Abstract
Cell penetrating peptides (CPP) have been widely used to increase the cellular delivery of their associated cargo. Multiple modes of uptake have been identified, however they cannot be predicted apriori. Elucidating these mechanisms is important for understanding peptide function as well as further optimizing cellular delivery. We have developed a class of MK2 inhibitor peptides, named FAK and YARA that utilize CPP domains to gain cellular access. In this study, we investigate the mechanism of endocytosis of these MK2 inhibitors by examining the uptake of fluorescently labeled peptide in human monocyte (THP-1) and mesothelial cells, and looking for colocalization with known markers of endocytosis. Our results indicate that uptake of the MK2 inhibitors was minimally enhanced by the addition of the fluorescent label, and that the type of endocytosis used by the inhibitor depends on several factors including concentration, cell type, and which CPP was used. We found that in THP-1 cells uptake of YARA occurred primarily via macropinocytosis, while FAK entered via all three mechanisms of endocytosis examined in this study. In mesothelial cells, uptake of YARA occurred via caveolae-mediated endocytosis, but became less specific at higher concentrations; while uptake of FAK occurred through clathrin-mediated endocytosis. In all cases, the delivery resulted in active inhibition of MK2. In summary, the results support endocytic uptake of fluorescently labeled FAK and YARA in two different cell lines, with the mechanism of uptake dependent on extracellular concentration, cell type, and choice of CPP.
Introduction
Cell penetrating peptides (CPPs) have successfully delivered a variety of cargo to the interior of cells, including peptides, antibodies [1, 2], nanoparticles [3], oliognucleotides [4], siRNA [5], adenoviruses [6], and DNA for more than two decades [7, 8]. CPPs circumvent the need for permeation methods like electroporation and chemical transfection that might damage the cell membrane. CPPs, which have also been referred to as protein transduction domains, are typically defined as less than 30 amino acids in length, are amphipathic, contain a net positive charge, and most importantly, can deliver cargo across the plasma cell membrane. Several hydrophobic CPP sequences have also been identified.
The most widely used CPP for cargo delivery is the human immunodeficiency virus-transactivator of transcription (HIV-Tat or simply, Tat). The ability of Tat to cross the cell membrane was discovered in 1988 by two separate research groups [9, 10]. Nine years later, Vives et al. identified the minimum amino acid sequence required for Tat to transduce the cell membrane [11]. Antennapedia (Antp), which is a 16-amino acid CPP from a Drosophilia transcription factor, was the first CPP used to demonstrate in vitro intracellular protein delivery [12]. In 1999, Schwareze et al. reported the ability of Tat to deliver cargo in vivo 13]. Once the potential of CPPs was discovered, investigators focused on expanding the repertoire of CPPs in an effort to maximize transduction efficiency of various cargo. Two reviews providing information on many additional CPPs include a recent review by Jones and Sayers [14] and one by Brugnano, et al. [15].
Uptake of CPPs is traditionally confirmed with visualization of fluorescently labeled peptides within cells, either with microscopy or fluorescence-activated cell sorting (FACs). However, the mechanism by which CPPs are able to translocate into the cell cytoplasm has been an issue of debate since CPPs were discovered. It is generally agreed that CPP uptake occurs via endocytosis; however, there is mounting evidence that, like antimicrobial peptides, some CPPs can translocate through the membrane using some mode of membrane perturbation [14, 16, 17]. While many CPPs enter cells through endocytosis, clarifying whether it occurs through clathrin, caveolae, macropinocytosis, or one of the newly discovered modes of endocytosis, including an apparent ligand-based mode [18] and other clatherin-independent modes[19] is still highly controversial. The specific pathway of endocytosis is dependent upon several factors including the amino acid sequence of the CPP, the cargo being delivered, the extracellular peptide concentration, and the proteoglycan concentration on the cell surface [20, 21]. Recently, Gump et al. investigated the effect of surface glycosaminoglycans and sialic acid on the transduction of Tat [22]. A deficiency in surface sialic acids and glycosaminoglycans significantly reduced Tat uptake but did not entirely inhibit transduction. Interestingly, it was the enzymatic removal of all cell surface proteins that completely inhibited the uptake of TAT[22]. The identity of these crucial cell surface proteins has yet to be discovered, and the importance of these proteins in the uptake of other CPPs has yet met minimal exploration.
Initially, pinpointing the mechanism of uptake of various cell-penetrating peptides was controversial. One of the reasons for this controversy in uptake was elegantly shown by Lebleu’s group in which they discovered that processing of cells, specifically fixing cells, dramatically altered the intracellular location of the cell penetrating peptides [23]. As described above, it has now become clear that CPP and cargo identity as well as other environmental conditions can affect the mode of uptake. It is also clear that CPPs can enter cells through endocytosis and through some means of membrane perturbation [24–26]. However, even in unfixed cells, appropriate measures must be taken to ensure that the signal observed is actually within the cell and not simply bound to cell surface. The positive charge of cell-penetrating peptides facilitates surface binding to cells, which often express negatively charged glycosaminoglycans on their surface [22]. Although the mechanism of transduction of CPPs is still debated, it is accepted that CPPs can successfully deliver cargo, like bioactive peptides, into a wide variety of cells. Several investigators have been interested in delivering pro-apoptotic peptides as a means of inhibiting the growth of cancer cells [27–29]. Others have used CPPs to inhibit kinases crucial for the proliferation of cancer cells [30, 31].
Our laboratory has designed a family of peptide therapeutics that utilize cell-penetrating peptides to gain intracellular access. These therapies consist of two domains: a cell penetrating peptide that delivers the cargo into the cell and a cargo domain, that was designed to inhibit the serine/threonine kinase mitogen activated protein kinase activated protein kinase 2 (MK2). We showed previously that the cell-penetrating peptide affected the specificity of the kinase inhibition [32]; we also showed a small effect of CPP on the peptide inhibition of MK2, with the FAK MK2 inhibitor peptide demonstrating an IC50 of 1.8 μM and the YARA MK2 inhibitor peptide exhibiting an IC50 of 5.6 μM [30]. These peptides have demonstrated promising results in in vitro, ex vivo, and in vivo systems [33–38], suggesting that the therapies gain intracellular access; however, neither visual confirmation of uptake of these peptides, nor investigation of the mode of uptake were investigated previously. We describe the uptake of two MK2-inhibitor peptides called YARA and FAK (Table 3) via live cell imaging using confocal microscopy and flow cytometry. In addition, inhibitors of endocytosis are used to show differential modes of uptake in differing cell types.
Materials and Methods
Peptide Synthesis
MK2-inhibitor peptides (Table 3.1) were synthesized on Knorr-amide resin (Synbiosci Corp.) using standard FMOC chemistry. Two different chemistries were used to couple each amino acid. The first coupling reagents were N-hydroxybenzotriazole (HoBt)/N, N’-diisopropylcarbodiimide (DIC) and the second coupling reagents were 2-(1Hbenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and lutidine. For FITC-labeled FAK, aminohexanoic acid was added to N-terminus to serve as a spacer for the addition of FITC isomer 1 (Molecular Probes). For FITC-labeled YARA, β-alanine was added to the N-terminus to serve as a spacer for the addition of the FITC isomer. The FITC isomer was solubilized in 12:7:5 pyridine/DMF/DCM and incubated with the deprotected peptide overnight. A ninhydrin test was used to check complete coupling of FITC to the peptide. Following synthesis, the peptide was cleaved from the resin with a trifluoroacetic acid-based cocktail, precipitated in ether and purified using an acetonitrile gradient with a constant concentration of 0.1% trifluoroacetic acid on an FPLC (ÄKTA Explorer, GE Healthcare) equipped with a 22/250 C18 prep-scale column (Grace Davidson). Molecular weight was confirmed by time-of-flight MALDI mass spectrometry using a 4800 Plus MALDI TOF/TOF™ Analyzer (Applied Biosystems).
