Abstract
Class A G-protein coupled receptors (GPCRs) are thought to have a common topology that includes seven transmembrane alpha helices (TMHs) that are arranged to form a closed bundle. This bundle forms the ligand binding pocket into which ligands are commonly thought to enter via the extracellular milieu. This ligand approach direction makes sense for GPCRs that have small positively charged ligands, such as the beta-2-adrenergic or the dopamine D2 receptor. However, there is a growing sub-group of Class A GPCRs that bind lipid-derived endogenous ligands, such as the cannabinoid CB1 and CB2 receptors (with endogenous ligands, N-arachidonoylethanolamine (anandamide) and sn-2-arachidonylglycerol (2-AG)) and the S1P1-5 receptors (with endogenous ligand, sphingosine-1-phosphate). Even the widely studied Class A GPCR, rhodopsin, binds a highly lipophillic chromophore (11-cis-retinal). For these receptors, ligand approach from the extracellular milieu has seemed unlikely given that the ligands of these receptors readily partition into lipid or are actually synthesized in the lipid bilayer. The recent X-ray-crystal structure of the sub-type 1 sphingosine-1-phosphate receptor (S1P1) provides important information on the key structural variations that may be the hallmarks for a Class A GPCR that binds lipid-derived ligands. These include an extracellular domain that is closed off to the extracellular milieu and the existence of an opening between transmembrane helices that may serve as a portal for ligand entry via the lipid bilayer. This review examines structural aspects that the cannabinoid receptors may share with the S1P1 receptor based upon sequence homology. This review also examines experimental and simulation results that suggest ligand entry via a lipid portal is quite likely for this emerging sub-group.
Keywords: Cannabinoid, Sphingosine-1-phosphate, GPCR, Crystal structure, Lipid portal
G protein-coupled receptors (GPCRs) are integral membrane proteins that serve as very important links through which cellular signal transduction mechanisms are activated. Class A GPCRs (rhodopsin-like) are thought to have a common topology that includes seven transmembrane alpha helices (TMHs) that are arranged to form a closed bundle. This bundle forms the ligand binding pocket into which ligands are commonly thought to enter via the extracellular milieu. This ligand approach direction makes sense for GPCRs that have small positively charged ligands, such as the beta-2-adrenergic or the dopamine D2 receptor. However, there is a growing sub-group of Class A GPCRs that bind lipid-derived endogenous ligands, such as the cannabinoid CB1 and CB2 receptors (Devane et al., 1988; Munro et al., 1993) (with endogenous ligands, N-arachidonoylethanolamine (anandamide) (Devane et al., 1992) and sn-2-arachidonylglycerol (2-AG))(Mechoulam et al., 1995) and the S1P1-5 receptors (Chun, 1999, 2005; Chun et al., 1999, 2000; Sanchez and Hla, 2004; Zhang et al., 1999) (with endogenous ligand, sphingosine-1-phosphate) (Choi et al., 2011; Graler, 2010; Hla and Brinkmann, 2011). Even the widely studied Class A GPCR, rhodopsin, binds a highly lipophillic chromophore (11-cis-retinal) (Palczewski et al., 2000). For these receptors, ligand approach from the extracellular milieu has seemed unlikely given that the ligands of these receptors readily partition into lipid or are actually synthesized in the lipid bilayer.
The recent X-ray-crystal structure of the sub-type 1 sphingosine-1-phosphate receptor (S1P1) (Hanson et al., 2012) provides important information on the key structural variations that may be the hallmarks for a Class A GPCR that binds lipid-derived ligands. These include an extracellular domain that is closed off to the extracellular milieu and the existence of an opening between transmembrane helices that may serve as a portal for ligand entry via the lipid bilayer. This review examines structural aspects that the cannabinoid receptors may share with the S1P1 receptor based upon sequence homology. This review also examines experimental and simulation results that suggest ligand entry via a lipid portal is quite likely for this emerging sub-group.
1. Cannabinoid receptors: ligands and signalling
1.1. CB1 receptor
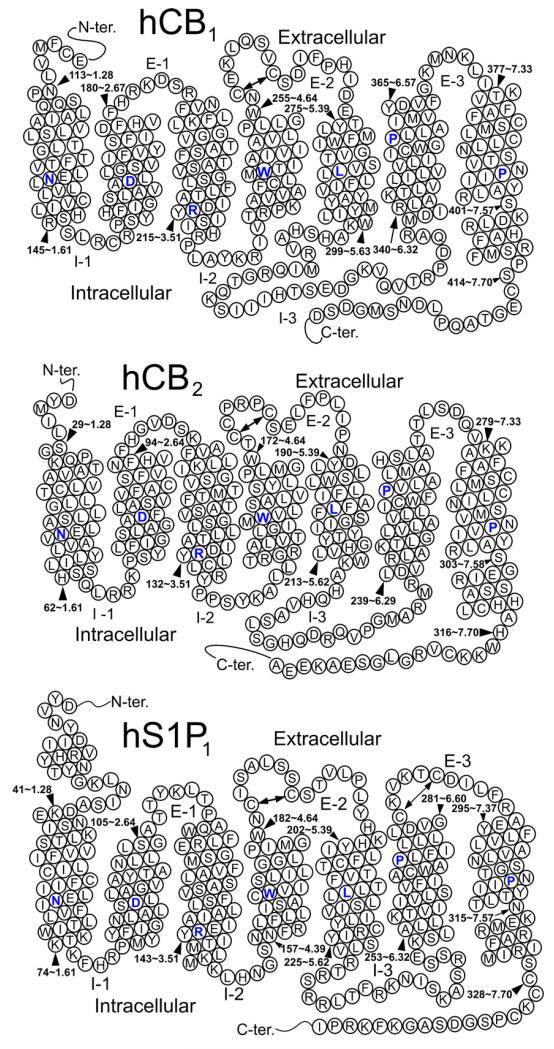
The cannabinoid CB1 and CB2 receptors (see Fig. 1) belong to the Class A (rhodopsin (Rho) family) of G-protein coupled receptors (GPCRs). CB1 was initially cloned from a rat cerebral cortex cDNA library (Matsuda et al., 1990) and early sequence analyses revealed that this receptor had highest homology with the endothelial differentiation gene (EDG) receptor family (now split into the lysophosphatidic acid (LPA) receptors and the spinghosine-1-phosphate (S1P) receptors) (Bramblett et al., 1995). CB1 receptors are expressed in the central nervous system (CNS) (Glass et al., 1997; Westlake et al., 1994) and are particularly rich in certain brain areas such as basal ganglia, cerebellum, and hippocampus (Pertwee, 1997). CB1 receptors are also found in the periphery, including human testis (Gerard et al., 1991), retina (Straiker et al., 1999), sperm cells (Schuel et al., 1999), colonic tissues (Wright et al., 2005), peripheral neurons (Ishac et al., 1996), adipocytes (Roche et al., 2006), and other organs including human adrenal gland, heart, lung, prostate, uterus, and ovary (Bouaboula et al., 1993; Galiegue et al., 1995; Rice et al., 1997).
Fig. 1.
The sequences of the human CB1, CB2 and S1P1 receptors are illustrated here in helix net diagrams.
