Abstract
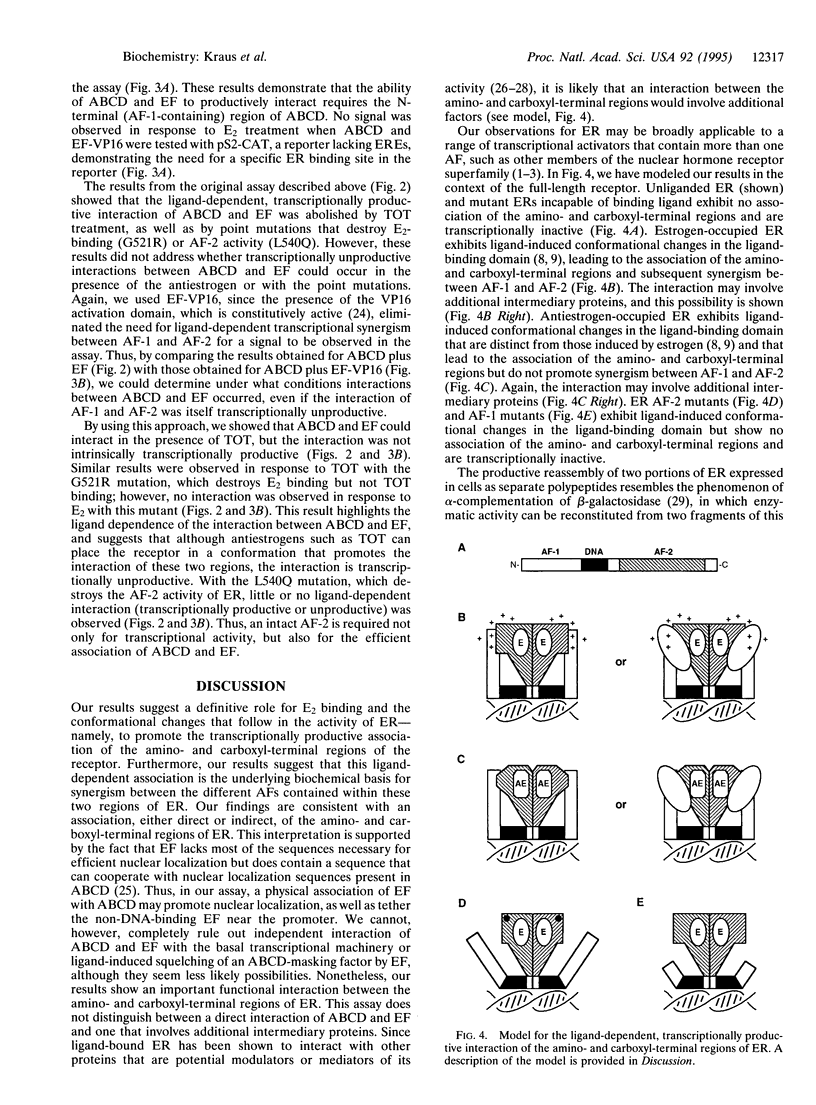
The estrogen receptor (ER), a 66-kDa protein that mediates the actions of estrogens in estrogen-responsive tissues, is a member of a large superfamily of nuclear hormone receptors that function as ligand-activated transcription factors. ER shares a conserved structural and functional organization with other members of this superfamily, including two transcriptional activation functions (AFs), one located in its amino-terminal region (AF-1) and the second located in its carboxyl-terminal, ligand-binding region (AF-2). In most promoter contexts, synergism between AF-1 and AF-2 is required for full ER activity. In these studies, we demonstrate a functional interaction of the two AF-containing regions of ER, when expressed as separate polypeptides in mammalian cells, in response to 17 beta-estradiol (E2) and antiestrogen binding. The interaction was transcriptionally productive only in response to E2, and was eliminated by point or deletion mutations that destroy AF-1 or AF-2 activity or E2 binding. Our results suggest a definitive mechanistic role for E2 in the activity of ER--namely, to alter receptor conformation to promote an association of the amino- and carboxyl-terminal regions, leading to transcriptional synergism between AF-1 and AF-2. The productive re assembly of two portions of ER expressed in cells as separate polypeptides demonstrates the evolutionarily conserved modular structural and functional organization of the nuclear hormone receptors. The ligand-dependent interaction of the two AF-containing regions of ER allows for the assembly of a complete activation function from two distinct regions within the same protein, providing a mechanism for hormonally regulated transcription.
