Abstract
The Proepicardium (PE) is an embryonic tissue that gives rise to multipotent vascular progenitors. Most notably the PE gives rise to the epicardium, cardiac fibroblasts, myocardium, and coronary vessels including both vascular smooth muscle and vascular endothelium. Much attention has been given to epicardial-derived cells that show the capacity to differentiate into a wide variety of vascular progenitors including cardiomyocytes. However, it is the PE itself that possesses the greatest potential as a source of multipotent vascular progenitors. We show here a simple method to manually isolate mouse PE at the ninth day of mouse embryonic development and culture highly pure PE tissue in serum-free conditions. This PE culture method allows for the ex vivo analysis of specific growth factors on PE and epicardial development with greater efficiency and precision than existing epicardial culture methods.
Keywords: proepicardium, stem cells, cardiovascular, progenitor cell culture, mouse
1. Introduction
The proepicardium (PE) is a source of multipotent vascular progenitors. As the PE uniquely develops in isolation from the beating heart tube, it lends to the possibility of extracting these progenitors for cell culture experiments. The PE is an epithelial-like mesodermal tissue (mesothelium) that develops through inductive interactions from the embryonic liver [1]. The PE and epicardium are distinguishable from the surrounding mesothelial tissue and heart by a unique “grape-like” tissue morphology and by the expression of Wilm’s tumor 1 (WT1), Tbx18 and Epicardin (Tcf21) beginning at the eight day of mouse embryonic development [2–6]. The PE is considered of cardiovascular origin as it derives from Nkx2.5 and Isl1-expressing cardiac progenitors that also form the primary myocardium [7]. However, despite being derived from early cardiovascular progenitors, the PE develops at a distance from the primary heart tube. The PE is located posterior to the heart tube over the embryonic liver and doesn’t contact the heart until later in the ninth day of mouse embryonic development (E9.5). After contact with the heart, the PE transfers onto the heart engulfing the myocardium in an epithelial covering, or epicardium, between E9.5 and E11 [8, 9]. In addition to forming the epicardium, PE-derived cells invade the myocardium giving rise to myocardial cells, fibroblasts and coronary vessel precursors (vascular smooth muscle and vascular endothelium) [10–14].
In addition to providing vascular progenitors during embryonic development, the epicardium can be reactivated in the mature heart by cardiac injury that stimulates epicardial cells to invade the myocardium giving rise to fibroblasts and smooth muscle. However, these cells do not differentiate into myocardium or coronary endothelium that would be necessary to rebuild an injured heart [15]. In zebrafish, the epicardium can form myocardium to repair and rebuild an injured heart suggesting that an evolutionary mechanism of epicardium-mediated heart repair may exist [16]. As culture conditions have been identified to stimulate mammalian and avian PE and epicardium to differentiate into a multitude of cardiovascular cell types, it raises the possibility that epicardial-derived cells can be redirected to form new coronary vessels or beating myocardium in the injured heart [17–22]. Mouse PE culture studies may help elucidate how epicardium or epicardial progenitors can be reprogrammed into multipotent progenitors and efficiently differentiated into specific cardiovascular cell lineages.
Currently methods only exist to isolate and culture epicardial cells in mouse despite the importance of proepicardium as a source of vascular progenitors. Essentially, the means to isolate epicardial cells involves either allowing the cells to migrate off an excised heart onto a culture dish in media containing high serum [23] and supplemented with Fibroblast growth factor 2 (basic FGF) [24] or Thymosin B4 (TB4) [25] or FACS sorted epicardium from enzymatically-digested heart tissue using epicardium-cell-specific transgenic expression of a fluorescent protein as a means to sort [26]. No method has been described to isolate mouse PE. However, in the avian embryo isolation and culture of the highly pure PE tissue has been established [27]. The PE of avian embryos is isolated with fine forceps as it protrudes away from the body cavity towards the heart. Additionally serum-free conditions have been successfully used in avian PE culture allowing for the routine testing of paracrine factors on PE development [27–29]. The potential of serum-free PE culture has not yet been realized in mouse. The mouse PE does not protrude to the same extent as avian embryos making the likelihood of PE excision at high purity seemingly not feasible. Additionally, current mouse epicardial culture methods require high serum media often supplemented with growth factors impinging on the ability to study individual growth factors effectively in epicardial cultures [23–25]. There is tremendous benefit to combine the power of mouse transgenesis with the ability to culture PE in defined conditions. We show here a reliable method to isolate mouse PE and culture in serum-free conditions.