CB1 receptors signal via multiple second messenger systems (for a review see Turu and Hunyady, 2009). CB1 receptor agonists inhibit forskolin-stimulated adenylyl cyclase by activation of a pertussis toxin-sensitive G-protein (Felder et al., 1995; Howlett et al., 1986). In heterologous cells, CB1 receptors inhibit N-, P-, and Q-type calcium channels and activate inwardly rectifying potassium channels (Felder et al., 1995; Mackie et al., 1995; Pan et al., 1996). PKA-dependent inhibition of voltage-gated N-type Ca2+ channels (N-type VGCCs) has also been detected in neuronal cells (Azad et al., 2008). Inhibition of calcium channels and enhancement of inwardly rectifying potassium currents is pertussis toxin-sensitive, but independent of cAMP inhibition, suggestive of a direct G protein mechanism (Mackie et al., 1995). In pertussis-pretreated cells, CB1 stimulation has also been shown to lead to adenylyl cyclase activation suggesting that in certain circumstances, CB1 can couple to Gs proteins (Abadji et al., 1999; Glass and Felder, 1997; Kearn et al., 2005). CB1 has been reported to induce receptor-mediated Ca2+ fluxes, however, the mechanism of this response is unclear. Evidence that such a Ca2+ signal may be Gq/PLC dependent in rat insulinoma beta-cells has been reported (De Petrocellis et al., 2007). CB1 stimulation in vitro and in vivo leads to activation of ERK1/2 kinases in a variety of cell types (Howlett, 2005) and β-arrestins have been reported to play a role in CB1 desensitization (Breivogel et al., 2008; Kouznetsova et al., 2002).
1.2. CB2 receptor
The second cannabinoid receptor sub-type, CB2 was cloned from a human promyelocytic leukemia cell HL60 cDNA library (Munro et al., 1993). The human CB2 receptor exhibits 78% homology to the human CB1 receptor within the transmembrane regions, 64% homology throughout the whole protein (Munro et al., 1993). Unlike the CB1 receptor, which is highly conserved across human, rat and mouse, the CB2 receptor is much more divergent. Sequence analysis of the coding region of the rat CB2 genomic clone indicates 93% amino acid identity between rat and mouse and 81% amino acid identity between rat and human.
The CB2 receptor is highly expressed throughout the immune system (Galiegue et al., 1995; Howlett et al., 2002) and is expressed in the CNS under both pathological (Benito et al., 2003) and physiological conditions (Van Sickle et al., 2005). Studies suggest that brain CB2 receptors modulate cocaine’s rewarding and locomoter-stimulating effects (Xi et al., 2011). The quite specific localization of CB2, as well as the fact that CB2 knock-out mice fail to respond to the immunomodulatory effects of classical cannabinoids (Buckley et al., 2000), suggest that CB2 receptor ligands would have potential therapeutic applications as immunomodulators for the treatment of inflammation and allergy. Several papers report the role of the CB2 receptor in modulating leukocyte migration (Franklin and Stella, 2003; Jorda et al., 2002; Kishimoto et al., 2003; Massi et al., 2000), activation (Kishimoto et al., 2004), and antigen processing (McCoy et al., 1999). Additional applications could arise from studies on bone physiology, as blockage of CB2 has been reported to protect from bone loss in ovariectomized mice (Idris et al., 2005). However, others have reported that CB2 activation is involved in protecting from bone loss (Ofek et al., 2006). Intracellular CB2-dependent signalling pathways include Gi/o-dependent inhibition of adenylyl cyclase, stimulation of mitogen-activated protein kinase (Bouaboula et al., 1996, 1999), phosphoinositide 3-kinase pathways (Sanchez et al., 2003), and activation of de novo ceramide production or cyclooxygenase-2 (COX-2) induction (Guzman et al., 2001).
1.3. Cannabinoid ligands
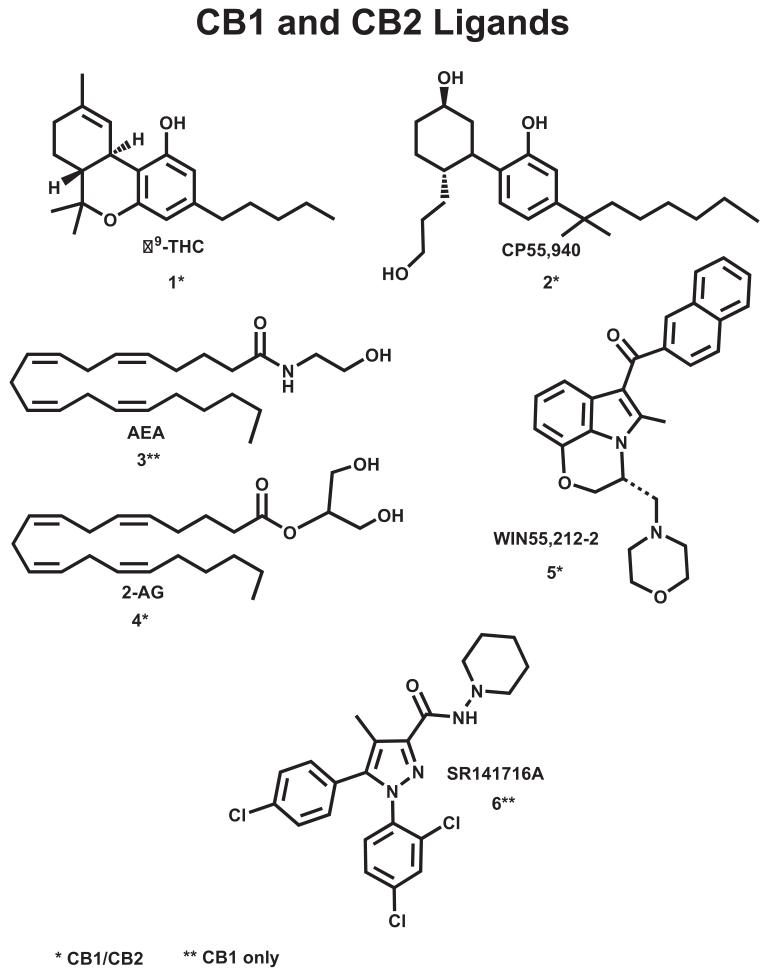
The cannabinoid (CB) receptors transduce signals in response to central nervous system-active constituents of Cannabis sativa and their synthetic analogs, such as the classical cannabinoid, (–) trans-Δ9-tetrahydrocannabinol (Δ9-THC 1; Chart 1) and to three other structural classes of ligands, the non-classical cannabinoids typified by (1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl) cyclohexan-1-ol (CP-55940; 2; Chart 1)), the endogenous cannabinoids, typified by N-arachidonoylethanolamine (anandamide, AEA) (3; Chart 1) and sn-2-arachidonylglycerol (2-AG; 4; Chart 1), and the aminoalkylindoles (AAIs) typified by (R)-[2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl](1-naphthalenyl)methanone (WIN55,212-2; 5; Chart 1) (Reggio, 2005). Mutation studies have shown that the classical/non-classical/endogenous cannabinoids bind in the TMH2-3-6-7 region (Kapur et al., 2007; Song and Bonner, 1996), while the AAIs, along with biaryl pyrazole antagonists typified by SR141716A (6; Chart 1) bind in the TMH3-4-5-6 region of CB1 (Hurst et al., 2006; McAllister et al., 2003).
Chart 1.
This figure illustrates the structures of ligands that bind to the CB1 and CB2 receptors.
All known cannabinoid ligands are highly lipophilic and endogenous cannabinoid ligands have been shown to be synthesized in the lipid bilayer. For example, the CB1/CB2 endogenous cannabinoid, 2-AG (4) (Mechoulam et al., 1995; Sugiura et al., 1995), is synthesized on demand from the lipid bilayer in a two step process in which phospholipase C-β hydrolyses phosphatidylinositol-4,5-bisphosphate to generate diacylglycerol, which is then hydrolyzed by diacylglycerol lipase to yield 2-AG (Di Marzo, 2008; Piomelli, 2003). After 2-AG interaction with the membrane-embedded CB receptor, it is hydrolyzed to arachidonic acid and glycerol by a membrane-associated enzyme, monoacylglycerol lipase (Dinh et al., 2002).
