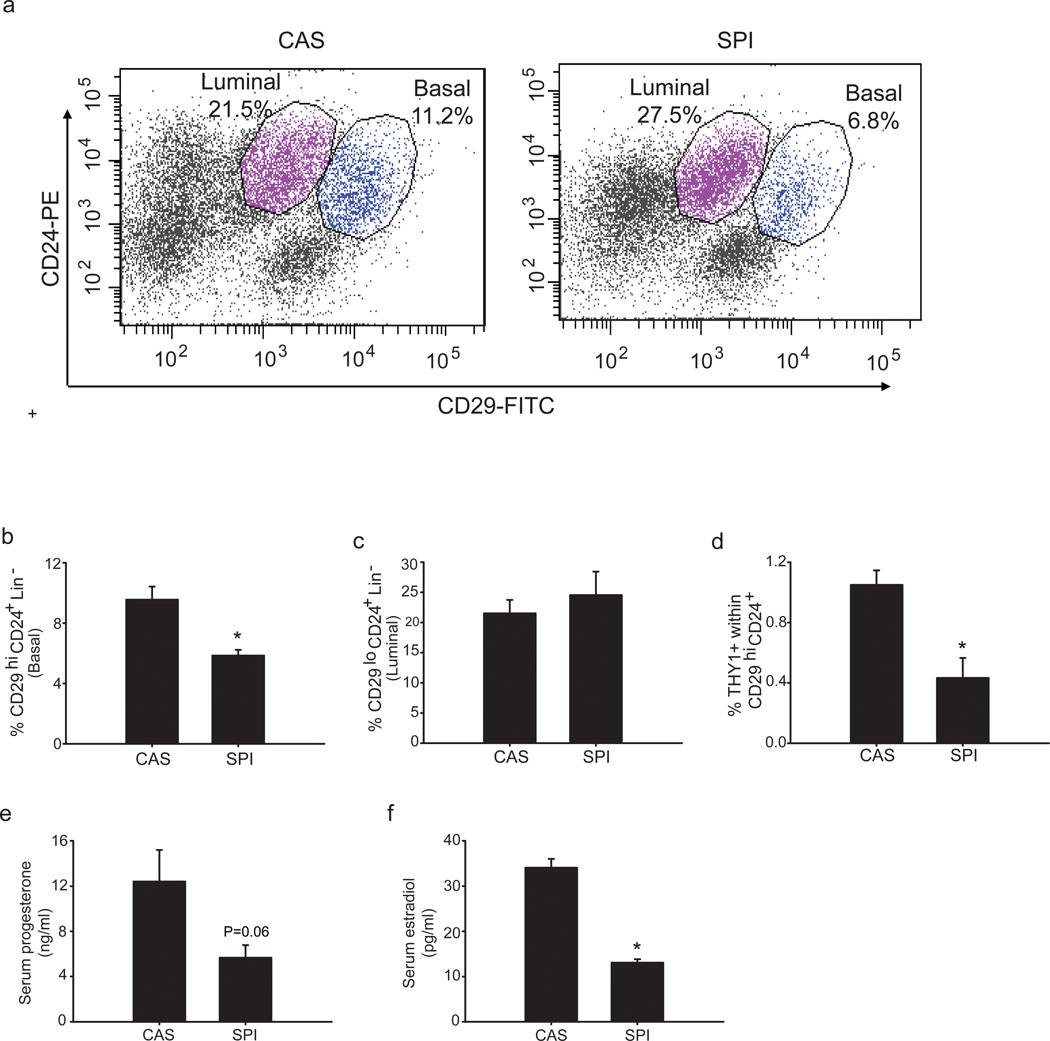
To investigate whether diet can alter the frequency of distinct epithelial subpopulations, we analyzed hyperplastic mammary glands of PND75 Wnt1-Tg virgin mice fed CAS (control) or SPI diets beginning at weaning. Epithelial subpopulations between the two diet groups were quantified by FACS, based on the expression of cell surface antigens CD29 (β1 integrin) and CD24 (heat stable antigen) within the Lineage negative (Lin−) population (CD45−, TER119−, CD31−); these markers have been previously shown to distinguish basal from luminal epithelial cells (Stingl et al., 2006; Clarke, 2008; Visvader, 2009). The basal (MaSC-enriched) subpopulation (CD29hiCD24+) was decreased by ~40% (P = 0.009) in hyperplastic mammary glands of Wnt1-Tg mice fed SPI relative to those fed CAS (Fig. 2a, 2b). By contrast, the luminal (CD29loCD24+) subpopulation did not differ between the dietary groups (Fig. 2a, 2c). Further fractionation of the MaSC-enriched population using the surface marker Thy1, which was shown previously to distinguish the CSC (tumor-initiating) population (CD29hiThy1+CD24+) in Wnt1-Tg mammary tumors (Cho et al., 2008), resulted in the isolation of a very small cell fraction (~1%) from the total bulk of hyperplastic epithelial cells in the CAS-fed group. This cell pool was reduced (by 2-fold; P = 0.012) in epithelial cells isolated from hyperplastic mammary glands of corresponding SPI-fed Wnt1-Tg mice (Fig. 2d). Results demonstrate that SPI diet can alter the relative frequency of distinct epithelial subpopulations in mouse mammary glands overexpressing Wnt1 oncogene.
Figure 2.
