Abstract
Immunotherapeutic approaches are currently in the spotlight for their potential as disease-modifying treatments for neurodegenerative disorders. The discovery that α-synuclein (α-syn) can transmit from cell to cell in a prion-like fashion suggests that immunization might be a viable option for the treatment of synucleinopathies. This possibility has been bolstered by the development of next-generation active vaccination technology with short peptides-AFFITOPEs® (AFF) that do not elicit a α-syn specific T-cell response. This approach allows the generation of long-term sustained, more specific, non-cross reacting antibodies suitable for the treatment of synucleinopathies such as Parkinson’s disease (PD). In this context, we screened a large library of peptides that mimic the c-terminus region of α-syn and discovered a novel set of AFF that identified α-syn oligomers. Next, the peptide that elicited the most specific response against α-syn (AFF 1) was selected for immunizing two different transgenic mouse models of PD and Dementia with Lewy bodies (DLB), the PDGF- and the mThy1-α-syn tg mice. Vaccination with AFF 1 resulted in high antibody titers in CSF and plasma, which crossed into the CNS and recognized α-syn aggregates. Active vaccination with AFF 1 resulted in decreased accumulation of α-syn oligomers in axons and synapses that was accompanied by reduced degeneration of TH fibers in the caudo-putamen nucleus and by improvements in motor and memory deficits in both in vivo models. Clearance of α-syn involved activation of microglia and increased anti-inflammatory cytokine expression, further supporting the efficacy of this novel active vaccination approach for synucleinopathies.
Keywords: vaccination, Parkinson’s Disease, immunotherapy, alpha-synuclein, AFFITOPE®, AFFITOME®
INTRODUCTION
Neurodegenerative conditions with α-synuclein (α-syn) accumulation are common causes of dementia and movement disorders in the aging population [48]. This heterogeneous group of disorders includes Parkinson’s Disease (PD), PD dementia (PDD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA) and others [74,25], characterized by specific patterns of α-syn accumulation (neurons in PD/DLB, oligodendrocytes in MSA) and neurodegeneration. It is estimated that over 10 million people over the world suffer from these conditions for which no cure is currently available [62]. α-syn is a primarily cytosolic intracellular protein [81] found at the presynaptic terminal [27] that may play a role in synaptic plasticity [53]. It has been proposed that abnormal α-syn accumulation in synaptic terminals and axons plays an important role in the pathogenesis of PD and related disorders [24,28,35,74,21,31,36,66]. Previous work has suggested that α-syn oligomers rather than fibrils might be the neurotoxic species [12,76].
The discovery in recent years that intracellular proteins such as α-syn [39,17,56] and Tau [29,16,41] can, under pathological conditions, transmit from cell to cell in a prion-like fashion suggests that targeting extracellular α-syn aggregates might be a viable option for the treatment of synucleinopathies [7,40,79]. Strategies include delivery of α-syn-degrading enzymes such as neurosin [71], promoting clearance via microglial cells [7] and autophagy [45], and targeting extracellular α-syn with antibodies [79,7,44,45]. We have previously shown that active immunization with full-length recombinant α-syn elicits an immune response that aids the clearance of α-syn in tg models [44]. The disadvantage of such approach is that the full-length molecule could cause α-syn-specific T-cell and autoimmune responses.
The need for discovering new active immunization approaches for DLB, PD and related disorders has recently been advanced by the development of a next-generation active vaccination technology with small peptides, or AFFITOPEs® [64]. These are short immunogenic (B-cell response) peptides that are too short for inducing a T-cell response (autoimmunity) and do not carry the native epitope but rather a sequence that mimics the original epitope (e.g. oligomeric α-syn) [64]. This methodology allows for the generation of a long term, sustained, more specific, non-cross reacting antibody responses suitable for the treatment of synucleinopathies such as PD.
AFFITOPEs® have shown promise in models of AD, and clinical phase II trials in AD patients are underway. In this context the main objective of this study was to investigate the potential therapeutical value of active vaccination with novel α-syn AFFITOPEs® (AFF) in two different tg models of PD and DLB-like pathology. We found that immunization with conjugate vaccines containing the small peptide AFF 1, that mimics the C-terminus of α-syn (110–130), specifically reduced the accumulation of α-syn oligomers, ameliorated the neurodegenerative pathology and rescued the motor and memory deficits in α-syn tg mice without triggering overt T-cell or inflammatory responses. We also describe a novel mechanism for α-syn clearance based on microglial activation, which could be mediated by increased fractalkine expression. Based on these results, a Phase I clinical trial for PD has been initiated.
MATERIALS AND METHODS
AFFITOPE® identification, peptide production and vaccine formulation
For identifying AFFITOPE® peptides, we screened phage display libraries (New England BioLabs, Ipswich, USA) with monoclonal antibodies that specifically bind the C-terminus of human α-syn. Peptides were synthesized by FMOC solid phase peptide synthesis (EMC microcollections GmbH, Tuebingen, Germany). For the generation of suitable vaccines, the AFFITOPEs® contain an additional N-terminal cysteine residue in order to conjugate peptides to the carrier protein Keyhole Limpet Hemocyanin (KLH, Biosyn GmbH, Fellbach, Germany) using N-gamma-Maleimidobutyryl-oxysuccinimide ester (GMBS, Thermo Scientific, Rockford, USA). AFFITOPE®-KLH conjugates were adsorbed to Aluminum hydroxide (Alum, Brenntag, Frederikssund, Denmark) as adjuvant. The dose used for vaccinating the animals was 30 μg peptide containing 0.1% Alum.
Selection of AFFITOPEs® and epitope mapping
C57BL/6J mice (n=6/group) received biweekly injections of α-syn-targeting vaccines (AFF 1–7-conjugates) or of a vaccine composed of the C-terminal α-syn peptide (110–130) with carrier/adjuvant backbone of the AFFITOPEs®. Pooled plasma samples, obtained two weeks after the third immunization, were subjected to ELISA analysis to measure the titers of antibodies recognizing the C-terminal α-syn peptide as well as antibodies recognizing the AFF peptides. These titers were compared to titers of antibodies against recombinant full-length α-syn and β-syn. All peptides were used as bovine serum albumin (BSA) conjugates (1 μM).
Mapping of the epitope detected by AFF 1-induced antibodies was also done by ELISA in plates coated either with recombinant human full-length human α-syn (1–140), recombinant human α-syn 1–95, recombinant human α-syn 96–140, recombinant human β-syn (2 μg/ml, all obtained from rPeptide) or α-syn 110–130-BSA conjugate (1 μM).
Isolation of AFF 1-induced antibodies and labeling
The AFF 1-induced monoclonal antibody mAb-AFF1 (mouse IgG1) directed against α-syn was created by repeated immunization of BALB/c mice using AFF 1 conjugate vaccine with Alum as adjuvant as previously described [43]. Fusion of spleen cells with Ag8.531 myeloma cells and cloning of the hybridoma was performed as previously described [30]. mAb-AFF1 was purified using a Protein G-sepharose column (HiTrap Sepharose, GE Healthcare) and labelled with Alexa-488 using Alexa Fluor® 488 Protein Labeling Kit (Life Technologies) according to manufacturer’s protocol. Alexa-labelled mAb-AFF1 was injected intravenously into 6 month-old mThy1-α-syn and PDGF-α-syn transgenic animals. For studies of antibody trafficking into the CNS, 6 month-old non-tg, PDGF-α-syn tg, and mThy1-α-syn tg mice were injected intravenously with the Alexa-488-labeled mAb-AFF1, or a non-immune control Alexa-488-tagged IgG1 at a concentration of 1 mg/kg. Mice were sacrificed at 0, 24, 48 and 72 h after injection (n=3/group).