2. S1P receptors: ligands and signalling
Sphingosine 1-phosphate (S1P) (7; Chart 2)) is a lipid signaling molecule that regulates the cardiovascular and immune systems and functions in numerous physiological and pathophysiological conditions (for reviews, see Refs. Choi et al., 2011; Graler, 2010; Hla and Brinkmann, 2011). The spinghosine-1-phosphate receptors (S1P1-5) are Class A GPCRs that bind the endogenous lipid, S1P. Along with the lysophosphatidic acid (LPA) receptors, the S1P receptors were originally named the endothelial differentiation gene (EDG) family of Class A GPCRs. There are five known S1P receptor subtypes, S1P 1-5, that are expressed on a wide range of cell types, including neural cells and lymphocytes (Chun, 1999, 2005; Chun et al., 1999, 2000; Sanchez and Hla, 2004; Zhang et al., 1999). S1P1-3 receptors are found widely distributed in the CNS, immune and cardiovascular systems. S1P1 is found on T and B lymphocytes (Im et al., 2000; Ishii et al., 2004). S1P4 receptor expression is confined to lymphoid and hematopoietic tissues, while S1P5 is found predominantly in the CNS white matter (Sanchez and Hla, 2004; Watterson et al., 2003). The activation and functional status of cells can alter the expression pattern of S1P receptors (Matloubian et al., 2004; Miron et al., 2008).
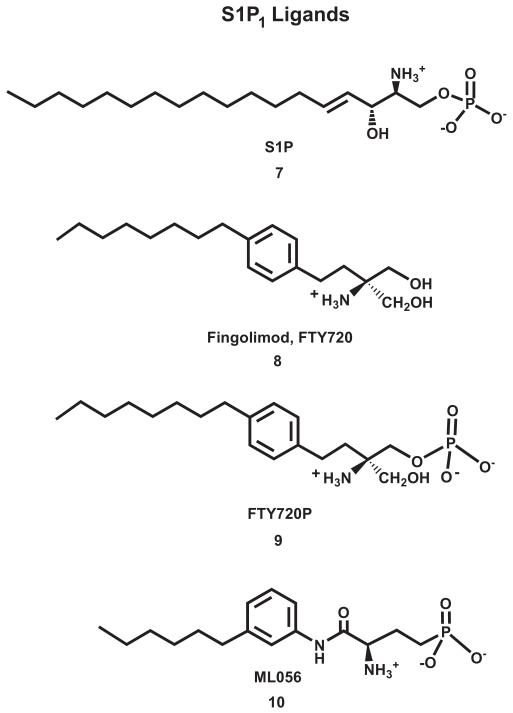
Chart 2.
This figure illustrates structures of agonists, produgs and antagonists that bind to the S1P1 receptor.
The five S1P receptor sub-types possess high overall sequence homology that ranges from 33% to 51% (Parrill et al., 2004). Autocrine or paracrine binding of S1P to the S1P1-5 receptors activates a variety of G proteins whose downstream signaling accounts for many of S1P’s important functions in cancer, inflammation and the cardiovascular system (Choi et al., 2011; Edmonds et al., 2011; Graler, 2010; Hla and Brinkmann, 2011). The fact that S1P action results in inhibition of lymphocyte recirculation (Chun and Hartung, 2010; Mandala et al., 2002) has been the basis for the development of the pro-drug, FTY720 (fingolimod, 8; Chart 2) which has recently been approved for the clinical treatment of relapsing-remitting multiple sclerosis (Brinkmann et al., 2010). This compound is phosphorylated by sphingosine kinase to produce FTY720P (9; Chart 2) which binds to the S1P receptors.
3. GPCR X-ray crystal structures
In the dicussion of receptor residues that follows, the Ballesteros–Weinstein numbering system is used (Ballesteros and Weinstein, 1995). In this numbering system, the label .50 is assigned to the most highly conserved Class A residue in each transmembrane helix (TMH). This is preceeded by the TMH number. In this system, for example, the most highly conserved residue in TMH6 is P6.50. The residue immediately before this would be labeled 6.49 and the residue immediately after this would be labeled 6.51.
The number of Class A GPCR/ligand complexes that have been crystallized is growing, but still represents only a handful of receptors. These include rhodopsin (Rho) (Li et al., 2004; Okada et al., 2002; Palczewski et al., 2000), meta-rhodopsin II (Choe et al., 2011), the β2-adrenergic receptor (β2-AR) (Cherezov et al., 2007; Rasmussen et al., 2007, 2011; Rosenbaum et al., 2007), the β1-adrenergic receptor (β1-AR) (Moukhametzianov et al., 2011; Warne et al., 2008), the adenosine A2A receptor (Jaakola et al., 2008; Lebon et al., 2011), the CXCR4 receptor (Wu et al., 2010), the dopamine D3 receptor (Chien et al., 2010), the histamine H1 receptor (Shimamura et al., 2011), the nociceptin/orphanin FQ receptor (Thompson et al., 2012), the μ (Manglik et al., 2012), delta (Granier et al., 2012) and kappa (Wu et al., 2012) opioid receptors and the M2-(Haga et al., 2012) and M3-muscarinic acetylcholine receptors (Kruse et al., 2012). These crystal structures reveal a common topology that includes: (1) an extracellular N terminus; (2) seven transmembrane alpha helices (TMHs) arranged to form a closed bundle; (3) loops connecting TMHs that extend intra- and extra-cellularly; and, except for the CXCR4 receptor (Wu et al., 2010), (4) an intracellular C terminus that begins with a short helical segment (Helix 8) oriented parallel to the membrane surface. Ligand binding occurs within the TMH bundle, with additional ligand interactions occurring with extracellular (EC) loop residues in some structures.
Within each Class A GPCR binding pocket, there is thought to be a set of residues that change conformation upon agonist binding. These are called “toggle switch” residues and typically include residue W6.48 of the TMH6 CWXP motif and another residue that interacts with W6.48. The β2-AR has an aromatic residue at 6.52 (F6.52) that is part of its toggle switch (Shi et al., 2002). The CB1 and CB2 receptors have no aromatic residue at 6.52. In CB1R, the “toggle switch” pair has been shown to be W6.48 and F3.36 (McAllister et al., 2004). The hallmark of Class A GPCR activation by an agonist is the “tripping” of the toggle switch within the binding pocket that allows TMH6 to flex in the CWXP hinge region and straighten. This straightening breaks the “ionic lock” between R3.50 and E/D6.30 at the intracellular end of the receptor. The result is the formation of an intracellular opening of the receptor, exposing residues that can interact with the C-terminus of the Gα sub-unit of the G protein (Hamm et al., 1988).