Dietary SPI reduces the frequency of the MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation in hyperplastic mammary glands of Wnt1-Tg mice. (a) Representative FACS plot of mammary epithelial cells (MECs) from hyperplastic mammary glands of PND75 Wnt1-Tg mice fed CAS (Left) or SPI (Right). Basal and luminal epithelial sub-populations were identified based on their expression of mouse cell surface markers CD29 and CD24 within the lineage negative (CD45−, TER119−, and CD31−) population after exclusion of dead cells (DAPI+). The gating strategy for FACS analyses is presented in Supplementary Figure S2. (b) SPI diet reduced MaSC-enriched (CD29hiCD24+) epithelial sub-population; * P=0.009. (c) Diet had no effect on luminal (CD29loCD24+) epithelial sub-population. (d) SPI reduced the Thy1+CD24+CD29hi (CSC) sub-population in hyperplastic mammary tissue of Wnt1-Tg mice. * P=0.012 relative to CAS. Data shown (for panels b–d) are from at least 5–7 independent experiments, with each experiment representing pooled MECs isolated from 2–3 mice for each diet group. Serum levels of progesterone (e) and estradiol 17-β (f) of PND75 Wnt1-Tg female mice fed CAS or SPI were quantified as described under Materials and methods. *P=0.001.
Dietary SPI exposure decreases systemic levels of progesterone and estradiol
While both human and mouse MaSCs lack estrogen receptor and progesterone receptor expression, MaSC activity is under hormonal regulation via paracrine signaling from the luminal compartment (Asselin-Labat et al., 2006, 2010; Joshi et al., 2010). To determine if the specific reduction in the frequency of the basal (MaSC) subpopulation by dietary SPI is correlated with systemic changes in the hormonal milieu, serum levels of estradiol-17β and progesterone were measured in PND75 Wnt1-Tg mice that were used as source of hyperplastic mammary glands for FACS analyses. Levels of progesterone were lower (by 2.2-fold) in SPI- than in CAS-fed Wnt1-Tg mice (Fig. 2e). Similarly, serum estradiol-17β concentrations were lower (by 2.6-fold) in SPI- than in the CAS-fed group (Fig. 2f). Thus, dietary SPI effects on the frequency of the basal (CD29hiCD24+) population may involve changes in systemic steroid hormone levels.
Dietary SPI protects against spontaneous mammary tumor formation in Wnt1-Tg mice
To evaluate whether the reduction in the frequency of the basal (CD29hiCD24+Lin−) population within hyperplastic mammary glands can be associated with lower risk for mammary tumors, Wnt1-Tg female mice fed CAS or SPI beginning at weaning and throughout their lifetime were followed for formation of tumors for a period of 8 months (Fig. 1a). The study closure was set a priori at age 8 months to allow for optimal tumor development, given that only ~50% of Wnt-Tg female mice develop mammary tumors by age 5–6 months (Tsukamoto et al., 1988). Approximately one-half (47%) of Wnt1-Tg mice fed SPI (from a total of 30 mice) were tumor-free during the 8 months of the study (Table 1). In contrast, the majority of CAS-fed mice (77%) harboring the Wnt1 transgene developed mammary tumors within this period. Interestingly, the decrease in tumor incidence with dietary SPI was accompanied by shorter tumor latency (SPI vs. CAS: 4.65 vs. 6.01 months, P = 0.015). Tumor growth (within two weeks after initial detection) and tumor weights at sacrifice were similar in both groups (Table 1). Histopathologic features of mammary tumors showed predominance of solid carcinoma (Cardiff et al., 2000) consistent with previous reports (Tsukamoto et al., 1988). While the frequency of solid carcinoma tended to be higher for SPI-fed relative to CAS-fed mice, the increase did not reach statistical significance (Table 1). There was no evidence of metastasis within the two-week post-tumor period. Transcript levels of Wnt1 in mammary tumors did not differ between diet groups and there was no evidence of increased Wnt activity as measured by tumor c-Myc expression (data not shown). Data indicate that the reduced frequency of the basal (CD29hiCD24+Lin−) epithelial pool in hyperplastic mammary glands with dietary SPI (Fig. 2) may relate to lower incidence (risk) of full-blown mammary tumor formation at a later age.
Table 1.
Mammary Tumor Parameters for Wnt1-Tg Mice fed CAS or SPI.
| Diet | Mice (n) | % Incidencea | Latencyb | Tumor growthc | Tumor weightd | % Solid Carcinomag |
|---|---|---|---|---|---|---|
| CAS | 30 | 77 | 6.01±0.33 | 45.38±9.14 | 0.0033±0.0001 | 50 |
| SPI | 30 | 47e | 4.65±0.40f | 46.96±7.81 | 0.0035±0.0001 | 70h |
Incidence measured at 8 months of age.
Mean tumor onset (months) = age of initial tumor appearance.
Tumor growth was measured within two weeks after initial tumor detection.
Tumor weight normalized to body weight.
P = 0.033 (Fisher's exact test; two-tailed).
P = 0.015 (t-test).
n=20/group.
P = 0.17 (Fisher's exact test; one-tailed).