Transgenic mouse models and active immunization protocol
Two animal mouse models over-expressing α-syn were vaccinated with the AFFITOPE® AFF 1. The PDGF-α-syn mice express human α-syn under the PDGF-β promoter [46,61], and present α-syn aggregates distributed through the temporal cortex and hippocampus similar to what has been described in human DLB, accompanied by behavioral deficits [47,60,2]. The mThy1-α-syn mice express human α-syn under the mThy1 promoter [46,61], and develop behavioral motor deficits [19], axonal pathology, and accumulation of full-length and C-terminus calpain-cleaved α-syn and aggregates in cortical and subcortical regions [21] mimicking PD and PD dementia [47,60]. However, although these mouse models recapitulate some of the α-syn-mediated functional and neuropathological alterations observed in PD and DLB, they do not completely reproduce the pathology of these diseases [11,61].
A total of 24 PDGF-α-syn tg mice (6 month old) and 24 mThy1-α-syn tg mice (3–4 month old) received biweekly to monthly sub-cutaneous injections for 6 months with either AFF 1 (30 μg per dose) plus adjuvant, or adjuvant alone (n=12/group). The animals did not present any significant change in body weight, eating habits or abnormal behavior as a result of the immunization protocol. Mice were bled once a month and antibody titers monitored by ELISA analysis. At the end of the studies, mice were tested for behavioral effects. Upon termination, the right hemibrain was post-fixed in 4% paraformaldehyde in PBS (pH 7.4) at 4°C for 48 h for neuropathological analysis, while the left hemibrain was snap-frozen and stored at −70°C for subsequent protein extraction.
Behavioral analysis
In order to evaluate the functional effects of active immunization on motor function in mice, animals were tested in a modified coat hanger test, the body suspension test [50,34]. Briefly, animals were suspended by their forelimbs on a metal bar located about 50 cm above ground. The use of the hindlimbs was quantified using a three-category scale: a score of 0 indicates the inability to use the hindlimbs, 1 reflects the ability to use one hindlimb, and a score of 2 indicates the use of both hindlimbs to support the body. Mice had to hold on with one or both hindlimbs for minimum of 5 seconds to score. The maximum time allowed for the test was 120 seconds, and each mouse was tested 3 times.
In order to evaluate the cognitive effects of active immunization treatment in mice, animals were tested in the water maze as previously described [47]. Briefly, mice were first trained to locate a visible platform (days 1 to 3) and then a submerged hidden platform (days 4 to 7) in three daily trials 2–3 min apart. Mice failing to find the hidden platform within 90 seconds were placed on it for 30 seconds. The same platform location was used for all sessions but the starting point was changed randomly between two alternative entry points. On the final day of testing the platform was removed and the time spent by mice in the correct quadrant was measured (probe test). Time to reach the platform (escape latency) was recorded with a Noldus Instruments EthoVision video tracking system (San Diego Instruments).
Immunoblot and ELISA analysis for human α-syn
Hemibrains were homogenized and divided into cytosolic and membrane fractions as previously described [72,14]. For immunoblot analysis, 20 μg of total protein per lane was loaded on 4–12% Bis-Tris SDS-PAGE gels and blotted onto polyvinylidene fluoride (PVDF) membranes. To determine the effects of the immunotherapy in levels of α-syn, blotted samples from immunized α-syn tg mice were probed with antibodies against full length human α-syn (1:1000, SYN211, Life Technologies). Additional analysis was performed with antibodies against β-syn (Abcam). Incubation with primary antibodies was followed by species-appropriate incubation with secondary antibodies tagged with horseradish peroxidase (1:5000, Santa Cruz Biotechnology), visualization with enhanced chemiluminescence, and analysis with a Versadoc XL imaging apparatus (BioRad). Analysis of β-actin (Sigma) levels was used as a loading control.
To determine human α-syn levels, an ELISA analysis was used (Life Technologies), performed following manufacturer’s instructions. This ELISA kit is designed to react with human α-syn specifically, without detectable cross-reactivity to mouse α-syn.
Immunohistochemical analysis
Analysis of α-syn accumulation was performed in serially-sectioned paraffin or vibratome sections from α-syn tg mice by incubating the sections overnight at 4°C with a monoclonal antibody against human α-syn (1:250, LB509, Invitrogen). Antibodies against MAP2 and synaptophysin (1:1000, Millipore) were used to examine the effects of immunization on the complexity and preservation of dendrites and pre-synaptic terminals [45]. Antibodies against fractalkine (1:250, Santa Cruz Biotechnology), Iba1 (1:1000, Wako) and GFAP (1:1000, Millipore) were used to examine the effects of immunization on microglial and astroglial cell activation respectively. Primary antibody incubation was followed by biotinylated secondary incubation (1:100, Vector Laboratories), Avidin-Biotin Complex (1:200, ABC Elite, Vector Laboratories) and developed by incubation with diaminobenzidine. Sections were analyzed with a bright field digital B50 Olympus microscope; serial digital images were analyzed with the Image J program (NIH) to determine counts and levels of immunoreactivity [7,77,78]. For immunofluorescence analyses, primary antibody binding was detected using a FITC-tagged secondary antibody or the Tyramide Signal Amplification™-Direct (Red) system (1:100, NEN Life Sciences). Sections were imaged with a Zeiss 63X objective on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 laser scanning confocal microscope (LSCM) system (BioRad) [46]. All sections were processed simultaneously under the same conditions and experiments were performed in triplicate in order to assess the reproducibility of results.
Analysis of cytokine levels
400 μg of protein from the cytosolic fraction of brain tissue homogenates (n=4 per condition) were used for analyzing relative levels of 40 different mouse cytokines using a Mouse cytokine panel array (R&D systems, Minneapolis, USA) following the instructions of the supplier. The binding of the detection antibody was detected in a VersaDoc gel-imaging machine (BioRad) and quantified using Quantity One software (BioRad). Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) protein levels were measured by immunoblot and immunocytochemical analysis in the cytosolic and particulate fractions of mouse brain homogenates. The antibodies used for both analyses were anti-CX3CL1 (M-18, Santa Cruz) and anti-CX3CR1 (Abcam). Analysis of β-actin (Sigma) levels was used as a loading control.
Statistical analysis
Values are expressed as average ± SEM. To determine the statistical significance we used one-way analysis of variance (ANOVA) with post-hoc Dunnett’s test when comparing to the control. Additional comparisons were done using Tukey-Kramer or Fisher post hoc tests. Two-way ANOVA analysis was used to analyze water maze and body suspension test data. The differences were considered to be significant if p values were less than 0.05.