3.1. S1P1 receptor X-ray crystal structure
The recently published X-ray crystal structure of the S1P1 receptor fused to T4-lysozyme (T4L) (Hanson et al., 2012) gives us the first opportunity to see the differences between the structures of lipid triggered Class A GPCRs vs those triggered by small aminergic ligands (such as the dopamine or histamine receptors) or peptides (such as the mu-, delta- and kappa-opioid receptors). In the S1P1 receptor crystal structure, the receptor is in complex with the selective antagonist sphingolipidmimic (R)-3-amino-(3 hexylphenyl-amino)-4-oxobutylphosphonic acid (ML056; 10, Chart 2) (Sanna et al., 2006). The resolution in the S1P1/T4L structure is 3.35Å using traditional X-ray diffraction data processing methods and 2.8Å using an experimental microdiffraction data assembly method to help process data of rapidly decaying micro-crystals (Hanson et al., 2012). The human S1P1-5 receptors have very high (62–64%) sequence homology with the human cannabinoid CB1 receptor in their transmembrane helix (TMH) regions. It is interesting that the S1P1-5 family of receptors have such high homology with the cannabinoid CB1/CB2 receptors, given that both receptors bind endogenous lipid-derived ligands. With this high sequence homology, it is likely that structural motifs seen in the recent S1P1 receptor X-ray crystal structure will have implications for the structures of the cannabinoid CB1 and CB2 receptors.
3.2. Unique and shared structural motifs between S1P1, and the CB1 and CB2 receptors
Fig. 1 provides the helix net diagrams for the human CB1, CB2 and S1P1 sequences. The human S1P1 receptor sequence has most of the highly conserved residue/sequence motifs found in Class A GPCRs: N1.50, D2.50, TMH3 ERY motif, W4.50, TMH6 motif CWXP (CWAP), and TMH7 motif NPXXY (NPIIY). Like the CB1 and CB2 receptors, S1P1 lacks the highly conserved proline in TMH5.
3.3. S1P1 ionic lock
The S1P1 “ionic lock” contains a substitution of an Asn for the negatively charged residue usually found at position 6.30. N6.30 in S1P1 is part of a hydrogen bond network that includes R3.50 and R78 in the intracellular loop-1 (IC-1).
4. S1P1 transmembrane domains
TMH1
TMH1 in the S1P1 structure leans away from the bundle, creating a space between TMH1 and TMH7. Like CB1 and CB2, S1P1 has an E at residue 1.49. This residue faces lipid, but is shielded by F1.45 and I7.51.
TMH2
TMH2 in the S1P1 structure has no proline or other helix deforming motif, so the helix is straight and does not lean toward TMH1 and TMH7. CB1 and CB2 also lack any helix deforming motif. In CB2, the accessibility of TMH2 residues to the binding pocket has been characterized with subsituted cysteine accessibility studies (Zhang et al., 2005).
TMH3
TMH3 in S1P1 has the Class A conserved D/ERY motif at residues 3.49-3.51 (E3.49-R3.50-Y3.51). CB1 and CB2 also have this motif, but the residue at 3.49 is an aspartate in both (i.e. D3.49-R3.50-Y3.51).
TMH4
The extracellular ends of TMH4 in S1P1 and CB1 have the GWNC motif (in CB2 this is GWTC). In this motif, W4.64 forms an aromatic stacking interaction with Y5.39 in the S1P1 structure. CB1 and CB2 have Y5.39 as well. This suggests that the extracellular ends of TMH4 and 5 may have an orientation in CB1 and CB2 that is similar to the S1P1 structure.
TMH5
In most Class A GPCRs, there is a proline at position 5.50. The S1P1 sequence is unusual in that it lacks a proline in TMH5. The result is that TMH5 in S1P1 is very straight. Both the CB1 and CB2 receptors also lack a proline in TMH5. It is interesting to note that if TMH5 in S1P1 had a P5.50, the motif that characterizes the tops of TMH4 and TMH5 (i.e., the W4.64/Y5.39 aromatic stacking interaction) would not be possible (see above).
TMH6
The S1P1 TMH6 has the Class A conserved CWXP sequence motif (CWAP). W6.48 is likely part of the “toggle switch” for ligand activation of S1P1. For this residue to change conformation, F6.52 would need to change conformation first. Thus, it is likely that F6.52 is part of the ligand binding pocket toggle switch. In CB1 and CB2, there are no aromatics flanking W6.48, but there is an aromatic residue on TMH3 (F3.36) that has an aromatic stacking interaction with W6.48 in the inactive state of CB1. Mutation studies suggest that F3.36/W6.48 is the toggle switch interaction in CB1 (McAllister et al., 2004).
TMH7
THM7 in S1P1 has the Class A conserved NPXXY motif (NPIIY). It is interesting that the CB1 receptor also has NPIIY, while the CB2 receptor has a homologous NPVIY motif.
5. S1P1 loops
Many Class A GPCRs have a Cys in the EC-2 loop that forms a disulfide bridge with C3.25. S1P1 lacks a Cys at 3.25, but forms an internal disulfide with a second Cys in the EC-2 loop. This same motif is present in the CB1 and CB2 sequences. S1P1 has a second disulfide loop between an EC-3 loop cysteine and C6.61 which is at the end of TMH6/beginning of the EC-3 loop. This causes TMH6 and TMH7 to pack closely. This second disulfide bridge is not possible for the CB1 and CB2 receptors, since they lack both the cysteine in the EC-3 loop and the cysteine at position 6.61. This suggests that the packing of TMH6/TMH7 in the CB1 and CB2 receptors will deviate from the orientation seen in the S1P1 structure.
6. S1P1 extracellular domain is closed
6.1. N terminus
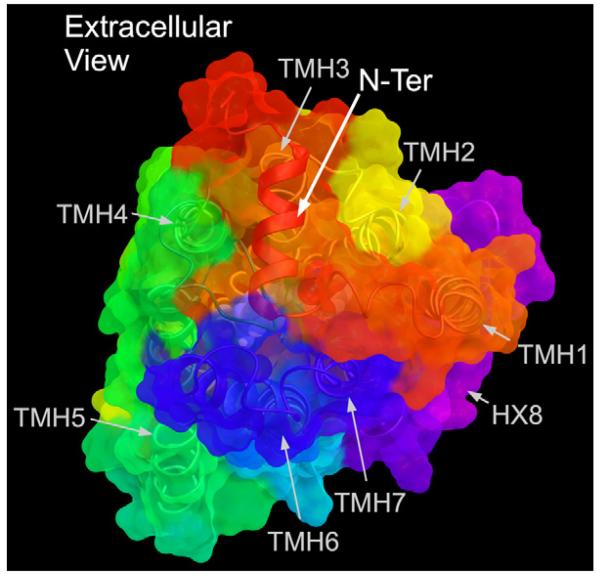
The most striking aspect of the S1P1 crystal structure is that the N-terminus, which contains a helical segment, is packed across the TMH bundle (from TMH3 to TMH6) with the EC-1 and EC-2 loops packing against the N-terminal helix (see Fig. 2). This arrangement occludes ligand access to the receptor from the extracellular milieu (Hanson et al., 2012). It is likely that this is similar in the CB receptors, particularly in CB1 since the N-terminus of CB1 is quite long (112 residues). Mutation and functional studies have shown thus far that the primary function of this long N-terminus in CB1 is to keep the receptor in the endoplasmic reticulum (ER), diminshing cell surface expression (Andersson et al., 2003).
Fig. 2.
The N-terminus of the S1P1 crystal structure (red) contains a helical segment that is packed across the TMH bundle (from TMH3 to TMH6) with the EC-1 and EC-2 loops packing against this N-terminal helix. This arrangement occludes ligand access to the receptor from the extracellular milieu (Hanson et al., 2012).
6.2. Binding site residues
The only positively charged residue facing into the binding pockets of CB1 and CB2 is K3.28. In CB1 this has been shown to be the primary intertaction site for classical and endogenous ligands (Song and Bonner, 1996). S1P1 has a positively charged amino acid facing into the binding pocket at position 3.28 as well (R3.28). However, in contrast to the CB receptors, S1P1 also has a negatively charged residue facing into the binding pocket, E3.29. The presence of these two charged residues suggest that S1P1 should be able to bind negatively charged, positively charged or zwitterionic ligands. In fact S1P, the S1P1 endogenous ligand (see 7, Chart 2), is a zwitterion.