MECs from SPI-fed Wnt1-Tg mice exhibit lower repopulating activity and mammosphere-forming efficiency
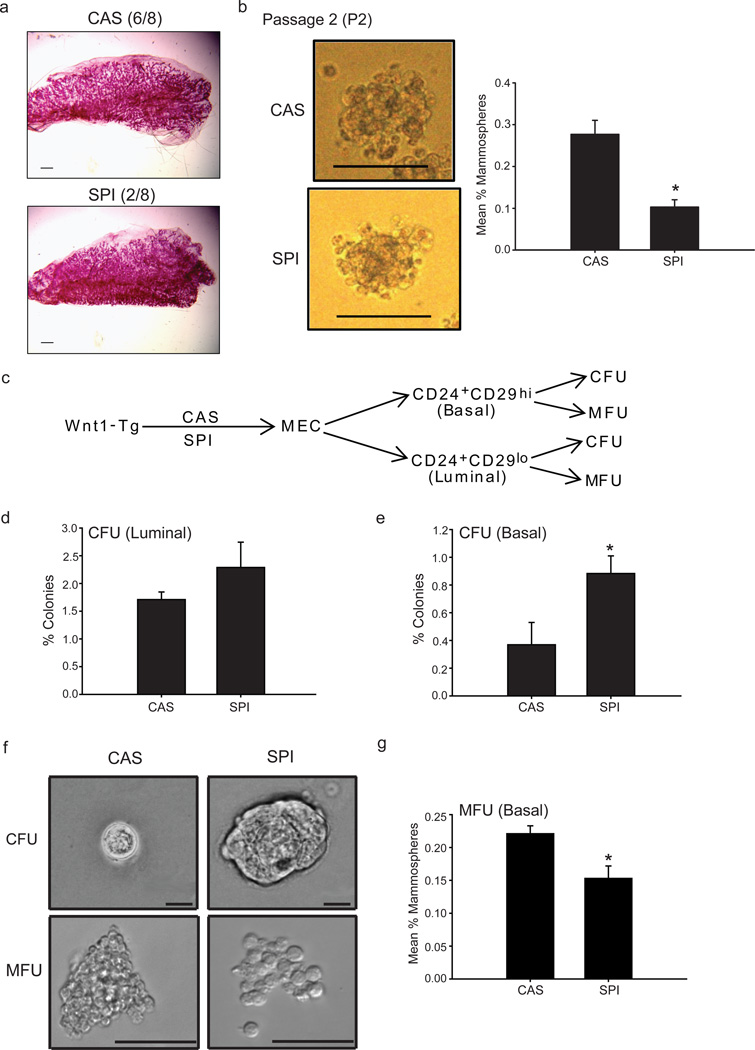
Transplantation of MECs into the cleared fat pads of recipient mice results in the reconstitution of a functional mammary gland, reflecting regenerative/renewal activity (Shackleton et al., 2006; Visvader, 2009). Previous studies showed that MECs from hyperplastic Wnt1-Tg mammary tissues exhibited high in vivo repopulating activity upon transplantation; this was attributed to an increase in the size of the MaSC population in Wnt1-Tg mammary glands (Liu et al., 2004). We determined whether SPI relative to CAS diet, affected mammary gland repopulation in vivo to further support the FACS results (Fig. 2). Isolated MECs from hyperplastic mammary glands of PND75 Wnt1-Tg mice fed either CAS or SPI beginning at weaning were transplanted (10,000 cells per injection) into three-week old syngeneic wildtype recipient mice fed CAS diet throughout the study. To preclude host effects in the analyses, each recipient mouse served as its own control, with right and left cleared mammary fat pads injected, respectively, with MECs isolated from PND75 Wnt1-Tg mice fed CAS- or SPI. Hyperplastic MECs from both CAS- and SPI-fed Wnt1-Tg mice showed the ability to generate a complete mammary epithelial tree (90–100% fat pad filled) 8 weeks after transplantation into cleared fat pads of wildtype recipient mice (Fig. 3a). However, while 6 of 8 MEC transplants from Wnt-Tg CAS-fed mice generated outgrowths in recipient mice, only 2 of 8 outgrowths were observed from MECs of corresponding SPI-fed mice. These findings indicate that dietary SPI exposure limits the repopulating activity of the MaSC-enriched (CD29hiCD24+Lin−) epithelial subpopulation within hyperplastic mammary glands.
Figure 3.
MECs from SPI-fed Wnt1-Tg mice have lower repopulating activity and mammosphere-forming ability. (a) Whole mounts depicting mammary outgrowths from transplantation of freshly isolated MECs (10,000 cells) from hyperplastic mammary glands of Wnt1-Tg mice fed CAS (6/8 outgrowths) or SPI (2/8 outgrowths). Scale bar, 1000 µm. Eight independent experiments were conducted, with each experiment representing an individual mouse for each donor and recipient. (b) MECs from SPI-fed Wnt1-Tg mice displayed lower ability to form mammospheres (from secondary passage, P2), quantified as mammosphere-forming units (MFU). Representative mammospheres are shown at day 7 of secondary passage (P2). Scale bar, 100 µm. Data represent the mean % MFUs ± SEM of seven independent experiments, where each experiment utilized mammary epithelial cells isolated from a different mouse per diet group and performed in 8–16 replicates. * P≤ 0.001 relative to CAS. (c) Schematic diagram for in vitro analysis of FACS-sorted MECs from CAS- and SPI-fed Wnt1-Tg mice. Basal (CD29hiCD24+) and luminal (CD29loCD24+) epithelial subpopulations were subjected to colony-forming and mammosphere-forming assays. (d) Diet did not influence the colony-forming ability of luminal epithelial subpopulations. Data (mean ± SEM) are from three independent experiments, with each experiment representing an individual mouse and carried out in triplicates. (e) Dietary exposure to SPI enhanced the clonogenic activity of MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation. Data (mean ± SEM) are from three independent experiments, with each experiment representing an individual mouse and carried out in triplicates. *P≤0.05 by t-test. (f) Representative colonies formed in matrigel (CFU) and mammospheres formed under non-adherent conditions (MFU) for FACS-sorted MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation from CAS-and SPI-fed Wnt1-Tg mice are shown. Scale bar, 250 µm. (g) Mammosphere-forming ability of MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation is reduced with dietary SPI exposure. Data (mean ± SEM) are from three independent experiments, with each experiment representing an individual mouse and carried out in triplicates. * P≤ 0.05, by t-test.
To extend these findings, we quantified the mammosphere-forming ability of MECs isolated from hyperplastic mammary glands of PND75 Wnt1-Tg mice of the two diet groups. This ex vivo assay, based on the competence of a small population of mammary stem/progenitor cells to grow in suspension as spheres (Dontu et al. 2003) constitutes another measure of regenerative potential. Representative mammospheres formed at passage 2 (P2) from MECs isolated from hyperplastic mammary glands of CAS- and SPI-fed Wnt-Tg mice are shown (Fig. 3b). MECs from SPI-fed mice displayed lower mammosphere numbers (P2, by 60%) than from control mice (Fig. 3b), consistent with inhibition by dietary SPI of the expansion of epithelial subpopulation(s) with renewal capacity.
MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation from SPI-fed mice exhibit enhanced clonogenic but reduced mammosphere-forming activities
The shorter mammary tumor latency with dietary SPI exposure (Table 1) suggests higher proliferation of tumor cells, once tumors are initiated, in SPI-fed mice. The mouse mammary luminal epithelial population (CD29loCD24hi) is composed of both luminal progenitors and differentiated luminal cells (Visvader, 2009), which could not be distinguished by the antigen markers used in our FACS assays (Fig. 2). Similar to transformed basal stem/progenitor cells, aberrant luminal progenitors exhibit tumorigenic capabilities that sustain mammary tumors (Vailliant et al., 2008; Lim et al., 2009). These phenotypes are quantifiable ex vivo by measuring the clonogenic activity of isolated cells, using the colony formation assay. To examine the possibility that expansion of the luminal progenitor subpopulation may underlie the observed decrease in tumor latency with dietary SPI exposure, despite the reduction in the size of the mammary CD29hiCD24+Lin− pool (Fig. 2) and lower tumor incidence (Table 1), MECs from Wnt1-Tg mice fed CAS or SPI diets were sorted into basal (CD29hiCD24+) and luminal (CD29loCD24hi) subpopulations by FACS. Each epithelial subpopulation was then subjected to both clonogenic and mammosphere-formation assays (Fig. 3c). The luminal subpopulation formed colonies upon culture in Matrigel that did not differ with diet (Fig. 3d), and showed no mammosphere-forming ability (data not shown). By contrast, the basal subpopulation isolated from MECs of SPI-fed Wnt-Tg mice displayed higher clonogenic activity (Fig. 3e) and formed colonies that were predominantly larger (diameter ≥100 mm) than those from corresponding mice fed CAS (diameter ≤ 50 μm). Representative colonies at higher magnification are shown (Fig. 3f). Consistent with that found for the unfractionated MECs (Fig. 3b), the mammosphere-forming ability of the basal subpopulation was lower for the SPI- than for the CAS-fed groups (Fig. 3g), although no significant changes in mammosphere sizes were observed between diet groups (Fig. 3f). Results indicate that dietary SPI modifies the clonogenic and mammosphere-forming behavior of the basal (CD29hiCD24+Lin−) subpopulation in the absence of corresponding effects on the luminal epithelial subpopulation.
The CD29hiCD24+Lin= epithelial subpopulation from SPI-fed Wnt1-Tg mice exhibits down-regulated expression of breast cancer stem/progenitor cell-related genes
In order to identify gene networks that may relate to lower mammary tumor incidence with dietary SPI intake (Table 1), we performed microarray analysis on FACS-sorted CD29hiCD24+Lin− epithelial subpopulation of hyperplastic mammary glands from PND75 Wnt1-Tg mice fed CAS or SPI. Unsupervised hierarchical clustering of gene expression indicated that CAS samples grouped separately from those of the SPI group (data not shown). A total of 907 genes were significantly regulated by at least 1.3-fold with dietary intake of SPI. Of these, 297 were up-regulated (Supplementary Table S4) and 610 were down-regulated (Supplementary Table S5). The list for SPI up-regulated genes included candidate tumor suppressor genes methylated or lost in several cancers, including breast cancer, such as basonuclin 1 (Bnc1); catenin alpha-like-1 (Ctnnal1); cytochrome P450, family 24, subfamily a, polypeptide 1 (Cyp24a1); FBJ osteosarcoma oncogene B (Fosb); krüppel-like factor 11 (Klf11); pyruvate dehydrogenase kinase, isoenzyme 4 (Pdk4); and WNK lysine deficient protein kinase 2 (Wnk2). Conversely, dietary SPI attenuated the expression of numerous genes previously shown to be enriched in breast CSCs; these include aldehyde dehydrogenase 1 (Aldh1); interleukin 6 (Il6); notch gene homolog 2 (Notch2); and thymus cell antigen 1 (Thy1) (Dontu et al., 2003; Cho et al., 2008; Fu et al., 2010, Park et al., 2010). Beta 2-microglobulin (B2m), a protein that is up-regulated in multidrug-resistant tumor cells (Scheffer et al., 2002), was also decreased in CD29hiCD24+Lin− from hyperplastic mammary glands of SPI-fed mice. SPI similarly down-regulated expression of signal transducer and activator of transcription 1 gene (Stat1), recently shown to mediate radiation resistance of human breast cancer stem cells (Zhang et al., 2011). Ingenuity Pathway Analysis (IPA) on the 907 annotated genes regulated by SPI (≥ 1.3-fold) revealed the top gene networks (Supplementary Table S6), functional processes (Supplementary Table S7), and canonical signaling pathways (Supplementary Table S8). These include those involved in organ development, cancer, metabolic disorders, and cell-cell communication.