2. Materials and methods
2.1 Embryo collection and PE isolation
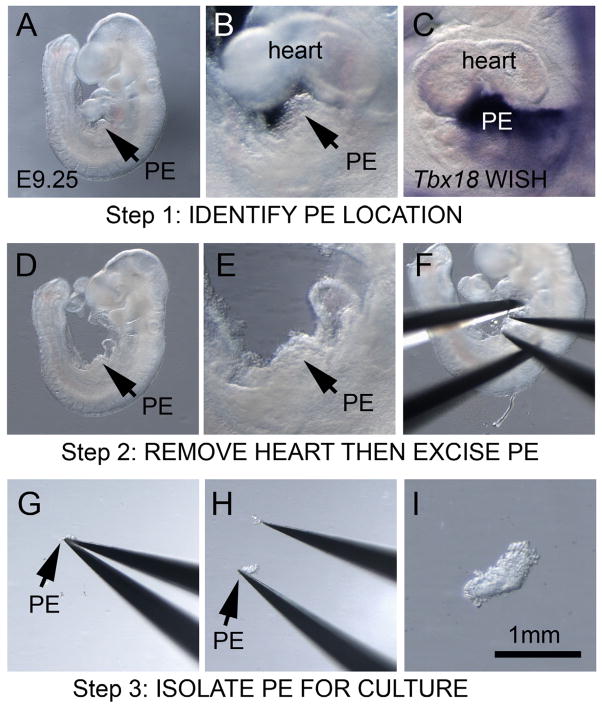
Mouse matings of NIH-Swiss mice (Charles River) produced pregnancies that were dissected at E9.0 or before E9.5 by placing the uterus in room temperature sterile 1x PBS to expose individual decidua. After E9.5 much of the PE had already transferred onto the heart and these stages were not useful for PE collection. Embryos were removed from the decidua in sterile room temperature 1x PBS using fine forceps (Fine Science tools, Dumont #5 cat. # 11252–20) in a plastic uncoated dish (Falcon, 60×15 mm cat. #351007). The yolk sac and all membranes covering the embryo and the pericardium covering the heart were removed to give access to the heart and PE using fine forceps. First, the PE was located in the embryo. The PE was best viewed from the left side of the embryo as a “grape-like” clustering of cells over liver just under the heart between the atria and the ventricle (Fig 1A,B). To increase confidence in PE identification, we examined the detection of the PE-expressed gene Tbx18 by whole-mount in situ hybridization (WISH) at E9.25 in fixed embryos as a reference (Fig 1C) [1, 6]. After the location of the PE was identified, the heart tube was removed to assist in visualizing the PE and to prevent the heart from interfering in PE excision (Fig 1D,E). The PE was removed by depressing a partially opened sharp fine forceps (Fine Science tools, Dumont #55 cat. # 11255-20) against the liver region with the PE positioned within the opening of the forceps (Fig 1F). Another pair of forceps, a blunted Dumont #5, was required to anchor the embryo in place during PE excision. By maintaining pressure on the liver and closing the forceps the PE was removed with the PE secured in the forceps. Retracting the closed forceps away from the embryo isolated the PE (Fig 1G).
Figure 1.
Isolation of PE tissue in E9.0–9.25 mouse embryos. (A) E9.25 mouse embryo showing location of PE and magnified image of PE region showing “grape-like” morphology of PE tissue (B). (C) Tbx18 whole-mount insitu hybridization detecting PE progenitors to illustrate the location of the PE. (D) Embryo with heart tube removed to make accessible the PE and magnified image of the PE region (E). (F) Position of forceps to excise PE with fine forceps closing on dorsal and ventral margins of the PE while the embryo is held in place with another set of forceps. (G) Excised PE in comparison to the size of the forceps. (H) PE showing adhesion to the forceps. (I) A magnified view of the excised PE.