7. S1P1 antagonist binding
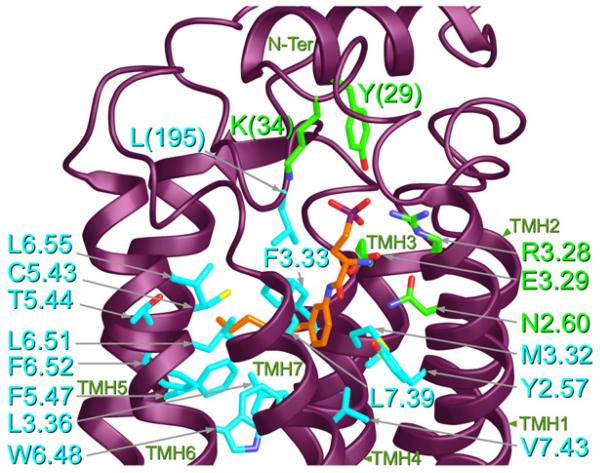
Fig. 3 illustrates the binding pocket for the antagonist, ML056 (10, Chart 2) in the S1P1 crystal structure (Hanson et al., 2012). This ligand is likely protonated at physiological pH, which results in a zwitterionic head group. Three charged residues have been commonly thought to bind the zwitterionic head of the endogenous agonist, S1P1. R3.28(120) and R7.35(292) have been proposed to ion pair with the phosphate group, while E3.29(121) has been proposed to ion pair with the ammonium moiety of sphingoshine 1-phosphate (Parrill et al., 2000). In the antagonist bound S1P1 receptor crystal structure, both R3.28(120) and E3.29(121) interact with the phosphonate and primary amine of the antagonist, ML056. However, R7.53(292) does not interact with the antagonist (this of course does not pre-clude that R7.53 may be important for agonist binding). Instead, ML056 has additional polar interactions with N2.60(101), S2.64(105) and with N-terminal residues, Y29 and K34. The aromatic and alkyl chain regions of ML056 are located in a lipophillic region of S1P1. This region includes Y2.57, M3.32, F3.33, L3.36, C5.43,T5.44, F5.47, W6.48, L6.51, F6.52, L6.55, L7.39, V7.43 and L195 on the EC-2 loop. In the binding site (see Fig. 3), the positioning of ML056 (orange) prevents the toggle switch residue, W6.48, from undergoing the χ1 g+ → trans conformational change characteristic of activation. Thus ML056, maintains S1P1 in its inactive state.
Fig. 3.
This figure illustrates the binding pocket for the antagonist, ML056 (10) in the S1P1 crystal structure (Hanson et al., 2012).
8. Ligand entry from lipid
8.1. S1P1 Receptor
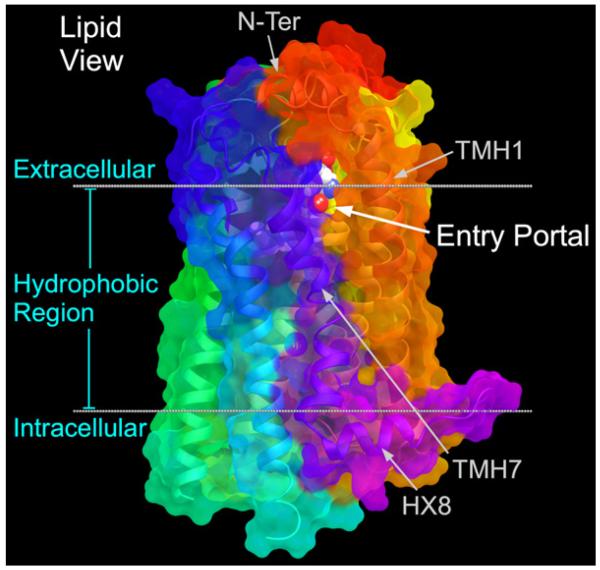
Why would there be limited acccess to the ligand binding pocket from the extracellular milieu? The answer seems to be that S1P1 is designed for ligand approach via the membrane bilayer. As suggested by Hanson and co-workers (Hanson et al., 2012), the S1P1 crystal structure shows a gap between TMH7 and TMH1 through which ligands may gain access to the binding pocket (see Fig. 4). This gap is produced by TMH1 leaning away from the TMH bundle, creating a TMH1/TMH7 gap. In the β2-adrenergic receptor (β2-AR) (Cherezov et al., 2007; Rasmussen et al., 2007), TMH1 also leans away from the TMH bundle creating space for an opening, however, in the β2-AR, this opening is filled by the top of TMH2 and by W7.40 and M1.39 which interact with each other and shield the bundle from the lipid bilayer. In S1P1, TMH2 is straight and does help fill the TMH1/TMH7 opening, but residues 7.40 and 1.39 are much smaller (V7.40 and F1.39). The net result is an opening to the lipid bilayer between TMH1 and TMH7 (see Fig. 4). The limited access to the ligand binding pocket from the extracellular milieu may explain why S1P1 ligands, including S1P, show slow saturation of receptor binding in the presence of excess ligand (Rosen et al., 2009).
Fig. 4.
The S1P1 crystal structure shows a gap between TMH7 and TMH1 through which ligands may gain access to the binding pocket (Hanson et al., 2012).