Cell Culture
Two different cell lines were used to characterize mechanism of uptake, an immortalized human monocyte cell line (THP-1, ATCC) and an immortalized human mesothelial cell line (ATCC CRL-9444). Cells were maintained at 37°C with 5% CO2 and were used between passages 3 and 12.
Mesothelial Cells
Mesothelial cell media consisted of Media 199 with Earle’s basic salt solution and 0.75mM L-glutamine (Mediatech, Inc.) supplemented with 1.25 g/L sodium bicarbonate (Sigma), 3.3nM epidermal growth factor (EGF; MBL International), 20 mM HEPES (Sigma), trace elements mixture B (Mediatech, Inc.), 10% fetal bovine serum (FBS; Mediatech, Inc.), and 1% penicillin/streptomycin (Mediatech, Inc.). For colocalization experiments, cells were seeded in 4- or 8-well chamber slides (Company) at a concentration of 150,000 cells/ml with a total of 200μl (8-well chamber slide) or 500μl (4 well chamber slide). For flow cytometry studies, cells were seeded in 12-well plates (Greiner-One) at a concentration of 250,000 cells/ml and a total of 2 ml was added per well. Cells were allowed to adhere and grow overnight.
THP-1 Cells
THP-1 cells were grown in RPMI 1640 with L-glutamine (Mediatech Inc, Manassas, VA) supplemented with 0.05 mM β-mercaptoethanol (Sigma-Aldrich), 10 mM HEPES (Mediatech Inc), 1 mM sodium pyruvate (Mediatech Inc), 10% fetal bovine serum (Hyclone) and 1% penicillin/streptomycin (Mediatech Inc). For colocalization experiments, cells were seeded in 4- or 8-well chamber slides at a concentration of 250,000 cells/ml with a total volume of 200μl (8-well chamber slide) or 500μl (4 well chamber slide). For flow cytometry experiments, cells were seeded in 12-well plates at a concentration of 250,000 cells/ml with a total volume of 2 ml added to each well. Cells were treated with 10 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for 48 hours to induce differentiation, which was confirmed by the monocytes becoming adherent.
Uptake Studies Using Confocal Microscopy: Colocalization Studies
To determine the type of endocytic uptake, markers of the three major types of endocytosis were identified (Table 2). The markers were used at a final concentration of 25μg/ml for texas red labeled transferrin (Invitrogen), 10 μg/ml for alexa fluor 594 labeled cholera toxin subunit B (CTXB; Invitrogen), and 1 mg/ml for texas red labeled dextran, MW 70,000 (Invitrogen). Cells were treated with FITC labeled peptide (30 μM for FITC-FAK and 100 μM for FITC-YARA) or with markers of endocytosis for 1 hour. After a one hour incubation, cells were washed three times with media, incubated with 100–200μl of trypan blue (0.4% solution; Sigma) to cover the bottom of the well and quench extracellular FITC signal for 1 minute. Cells were then washed five times with media before imaging live using an FV1000 confocal microscope equipped with ASW-10 software (Olympus). To minimize crosstalk between the FITC and texas red/alexa fluor 594 fluorophores, laser gain and PMT voltage were optimized on controls consisting of cells treated with only a single fluorophore and sequential activation of the 488nm and 543nm lasers was used. For all confocal imaging experiments, controls consisting of live, unlabeled cells were included to ensure cells did not autofluoresce. The plane of focus was chosen to be above the plasma membrane in all cases to ensure the focal plane captured the cytoplasm.
Table 2.
Reagents used to mark or inhibit different types of endocytosis.
| Endocytosis Pathway |
Marker of Endocytosis |
Inhibitor of Endocytosis | Mechanism of Inhibitor |
|---|---|---|---|
| macropinocytosis | dextran | 5-(N-ethyl-N- isopropyl)amiloride (EIPA) |
Ion-channel inhibitor |
|
caveolae- mediated |
cholera toxin subunit B (CTXB) |
methyl-β-cyclodextrin (MβCD) |
Extracts cholesterol from the plasma membrane and disrupts formation of lipid rafts |
|
clathrin- mediated |
transferrin | chlorpromazine (chlor) |
Prevents assembly of clathrin coated pits at the plasma membrane |
Uptake Studies Using Flow Cytometry: Inhibiting Endocytic Uptake
To confirm the type of endocytic uptake, pharmacological inhibitors of the three major types of endocytosis were identified (Table 2). All inhibitors were purchased from Sigma. Cells were exposed to several different concentrations of the inhibitors for four hours, then subjected to an MTT-based assay (CellTiter 96 AQueous One Proliferation Assay Reagent; Promega) to determine the range of toxicity, as determined by a decrease in metabolic activity measured by the MTT assay (data shown in Supplemental Information). The maximum non-toxic concentration of inhibitor was chosen to evaluate endocytic inhibition of MK2-inhibitor peptide. To evaluate inhibition of FITC-peptide uptake, cells were treated with inhibitors for one hour followed by treatment with the MK2-inhibitor peptide for one hour. Cells were washed with phosphate buffered saline (PBS; Mediatech), incubated with trypsin for 5–7 minutes, neutralized in serum-containing media, and spun down at 300xg for 5 minutes. Following another wash with PBS, extracellular FITC-signal was quenched with the addition of 100 μl trypan blue for 1 minute. Cells were washed with PBS 4–5 times to remove excess trypan blue. After the final wash, cells were fixed in 1X cytofix buffer (BD). Cells were stored in the dark at 4°C until samples could be run on the Cytomics FC500 MPL flow cytometer (Beckman Coulter) equipped with MXP Cytometry List Mode Data Acquisition and Analysis Software. Data acquisition required 10,000 events. Figures were obtained using Flow Jo software.
Functional Assay
To test whether the addition of the FITC label altered the function of the peptides, the FITC-labeled peptides were analyzed for their ability to knockdown proinflammatory cytokine production. Mesothelial cells were seeded in 96-well plates at a concentration of 150,000 cells/ml with a volume of 200 μl per well, in phenol red free media (Gibco) containing the same supplements listed above. Once the cells reached confluency, the cells were treated a final concentration of 1ng/ml IL-β (positive control) to induce proinflammatory cytokine production or 1ng/ml IL-1β + various concentrations of peptide to evaluate the ability of the peptide to decrease proinflammatory cytokines, or PBS only (negative control). After 24 hours, media was collected for cytokine analysis. THP-1 cells were seeded in 96-well plates at a concentration of 250,000 cells/ml with a total volume of 200 μl per well. The number of living cells was determined using the CellTiter 96 AQueous One Proliferation Assay Reagent (Promega) according to the manufacturer instructions. Briefly, 20 μl of reagent was added directly to 100 μl of cells and media. After one hour (mesothelial cells) or two hours (THP-1 cells) of incubation at 37°C, the absorbance was read at 490 nm with a correction at 650 nm using an M5 Spectrophotometer equipped with Softmax Pro software.