2.2 PE transfer, culture dishes and culture conditions
Isolated PE would stick to the forceps requiring another pair of forceps to dislodge it (Fig 1H). The resulting excised tissue PE was grapelike in appearance less than 1 mm in length (Fig 1I). PE was transferred directly into a culture dish containing media using a pipette or capillary retention using the forceps. PE explants were cultured under similar conditions as previously described for avian PE culture using uncoated plastic culture dishes in basic media either Dulbecco’s Modified Eagle Medium (DMEM) (Gibco cat. # 11965) or Medium 199 (Gibco cat. # 11150-059) with 50 units/ml Penicillin/Streptomycin (Gibco cat. # 15070-063) without serum [27]. To adhere the PE to the bottom of the dish the isolated PE was gently pressed against the plastic surface and the inherent “stickiness” of the explants facilitated placement. Gelatin coated culture dishes were not necessary for PE attachment however excessive movement of the PE culture dish or culture media can result in detachment of the PE. PE explants were cultured in a humidified 37°C, 5% Co2 incubator. Standard plastic dishes were sufficient for brightfield imaging and fluorescent imaging of PE cultures using stereo dissecting microscope. Fluorescent imaging using upright or inverted microscopes required high optical quality plastics dishes (m-Slide 8 well ibiTreat, cat. #80826, ibidi). 10 ng/ml rat recombinant Platelet-derived growth factor PDGF-BB (R&D Systems, cat. # 520-BB) was directly added to the media or bovine serum albumen (BSA) for controls as previously described [27]. The 10ng/ml PDGF-BB dose was sufficient to induce changes in cell behavior across the PE explant and chosen based off the 1.5–6 ng/ml effective dose (ED50) range previously shown to elicit behavioral changes 3T3 cultured fibroblast [30].
2.3 PE explants fixation and immunodetection of PE markers
Cultured PE explants were first rinsed in 1x PBS and fixed for analysis in 4% paraformaldehyde solution in 1x PBS at room temperature for 10 minutes directly in the culture dish. Care was taken to gently exchange the solutions as to not dislodge the explants. After fixation the explants were washed in PBT (1x PBS with 0.1% (v/v) Tween-20) to remove the fixative and then permeabilized with 0.2% (v/v) TritionX100 in PBT for 20 minutes. The explants were washed twice in PBT and the antibody solution was added (0.2% Roche blocking reagent in PBT with either 1:100 dilution of WT1 antibody (Santa Cruz cat. # sc-192, Rabbit monoclonal) or Epicardin (Tcf21) antibody (Abcam cat. # ab49475, Rabbit polyclonal), ZO1 (Invitrogen cat. # 33-9100, mouse monoclonal) or phospho-Histone H3 (Cell Signaling cat. # 9701, mouse monoclonal)). An extensive list of antibodies useful in PE and epicardial biology has previously been summarized [25]. Antibody incubation occurred overnight at 4°C in a humidified chamber. Excess primary antibody was removed with three 5 minute washes of PBT followed by the addition of an appropriate fluorochrome-conjugated secondary antibody at a dilution of 1:200 in blocking solution. Secondary antibodies were incubated at room temperature for 2 hours in the dark. The secondary antibody was removed with one 5 minute wash in PBT prior to DAPI-staining (1 μM DAPI in PBT) (DAPI, 4′,6-diamidino-2-phenylindole, dihydrochlorid, Invitrogen cat. # D21490) for 20 minutes at room temperature. The stain solution was then removed with three 5 minute washes of PBT prior to imaging explants on a Leica MZFLIII dissecting stereoscope with a Planapo 1.6x objective using a Zeiss Axiovision HRc digital camera operating Zeiss Axiovision imaging software (version 4.5). An Olympus Fluoview FV1000 Confocal laser scanning microscope was used to image ZO1-stained cell contacts.