RESULTS
Selection of AFFITOPEs®
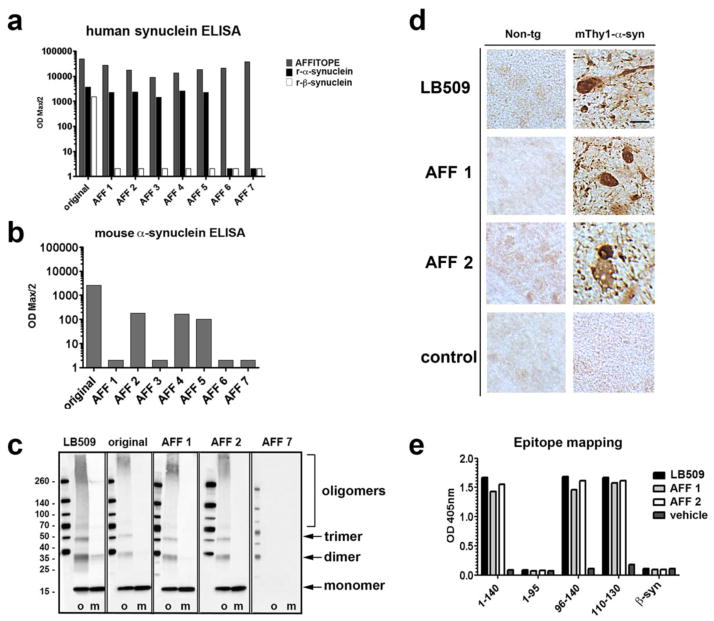
A total of 7 AFFITOPEs® that mimic the C-terminus of α-syn were tested in C57BL/6J mice by comparing titer levels of induced antibodies against α-syn and β-syn (Figure 1). As control condition, mice were immunized with the original C-terminal α-syn peptide (α-syn 110–130) in combination with the carrier/adjuvant, or with the adjuvant alone (not shown). All 8 conjugate vaccines were able to mount an immune response against the injected peptide moiety (AFFITOPE®) and the carrier molecule demonstrating the immunogenicity of all vaccines tested. The original C-terminal α-syn peptide (α-syn 110–130), as well as peptides AFF 1 to 5, elicited antibody titers against full-length human α-syn (Figure 1a); in contrast, mice immunized with AFF 6 or AFF 7 showed no reactivity to α-syn. Antibodies generated by injecting the original C-terminal α-syn peptide (α-syn 110–130) recognized both full length human α-syn and β-syn, while AFF 1 to 5 only recognized human α-syn and did not cross-react with human β-syn (Figure 1a). The LB509 monoclonal antibody against human α-syn was used as positive control and a non-immune IgG was used as a negative control (not shown).
Fig. 1. Immune response of mice vaccinated with AFFITOPEs®.
BALB/c mice were vaccinated three times and the titer of antibodies against different peptides was assessed by peptide ELISA. (a) Titer of antibodies against the injected peptide (AFFITOPE®) as well as against recombinant full-length human α-syn or β-syn. The original C-terminus of human α-syn (original, 110–130) was used as control. Antibody titers are depicted as ODmax/2. (b) Titer of antibodies against recombinant full-length murine α-syn. The original C-terminus of human α-syn (original, 110–130) was used as control. Antibody titers are depicted as ODmax/2. (c) Detection of oligomeric (o) and monomeric (m) α-syn by immunoblotting using plasma of single-vaccinated non-tg mice. The α-syn-specific antibody LB509 was used as positive control. (d) AFF 1 or AFF 2-induced antibodies were used to recognize human α-syn in brain sections of non-tg mice and of mThy1-α-syn tg mice. LB509 was used as positive control. Scale bar = 5 μm. (e) Epitope mapping of AFF 1 and AFF 2-induced antibodies. LB509 was used as control antibody. Maximum OD at a dilution of 1:100 is depicted
Next, sera from mice immunized with AFFITOPEs® were tested for their reactivity against mouse α-syn (Figure 1b). Reactivity towards mouse α-syn was strongest in the group immunized with the original epitope. Vaccination with AFF 2, AFF 4 and AFF 5 also induce some reactivity against murine α-syn, whereas animals vaccinated with AFF 1 or AFF 3 failed to mount such antibody response (Figure 1b).
To determine which species of α-syn the antibodies elicited by AFFITOPE® immunization recognize, immunoblot analysis was performed with monomeric and aggregated α-syn (Figure 1c) using 4-hydroxy-2-nonenal [58]. This analysis showed that some AFFITOPE®-induced antibodies (e.g.: AFF 1, AFF 2) detected oligomerized α-syn as well as α-syn monomers, in contrast to other AFFITOPEs® such as AFF 7, which failed to induce the production of antibodies against any α-syn species (Figure 1c). Immunohistochemical analysis of brain sections from non-tg and mThy1-α-syn tg mice confirmed that AFF 1 and AFF 2-induced antibodies detected intracellular and axonal aggregates in the substantia nigra of the tg mice but not in the substantia nigra of non-tg animals (Figure 1d). No immunoreactivity was observed with sera of mice immunized with adjuvant alone (control). Given the characteristics of the immune response elicited by AFF 1 and AFF 2, studies of epitope mapping were performed with plasma from these two groups (Figure 1e). These studies confirmed that the antibodies elicited by AFF 1 and AFF 2 recognize the C-terminus of α-syn (α-syn 110–130), but are also able to bind to full-length and N-terminal-truncated forms of α-syn, such as α-syn 96–140. No reactivity was detected for α-syn 1–96 and β-syn, respectively (Figure 1e).
Summarizing, antibodies generated by AFFITOPEs® such as AFF 1 recognize specifically α-syn, spare β-syn, have selectivity for α-syn oligomers and axonal aggregates, and the epitope is in the C-terminus of α-syn. Interestingly, vaccines based on the original α-syn do not induce similar discriminating antibodies. In addition, AFF 1 induces an immune response specific for human α-syn.
T-cell responses to immunization with AFFITOPEs®
We investigated the effects of AFFITOPE® immunization on cellular autoimmune responses directed against α-syn. AFFITOPEs® peptides are 8 amino acids long, therefore they should be too short to bind to MHCI/II molecules and activate α-syn-specific T cells. In addition, their amino acid sequence differs from the one of the native C-terminus of α-syn. To test whether AFF 1 or AFF 2 would activate a α-syn-specific T cell response, we analyzed splenocytes of immunized non-tg animals by ELISPOT (Supplemental Figure 1). Single cell suspensions from spleen and draining lymph nodes were isolated and stimulated in vitro with the carrier, α-syn, or AFFITOPE® peptides. Cultures were assessed for IFN-γ or IL-4-producing cells, reflecting T lymphocytes that had been primed during vaccination (Supplemental Figure 1a, 1b). Re-stimulation with the carrier protein demonstrated that both AFF 1 and AFF 2 had led to the induction of a carrier-specific T cell response (Supplemental Figure 1a, 1b). However, in vitro stimulation of splenocytes derived from AFF 1 and AFF 2-immunized animals with α-syn or the AFFITOPE® peptides did not yield a signal over background, confirming the expected inability of the two AFFITOPEs® to activate AFFITOPE® and α-syn-specific T cells (Supplemental Figure 1a, 1b). Consistent with these findings, immunohistochemical analysis of sections from mice immunized with adjuvant alone or AFFITOPEs® were analyzed for the presence of CD4-positive cells in the perivascular space (Supplemental Figure 1c). In the positive control animals (Experimental autoimmune encephalomyelitis, EAE), CD4-positive T-cells were detected around the blood vessels; in contrast, in the adjuvant alone or AFFITOPE®-immunized mice only rare CD4-positive cells were found (Supplemental Figure 1c). These results confirm that AFF 1 and AFF 2 do not induce cellular T cell responses. Next, a more detailed characterization of AFFITOPE® vaccination was performed using AFF 1 for efficacy studies in two tg models of synucleinopathy.