Results
Characterizing MK2-inhibitor peptide uptake
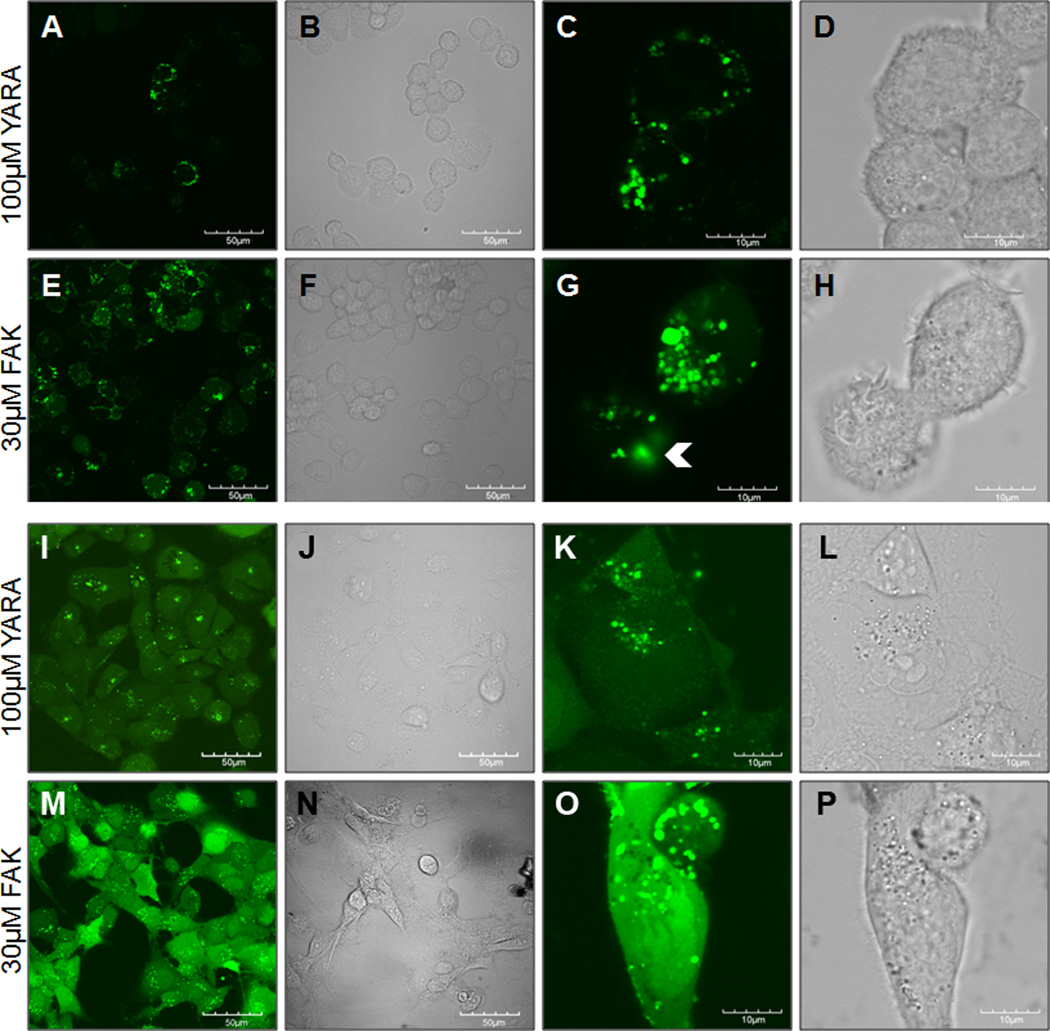
To confirm that the two MK2-inhibitor peptides gained intracellular access, the peptides were modified with an N-terminal FITC label and visualized using confocal microscopy. Intracellular uptake of both FITC-FAK and FITC-YARA was demonstrated in THP-1 (Figure 1 A-H) and mesothelial cells (Figure 1 I-P). In both cell types, FITC-FAK was taken up more efficiently than FITC-YARA even at lower FITC-FAK concentrations. In THP1 cells, FITC-YARA was not taken up uniformly by all cells (Figure 1A-D). Uptake appeared to occur via endocytosis, based on the shape of the FITC-containing vesicles. However, FITC-FAK uptake in mesothelial cells (Figure 2 M,O) had high levels of diffuse cytoplasmic staining in addition to uptake in vesicles. Furthermore, there is some evidence of FITC-FAK endocytic escape (Figure 1G, white arrowheads), which is required for the therapeutic to have a functional effect. While the evidence of endosomal escape, the rapid appearance of diffuse cytoplasmic staining could indicate that there is some level of membrane disruption that supports direct membrane translocation in addition to endosomal uptake. Multiple modes of uptake for FITC-FAK could also help explain the lower levels of peptide required for efficient inhibition of MK2.
Figure 1.
Figure 2.
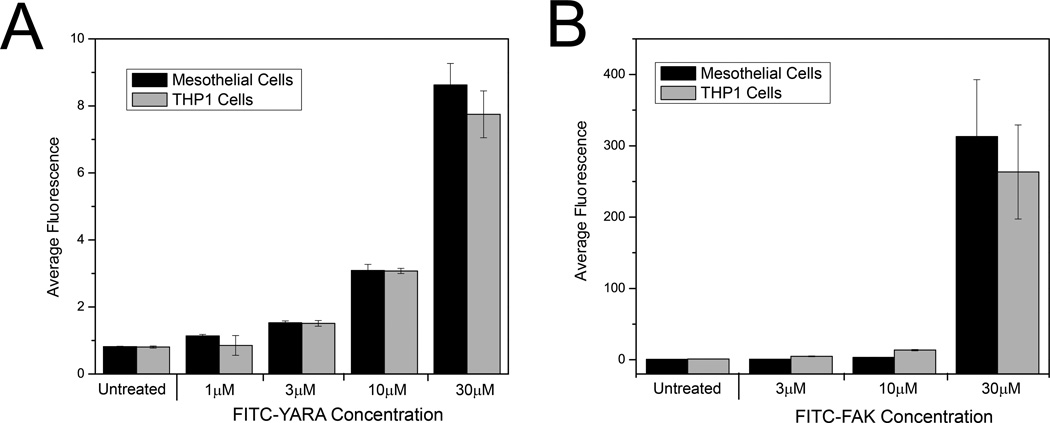
Uptake of the MK2-inhibitor peptides was additionally characterized utilizing flow cytometry, a technique that lends itself to more quantitative analyses. Uptake was confirmed in both cell lines for increasing concentrations of FITC-FAK and FITC-YARA compared to untreated controls, corroborating confocal data (Figure 2). Treating cells with higher concentration of peptide resulted in increased uptake. In addition, the increased efficiency of FITC-FAK uptake in both cells compared to FITC-YARA was confirmed by an increase in average fluorescence intensity as quantified by flow cytometry, as cells treated with 30μM FITC-YARA have an average fluorescence intensity of ~8, while cells treated with 30μM FITC-FAK have an average fluorescence intensity of ~300. There is also a large increase in intracellular fluorescence in cells treated with 30μM FITC-FAK compared to 10μM FITC-FAK (Figure 2B).