3 Results and Discussion
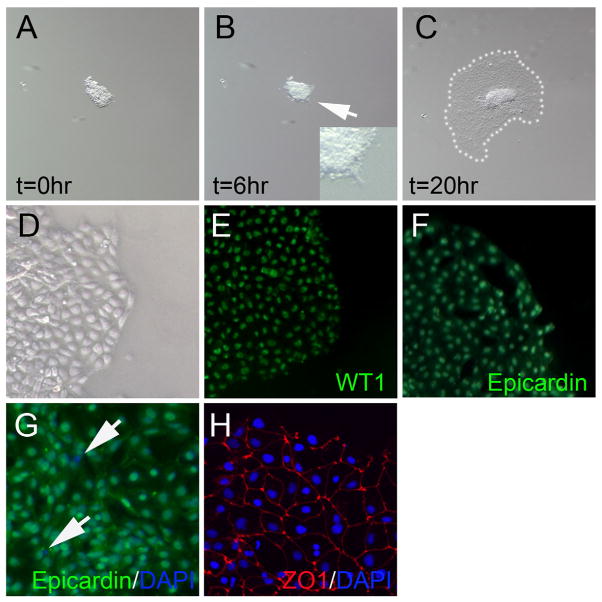
The mouse PE explants underwent spreading behavior in serum-free culture conditions (Fig 2A–C). Spreading of PE explants considered a normal PE behavior mimicking the spreading of the epicardium over the surface of the heart or protrusive behavior of the PE during contact with the heart[27]. Spreading was first evident after 6 hours of culture at the periphery of the PE explant (Fig 2B). After 20 hours of culture the explant had substantially spread and resulted in a “fried egg” appearance in most explants with spread proepicardial cells surrounding a raised surface at the site of initial PE explant attachment (Fig 2C). Higher magnification of the periphery of the spread explant showed that the proepicardial cells are epithelia-like in appearance (Fig 2D). To test for purity of PE explant isolation we used WT1 (Fig 2E) and Epicardin (Fig 2F) antibodies to mark PE cells verses non-PE mesothelium or other contaminating cell types. Immunodetection of these markers in the 20 hour cultured PE indicated that essentially all spreading cells were of PE origin. Quantification by dividing the number of epicardin positive PE-specific cells divided by the total number of DAPI positive cells indicated that 98 % (+/− 0.9 standard deviations) of spreading epithelial tissue was of PE origin assayed in three separate PE samples (Fig 2G). Purity of PE explants was however dependent quality of dissection and experience of the individual. Furthermore, the spread PE tissue could be confirmed to be epithelial in nature by the presence of ZO1-stained junctions (Fig 2H), like the native PE and epicardium, rather than non-specific cell association. This data shows that our method of PE isolation can produce high purity PE tissue for study in serum-free conditions.
Figure 2.
Culture of PE in serum-free media. (A) PE explant adhered to plastic dish (time=0 hours). (B) PE after 6 hours of culture showing initial spreading. Spreading PE explant magnified in inset image. (C) PE after 20 hours of culture showing spreading area highlighted by white dots. (D) Magnified view of periphery of PE explant. (E) WT1 detection in PE explant. (F) Epicardin (Tcf21) detection in PE explant. (G) ZO1 (red) and DAPI (blue) detection in PE explant showing intact tight junction contacts between cells. (H) DAPI (blue) and Epicardin (green) detection showing that most cells are PE-derived (green) however some cells are not of PE origin (blue) indicated with white arrows.
2.4 Treatment of PE explants with paracrine factors
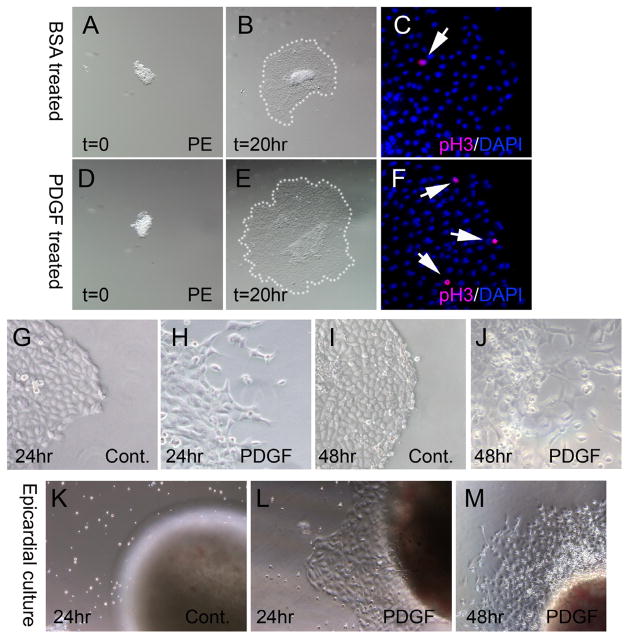
To directly test if our serum-free culture condition lend to study of specific paracrine factors on mouse PE development we used 10 ng/ml platelet-derived growth factor (PDGF) to elicit a predicted cell proliferative and epithelial to mesenchymal transition (EMT) response [31–33] as shown previous in serum free avian PE explant culture [27]. PDGF-treated explants showed more than doubling in spread area than BSA treated control PE explants (37.5 +/−5.66 verses 87.6 +/−6.19 arbitrary units respectively; n=3 explants per treatment (Fig. 3B,E). This difference in sped area was significant at 98% confidence by T-test. The increased size of the PDGF explants occurred with an increase in cell proliferation measured by detection of phospho-Histone H3 by immunofluorescence (Fig. 3C,F). PDGF treated explants had a near doubling in phospho-Histone H3 stained nuclei (1.3% of nuclei, n=3) compared to control explants (0.7% of nuclei, n=5). The observed increase in cell proliferation did not meet significance at 95% confidence level by T-test (p=0.06) in our sample set however the difference was highly suggestive of an induced proliferative change induced by PDGF. To assess EMT the explants were further cultured for a total of 24 hours when mesenchymal cells were frequently observed at the periphery of the spread PE explant treated with PDGF (Fig 3G) but not in control BSA-treated explants (Fig 3H). Over 48 hours of culture substantial mesenchymal cells were evident at the periphery of PE explants treated with PDGF (Fig 3J). No mesenchymal cells were observed in control PE explants cultured 48 hours (Fig 3I). This data effectively demonstrates that mouse PE culture can be used to successfully demonstrate altered behavior stimulated by a single growth factor over 20 hours. A limitation of mouse PE culture in treated samples is mouse litter size and precise staging. Precise comparisons of littermate PE cultures ultimately reduce the number of samples to around 10 samples per experiment. PE culture will show value in identifying factors that influence specific biological activity such as spreading behavior or EMT.