8.2. Rhodopsin/Opsin
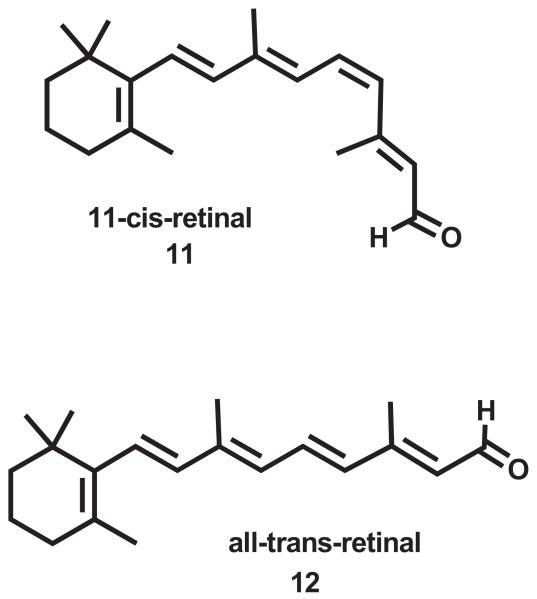
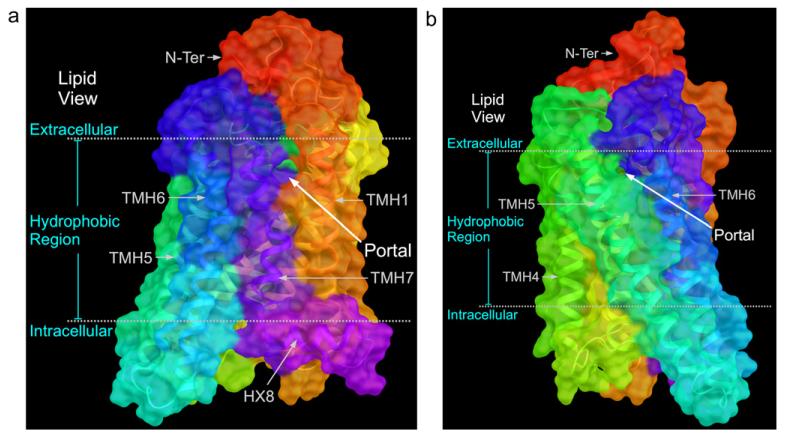
The light activated Class A GPCR, rhodopsin, has an inverse agonist in its ligand binding pocket, 11-cis-retinal (11; Chart 3) which is covalently bound by a protonated Schiff base to K296 in TMH7. Light triggered, 11-cis-retinal isomerization (to all-transretinal (12; Chart 3)) leads to the Schiff-base deprotonated active Meta II intermediate. With Meta II decay, the Schiff-base bond is hydrolyzed, all-trans-retinal is released from the pocket, and the apoprotein, opsin is reloaded with new 11-cis-retinal (Hildebrand et al., 2009). The ligand free apoprotein opsin crystal structure (Park et al., 2008) and the ligand-free opsin structure stabilized by a high affinity peptide derived from the C terminus of the alpha-subunit of the G protein (Ops*-GaCT) (Scheerer et al., 2008) both exist in activated conformations. Here the R3.50/E6.30 ionic lock is broken due to the movement of the intracellular end of TMH6 away from the bundle. Both of these crystal structures show two lipid bilayer-exposed openings between TMH1 and TMH7 and between TMH5 and TMH6 (see Fig. 5A and B) (Hildebrand et al., 2009; Schadel et al., 2003) that have been proposed to represent entry and exit pathways for 11-cis-retinal/trans-retinal as they are shuttled from the lipid bilayer into or out of the protein (Hildebrand et al., 2009). This entry and exit via the lipid bilayer is consistent with the high lipophilicity of the ligands (11 and 12). It is also important to add that the crystal strucures of rhodopsin (Palczewski et al., 2000) and opsin (Park et al., 2008; Scheerer et al., 2008) possess extracellular domains that shield the protein from ligand entrance via the extracellular space. Thus, rhodopsin/opsin represents another case of a GPCR whose lipophilic ligands may gain access to the ligand binding pocket via the lipid bilayer.
Chart 3.
This figure illustrates the structure of the chromophore for rhodopsin, 11-cis-retinal (11), and the structure to which this chromophore is converted by light, all trans-retinal (12).
Fig. 5.
The ligand free apoprotein opsin crystal structure (Park et al., 2008) is illustrated here. In (A), the view point is from the lipid bilayer looking towards TMH7 and TMH1. Here one can see that an opening between TMH7 and TMH1 exists. In (B), the view point is from the lipid bilayer looking towards TMH5 and TMH6. Here one can see that an opening between TMH5 and TMH6 also exists The openings illustrated in (A) and (B) have been proposed to be portals such that ligand movement from the lipid bilayer into opsin would be possible (Hildebrand et al., 2009; Schadel et al., 2003).
8.3. Cannabinoid receptors
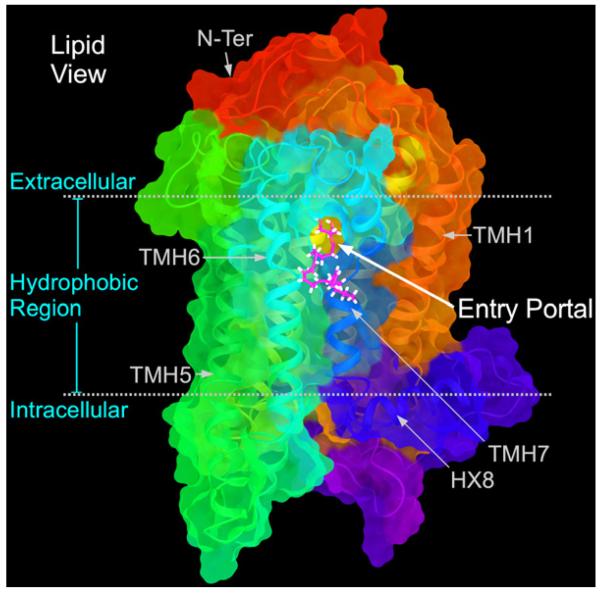
Experimental and computational studies of the CB receptors have also suggested a lipid portal for cannabinoid ligand entry. Isothiocyanate covalent labeling studies have suggested that a classical cannabinoid, (–)-7′-isothiocyanato-11-hydroxy-1′,1′ dimethylheptyl-hexahydrocannabinol (AM841), enters the cannabinoid CB1 receptor via the lipid bilayer at the level of C6.47 (Picone et al., 2005) (a CWXP motif residue that faces lipid), forming a covalent bond with this residue. Similar results were found for the CB2 receptor (Pei et al., 2008). Microsecond timescale molecular dynamics simulations of the interaction of the endogenous cannabinoid, 2-AG (4, Chart 1) with the CB2 receptor in a palmitoyl-oleoyl-phosphatidylcholine (POPC) lipid bilayer have suggested that (1) 2-AG first partitions out of bulk lipid at the TMH6/7 interface; (2) 2-AG then enters the CB2 receptor binding pocket by passing between TMH6/7; (3) the entrance of the 2-AG head group into the CB2 binding pocket is sufficient to trigger activation of CB2 (Hurst et al., 2010). Fig. 6 illustrates the opening that forms between TMH6 and TMH7 in CB2 as 2-AG is poised to enter the receptor from lipid. Fig. 7 illustrates the progress that 2-AG makes into the CB2 binding site over the microsecond long molecular dynamics simulation. Taken together, these studies suggest that the lipid portal for cannabinoids is TMH6/7.
Fig. 6.
This figure illustrates the result of molecular dynamics simulations of endogenous cannabinoid, 2-AG (4) binding to the membrane embedded CB2 receptor. Here an opening forms between TMH6 and TMH7 in the CB2 receptor as 2-AG (magenta) is poised to enter the receptor from the lipid bilayer (Hurst et al., 2010).
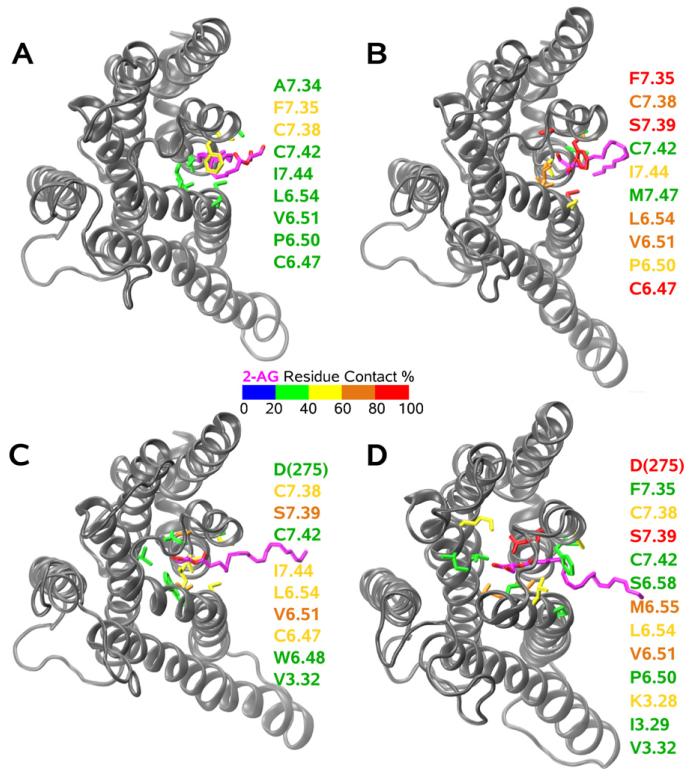
Fig. 7.