Characterizing if FITC label affects function
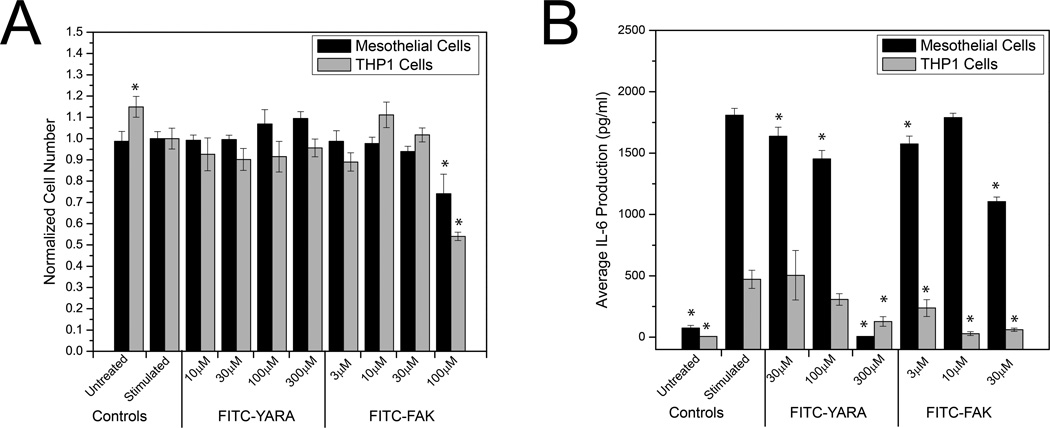
Previous work in our lab has demonstrated that changing a single amino acid can have a dramatic effect on peptide specificity [32]. We have also shown that addition of FITC to a CPP increased the affinity of the CPP to heparin [39], thus potentially increasing the peptide’s ability to bind to the cell surface and entering the cell. Therefore, we were interested in determining whether the addition of the FITC label and spacer amino acid affected the ability of the peptide to inhibit MK2 activity. To assess MK2 activity, we investigated IL-6 production, because we have previously shown that inhibition of MK2 activity suppresses IL-6 production due to a decrease in phosphorylation of heterogeneous nuclear ribonuclearproteain A0 (hnRNPA0) [34]. Cells were first screened for toxicity with the FITC-labeled peptides and then subjected to an IL-6 ELISA, which demonstrated that the MK2-inhibitor peptides are functional even with the addition of the FITC label (Figure 3). As expected, the addition of the FITC label to the peptides reduced the concentration of peptide required to suppress IL-6 production. In the absence of FITC, the YARA peptide requires 1000–3000 μM to suppress IL-6 in mesothelial cells [34], and FAK requires 30 μM to suppress IL-6 in THP1 cells [33]. This may be due to the enhanced binding of the peptide to the cell surface glycosaminoglycans. There was no evidence of toxicity in either cell type treated with concentrations of FITC-YARA up to 300μM; however, both cell types demonstrated toxicity when treated with 100μM FITC-FAK (Figure 3A, p>0.05; one-way ANOVA within each cell type). A dose dependent decrease in IL-6 production is observed with peptide treatment (Figure 3B). In THP-1 cells, FITC-YARA shows a significant decrease in IL-6 production at a concentration of 300μM and FITC-FAK decreases proinflammatory cytokines at a concentration of 3μM. In contrast, mesothelial cells require concentrations of 30μM for FITC-YARA and 30μM for FITC-FAK to significantly decrease IL-6 production. Finally, from unpublished data, we have established that the cargo portion of the peptide is unable to inhibit any MK2 activity in THP-1 and mesothelial cells even at concentrations in excess of 3 mM, thus suggesting that in the absence of the CPP, the cargo is not able to enter cells.
Figure 3.
MK2-inhibitor uptake: Active cellular temperature dependence
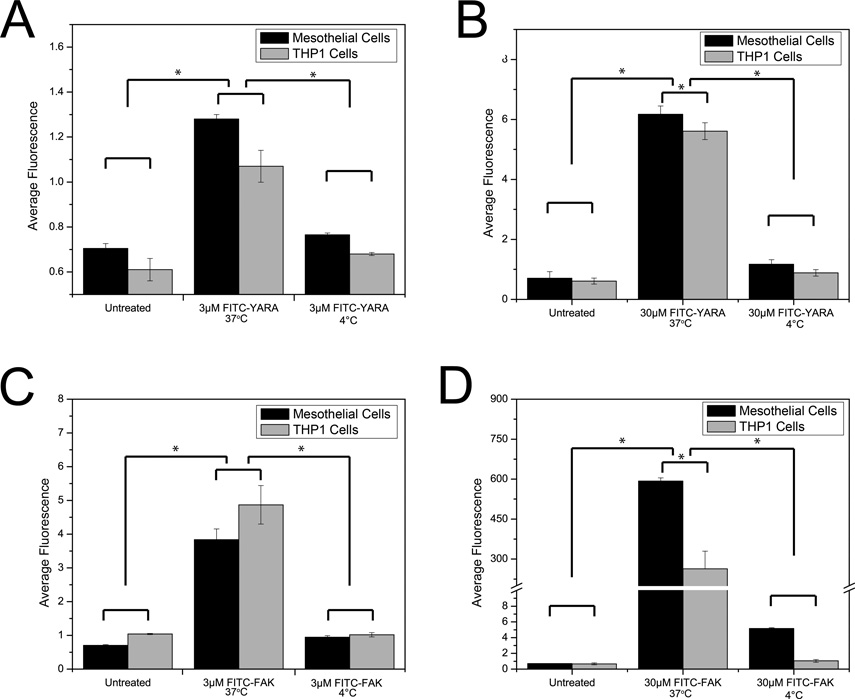
To evaluate whether uptake of the MK2-inhibitor peptides was dependent on active cellular processes of endocytosis, referred to as “energy dependent” in the CPP literature, intracellular access was monitored while the cells were incubated at 4°C. Flow cytometry showed that there was no difference between cells treated at 4°C with peptide compared to untreated cells for all peptides and cell types tested (Figure 4). However, when incubated at 37°C, peptides were taken up intracellularly as expected. This suggests that the majority of the CPP is taken up through an active process. Of note, it is still possible that some FITC-FAK enters cells through membrane disruption, which would also be suppressed at 4 °C due to reduced fluidity of the plasma membrane.
Figure 4.