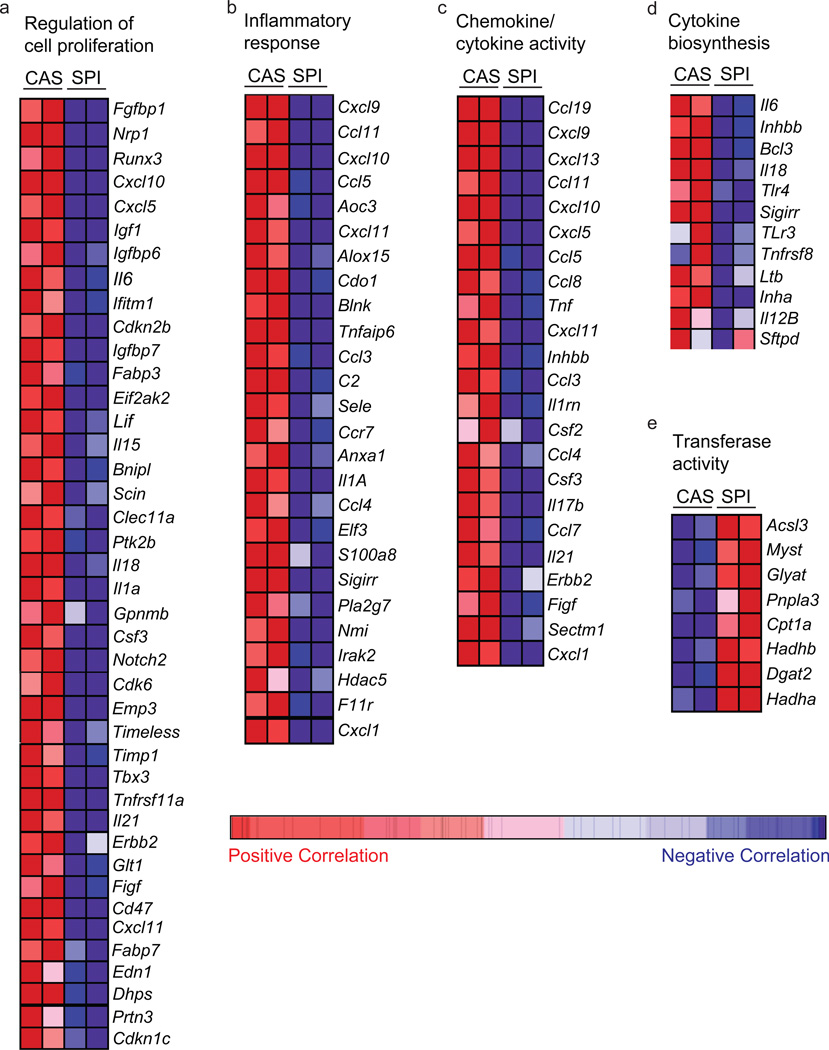
Inflammatory response, chemokine, and cytokine network genes are potential downstream targets of dietary SPI in the CD29hiCD24+Lin− epithelial population
We performed gene set enrichment analysis (False Discovery Rate (FDR) ≤ 0.25) to distinguish which of the MaSC- and/or breast CSC-associated genes previously identified to be associated with normal and aberrant stem cell renewal were most highly correlated with dietary SPI effects on the CD29hiCD24+Lin− epithelial population. The major gene functions down-regulated by SPI in this epithelial subpopulation were related to chemokine and cytokine activity (FDR<0.001); inflammation (FDR < 0.004), proliferative response (FDR <0.094) and oxidative stress (FDR<0.170). The heat maps of genes important to inflammation, cytokine and chemokine signaling, and proliferation which are down-regulated with dietary SPI and are previously linked to breast CSC regulation (Korkaya et al., 2011) are shown in Figure 4 (a–d). Genes in these categories include those for proinflammatory cytokines tumor necrosis factor (Tnf) and interleukin 6 (Il6), and for the chemokine (C-C or C-X-C motifs) ligands Ccl5, Ccl7, and Cxcl5. By contrast, there were only two gene functions up-regulated with SPI diet; these were tube development (FDR<0.151) and transferase activity (FDR<0.230); the heat map of genes in the latter category is shown (Fig. 4e).
Figure 4.
Dietary SPI downregulates inflammatory, chemokine, cytokine and proliferation networks in MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation. Heat map representation of SPI-attenuated (a–d) and upregulated (e) MaSC transcripts. (a) Cellular proliferation, (b) Inflammatory response; (c) Chemokine/cytokine activity, (d) Cytokine biosynthesis and (e) transferase activity. Red indicates positive correlation; blue, negative correlation.
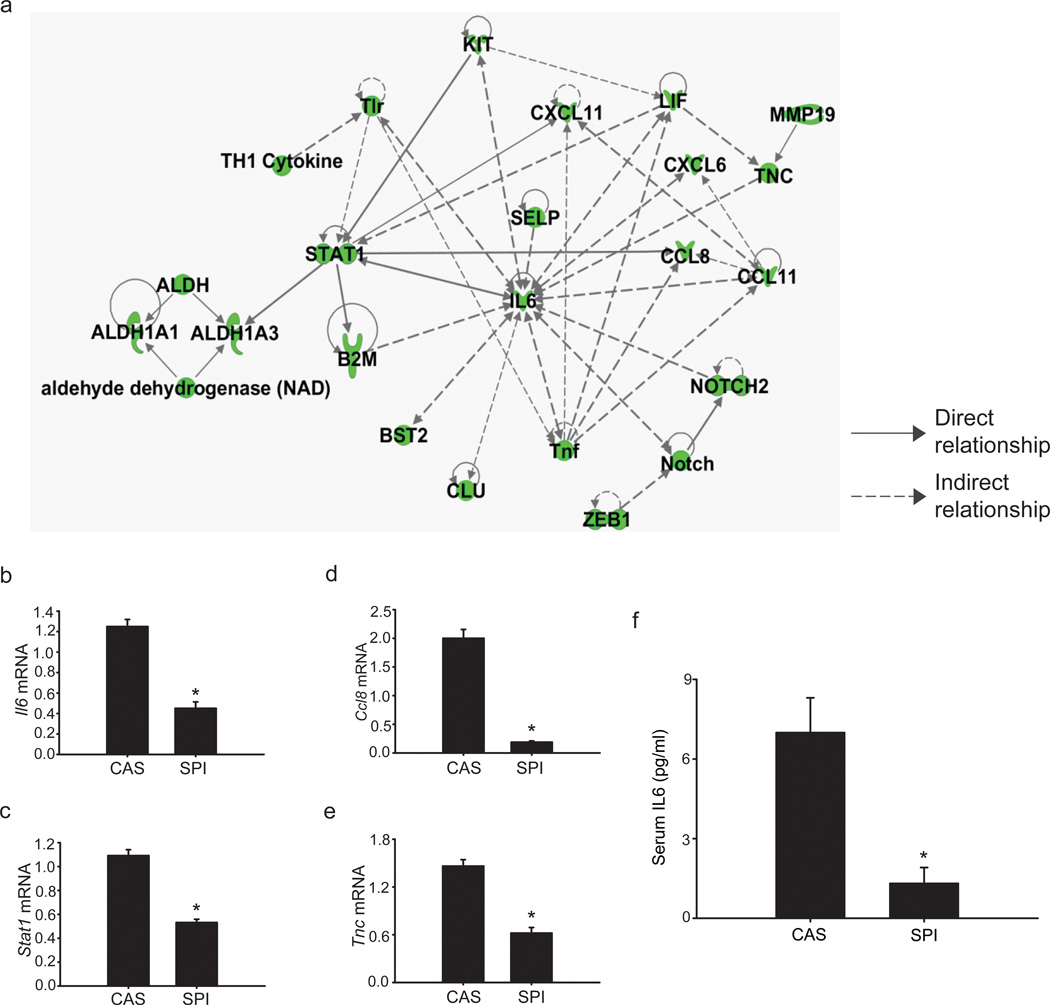
The overlapping networks of genes associated with MaSC and/or breast CSC self-renewal (Aldh, B2m, Il6, Notch2, Stat1, Thy1), inflammatory response/chemokine signaling (Ccl8, Ccl11, Ccl21, Cxcl6, Cxcl10, Cxcl11, Tlr, Tnf), and migration/EMT/metastasis (Bst2, Ezh2, Selp, Tnc, Zeb1), and whose expression are potentially influenced (directly or indirectly) by dietary SPI in MaSC-enriched populations, based on gene array analyses are summarized in Figure 5a. Altered expression of a subset of these genes implicated in stem cell survival (Il6, Stat1), inflammation/chemokine signaling (Il6, Ccl8) and metastasis (Tnc) (Iliopoulos et al., 2009; Ginestier et al., 2010; Liu et al., 2011; Oskarsson et al., 2011) were confirmed by QPCR (Figure 5b–e). The decrease in IL6 transcripts noted in MaSC-enriched (CD29hiCD24+Lin−) epithelial subpopulation of mammary glands from PND75 Wnt1-Tg mice fed SPI, relative to those fed CAS was accompanied by a parallel reduction in serum IL6 levels (Figure 5f). Data indicate that dietary SPI negatively regulates local (CD29hiCD24+Lin− epithelial population) as well as systemic IL6 production.