This figure illustrates the progress of 2-AG into the CB2 binding pocket. The color scale represents the percentage of the trajectory in which any portion of 2-AG is within 4Å of residues on CB2 (defined here as within contact distance). Residues within contact distance are listed on the right and are color coded according to this scale. The view is from the extracellular side of the receptor. (A) 2-AG has partitioned out of bulk lipid and contacts residues in or near the TMH6/7 interface. Highest contact is with F7.35(281) and C7.38(284). (B) 2-AG interaction with residues in the TMH6/7 interface increases with greater than 80% contact ocurring with F7.35(281), S7.39(285) and C6.47(257). (C) 2-AG begins to contact binding pocket residues on TMH3 (V3.32(113)), TMH6 (W6.48(258)), TMH7 (C7.42(288)) and the EC-3 loop (D(275)). (D) Subsequent to protonation, 2-AG contacts multiple residues on TMH3/6/7 and the EC-3 loop with formation of hydrogen bonds with D(275) in the EC-3 loop and to a lesser extent with S7.39(285) (Hurst et al., 2010).
A recent paper from the D.E. Shaw group (Dror et al., 2012) that explored ligand entry into the β1- and β2-AR, reported the existence of a “vestibule” in the EC regions of the β1- and β2-ARs to which ligands bound before entering the binding pocket. An analogy of this appears to exist for “lipid binding pathway” receptors like the cannabinoid receptors as our simulations have suggested that 2-AG initially partitions out of bulk lipid and associates with the lipid face of TMH6/TMH7 first, before entry into CB2 (Hurst et al., 2010).
9. Conclusions
Although they all share a common topology, every Class A GPCR has sequence dictated conformational differences in their transmembrane helix domains that make each receptor structure unique. Having said this, the S1P1 X-ray crystal structure has two important features that would appear to be important for CB1 and CB2 structures: (1) a closed extracellular domain and (2) an opening between transmembrane helices that allows ligand to pass from the lipid bilayer and into the ligand binding pocket.
Acknowledgements
This work was supported by NIDA grants RO1 DA003934 and KO5 DA021358.
Abbreviations
- MD
molecular dynamics
- 2-AGPI
2-arachidonoyl-sn-glycero-3-phosphoinositol
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- AEA
N-arachidonoylethanolamine
- TMH
transmembrane helix
- GPCR
G protein-coupled receptor
- EDG
endothelial differentiation gene
- LPA
lysophosphatidic acid
- S1P
spinghosine-1-phosphate
References
- Abadji V, Lucas-Lenard JM, Chin C, Kendall DA. Involvement of the carboxyl terminus of the third intracellular loop of the cannabinoid CB1 receptor in constitutive activation of Gs. Journal of Neurochemistry. 1999;72:2032–2038. doi: 10.1046/j.1471-4159.1999.0722032.x. [DOI] [PubMed] [Google Scholar]
- Andersson H, D’Antona AM, Kendall DA, Von Heijne G, Chin CN. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Molecular Pharmacology. 2003;64:570–577. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- Azad SC, Kurz J, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of CB1 specifically located on GABAergic interneurons inhibits LTD in the lateral amygdala. Learning and Memory. 2008;15:143–152. doi: 10.1101/lm.741908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros J, Weinstein H. Integrated methods for the construction of three-dimensional models and computational modeling of structure-function relations in G-protein-coupled receptors. Methods in Neurosciences. 1995;25:366–428. [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. Journal of Neuroscience. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Molecular Pharmacology. 1999;55:473–480. [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. European Journal of Biochemistry. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. European Journal of Biochemistry. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Bramblett RD, Panu AM, Ballesteros JA, Reggio PH. Construction of a 3D model of the cannabinoid CB1 receptor: determination of helix ends and helix orientation. Life Sciences. 1995;56:1971–1982. doi: 10.1016/0024-3205(95)00178-9. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Lambert JM, Gerfin S, Huffman JW, Razdan RK. Sensitivity to delta9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2 −/− mice. Behavioural Pharmacology. 2008;19:298–307. doi: 10.1097/FBP.0b013e328308f1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Reviews Drug Discovery. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. European Journal of Pharmacology. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC. Structure of the human dopamine d3 receptor in complex with a d2/d3 selective antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J. Lysophospholipid receptors: implications for neural signaling. Critical Reviews in Neurobiology. 1999;13:151–168. doi: 10.1615/critrevneurobiol.v13.i2.20. [DOI] [PubMed] [Google Scholar]
- Chun J. Lysophospholipids in the nervous system. Prostaglandins and Other Lipid Mediators. 2005;77:46–51. doi: 10.1016/j.prostaglandins.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Chun J, Contos JJ, Munroe D. A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs) Cell Biochemistry and Biophysics. 1999;30:213–242. doi: 10.1007/BF02738068. [DOI] [PubMed] [Google Scholar]
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Weiner JA, Fukushima N, Contos JJ, Zhang G, Kimura Y, Dubin A, Ishii I, Hecht JH, Akita C, Kaushal D. Neurobiology of receptor-mediated lysophospholipid signaling, from the first lysophospholipid receptor to roles in nervous system function and development. Annals of the New York Academy of Sciences. 2000;905:110–117. doi: 10.1111/j.1749-6632.2000.tb06543.x. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Marini P, Matias I, Moriello AS, Starowicz K, Cristino L, Nigam S, Di Marzo V. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma beta-cells. Experimental Cell Research. 2007;313:2993–3004. doi: 10.1016/j.yexcr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nature Reviews Drug Discovery. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror RO, Pan AC, Arlow DH, Borhani DW, Maragakis P, Shan Y, Xu H, Shaw DE. Pathway and mechanism of drug binding to G-protein-coupled receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;108:13118–13123. doi: 10.1073/pnas.1104614108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds Y, Milstien S, Spiegel S. Development of small-molecule inhibitors of sphingosine-1-phosphate signaling. Pharmacol Ther. 2011;132:352–360. doi: 10.1016/j.pharmthera.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Molecular Pharmacology. 1995;48:443–450. [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. European Journal of Pharmacology. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochemical Journal. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. Journal of Neuroscience. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graler MH. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cellular Physiology and Biochemistry. 2010;26:79–86. doi: 10.1159/000315108. [DOI] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M, Galve-Roperh I, Sanchez C. Ceramide: a new second messenger of cannabinoid action. Trends in Pharmacological Sciences. 2001;22:19–22. doi: 10.1016/s0165-6147(00)01586-8. [DOI] [PubMed] [Google Scholar]
- Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, Weis WI, Okada T, Kobilka BK, Haga T, Kobayashi T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547–551. doi: 10.1038/nature10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm HE, Deretic D, Arendt A, Hargrave PA, Koenig B, Hofmann KP. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988;241:832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand PW, Scheerer P, Park JH, Choe HW, Piechnick R, Ernst OP, Hofmann KP, Heck M. A ligand channel through the G protein coupled receptor opsin. PLoS ONE. 2009;4:e4382. doi: 10.1371/journal.pone.0004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handbook of Experimental Pharmacology. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Molecular Pharmacology. 1986;29:307–313. [PubMed] [Google Scholar]
- Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, McAllister S, Fleischer D, Kapur A, Abood M, Shi S, Jones J, Lewis D, Reggio P. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine 3.28(192) interaction. Journal of Medicinal Chemistry. 2006;49:5969–5987. doi: 10.1021/jm060446b. [DOI] [PubMed] [Google Scholar]