Titers and trafficking of AFF 1-induced antibodies in mThy1-α-syn tg mice
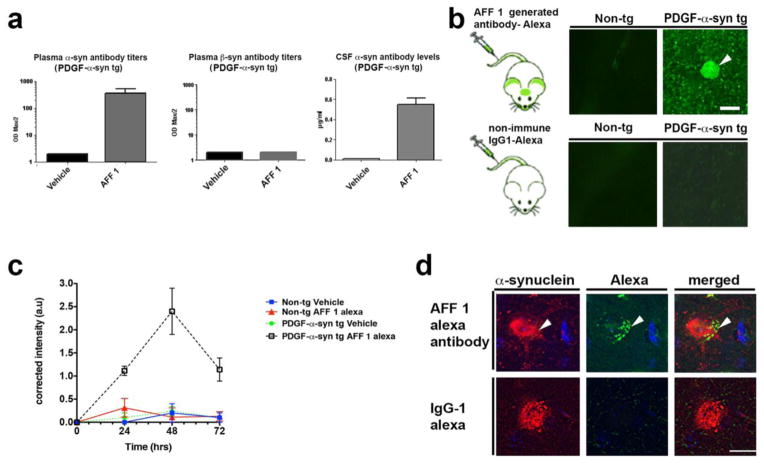
In addition to the analysis in BALB/c mice, reactivity of AFF1-induced antibodies was tested in mThy1-α-syn tg mice. Plasma from vehicle or AFF 1-treated tg animals were analyzed after three vaccinations (Figure 2a). Animals displayed relevant anti α-syn titers both in plasma and CSF, with CSF antibody levels ranging from approx 0.1–0.3% of the respective plasma levels in the animals tested. Furthermore, AFF 1 immunization did not elicit formation of antibodies against β-syn (Figure 2a).
Fig. 2. Reactivity of antibodies generated after vaccination with AFF 1 in mThy1-α-syn tg mice.
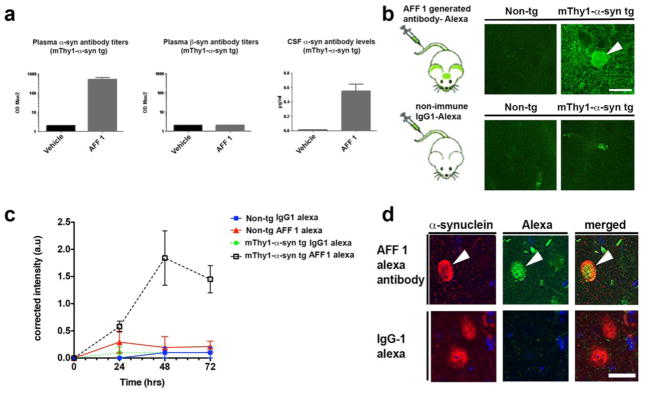
(a) Plasma or CSF of mThy1-α-syn mice treated with AFF 1 or vehicle were analyzed for the presence of α-syn or β-syn-specific antibodies after repeated vaccinations. Antibody titers are depicted as ODmax/2. (b) AFF 1-induced antibodies were tagged with Alexa-488 and administered to non-tg or mThy1-α-syn mice. AFF 1-induced antibodies bound α-syn in neuronal bodies (arrow-head) and neuropil. As negative control, a non-immune IgG1 was used and no binding was observed. Scale bar = 5 μm. (c) AFF 1-induced antibodies or non-immune IgG were tagged with Alexa-488 and administered to non-tg or mThy1-α-syn mice. A time course analysis was performed, showing that green fluorescence was only increased in brain sections of mThy1-α-syn tg animals injected with Alexa-488-tagged AFF 1 induced antibodies. Results are shown as corrected intensity values. (d) AFF 1-induced antibodies were tagged with Alexa-488 and used for immunofluorescence staining of brain sections of mThy1-α-syn tg mice (green), together with an antibody against α-syn (red). Colocalization was observed in neuronal cell bodies (arrow-head). As negative control, non-immune IgG1 was used and no α-syn staining was observed. Scale bar = 5 μm. Results are expressed as average ± SEM
In order to study the trafficking of AFF 1-induced antibodies into the CNS, a monoclonal antibody (mAb-AFF1) derived from an animal undergoing repeated AFF 1 immunization was produced according to standard procedures [43], subsequently tagged with Alexa-488 and injected intravenously into non-tg and mThy1-α-syn tg mice (Figure 2b). Vibratome brain sections were analyzed by confocal microscopy 0 to 72 h after injection. No labeling was observed in the non-tg mice injected with mAb-AFF1. In contrast, the mThy1-α-syn tg mice injected with mAb-AFF1 showed binding of the Alexa-488-tagged antibodies to α-syn aggregates in the neuropil and in neuronal cell bodies (48 h after injection; Figure 2b), likely after a process of antigen-antibody complex internalization [7,44]. Time-course analysis showed that the highest binding level was observed after 48 h, with a decline at 72 h (Figure 2c). Co-localization studies of brain sections from mThy1-α-syn tg mice treated with Alexa-488-tagged mAb-AFF1 demonstrated that the antibodies (green) co-localized with neuronal cells and neuronal processes containing human α-syn (red) as detected by SYN211 antibody staining (Figure 2d). Therefore, it can be concluded that the AFFITOPE® AFF 1 generates high titers of α-syn specific antibodies that are able to traffic into the CNS.
Immunization with AFF 1 reduces the accumulation of α-syn aggregates and motor deficits in mThy1-α-syn tg mice
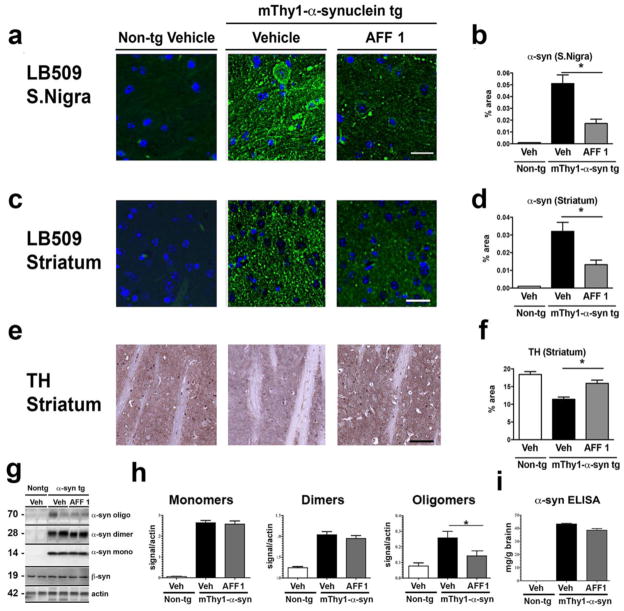
For the analysis of the preclinical efficacy of AFF 1-conjugate vaccine, mThy1-α-syn tg mice were immunized six times in biweekly and monthly intervals with AFF 1. Treatment started at 3 months of age, when only minor signs of PD-like alterations can be detected. After completion of the immunization protocol (10-month old), the efficacy of AFF 1 immunization was assessed by histological, biochemical and behavior analysis. The α-syn burden was analyzed by immunofluorescence in the areas most affected by transgene over-expression, including the substantia nigra and the striatum (Figure 3a, 3c), using the α-syn-specific monoclonal antibody LB509. The binding of the LB509 antibody to α-syn was not affected by the binding of AFF 1-induced antibodies, and they both colocalized within the same cells, as it demonstrated by double staining (Supplemental figure 2). The area occupied by α-syn positive structures was measured, using brain sections from non-tg littermates as background control (Figure 3b, 3d). The analysis revealed a significant reduction (75%) of the area occupied by α-syn-positive deposits in the substantia nigra following immunotherapy (Figure 3b). A similar analysis of α-syn staining in the neuropil, indicative of α-syn pathology, revealed a reduction of approximately 45% in the striatum (Figure 3d). Importantly, quantitative densitometry analysis of TH immunostaining also showed an increase in TH-positive structures in the striatum (Figure 3e, 3f).