Figure 5.
Dietary SPI regulation of molecules related to breast CSC survival, inflammation, and metastasis in MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation within hyperplastic mammary tissues fromWnt1-Tg mice. (a) Molecular network from pathway analysis indicating dietary SPI suppression of molecules involved in MaSC and/or breast CSC survival, inflammatory/chemokine network, and migration/metastasis. Green indicates downregulation. QPCR analyses of transcripts for Il6 (b), Stat1 (c), Ccl8 (d), and Tnc (e) in MaSC-enriched population (n=4–6 independent samples, where each sample represents RNAs pooled from 2–3 mice per diet group) as a function of dietary CAS or SPI exposure. 18s rRNA was used as normalizing control; *P≤0.05 relative to CAS. (f) IL6 levels were quantified in sera of PND75 Wnt1-Tg mice fed CAS or SPI; n=13 mice per diet group; * P ≤ 0.001 relative to CAS.
Discussion
To the best of our knowledge, the present study constitutes the first report describing diet-mediated inhibition of the expansion of the MaSC-enriched (CD29hiCD24+Lin−) epithelial subpopulation in vivo that may relate to decreased susceptibility to mammary tumor formation. Studies to date have largely demonstrated dietary factor effects on MaSCs in vitro using established breast cancer cell lines (Kakarala et al., 2010; Montales et al., 2012). Using Wnt1-Tg mice, which develop spontaneous mammary tumors previously shown to originate from dys-regulation of mammary stem/progenitor cells (Li et al., 2003; Liu et al., 2004), we show that diet may modify mammary tumor risk and outcome in part, by influencing the expansion of a distinct epithelial subpopulation with self-renewal capacity. The reduction in MaSC-enriched population in the hyperplastic mammary glands by dietary soy (by ~40%) was recapitulated in a similar reduction in the CSC (Thy+) population (by ~60%) but not in the luminal subpopulation. While speculative, the lack of dietary SPI effects on the luminal subpopulations could mean that soy-associated bioactive factors may (directly or indirectly) preferentially target cells that give rise to tumors with non-luminal A molecular subtype (Sørlie et al., 2001). We further show that the limited expansion of the basal (CD29hiCD24+Lin−) epithelial subpopulation with dietary SPI is associated with suppression of pro-inflammatory cytokine, chemokine and proliferative gene expression in this epithelial subpopulation and with reductions in systemic levels of the steroid hormones estradiol and progesterone and the cytokine IL-6, all of which are considered to promote breast cancer risk. Our collective data suggest that diet-regulated hormonal cues can impact MaSC self-renewal programs and by so doing, limit the transformation of MaSC to cancer stem cells for tumor initiation.