Mechanism of Endocytic Uptake Using Markers of Endocytosis
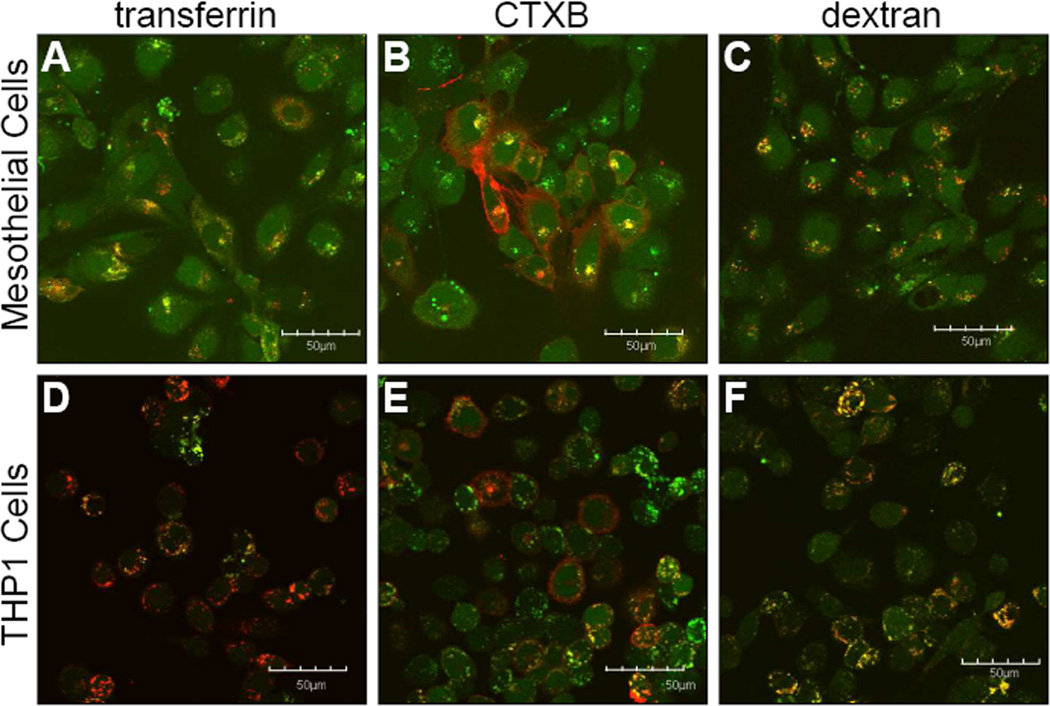
To determine the mechanism of endocytosis used for uptake, cells were co-incubated with markers that are known to be taken up by a specific mechanism of endocytosis. Colocalization was visually assessed by looking for color overlap (yellow) between the FITC-labeled MK2 inhibitor (green) and the marker of endocytosis (red) (Figures 5, 6). FITC-YARA appears to be taken up by all three mechanisms of endocytosis in mesothelial cells as there is significant overlap with all three endocytic markers (Figure 5A-C). However, in THP1 cells, FITC-YARA shows most colocalization with dextran, the marker of macropinocytosis (Figure 5F). Uptake of FITC-YARA in THP1 cells shows little colocalization with CTXB or transferrin, as with these markers most cells appear to independently uptake marker or peptide (Figure 5D&E).
Figure 5.
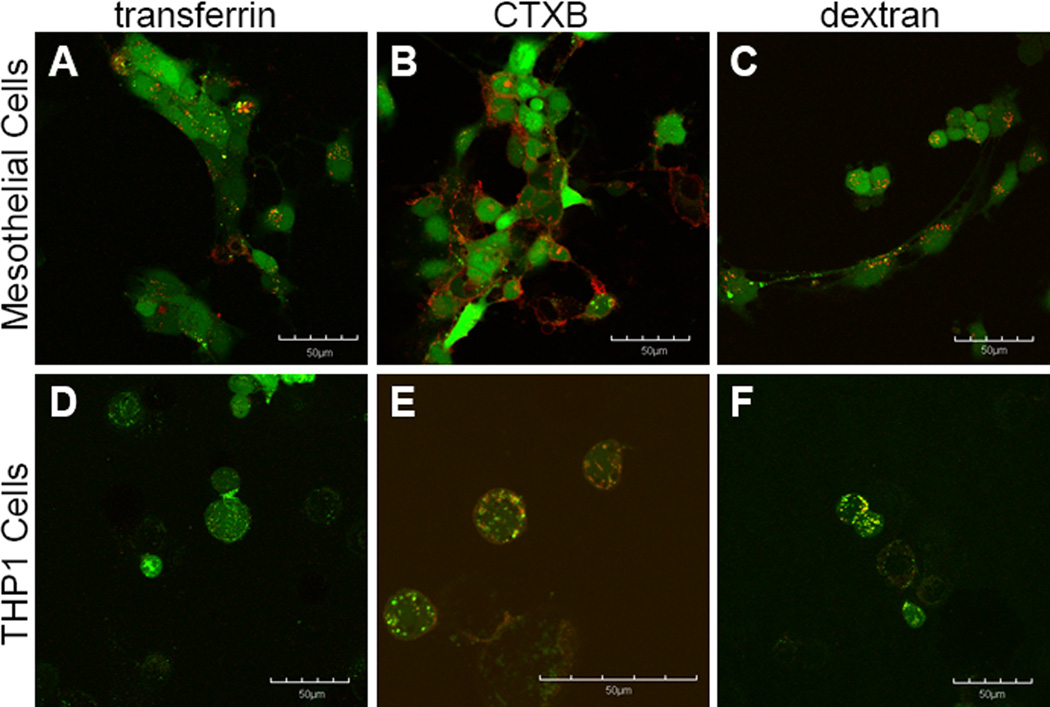
Figure 6.
FITC-FAK, by contrast, appears to be taken up by all three mechanisms of endocytosis in THP1 cells, with the most predominant colocalization occurring with CTXB and dextran, and small amounts of colocalization being observed with transferrin (Figure 6D-E). In mesothelial cells, FITC-FAK shows some colocalization with transferrin, the marker for clathrin-mediated endocytosis (Figure 6A), and a significant amount of diffuse cytoplasmic distribution. In mesothelial cells, negligible colocalization is observed with FITC-FAK and CTXB or dextran (Figure 6B&C).
Method of Endocytic Uptake Using Inhibitors of Endocytosis
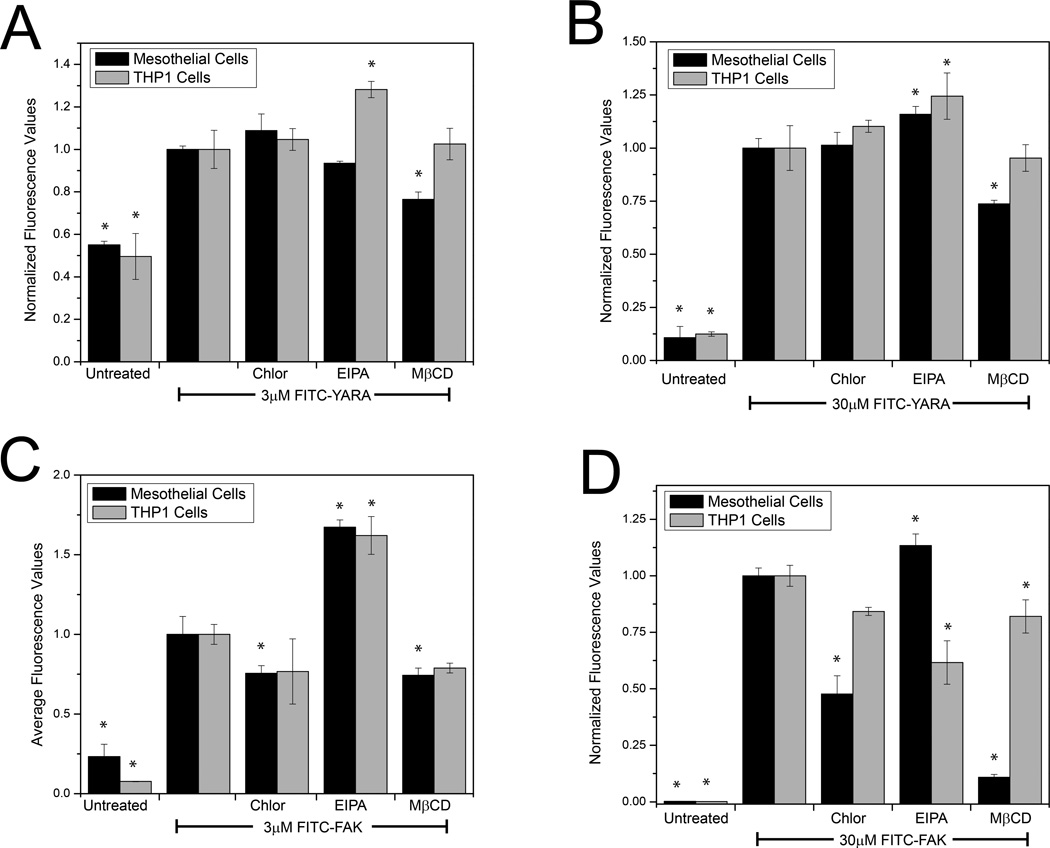
To confirm the mechanism of endocytic uptake, cells were treated with pharmacological inhibitors of endocytosis and uptake of MK2-inhibitor peptides was evaluated with flow cytometry. Mesothelial cells were treated with 100 μM EIPA and chlorpromazine and 10 mM MβCD, while THP-1 cells were treated with 50 μM EIPA, 100μM chlorpromazine, and 1mM MβCD.