Fig. 3. Immunization with AFF 1 reduced α-syn load in mThy1-α-syn tg mice.
α-syn levels were measured in non-tg mice and mThy1-α-syn tg mice immunized either with vehicle or AFF 1. (a) α-syn immunostaining of substantia nigra using the α-syn antibody LB509 (green). Cell nuclei were stained with DAPI (blue). Scale bar = 10 μm. (b) Quantification of the percentage of neuropil area positive for α-syn in substantia nigra. (c) α-syn immunostaining of striatum using the α-syn antibody LB509 (green). Cell nuclei were stained with DAPI (blue). Scale bar = 10 μm. (d) Quantification of the percentage of neuropil area positive for α-syn in striatum. (e) Tyrosine hydroxylase (TH) immunostaining of striatum. Scale bar = 10 μm. (f) Quantification of the percentage of neuropil area positive for TH in striatum. (g) Immunoblot analysis of α-syn species (oligomers, dimers, and monomers). Levels of β-syn did not change with any of the treatments. β-actin was used as loading control. (h) Densitometric analysis of immunoblot results. (i) ELISA analysis of total levels of human α-syn. Results are expressed as average ± SEM. (*) p<0.05
The effect of vaccination with AFF 1 on the levels of different α-syn species in the brain was determined by western blot. Brain homogenates from mThy1-α-syn tg mice immunized with vehicle or AFF 1, and vehicle-treated non-tg littermates were assessed for the content of α-syn monomers, dimers and oligomers using an antibody against human α-syn (Figure 3g). Quantification of α-syn monomers revealed similar levels in AFF 1 as well as in vehicle-treated animals (Figure 3h). Non-tg littermates did not show any detectable signal. The quantification of dimeric α-syn revealed a slight, but not significant reduction of human α-syn dimers in AFF 1 compared to vehicle-treated mice (Figure 3h). However, analysis of oligomeric α-syn showed a statistically significant reduction of 45% of α-syn oligomers in AFF 1-immunized mThy1-α-syn tg mice compared to vehicle-treated animals. Total levels of human α-syn remained unchanged after vaccination (Figure 3i). This result confirms that AFF 1 immunization induces antibodies able to specifically bind to and reduce neurotoxic oligomeric/aggregated α-syn.
In order to assess the preservation of motor function, a body suspension test [50,33] was performed at 9 months of age (Figure 4) measuring both, the time until the animal loses its grip and the use of the hindlimbs (quantified using a three-category scale as described in Materials and Methods). Figure 4 shows that mThy1-α-syn tg animals receiving adjuvant alone were nearly unable to fulfill the test as assessed by both parameters. Animals were holding on to the wire for 3.1 sec on average, whereas AFF 1-immunized animals were able to hold on for 19 secs. Non-tg littermates fulfilled this task in 53 secs on average (Figure 4a). Regarding hindlimb function, mThy1-α-syn tg mice were unable to use either of the two hindlimbs (Figure 4b). In contrast, AFF 1-treated animals showed a better conservation of hindlimb function, indicative for a statistically significant beneficial effect of immunotherapy on motor function in this mouse line. Taken together, these results confirm that immunization with AFF 1 reduced motor and dopaminergic deficits and accumulation of α-syn oligomers in the mThy1-α-syn tg mice.
Fig. 4. Effect of vaccination with AFF 1 on motor dysfunction in mThy1-α-syn tg mice.
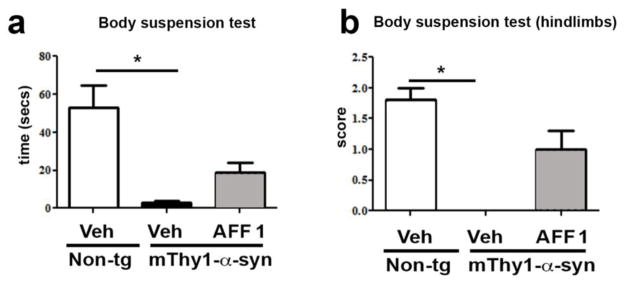
Non-tg mice and mThy1-α-syn tg mice immunized either with vehicle or AFF 1 were analyzed for motor dysfunction using the body suspension test at 9 months of age. (a) Latency to fall measured as the time the mouse is suspended until it falls from the bar. (b) The ability to use the hindlimbs was quantified using a three-category scale: a score of 0 indicates the inability to use the hindlimbs, 1 reflects the ability to use one hindlimb, and a score of 2 indicates the use of both hindlimbs to support the body. Results are expressed as average ± SEM. (*) p<0.05
Titers and trafficking of AFF 1-induced antibodies in PDGF-α-syn tg mice
Following the same procedure used for mThy1-α-syn mice, PDGF-α-syn tg mice and their non-tg littermates were immunized with AFF 1-conjugate vaccine and antibody titers were determined by ELISA (Figure 5a). Animals displayed high antibody titers in plasma and CSF, with CSF antibody levels ranging from approx. 0.1–0.2% of the respective plasma levels in the animals tested. Again, AFF 1 immunization did not elicit formation of antibodies against β-syn (Figure 5a).
Fig. 5. Reactivity of antibodies generated after vaccination with AFF 1 in PDGF-α-syn tg mice.
(a) Plasma or CSF of PDGF-α-syn mice treated with AFF 1 or vehicle were analyzed for the presence of α-syn or β-syn-specific antibodies after repeated vaccinations. Antibody titers are depicted as ODmax/2. (b) AFF 1-induced antibodies were tagged with Alexa-488 and administered to non-tg or PDGF-α-syn mice. AFF 1-induced antibodies bounded α-syn in neuronal bodies (arrow-head) and neuropil. As negative control, a non-immune IgG1 was used and no binding was observed. Scale bar = 5 μm. (c) AFF 1-induced antibodies or non-immune IgG were tagged with Alexa-488 and administered to non-tg or PDGF-α-syn mice. A time course analysis was performed, showing that green fluorescence was only increased in brain sections of PDGF-α-syn tg animals injected with Alexa-488-tagged AFF 1 induced antibodies. Results are shown as corrected intensity values. (d) AFF 1-induced antibodies were tagged with Alexa-488 and used for immunofluorescence staining of brain sections of PDGF-α-syn tg mice (green), together with an antibody against α-syn (red). Colocalization was observed in neuronal cell bodies (arrow-head). As negative control, non-immune IgG1 was used and no α-syn staining was observed. Scale bar = 5 μm. Results are expressed as average ± SEM
Analysis of antibody trafficking into the CNS was also analyzed using Alexa-488-labeled mAb-AFF1 by intravenous injection into non-tg and PDGF-α-syn tg mice (Figure 5b). PDGF-α-syn tg mice demonstrated binding to aggregates in the neuropil and in neuronal cell bodies (48h post injection), and time-course analysis (Figure 5c) confirmed similar uptake kinetics of mAb-AFF1 as described for mThy1-α-syn tg mice. Co-localization analysis also showed that the Alexa-488-tagged mAb-AFF1 (green) was internalized and co-localized with neuronal cells and neuronal processes containing human α-syn (red), detected by using the SYN211 antibody (Figure 5d).