A major feature of the SPI-altered gene network in the CD29hiCD24+Lin− epithelial subpopulation is a suppressed cytokine and chemokine transcriptional signature. Based on previously reported signaling pathways involving cytokines in this gene signature, we identify IL6 as a candidate target of SPI effects within the MaSC population. By reducing systemic levels and MaSC-enriched production of IL6, dietary SPI may effectively block a number of critical pathways in tumor initiation and subsequent tumor survival and metastasis. While the mechanistic association between SPI-induction of IL6 and inhibition of MaSC expansion was not evaluated in the present study, our findings are consistent with previous reports that IL6 promotes breast CSC self-renewal (Iliopoulos et al., 2009) and fosters the positive feedback loop between MaSCs and breast CSCs (Iliopoulos et al., 2011). Moreover, our findings that SPI diet reduced the expression of a preponderance of genes with pro-inflammatory activities in the CD29hiCD24+Lin− epithelial subpopulation highlight the functional importance of controlling the inflammatory milieu for breast cancer prevention (Cabodi and Taverna, 2010). Further, they support the clinical importance of an extensive search for bioactive food components with anti-inflammatory activities for further investigations into dietary cues for breast cancer prevention.
Our findings of decreased systemic progesterone, taken together with the observed reduction of the MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation and lower tumor incidence with dietary SPI intake, are consistent with the reduced incidence of mammary tumors in mice null for progesterone receptor when administered the chemical carcinogen DMBA (Lydon et al., 1999). Moreover, the transient breast cancer risk that lasts for a few years after a full-term pregnancy (Brit et al., 2007) has been attributed to the expansion of mammary stem cells during this period of high progesterone exposure (Tiede et al., 2009). Similarly, the lower levels of estradiol-17β in Wnt1-Tg mice fed SPI are consistent with high circulating estrogens as a risk factor for breast cancer (Litton et al., 2012). While MaSCs lack expression of receptors for estrogen and progesterone (Asselin-Labat et al., 2006, 2010; Joshi et al., 2010), they are subject to estrogen- and progesterone-induced expansion involving the stem cell niche via paracrine signaling. The pathway(s) linking the decline in systemic levels of progesterone, estrogen and IL6 by dietary SPI with the reduction in the frequency of a distinct epithelial subpopulation with renewal capacity remains to be determined and is the subject of current investigations in our group.
A paradoxical outcome of dietary SPI exposure is the shorter latency of Wnt1-generated mammary tumors, despite the significant reduction in tumor incidence in SPI-fed mice. We previously reported that in female rats exposed to SPI and administered the direct acting carcinogen N-methyl-nitrosourea (to induce mammary tumors), SPI reduction of tumor incidence was associated with higher grade tumors in rats that developed tumors (Simmen et al., 2005). Herein, we suggest a plausible explanation for this paradox based on the distinct functional responses of the MaSC-enriched (CD29hiCD24+Lin) epithelial subpopulation to diet. We found that relative to the control diet, exposure to SPI can reduce basal (MaSC-enriched) cell expansion (measured by mammosphere-forming ability) while enhancing its proliferative phenotype (measured by colony-formation assay). The molecular basis for the opposing actions of dietary soy on the same target population is currently unknown, but may involve distinct biological effects of associated bioactive components. Our findings highlight the complex nature of foods and their context-dependent effects and provide a cautionary note when considering dietary/nutritional strategies for the management of breast cancer patients or in women at high risk for the disease.
Our approach of utilizing hyperplastic MEC cells for evaluating dietary effects on the expansion of the basal-enriched (CD29hiCD24+Lin−) MaSC and CSC (CD29hiThy+CD24+) populations allowed us to demonstrate a potential (predictive) correlation between the sizes of these populations and subsequent risk for mammary tumors. Given this focus, dietary effects on the size of the CSC population in mammary tumors were not assessed. In a recent report, early Wnt1 overexpression was found to drastically alter the epithelial ontogeny of the mammary gland and hence, the developmental fate of mammary stem and progenitor cells (Van Amerongen et al., 2012). Hence, future studies will address whether the dietary effects shown here are recapitulated in normal MaSC of adult mammary glands in wildtype mice, utilizing similar approaches described herein, including limiting dilution transplantation studies for quantitative determination of stem cell frequency. We consider the absence of the latter data as a limitation to the present study.
In summary, our in vivo and ex vivo studies provide evidence for the functional impact of diet on specific epithelial subpopulations that may relate to breast cancer risk. Moreover, our findings raise the interesting possibility that by blocking key regulatory nodes linking steroid hormone signaling and inflammation, early dietary interventions can interfere with the expansion of specific epithelial subpopulations with high regenerative/renewal capacity. Our results suggest that diet-regulated cues can be further explored for breast cancer risk assessment and prevention.
Supplementary Material
Acknowledgments
The authors thank Dr. Jeffrey M. Rosen (Baylor College of Medicine) for valuable insights and training on mammary stem cell biology and for critical comments on this manuscript, Dr. Frank A. Simmen (UAMS) for helpful discussions during the course of this study, Dr. Andrea Harris (UAMS Flow Core) for assistance with flow cytometry, and Dr. Kartik Shankar (UAMS/ACNC) for technical assistance with gene array analyses. This study was supported by grants from the United States Department of Agriculture [CRIS 6251-5100002-06S, Arkansas Children’s Nutrition Center]; Department of Defense Breast Cancer Research Program (W81XWH-08-1-0548); University of Arkansas for Medical Sciences (UAMS) Children's University Medical Group; and UAMS Translational Research Institute (UL1 RR029884) to R.C.M.S. O.M.R. was supported by a pre-doctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-10-1-0047).