Each pharmacological inhibitor was evaluated for its effect on uptake of the FITC-labeled peptide (Figure 7). FITC-YARA uptake is inhibited in mesothelial cells by MβCD (inhibitor of caveolae-mediated endocytosis) at both low and high concentrations, while uptake in THP1 cells is increased by EIPA (an inhibitor of macropinocytosis) (Figure 7A&B). In contrast, FITC-FAK in mesothelial cells shows differential uptake compared to the positive control by all three inhibitors at both high and low concentrations, while THP1 cells show increased uptake of FITC-FAK with EIPA (Figure 7C&D).
Figure 7.
Discussion
Determining the mechanism of uptake of these MK2-inhibitor peptides is critical in advancing our understanding of peptide function, and if their mechanism of delivery can be further optimized. Although our previous work has demonstrated promising biological effects with these peptides for overcoming chronic inflammation [33], preventing the formation of abdominal adhesions [34, 35], inhibiting pulmonary fibrosis [40], and limiting intimal hyperplasia following vein graft bypass [36], the characterization of the mechanism of intracellular uptake has never been investigated. To visually verify intracellular delivery, we modified the peptides with an N-terminal FITC-label.
By comparing the uptake of two different MK2-inhibitor peptides (that differ only in their cell-penetrating peptide domain) in two different cell lines, we confirmed intracellular delivery and demonstrated that uptake occurs via endocytosis, but that it differs between the two cell lines analyzed and between the two cell-penetrating peptides. Visual confirmation of intracellular peptide by both confocal microscopy and flow cytometry (Figures 1,2) validated previous biological data [33, 34] suggesting that the peptides gained intracellular access.
To understand how the addition of the FITC –label might affect the function of the peptide, we tested the ability of FITC-labeled MK2 inhibitor peptides to decrease proinflammatory cytokines. The FITC-label did not impede the peptide’s ability to decrease in proinflammatory cytokine production (Figure 3). However, a comparison between concentrations required for efficacy (defined as the concentration required to see a statistically significant decrease compared to the positive control) shows that the FITC-labeled inhibitors demonstrate efficacy at much lower concentrations as compared to unlabeled peptides. Unlabeled MK2-inhibitors demonstrate efficacy in THP-1 cells at 30 μM for FAK [33] and 1000 μM for YARA [33], while concentrations of 3000 μM of YARA are required to see efficacy in mesothelial cells [34]. FAK has never demonstrated efficacy in mesothelial cells (data not shown). Thus, the addition of the FITC-label enhances the peptide’s function, either by increasing the amount of peptide that gains intracellular access, altering the mode of endocytosis, increasing the amount of peptide that undergoes endosomal escape into the cell cytoplasm, or changing the specificity or IC50 of the peptide. Previous work in our laboratory has shown that changing a single amino acid within a peptide sequence can dramatically change the specificity and IC50 of the MK2 inhibitor peptide [32]. Additionally, these functional changes could be the result of a change in peptide structure, as the addition of a fluorescent label to cell-penetrating peptides has been shown to increase their ability to bind heparin by changing the helical structure of the peptide to provide more sites of interaction between the peptide and heparin [41]. This structural change could enhance the peptide’s ability to gain intracellular access. Importantly, this functional data indicates that the peptides are able to escape the endosomes and access the cytoplasm before the vesicles fuse with lysosomes and the contents become degraded, or before the endosomes are trafficked back to the membrane. We have not yet looked at characterizing the mechanism of escape from endosomes; however, we suspect that YARA, which is an optimized version of the HIV-TAT cell penetrating peptide [42], has a built-in mechanism of endocytic escape. The FAK cell penetrating peptide domain was derived from the heparin-binding domain of anti-thrombin III [43], and may also have an inherent mechanism for endosomal escape, which would be consistent with the possibility that FITC-FAK may be able to induce some level of membrane disruption.
Uptake of the FITC-peptides occurs primarily via endocytosis. Not only is intracellular uptake of these MK2-inhibitor peptides completely temperature dependent (Figure 4), but uptake occurs via endocytic-looking vesicles (Figures 1, 5 and 6) which colocalize with known markers of endocytosis (Figures 5 and 6). Further, uptake can be altered with the use of pharmacologic inhibitors of endocytosis (Figure 7). We found that FITC-YARA was taken up by macropinocytosis in THP-1 cells as supported by its colocalization with dextran (Figure 5F) and the change in uptake with EIPA (Figure 7A&B). When treating with inhibitors of endocytosis, we anticipated observing a decrease in uptake; however, the opposite was observed with an increase in FITC-YARA uptake with EIPA treatment. According to Doherty, the inhibition of one pathway of endocytosis can result in the upregulation of other pathways of endocytosis that might not typically function at high levels within cells [44], which may explain the observed increase in FITC-YARA uptake.
In mesothelial cells the mechanism of uptake of FITC-YARA appears to be mainly through caveolae-mediated endocytosis; however the evidence is not as clear because uptake varies based on concentration. At low concentrations (3μM), FITC-YARA uptake was dependent upon cholesterol as MβCD inhibited uptake (Figure 7A). At higher concentrations (30μM), however, FITC-YARA uptake was inhibited by both MβCD and EIPA (Figure 7B), indicating that uptake became non-specific as concentration increased. Increasing FITC-YARA even more (100μM) demonstrated that FITC-YARA gained intracellular access through all three mechanisms of endocytosis, as FITC-YARA showed colocalization with all markers of endocytosis (Figure 5D-F). This is consistent with other literature reports that concentration of the CPP alone can affect the mode of endocytosis [45] [46, 47]. It is also consistent with the idea that CPPs bind to different proteins, carbohydrates or lipids with varying affinity and that the identity of the binding partner will effect the mode of endocytosis, i.e. at low concentrations the CPP binds only to high affinity partners, but at high concentrations may binding to multiple binding-partners and trigger multiple modes of uptake [48]. Internalization of the YARA CPP domain has been investigated in human keloid fibroblasts delivering a different peptide cargo [37]. It was reported that this peptide gained intracellular access through caveolae-mediated endocytosis based on colocalization with CTXB, caveolin-1, Rab7, and Rab9 [37]. However, MβCD was unable to inhibit uptake of the peptide, but much lower concentrations of inhibitor (5μM [37] vs 10mM used in this study) were used to inhibit uptake.