AFF 1 immunization reduces the accumulation of α-syn and rescues memory deficits in PDGF-α-syn tg mice
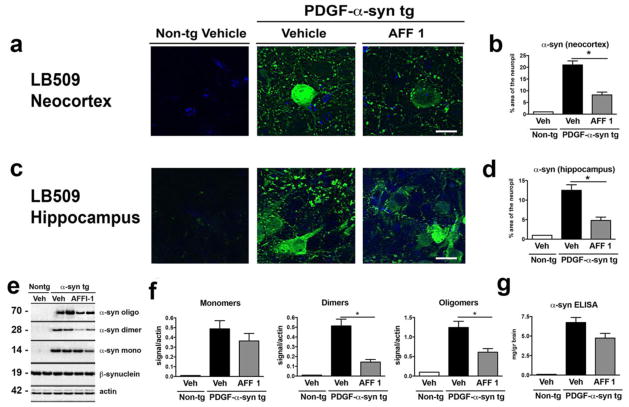
The effect of AFF 1 immunization on the α-syn load of PDGF-α-syn tg mice was measured by immunohistochemical and biochemical analysis. Immunofluorescence analysis of α-syn in neocortex and hippocampus showed that immunization with AFF 1 reduced α-syn levels in neuronal bodies and neuropil of both brain structures (Figure 6a, 6c). Quantification of the percentage of neuropil area stained by the LB509 antibody showed that AFF 1 immunization reduced α-syn levels from 20% to 8% in neocortex (Figure 6b), and from 12% to 5% in hippocampus of PDGF-α-syn tg mice (Figure 6d). Immunoblot analysis of α-syn (monomers, dimers and oligomers) was performed to determine if AFF 1 immunization reduced a specific α-syn species (Figure 6e, 6f). Vaccination with AFF 1 significantly reduced dimeric and oligomeric α-syn, but did not reduce the levels of monomeric α-syn, suggesting that the epitope recognized by AFF 1-induced antibodies could be specific for the aggregated species. Total levels of human α-syn, as measured by ELISA analysis, were slightly decreased (Figure 6g), but this reduction was not statistically significant.
Fig. 6. Immunization with AFF 1 reduced α-syn load in PDGF-α-syn tg mice.
α-syn levels were measured in non-tg mice and PDGF-α-syn tg mice immunized either with vehicle or AFF 1. (a) α-syn immunostaining of neocortex using the α-syn antibody LB509 (green). Cell nuclei were stained with DAPI (blue). Scale bar = 5 μm. (b) Quantification of the percentage of neuropil area positive for α-syn in neocortex. (c) α-syn immunostaining of hippocampus using the α-syn antibody LB509 (green). Cell nuclei were stained with DAPI (blue). Scale bar = 5 μm. (d) Quantification of the percentage of neuropil area positive for α-syn in hippocampus. (e) Immunoblot analysis of α-syn species (oligomers, dimers, and monomers). Levels of β-syn did not change with any of the treatments. β-actin was used as loading control. (f) Densitometric analysis of immunoblot results. (I) ELISA analysis of total levels of human α-syn. Results are expressed as average ± SEM. (*) p<0.05
To evaluate the effects of AFF 1 immunization on learning and memory in the PDGF-α-syn tg mice, the animals were tested in the water maze (Figure 7). The tg mice showed increased escape latencies during the learning period (Figure 7a), and spent significantly less time in the platform quadrant during the probe test than non-tg animals (Figure 7b). Immunization with AFF 1 improved learning and memory in the PDGF-α-syn tg mice, as AFF 1-immunized mice showed reduction of the escape latency during the training period, with time values similar to non-tg controls. AFF 1-immunized mice also swam for significantly more time in the platform quadrant during the probe test than vehicle treated mice. No difference in swim speed of vehicle and AFF 1-treated animals could be detected, indicating no significant influence of motor problems in this paradigm (not shown). Therefore, it can be concluded that immunization with AFF 1 reduces behavioral deficits in the PDGF-α-syn tg mice associated with learning and memory.
Fig. 7. Effect of vaccination with AFF 1 on memory deficits in PDGF-α-syn tg mice.
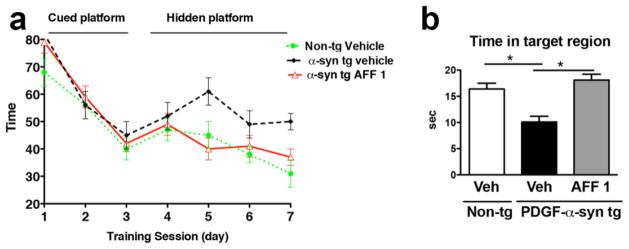
Non-tg mice or PDGF-α-syn tg mice were immunized either with vehicle or AFF 1, and spatial memory was measured using the water maze. (a) Time elapsed for finding the platform (escape latency), which was either cued (day 1–3) or hidden (day 4–7). (b) Time spent by the mice in the platform quadrant (target region) during the probe test. Results are expressed as average ± SEM. (*) p<0.05
Immunization with AFF 1 ameliorates the neurodegenerative pathology in PDGF-α-syn tg mice
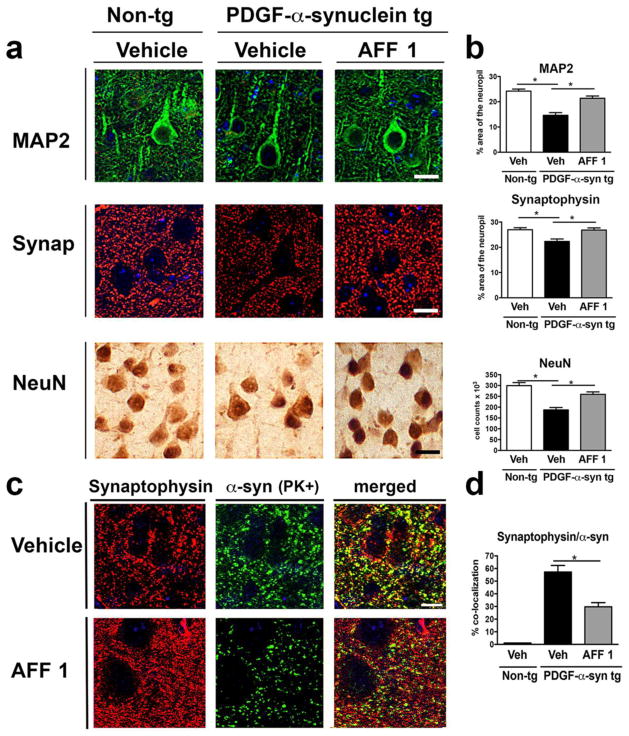
Effects of AFF 1 immunization on synaptic and neurodegenerative pathology were assessed in PDGF-α-syn tg mice. Sections from non-tg mice treated with vehicle, or PDGF-α-syn tg mice treated with vehicle or AFF 1, were immunostained with antibodies against dendritic and synaptic markers (MAP2, synaptophysin), as well as neuronal markers (NeuN) (Figure 8a, 8b). PDGF-α-syn tg mice showed a significant decrease in MAP2 and synaptophysin staining in the neuropil, which indicates dendritic and synaptic loss in these mice. Immunization with AFF 1 increased MAP2 and synaptophysin levels back to the levels similar to non-tg controls. PDGF-α-syn tg mice are also characterized by the presence of abundant neuronal cell death, measured by the loss NeuN staining (Figure 8a), and this neurodegeneration was also prevented by immunization with AFF 1. These results suggest that AFF 1-mediated α-syn clearance is reducing synaptic pathology and neuronal cell death.