Footnotes
Author Contributions: O.R.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; H.M.: collection and assembly of data, data analysis and interpretation, final approval of manuscript; M.T.E., J.M.P.P. and M.E.H.: collection and assembly of data, data analyses and interpretation, final approval of manuscript; S.N.: data analysis and interpretation, final approval of manuscript; R.C.M.S: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support, overall study supervision
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J. Natl. Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 3.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 4.Balko JM, Miller TW, Morrison MM, Hutchinson K, Young C, Rinehart C, Sánchez V, Jee D, Polyak K, Prat A, Perou CM, Arteaga CL, Cook RS. The receptor tyrosine kinase ErbB3 maintains the balance between luminal and basal breast epithelium. Proc Natl. Acad. Sci. U.S.A. 20102;109:221–226. doi: 10.1073/pnas.1115802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brit K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr. Rel. Cancer. 2007;14:907–933. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 6.Cabodi S, Taverna D. Interfering with inflammation: a new strategy to block breast cancer self-renewal and progression? Breast Cancer Res. 2010;12:305–306. doi: 10.1186/bcr2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 8.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–371. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 10.Clarke MF. What can we learn about breast cancer from stem cells? Adv. Exp. Med. Biol. 2008;458:780–783. doi: 10.1007/978-0-387-69080-3_2. [DOI] [PubMed] [Google Scholar]
- 11.Dave B, Eason RR, Till SR, Geng Y, Velarde MC, Badger TM, Simmen RC. The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis. 2005;26:1793–1803. doi: 10.1093/carcin/bgi131. [DOI] [PubMed] [Google Scholar]
- 12.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farnie G, Clarke RB. Mammary stem cells and breast cancer- role of Notch signaling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 14.Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Gaudet MM, Abrahamson PE, Bell P, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol. Biomarkers Prev. 2007;16:2285–2292. doi: 10.1158/1055-9965.EPI-07-0245. [DOI] [PubMed] [Google Scholar]
- 15.Fu YP, Edvarsen H, Kaushiva A, Arhancet JP, Howe TM, Kohaar I, Porter-Gill P, Shah A, Landmark-Høyvik H, Foss SD, Ambs S, Naume B, Børrensen_Dale AL, Kristensen VN, Prokunina-Ollson L. Notch2 in breast cancer: association of SNP rs11249433 with gene expression in ER-positive breast tumors without Tp53 mutations. Mol Cancer. 2010;9:113. doi: 10.1186/1476-4598-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc. Natl. Acad. Sci. USA. 2011;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 19.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korde LA, Wu AH, Fears T, Nomura AM, West DM, Kolonel LN, Pike MC, Hoover RN, Ziegler RG. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–1059. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
- 21.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-Catenin signaling. PLoS Biol. 2009;7:1–14. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMarca HL, Visbal AP, Creighton CJ, Liu H, Zhang Y, Behbod F, Rosen JM. CCAAT/enhancer binding protein beta regulates stem cell activity and specifies luminal cell fate in the mammary gland. Stem Cells. 2010;28:535–544. doi: 10.1002/stem.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl. Acad. Sci. USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 26.Litton JK, Arun BK, Brown PH, Hortobagyi GN. Aromatase inhibitors and breast cancer prevention. Expert Oncol Pharmacother. 2012;13:325–331. doi: 10.1517/14656566.2012.651459. [DOI] [PubMed] [Google Scholar]
- 27.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl. Acad. Sci. USA. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lydon JP, Ge G, Kittrell FS, Medina D, O’Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59:4276–4284. [PubMed] [Google Scholar]
- 31.Montales MT, Rahal OM, Kang J, Rogers TJ, Prior RL, Wu X, Simmen RC. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33:652–660. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- 32.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pabona JM, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RC. Kruppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2012;97:E376–E392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SY, Gönen M, Kim HJ, Michor F, Polyak K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J Clin Invest. 2010;120:636–644. doi: 10.1172/JCI40724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahal OM, Simmen RC. PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis. 2010;31:1491–1500. doi: 10.1093/carcin/bgq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahal OM, Pabona JMP, Kelly T, Huang Y, Hennings LJ, Prior RL, Al-Dwairi A, Simmen FA, Simmen RCM. Suppression of Wnt1-induced mammary tumor growth and lower serum insulin in offspring exposed to maternal blueberry diet suggest early dietary influence on developmental programming. Carcinogenesis. 2013;34:464–474. doi: 10.1093/carcin/bgs353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheffer GL, de Jong MC, Monks A, Flens MJ, Hose CD, Izquierdo MA, Shoemaker RH, Scheper RJ. Increased expression of beta 2-microglobulin in multidrug- resistant tumour cells. Br. J. Cancer. 2002;86:1943–1950. doi: 10.1038/sj.bjc.6600354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 39.Simmen RC, Eason RR, Till SR, Chatman L, Jr, Velarde MC, Geng Y, Korourian S, Badger TM. Inhibition of NMU-induced mammary tumorigenesis by dietary soy. Cancer Lett. 2005;224:45–52. doi: 10.1016/j.canlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 42.Su Y, Eason RR, Geng Y, Till SR, Badger TM, Simmen RC. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28:1046–1051. doi: 10.1093/carcin/bgl240. [DOI] [PubMed] [Google Scholar]
- 43.Su Y, Simmen RCM. Soy isoflavone genistein upregulates epithelial adhesión molecule E-cadherin expression and attenuates β-catein signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–339. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gilette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiede BJ, Owens LA, Li F, DeCosta C, Kang Y. A novel mouse model for non- invasive single marker tracking of mammary stem cells in vivo reveals stem cell dynamics throughout pregnancy. PLoS One. 2009;4:e8035. doi: 10.1371/journal.pone.0008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsukamoto AS, Grosschedl R, Guzman RC, Parlow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 47.Vaillant FA, Asselin-Labat M-L, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/β3 identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 48.Van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-Catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:1–14. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–194. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 50.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang JF, Chen LH, Yuan YW, Xie GZ, Sun AM, Liu Y, Chen ZX. STAT1 promotes radioresistance of CD44(+)/CD24(−/low) cells in breast cancer. Exp. Biol. Med. 2011;236:418–422. doi: 10.1258/ebm.2011.010287. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, III, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.