Figure 3.
Mitogen induced behavioral changes of PE and epicardial cultures. (A–C) BSA control treated and (D–F) 10 ng/ml PDGF-treated PE cultures. (A,D) PE explants at 0 hours of culture showing similar size of explanted tissue. (B) Control PE after 20 hours culture compared to (E) PDGF-treated PE after 20 hours showing greater spread area indicated with white dots. (C,F) Phospho-Histone H3 (pink) and DAPI (blue) detection showing greater cell proliferation in (F) PDGF-treated vs (C) control samples. (G) 24 hour cultured control PE. (H) 24 hour cultured PDGF-treated PE showing mesenchymal cells at periphery of explant. (I) 48 hour cultured control PE. (J) 48 hour cultured PDGF-treated PE showing many mesenchymal cells. (K–M) Epicardial cultures of E13.5 heart fragments. (K) 24 hour cultured control heart showing an absence of spreading epicardium. (L) 24 hour PDGF-treated heart showing epicardial spreading without mesenchymal cells at periphery. (M) 48 hour PDGF-treated heart showing epicardial spreading and a few mesenchymal cells at the periphery.
The use of mouse epicardial cultures to model formation of epicardium from proepicardium or entry of epicardial cells into the heart to form coronary vessels has not been validated in the absence of mouse PE cultures for comparison. We compared our PE cultures to primary epicardial cultures with and without stimulation with PDGF to induce EMT that normally occurs during entry of epicardium to form coronary vessels. For isolating epicardium, we used E13.5 heart tissue similar to previously described culture methods [24] however we employed similar serum-free culture conditions shown successful in our PE culture method. Unlike PE culture that showed cell adherence and spreading directly on a plastic substrate in serum-free conditions, no adhesion or spreading was evident from the epicardium of E13.5 hearts after 24 (Fig 3K) or 48 hours (data not shown). It was only in PDGF-treated heart cultures that epicardial adherence and spreading onto the culture dish was observed over 24 hours of culture (Fig 3L) as observed in untreated PE cultures (Fig 2H). It was only after 48 hours of culture that PDGF-treated epicardial cultures showed any separation of individual cells from the edge of the epithelia indicating EMT (Fig 3M). This data shows that mouse PE culture was substantially more effective in demonstrating EMT induction by PDGF than epicardial primary cultures. Different sensitivity between PE and epicardial culture likely reflects a more advanced differentiation state of the epicardium. Although we demonstrate a greater responsiveness of PE culture, it is unlikely that PE cultures could replace epicardial cultures as each system has specific utility to answer different biological questions.
A potential benefit of PE cultures is that they retain a higher differentiation potential than epicardial culture consistent with developmentally regulated changes of “stem-ness”. For example, PE cultures may be a more effective means to study factors necessary for differentiation into specific vascular tissue types. The PE like other progenitor populations loses multipotency as development proceeds. Although differentiation of the PE and epicardium is evident by the loss of specific markers (WT1, Tbx18 and Tcf21) during invasion into the heart [5, 6], it is unclear how much potential is lost during differentiation from the PE to epicardium. Recently lineage tracing experiments have shown that Tcf21 (epicardin) expressing epicardium can initially form coronary vessel progenitors and cardiac fibroblasts but after the thirteenth day of embryonic development these cells can only give rise to cardiac fibroblast [34]. PE culture may serve as a mechanism to avoid potential loss of potency in epicardial cell culture assays and provide an easily amenable system to study differentiation of vascular progenitors.