The uptake of FITC-FAK in these cells also appears to be via endocytosis, as uptake was found to be both temperature dependent (Figure 4C&D) and affected by endocytic inhibitors (Figure 7C&D). In mesothelial cells, FITC-FAK showed uptake via clathrin-mediated endocytosis at high concentrations while showing negligible uptake via caveolae-mediated endocytosis or macropinocytosis. Interestingly, in THP-1 cells, FITC-FAK uptake was less specific, showing uptake through all three mechanisms of endocytosis at concentrations of 30μM. This is consistent with the images in Figure 1, which shows large cytoplasmic distribution of the FITC labeled peptide and indicates that uptake of FITC-FAK is extremely efficient. It should again be noted that the concentration dependence of FITC-FAK uptake coupled with the diffuse cytoplasmic staining could also be indicative of some level of membrane disruption, thus adding a fourth mode of uptake in THP-1 cells. Regardless, the uptake is both temperature dependent and can be altered with inhibitors of endocytosis.
Although the markers of endocytosis used in this study are well characterized, it must be acknowledged that the inhibitors of endocytosis may not be as specific for one mechanism of endocytosis as desired. For example, MβCD is known to remove cholesterol from the cell membrane[49] and this is typically associated with caveolae which are enriched with cholesterol [50]. However, MβCD is not selective in cholesterol removal from the membrane, and it has been shown to affect clathrin-mediated endocytosis [51].
In conclusion, these results support endocytic uptake of both FITC-FAK and FITC-YARA in two different cell lines. Mechanism of uptake is demonstrated to be dependent upon extracellular concentration, cell type, and choice of CPP. The functional data definitively shows that both peptides enter the cytoplasm where they inhibit MK2 activity. For the FITC-YARA peptide, entrance into the cytoplasm appears to be primarily through endosomal escape, as there is little evidence of uptake via membrane disruption. While there is clear evidence of FITC-FAK endosomal uptake and even endosomal escape, the rapid appearance of this peptide in the cytoplasm of mesothelial cells leaves open the possibility that there is some uptake via membrane disruption in this cell type. The clear multiple uptake modes highlight the difficulty of fully defining CPP mode of uptake and shows that each peptide-cargo combination may have its own unique blend of multiple modes of cellular uptake for different cell types.
Supplementary Material
Table 1.
Peptide Identifiers and Single Amino Acid Sequences.
| Identifier | Peptide Sequence |
|---|---|
| FITC-FAK | FITC- (ε-Ahx)- FAKLAARLYRKALARQLGVAA |
| FITC -YARA | FITC- βA- YARAAARQARAKALARQLGVAA |
Acknowledgments
Grant NIH R01HL106792
Abbreviations
- CPP
cell penetrating peptide
- Tat
transactivator of transcription
- Antp
Antennapedia
- FACS
or fluorescence-activated cell sorting
- MK2
mitogen activated protein kinase activated protein kinase 2
- HoBt
N-hydroxybenzotriazole
- DIC
N, N’-diisopropylcarbodiimide
- HBTU
2-(1Hbenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- PMA
phorbol 12-myristate 13-acetate
- CTXB
cholera toxin subunit B
References
- 1.Kameyama S, Horie M, Kikuchi T, Omura T, Takeuchi T, Nakase I, Sugiura Y, Futaki S. Effects of cell-permeating peptide binding on the distribution of 125I-labeled Fab fragment in rats. Bioconjug Chem. 2006;17(3):597–602. doi: 10.1021/bc050258k. [DOI] [PubMed] [Google Scholar]
- 2.Kameyama S, Okada R, Kikuchi T, Omura T, Nakase I, Takeuchi T, Sugiura Y, Futaki S. Distribution of immunoglobulin Fab fragment conjugated with HIV-1 REV peptide following intravenous administration in rats. Mol Pharm. 2006;3(2):174–180. doi: 10.1021/mp050064m. [DOI] [PubMed] [Google Scholar]
- 3.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999;10(2):186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 4.Abes R, Arzumanov AA, Moulton HM, Abes S, Lvanciva GD, Lversen PL, Gait MJ, Lebleu B. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochemical Society Transactions. 2007;35:775–779. doi: 10.1042/BST0350775. [DOI] [PubMed] [Google Scholar]
- 5.Eguchi A, Dowdy SF. siRNA delivery using peptide transduction domains. Trends Pharmacol Sci. 2009;30(7):341–345. doi: 10.1016/j.tips.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Eto Y, Yoshioka Y, Asavatanabodee R, Kida S, Maeda M, Mukai Y, Mizuguchi H, Kawasaki K, Okada N, Nakagawa S. Transduction of adenovirus vectors modified with cell-penetrating peptides. Peptides. 2009;30(8):1548–1552. doi: 10.1016/j.peptides.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Sawant R, Torchilin V. Intracellular transduction using cell-penetrating peptides. Mol Biosyst. 2010;6(4):628–640. doi: 10.1039/b916297f. [DOI] [PubMed] [Google Scholar]
- 8.Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27(2):85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55(6):1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 10.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 11.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272(25):16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 12.Perez F, Joliot A, Bloch-Gallego E, Zahraoui A, Triller A, Prochiantz A. Antennapedia homeobox as a signal for the cellular internalization and nuclear addressing of a small exogenous peptide. J Cell Sci. 1992;102(Pt 4):717–722. doi: 10.1242/jcs.102.4.717. [DOI] [PubMed] [Google Scholar]
- 13.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 14.Jones AT, Sayers EJ. Cell entry of cell penetrating peptides: tales of tails wagging dogs. Journal of controlled release : official journal of the Controlled Release Society. 2012;161(2):582–591. doi: 10.1016/j.jconrel.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Brugnano J, Ward BC, Panitch A. Cell penetrating peptides can exert biological activity: a review. Biomolecular Concepts. 2010;1(2):109–116. doi: 10.1515/bmc.2010.016. [DOI] [PubMed] [Google Scholar]
- 16.Lorents A, Kodavali PK, Oskolkov N, Langel U, Hallbrink M, Pooga M. Cell-penetrating peptides split into two groups based on modulation of intracellular calcium concentration. The Journal of biological chemistry. 2012;287(20):16880–16889. doi: 10.1074/jbc.M111.318063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobin M-L, Bonnafous P, Temsamani H, Dole F, Grélard A, Dufourc EJ, Alves ID. The enhanced membrane interaction and perturbation of a cell penetrating peptide in the presence of anionic lipids: Toward an understanding of its selectivity for cancer cells. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2013;1828(6):1457–1470. doi: 10.1016/j.bbamem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Schelhaas M, Shah B, Holzer M, Blattmann P, Kuhling L, Day PM, Schiller JT, Helenius A. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS pathogens. 2012;8(4):e1002657. doi: 10.1371/journal.ppat.1002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 20.Jiao CY, Delaroche D, Burlina F, Alves ID, Chassaing G, Sagan S. Translocation and Endocytosis for Cell-penetrating Peptide Internalization. Journal of Biological Chemistry. 2009;284(49):33957–33965. doi: 10.1074/jbc.M109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vives E. Cellular uptake [correction of utake] of the Tat peptide: an endocytosis mechanism following ionic interactions. J Mol Recognit. 2003;16(5):265–271. doi: 10.1002/jmr.636. [DOI] [PubMed] [Google Scholar]