Full text
PDF
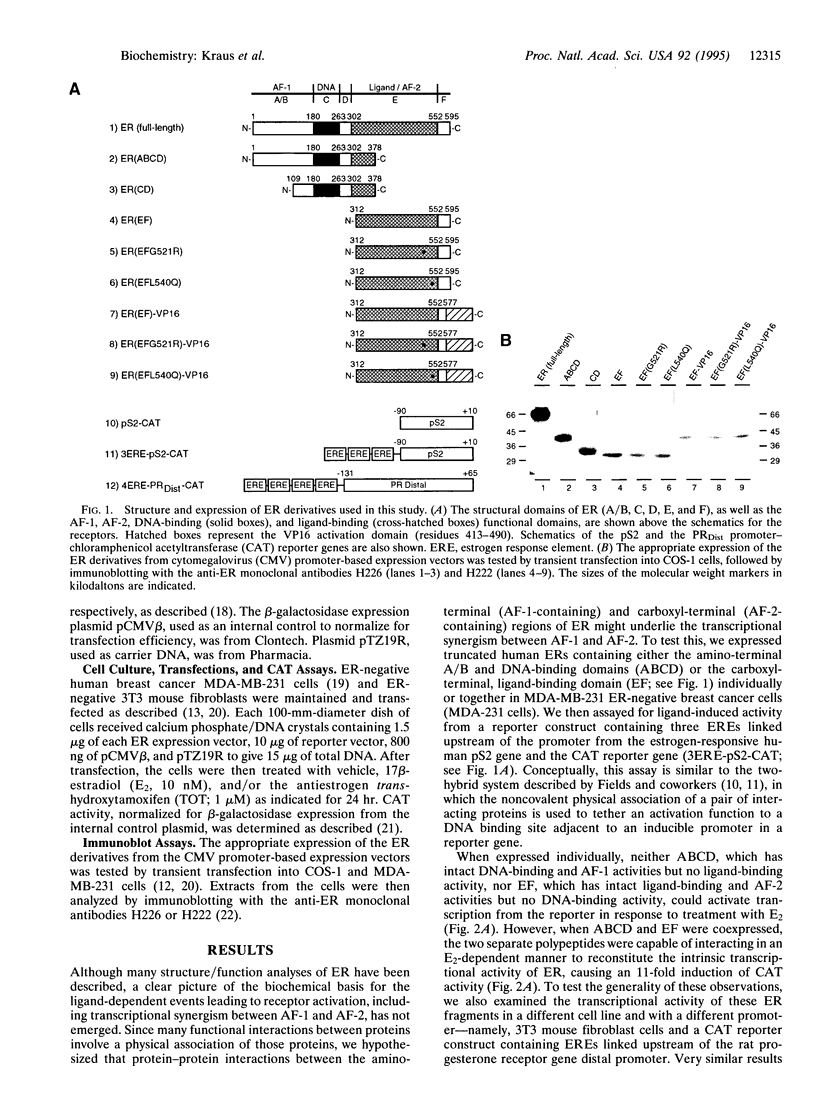
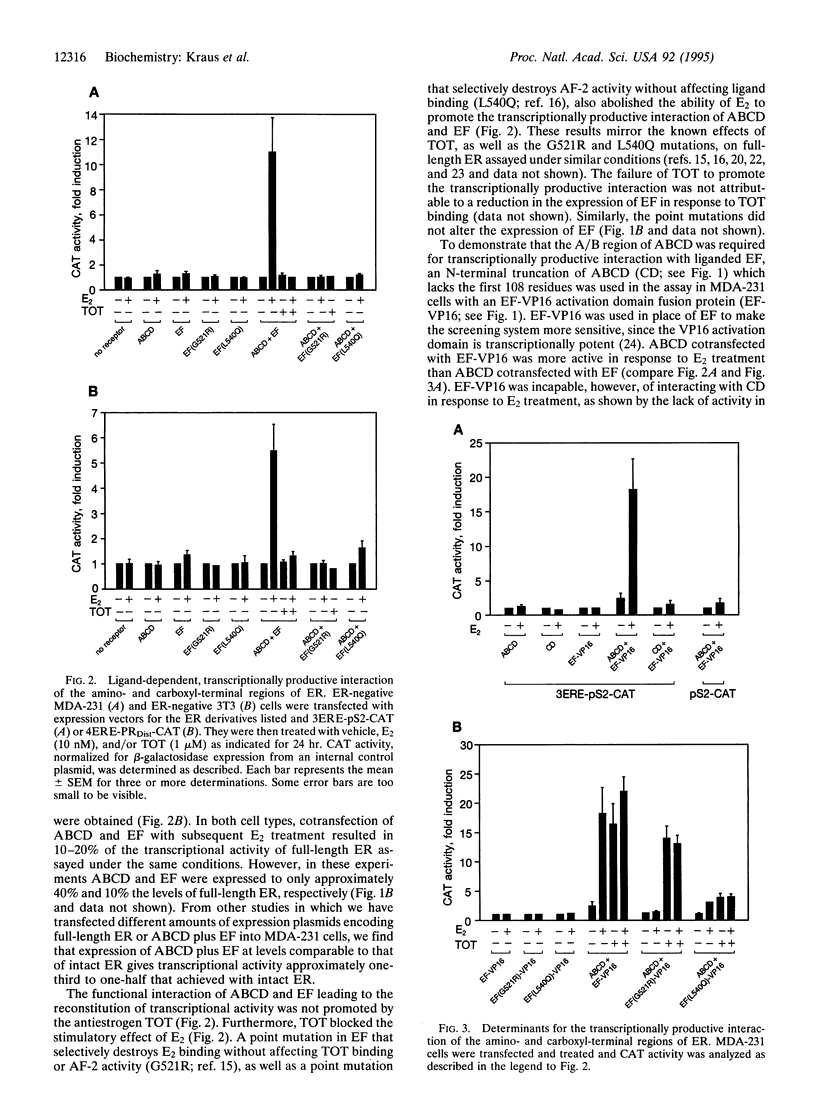

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beekman J. M., Allan G. F., Tsai S. Y., Tsai M. J., O'Malley B. W. Transcriptional activation by the estrogen receptor requires a conformational change in the ligand binding domain. Mol Endocrinol. 1993 Oct;7(10):1266–1274. doi: 10.1210/mend.7.10.8264659. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993 Nov;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Cavaillès V., Dauvois S., Danielian P. S., Parker M. G. Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10009–10013. doi: 10.1073/pnas.91.21.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P. S., White R., Hoare S. A., Fawell S. E., Parker M. G. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993 Feb;7(2):232–240. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fritsch M., Leary C. M., Furlow J. D., Ahrens H., Schuh T. J., Mueller G. C., Gorski J. A ligand-induced conformational change in the estrogen receptor is localized in the steroid binding domain. Biochemistry. 1992 Jun 16;31(23):5303–5311. doi: 10.1021/bi00138a009. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Halachmi S., Marden E., Martin G., MacKay H., Abbondanza C., Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994 Jun 3;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Hardie G. Pseudosubstrates turn off protein kinases. Nature. 1988 Oct 13;335(6191):592–593. doi: 10.1038/335592a0. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Zava D. T., Thilagar A. K., Jensen E. M., McGuire W. L. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978 Aug;38(8):2434–2437. [PubMed] [Google Scholar]
- Jordan V. C., Murphy C. S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990 Nov;11(4):578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Montano M. M., Katzenellenbogen B. S. Cloning of the rat progesterone receptor gene 5'-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993 Dec;7(12):1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Montano M. M., Katzenellenbogen B. S. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994 Aug;8(8):952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Weis K. E., Katzenellenbogen B. S. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol. 1995 Apr;15(4):1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Le Douarin B., Zechel C., Garnier J. M., Lutz Y., Tora L., Pierrat P., Heery D., Gronemeyer H., Chambon P., Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995 May 1;14(9):2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M. M., Müller V., Trobaugh A., Katzenellenbogen B. S. The carboxy-terminal F domain of the human estrogen receptor: role in the transcriptional activity of the receptor and the effectiveness of antiestrogens as estrogen antagonists. Mol Endocrinol. 1995 Jul;9(7):814–825. doi: 10.1210/mend.9.7.7476965. [DOI] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Differential DNA-binding abilities of estrogen receptor occupied with two classes of antiestrogens: studies using human estrogen receptor overexpressed in mammalian cells. Nucleic Acids Res. 1991 Dec 11;19(23):6595–6602. doi: 10.1093/nar/19.23.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Mutagenesis of cysteines in the hormone binding domain of the human estrogen receptor. Alterations in binding and transcriptional activation by covalently and reversibly attaching ligands. J Biol Chem. 1991 Jun 15;266(17):10880–10887. [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. S. Protein kinases: a diverse family of related proteins. Bioessays. 1987 Jul;7(1):24–29. doi: 10.1002/bies.950070106. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., LaMarco K. L., McKnight S. L. Evidence of DNA: protein interactions that mediate HSV-1 immediate early gene activation by VP16. Genes Dev. 1988 Jun;2(6):730–742. doi: 10.1101/gad.2.6.730. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tzukerman M. T., Esty A., Santiso-Mere D., Danielian P., Parker M. G., Stein R. B., Pike J. W., McDonnell D. P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994 Jan;8(1):21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Wrenn C. K., Katzenellenbogen B. S. Cross-linking of estrogen receptor to chromatin in intact MCF-7 human breast cancer cells: optimization and effect of ligand. Mol Endocrinol. 1990 Nov;4(11):1647–1654. doi: 10.1210/mend-4-11-1647. [DOI] [PubMed] [Google Scholar]
- Wrenn C. K., Katzenellenbogen B. S. Structure-function analysis of the hormone binding domain of the human estrogen receptor by region-specific mutagenesis and phenotypic screening in yeast. J Biol Chem. 1993 Nov 15;268(32):24089–24098. [PubMed] [Google Scholar]
- Ylikomi T., Bocquel M. T., Berry M., Gronemeyer H., Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 1992 Oct;11(10):3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]