- Hurst DP, Grossfield A, Lynch DL, Feller S, Romo TD, Gawrisch K, Pitman MC, Reggio PH. A lipid pathway for ligand binding is necessary for a cannabinoid G protein-coupled receptor. Journal of Biological Chemistry. 2010;285:17954–17964. doi: 10.1074/jbc.M109.041590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AI, van’t Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, Ralston SH. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nature Medicine. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Heise CE, Ancellin N, O’Dowd BF, Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. Journal of Biological Chemistry. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. British Journal of Pharmacology. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annual Review of Biochemistry. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- Kapur A, Hurst DP, Fleischer D, Whitnell R, Thakur GA, Makriyannis A, Reggio PH, Abood ME. Mutation studies of S7.39 and S2.60 in the human CB1 cannabinoid receptor: evidence for a serine induced bend in CB1 transmembrane helix 7. Molecular Pharmacology. 2007;71:1512–1524. doi: 10.1124/mol.107.034645. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Molecular Pharmacology. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, Sugiura T. 2-arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. Journal of Biological Chemistry. 2003;278:24469–24475. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Kobayashi Y, Oka S, Gokoh M, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. Journal of Biochemistry. 2004;135:517–524. doi: 10.1093/jb/mvh063. [DOI] [PubMed] [Google Scholar]
- Kouznetsova M, Kelley B, Shen M, Thayer SA. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Molecular Pharmacology. 2002;61:477–485. doi: 10.1124/mol.61.3.477. [DOI] [PubMed] [Google Scholar]
- Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, Shaw DE, Weis WI, Wess J, Kobilka BK. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. Journal of Molecular Biology. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. Journal of Neuroscience. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the Muopioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Fuzio D, Vigano D, Sacerdote P, Parolaro D. Relative involvement of cannabinoid CB(1) and CB(2) receptors in the Delta(9)-tetrahydrocannabinol-induced inhibition of natural killer activity. European Journal of Pharmacology. 2000;387:343–347. doi: 10.1016/s0014-2999(99)00860-2. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Hurst DP, Barnett-Norris J, Lynch D, Reggio PH, Abood ME. Structural mimicry in class A G protein-coupled receptor rotamer toggle switches: the importance of the F3.36(201)/W6.48(357) interaction in cannabinoid CB1 receptor activation. Journal of Biological Chemistry. 2004;279:48024–48037. doi: 10.1074/jbc.M406648200. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Rizvi G, Anavi-Goffer S, Hurst DP, Barnett-Norris J, Lynch DL, Reggio PH, Abood ME. An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. Journal of Medicinal Chemistry. 2003;46:5139–5152. doi: 10.1021/jm0302647. [DOI] [PubMed] [Google Scholar]
- McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. Journal of Pharmacology and Experimental Therapeutics. 1999;289:1620–1625. [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Annals of Neurology. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- Moukhametzianov R, Warne T, Edwards PC, Serrano-Vega MJ, Leslie AG, Tate CG, Schertler GF. Two distinct conformations of helix 6 observed in antagonist-bound structures of a {beta}1-adrenergic receptor. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8228–8232. doi: 10.1073/pnas.1100185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Fujiyoshi Y, Silow M, Navarro J, Landau EM, Shichida Y. Functional role of internal water molecules in rhodopsin revealed by X-ray crystallography. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Molecular Pharmacology. 1996;49:707–714. [PubMed] [Google Scholar]
- Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- Parrill AL, Sardar VM, Yuan H. Sphingosine 1-phosphate and lysophos-phatidic acid receptors: agonist and antagonist binding and progress toward development of receptor-specific ligands. Seminars in Cell and Developmental Biology. 2004;15:467–476. doi: 10.1016/j.semcdb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Parrill AL, Wang D, Bautista DL, Van Brocklyn JR, Lorincz Z, Fischer DJ, Baker DL, Liliom K, Spiegel S, Tigyi G. Identification of Edg1 receptor residues that recognize sphingosine 1-phosphate. Journal of Biological Chemistry. 2000;275:39379–39384. doi: 10.1074/jbc.M007680200. [DOI] [PubMed] [Google Scholar]
- Pei Y, Mercier RW, Anday JK, Thakur GA, Zvonok AM, Hurst D, Reggio PH, Janero DR, Makriyannis A. Ligand-binding architecture of human CB2 cannabinoid receptor: evidence for receptor subtype-specific binding motif and modeling GPCR activation. Chemistry and Biology. 2008;15:1207–1219. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacology and therapeutics. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Picone RP, Khanolkar AD, Xu W, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (–)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Molecular Pharmacology. 2005;68:1623–1635. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta 2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Rasmussen SGF, Choi H-J, Fung JJ, Pardon E, Casarosa P, Chae PS, DeVree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the beta-2-adrenoreceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio PH. Cannabinoid receptors and their ligands: ligand-ligand and ligand-receptor modeling approaches. Handbook of Experimental Pharmacology. 2005;247:281. [PubMed] [Google Scholar]
- Rice W, Shannon JM, Burton F, Fiedeldey D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar Type II cells in the lung: regulation by hydrocortisone. European Journal of Pharmacology. 1997;327:227–232. doi: 10.1016/s0014-2999(97)89665-3. [DOI] [PubMed] [Google Scholar]
- Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF, Haffaf Y, Cesari M, Festy F. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochemistry and Cell Biology. 2006;126:177–187. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annual Review of Biochemistry. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cellular Signalling. 2003;15:851–859. doi: 10.1016/s0898-6568(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. Journal of Cellular Biochemistry. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chemical Biology. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Schadel SA, Heck M, Maretzki D, Filipek S, Teller DC, Palczewski K, Hofmann KP. Ligand channeling within a G-protein-coupled receptor, the entry and exit of retinals in native opsin. Journal of Biological Chemistry. 2003;278:24896–24903. doi: 10.1074/jbc.M302115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Schuel H, Chang MC, Burkman LJ, Picone RP, Makriyannis A, Zimmerman AM, Zimmerman S. Cannabinoid Receptors in Sperm Marijuana and Medicine. Humana Press; Totowa: 1999. pp. 335–345. [Google Scholar]
- Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA. Beta2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. Journal of Biological Chemistry. 2002;277:40989–40996. doi: 10.1074/jbc.M206801200. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, Kobayashi T, Stevens RC, Iwata S. Structure of the human histamine H1 receptor complex with doxepin. Nature. 2011;475:65–70. doi: 10.1038/nature10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZH, Bonner TI. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Molecular Pharmacology. 1996;49:891–896. [PubMed] [Google Scholar]
- Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Investigative Ophthalmology and Visual Science. 1999;40:2442–2448. [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochemical and Biophysical Research Communications. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, Roth BL, Cherezov V, Stevens RC. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature. 2012;485:395–399. doi: 10.1038/nature11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. Journal of Molecular Endocrinology. 2009;44:75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta-1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson K, Sankala H, Milstien S, Spiegel S. Pleiotropic actions of sphingosine-1-phosphate. Progress in Lipid Research. 2003;42:344–357. doi: 10.1016/s0163-7827(03)00015-8. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nature Neuroscience. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Contos JJ, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- Zhang R, Hurst DP, Barnett-Norris J, Reggio PH, Song ZH. Cysteine 2.59(89) in the Second transmembrane domain of human Cb2 receptor is accessible within the ligand binding crevice: evidence for possible Cb2 deviation from a rhodopsin template. Molecular Pharmacology. 2005;68:69–83. doi: 10.1124/mol.104.007823. [DOI] [PubMed] [Google Scholar]