- 22.Gump JM, June RK, Dowdy SF. Revised role of glycosaminoglycans in TAT protein transduction domain-mediated cellular transduction. J Biol Chem. 2010;285(2):1500–1507. doi: 10.1074/jbc.M109.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides - A reevaluation of the mechanism of cellular uptake. Journal of Biological Chemistry. 2003;278(1):585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 24.Hirose H, Takeuchi T, Osakada H, Pujals S, Katayama S, Nakase I, Kobayashi S, Haraguchi T, Futaki S. Transient Focal Membrane Deformation Induced by Arginine-rich Peptides Leads to Their Direct Penetration into Cells. Mol Ther. 2012;20(5):984–993. doi: 10.1038/mt.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomone F, Cardarelli F, Di Luca M, Boccardi C, Nifosì R, Bardi G, Di Bari L, Serresi M, Beltram F. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. Journal of Controlled Release. 2012;163(3):293–303. doi: 10.1016/j.jconrel.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends in molecular medicine. 2012;18(7):385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002;277(46):44236–44243. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]
- 28.Kashiwagi H, McDunn JE, Goedegebuure PS, Gaffney MC, Chang K, Trinkaus K, Piwnica-Worms D, Hotchkiss RS, Hawkins WG. TAT-Bim induces extensive apoptosis in cancer cells. Ann Surg Oncol. 2007;14(5):1763–1771. doi: 10.1245/s10434-006-9298-z. [DOI] [PubMed] [Google Scholar]
- 29.Watkins CL, Brennan P, Fegan C, Takayama K, Nakase I, Futaki S, Jones AT. Cellular uptake, distribution and cytotoxicity of the hydrophobic cell penetrating peptide sequence PFVYLI linked to the proapoptotic domain peptide PAD. J Control Release. 2009;140(3):237–244. doi: 10.1016/j.jconrel.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Giorello L, Clerico L, Pescarolo MP, Vikhanskaya F, Salmona M, Colella G, Bruno S, Mancuso T, Bagnasco L, Russo P, Parodi S. Inhibition of cancer cell growth and c-Myc transcriptional activity by a c-Myc helix 1-type peptide fused to an internalization sequence. Cancer Res. 1998;58(16):3654–3659. [PubMed] [Google Scholar]
- 31.Mutoh M, Lung FD, Long YQ, Roller PP, Sikorski RS, O'Connor PM. A p21(Waf1/Cip1)carboxyl-terminal peptide exhibited cyclin-dependent kinase-inhibitory activity and cytotoxicity when introduced into human cells. Cancer Res. 1999;59(14):3480–3488. [PubMed] [Google Scholar]
- 32.Ward B, Seal BL, Brophy CM, Panitch A. Design of a bioactive cell-penetrating peptide: when a transduction domain does more than transduce. J Pept Sci. 2009;15(10):668–674. doi: 10.1002/psc.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brugnano JL, Chan BK, Seal BL, Panitch A. Cell-penetrating peptides can confer biological function: regulation of inflammatory cytokines in human monocytes by MK2 inhibitor peptides. J Control Release. 2011;155(2):128–133. doi: 10.1016/j.jconrel.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Ward BC, Kavalukas S, Brugnano J, Barbul A, Panitch A. Peptide inhibitors of MK2 show promise for inhibition of abdominal adhesions. J Surg Res. 2011;169(1):e27–e36. doi: 10.1016/j.jss.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavalukas SL, Uzgare AR, Panitch A, Ward BC, Barbul A. MK2 inhibitor peptide reduces adhesion formation without affecting colonic anastomotic healing. Journal of the American College of Surgeons. 2009;209(3 Supplement):S17. [Google Scholar]
- 36.Muto A, Panitch A, Kim N, Park K, Komalavilas P, Brophy CM, Dardik A. Inhibition of Mitogen Activated Protein Kinase Activated Protein Kinase II with MMI-0100 reduces intimal hyperplasia ex vivo and in vivo. Vascul Pharmacol. 2012;56(1–2):47–55. doi: 10.1016/j.vph.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopes LB, Brophy CM, Flynn CR, Yi Z, Bowen BP, Smoke C, Seal B, Panitch A, Komalavilas P. A novel cell permeant peptide inhibitor of MAPKAP kinase II inhibits intimal hyperplasia in a human saphenous vein organ culture model. J Vasc Surg. 2010;52(6):1596–1607. doi: 10.1016/j.jvs.2010.06.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes LB, Flynn C, Komalavilas P, Panitch A, Brophy CM, Seal BL. Inhibition of HSP27 phosphorylation by a cell-permeant MAPKAP Kinase 2 inhibitor. Biochem Biophys Res Commun. 2009;382(3):535–539. doi: 10.1016/j.bbrc.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules. 2003;4(6):1572–1582. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 40.Vittal R, Fisher A, Gu H, Mickler EA, Panitch A, Lander C, Cummings OW, Sandusky GE, Wilkes DS. Peptide-mediated Inhibition of MK2 Ameliorates Bleomycin-Induced Pulmonary Fibrosis. American journal of respiratory cell and molecular biology. 2013 doi: 10.1165/rcmb.2012-0389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seal BL, Panitch A. Viscoelastic behavior of environmentally sensitive biomimetic polymer matrices. Macromolecules. 2006;39(6):2268–2274. [Google Scholar]
- 42.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61(2):474–477. [PubMed] [Google Scholar]
- 43.Tyler-Cross R, Sobel M, McAdory LE, Harris RB. Structure-function relations of antithrombin III-heparin interactions as assessed by biophysical and biological assays and molecular modeling of peptide-pentasaccharide-docked complexes. Arch Biochem Biophys. 1996;334(2):206–213. doi: 10.1006/abbi.1996.0448. [DOI] [PubMed] [Google Scholar]
- 44.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 45.Alves ID, Jiao CY, Aubry S, Aussedat B, Burlina F, Chassaing G, Sagan S. Cell biology meets biophysics to unveil the different mechanisms of penetratin internalization in cells. Biochim Biophys Acta. 2010;1798(12):2231–2239. doi: 10.1016/j.bbamem.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278(33):31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 47.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8(7):848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 48.Walrant A, Bechara C, Alves ID, Sagan S. Molecular partners for interaction and cell internalization of cell-penetrating peptides: how identical are they? Nanomedicine. 2012;7(1):133–143. doi: 10.2217/nnm.11.165. [DOI] [PubMed] [Google Scholar]
- 49.Irie T, Fukunaga K, Pitha J. Hydroxypropylcyclodextrins in parenteral use. I: Lipid dissolution and effects on lipid transfers in vitro. Journal of pharmaceutical sciences. 1992;81(6):521–523. doi: 10.1002/jps.2600810609. [DOI] [PubMed] [Google Scholar]
- 50.Smart EJ, Anderson RG. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 2002;353:131–139. doi: 10.1016/s0076-6879(02)53043-3. [DOI] [PubMed] [Google Scholar]
- 51.Ivanov A. Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful? In: Ivanov A, editor. Exocytosis and Endocytosis. Humana Press; pp. 15–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.