Fig. 8. Effect of vaccination with AFF 1 on the synaptic pathology of PDGF-α-syn tg mice.
(a) Immunostaining of synaptic (MAP2, green; synaptophysin, red) and neuronal markers (NeuN) in brain sections of non-tg mice or PDGF-α-syn tg mice immunized either with vehicle or AFF 1. Scale bar = 5 μm. (b) Quantification of the percentage of the MAP2- or synaptophysin-positive area of neuropil, and NeuN cell counts. (c) Double immunostaining for synaptophysin (red) and proteinase K (PK)-resistant α-syn (green) in vehicle and AFF 1-treated PDGF-α-syn tg mice. Scale bar = 5 μm. (d) Quantification of the percentage of co-localization between synaptophysin and PK-resistant α-syn. Results are expressed as average ± SEM. (*) p<0.05
Finally, co-localization of synaptophysin with aggregated α-syn was analyzed by double immunofluorescence (Figure 8c, 8d). In PDGF-α-syn tg mice treated with vehicle, there was a high level of co-localization of proteinase K (PK)-resistant α-syn with the synaptic marker synaptophysin, which indicated the accumulation of aggregated α-syn in synaptic terminals. In the AFF 1-immunized tg mice, a reduction in co-localization of PK-resistant α-syn and synaptophysin was observed, together with an increase in synaptophysin staining (more synaptic buttons) and a reduction in total α-syn levels. Taken together, these results suggest that immunization with AFF 1 is reducing synaptic pathology by reducing the accumulation of α-syn in the synaptic terminals of the PDGF-α-syn tg mice.
Immunization with AFF 1 promotes microglial α-syn clearance and anti-inflammatory cytokine production in PDGF-α-syn tg mice
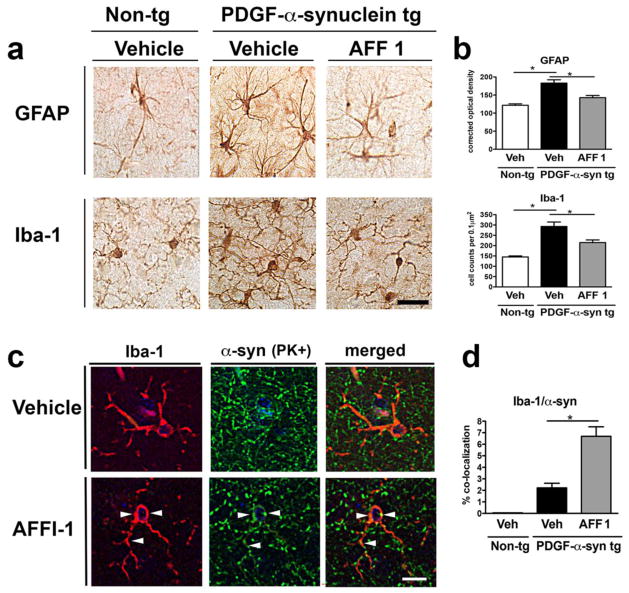
In order to analyze if AFF 1 immunization affects neuroinflammation associated with α-syn accumulation, immunohistochemical analysis of astrogliosis and microgliosis was performed in non-tg and PDGF-α-syn tg mice treated with vehicle or AFF 1 (Figure 9a) using the astroglial marker GFAP and the microglial marker Iba1. PDGF-α-syn tg mice showed significant astrogliosis and microgliosis when compared to non-tg controls, observed as an increase in the number of glial projections and intensity of the staining. AFF 1 immunization significantly reduced both astroglial and microglial reactivity, to values similar to those observed in non-tg animals (Figure 9b). We also studied the colocalization of Iba1 and PK-resistant α-syn (Figure 9c). Microglial cells have the ability to phagocytose α-syn and clear out extracellular α-syn aggregates [38]. We observed that the percentage of co-localization between Iba1 and PK-resistant α-syn was low in PDGF-α-syn tg mice (2%), and the percentage increased up to 7% in PDGF-α-syn tg mice immunized with AFF 1 (Figure 9d). This increase in α-syn-positive microglial cells suggests that immunization using AFF 1 is stimulating the clearance of α-syn by microglial cells.
Fig. 9. Effect of vaccination with AFF 1 on astrogliosis and microgliosis in PDGF-α-syn tg mice.
Non-tg mice or PDGF-α-syn tg mice were immunized either with vehicle or AFF 1, and glial markers were analyzed by immunohistochemistry. (a) Immunostaining of the astroglial marker GFAP and the microglial marker Iba1. Scale bar = 25 μm. (b) Quantification of the intensity of GFAP staining and Iba1 cell counts. (c) Double immunostaining for Iba1 (red) and proteinase K (PK)-resistant α-syn (green) in vehicle and AFF 1 treated PDGF-α-syn tg mice. Co-localization between both markers is denoted by arrow-heads. Scale bar = 5 μm. (d) Quantification of the percentage of colocalization between Iba1 and PK-resistant α-syn. Results are expressed as average ± SEM. (*) p<0.05
Astrogliosis and microgliosis are typically associated with an imbalance in brain cytokine levels towards an increase of pro-inflammatory cytokines and a decrease of anti-inflammatory cytokines. An analysis of relative levels of 40 different cytokines in the soluble fraction of brain homogenates showed that AFF 1 immunization significantly increased the levels of the anti-inflammatory cytokines IL-1Ra, IL-2 and IL-27 (Figure 10a) compared to vehicle-treated tg mice. Interestingly, immunization with AFF 1 also upregulated the protein levels of SDF-1, a chemokine involved in lymphocyte attraction that regulates production of the anti-inflammatory cytokine fractalkine (CX3CL1) by neuronal cells. Fractalkine is constitutively expressed by neurons and is able to reduce microglial activity via binding to the G protein-coupled receptor CX3CR1 expressed on glial cells. Therefore, we investigated if AFF 1 immunization modulated fractalkine levels in PDGF-α-syn tg mice. Immunoblot analysis of fractalkine and its receptor, CX3CR1, showed that AFF 1 immunization significantly increased the protein levels of insoluble, membrane-associated, fractalkine (Figure 10b, 10c). Levels of soluble fractalkine were also elevated, but this increase was not statistically significant (Figure 10b, 10c). AFF 1 immunization did not affect the protein levels of the receptor CX3CR1 (Figure 10b, 10c). Finally, in order to confirm the effect of immunotherapy on neuroinflammation, we measured the levels of inducible nitric oxide synthase (iNOS) by immunoblot (Supplemental Figure 3). iNOS is elevated in PD brains and it is a maker of oxidative stress and inflammation [55,4]. Consistent with the previous results on neuroinflammation, iNOS levels were elevated in the brain of PDGF-α-syn tg mice and they were reduced to non-tg levels by vaccination with AFF 1 (Supplemental Figure 3). These results suggest that immunization with AFF 1 reduce neuroinflammation in the PDGF-α-syn tg mouse model by stimulating microglial α-syn clearance and by increasing the production of anti-inflammatory cytokines.