Highlights.
Method for isolation of mouse PE for cell culture experiments.
PE cells are vascular progenitors with potential to form all cardiac tissue types.
A model to study factors necessary heart formation and cardiac repair.
Acknowledgments
This research was assisted by Kim Peifley of the Optical Microscopy and Analysis Laboratory, SAIC-Frederick. Thanks to Mark Kennedy and Raviindra Chalamalasetty for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishii Y, Langberg JD, Hurtado R, Lee S, Mikawa T. Development. 2007;134:3627–3637. doi: 10.1242/dev.005280. [DOI] [PubMed] [Google Scholar]
- 2.Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 3.Haenig B, Kispert A. Dev Genes Evol. 2004;214:407–411. doi: 10.1007/s00427-004-0415-3. [DOI] [PubMed] [Google Scholar]
- 4.Kraus F, Haenig B, Kispert A. Mech Dev. 2001;100:83–86. doi: 10.1016/s0925-4773(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 5.Robb L, Mifsud L, Hartley L, Biben C, Copeland NG, Gilbert DJ, Jenkins NA, Harvey RP. Dev Dyn. 1998;213:105–113. doi: 10.1002/(SICI)1097-0177(199809)213:1<105::AID-AJA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Zeng B, Ren XF, Cao F, Zhou XY, Zhang J. J Biomed Sci. 2011;18:67. doi: 10.1186/1423-0127-18-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B, von Gise A, Ma Q, Rivera-Feliciano J, Pu WT. Biochem Biophys Res Commun. 2008;375:450–453. doi: 10.1016/j.bbrc.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers LS, Lalani S, Runyan RB, Camenisch TD. Dev Dyn. 2008;237:145–152. doi: 10.1002/dvdy.21378. [DOI] [PubMed] [Google Scholar]
- 9.Viragh S, Challice CE. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. Dev Cell. 2012;22:639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikawa T, Gourdie RG. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 13.Reese DE, Mikawa T, Bader DM. Circ Res. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- 14.Mikawa T, Borisov A, Brown AM, Fischman DA. Dev Dyn. 1992;193:11–23. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- 15.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Pu WT. Regen Med. 2008;3:633–635. doi: 10.2217/17460751.3.5.633. [DOI] [PubMed] [Google Scholar]
- 18.Smart N, Dube KN, Riley PR. Vascul Pharmacol. 2012 doi: 10.1016/j.vph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Smart N, Riley PR. Future Cardiol. 2012;8:53–69. doi: 10.2217/fca.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien KR, Domian IJ, Parker KK. Science. 2008;322:1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 22.Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. Dev Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Chen TH, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, Burch JB, Eid H, Sucov HM. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 24.Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Circ Res. 2008;103:1393–1401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart N, Riley PR. Curr Protoc Stem Cell Biol, Chapter. 2009;2(Unit2C):2. doi: 10.1002/9780470151808.sc02c02s8. [DOI] [PubMed] [Google Scholar]
- 26.Zhou B, Pu WT. Methods Mol Biol. 2012;843:155–168. doi: 10.1007/978-1-61779-523-7_15. [DOI] [PubMed] [Google Scholar]
- 27.Ishii Y, Garriock RJ, Navetta AM, Coughlin LE, Mikawa T. Dev Cell. 2010;19:307–316. doi: 10.1016/j.devcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlueter J, Manner J, Brand T. Dev Biol. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Torlopp A, Schlueter J, Brand T. Dev Dyn. 2010;239:2393–2403. doi: 10.1002/dvdy.22384. [DOI] [PubMed] [Google Scholar]
- 30.Raines EW, Ross R. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- 31.Kang J, Gu Y, Li P, Johnson BL, Sucov HM, Thomas PS. Dev Dyn. 2008;237:692–701. doi: 10.1002/dvdy.21469. [DOI] [PubMed] [Google Scholar]
- 32.Smith CL, Baek ST, Sung CY, Tallquist MD. Circ Res. 2011;108:e15–26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Dev Biol. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 34.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]