Fig. 10. Effect of vaccination with AFF 1 on cytokine levels in PDGF-α-syn tg mice.
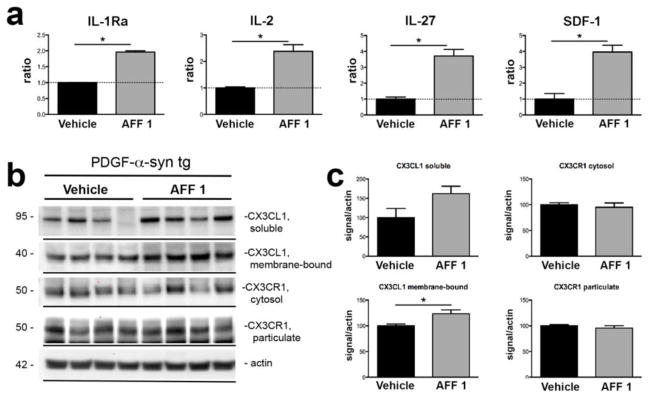
(a) PDGF-α-syn tg mice were immunized either with vehicle or AFF 1, and cytokine levels were analyzed using a mouse cytokine array. Significant changes were observed for the cytokines IL-1Ra, IL-2, IL-27 and SDF-1. Results are expressed as densitometry analysis relative to the control condition of vehicle-treated PDGF-α-syn tg mice. (b) Immunoblot analysis of fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) levels in the soluble and insoluble fractions of brain homogenates of PDGF-α-syn tg mice immunized either with vehicle or AFF 1. β-actin was used as loading control. (c) Densitometric analysis of the immunoblot results. Results are expressed as average ± SEM. (*) p<0.05
Taken together, our results suggest that active immunization using AFFITOPE® vaccines including AFF 1 could be a therapeutic alternative for the treatment of PD and related synucleinopathies.
DISCUSSION
The present study showed that immunization with the next generation AFFITOPE® vaccine AFF 1 reduces accumulation of α-syn oligomers (but not monomers) in two different tg models of synucleinopathies and ameliorates the behavioral and neurodegenerative pathology in these tg mice. AFF 1 was selected for the in vivo studies because it demonstrated a high ability to elicit α-syn specific antibodies recognizing oligomers, does not cross-react with other members of the synuclein family, and does not promote α-syn specific T-cell responses. This is a novel approach, as we used short, α-syn-mimicking peptides, foreign to the murine/human proteome, to target α-syn without the need to break tolerance against endogenous α-syn. This methodology allows for the generation of long term, sustained, more specific, antibody responses suitable for the treatment of synucleinopathies such as PD.
Previous active and passive immunization studies have shown that immunization reduces the pathology in α-syn tg models of PD. Active immunization of PDGF-α-syn mice with full-length human α-syn induced the production of high affinity antibodies, a decrease in α-syn accumulation in neuronal bodies and synapses, and a reduction of neurodegeneration [44]. These results are consistent with studies showing that vaccination is effective experimentally in other mouse models of neurodegenerative diseases, reducing the accumulation of Aβ [6,63,52,69], tau [75,5], PrP [67] and huntingtin [42,51]. Importantly, active vaccination with human α-syn did not trigger inflammatory responses in immunized mice, as confirmed by Iba1 (microglial) and GFAP (astroglial) staining. Likewise, immunization using AFF 1-conjugate vaccine did not elicit T-cell responses, normally associated with autoimmunity [68], which is relevant as active immunization has been associated previously with vasculitis and autoimmune responses [49,82]. These results suggest that AFF 1-induced antibodies do not interfere with the physiological activity of endogenous murine α-syn, but they selectively reduce aggregated, toxic species of human α-syn in tg animals. Interestingly, the high affinity antibodies produced by mice immunized with full-length human α-syn recognized epitopes within the C-terminal region of human α-syn, including amino acids 85–99, 109–123, 112–126, and 126–138 [44]. Epitope mapping of AFF 1-induced antibodies showed that the antibodies generated also recognized an area in the C-terminal region of α-syn (110–130), supporting the critical involvement of this part of the protein in antibody-mediated targeting and clearance.
Passive immunization studies with antibodies against the C-terminus of α-syn further confirmed this hypothesis [45]. Administration of antibodies against human α-syn reduced behavioral and neuropathological deficits in the PDGF-α-syn mouse model. Of those antibodies, the most specific for human α-syn was 9E4, which recognizes an epitope in the C-terminal region of the protein (118–126). A possible explanation is that the C-terminal portion of the protein penetrates the membrane and is exposed to the extracellular medium, where antibodies could recognize it. Passive immunization with 9E4 ameliorated motor behavior and learning deficits and improved synaptic pathology in the transgenic mice as confirmed by electron microscopy, PSD95 and synapsin I immunoreactivity. Similarly, AFF 1-induced antibodies also crossed the brain blood barrier and co-localized with α-syn in two different models of synucleinopathy, reducing oligomeric α-syn levels and protecting from synaptic deterioration, as observed by MAP2 and synaptophysin staining. The mechanism through which the antibody-antigen complexes are internalized is not completely understood, but might include interaction with Fcγ receptors [7] and endolysosomal trafficking for autophagy degradation [44]. Taken together, these results suggest that antibodies specific against the C-terminus of α-syn, regardless of whether they are generated after active immunization with AFF 1 or passively administered, are equally able to reduce the pathology in two α-syn mouse models without eliciting autoimmune responses.
However, the present study differs in a number of aspects with respect to other active immunization approaches against α-syn. The AFFITOPE® peptides used in this manuscript constitute a second-generation strategy of active immunization, designed to improve and advance the development of new immunotherapeutic alternatives for PD and related synucleinopathies. The AFFITOME® technology was developed to reduce the autoimmune responses that are traditionally associated with targeting self-proteins [64]. This technology aims at targeting the specific conformation of the protein driving disease pathology. For that purpose, AFFITOPEs® are developed as short peptides mimicking parts of the native structure of a pathologically relevant epitope. AFFITOPE® AD02, designed after β-amyloid for the treatment of AD, is currently undergoing phase II clinical trials. For PD’s α-syn, AFFITOPEs® were selected following the specific criteria of reactivity against α-syn but not β-syn [65].
In order to study the effect of AFFITOPE® vaccine-induced antibodies on synucleinopathies, two different α-syn transgenic models were used, which express human α-syn under the control of two different promoters: PDGF [46] and mThy1 [61,80]. The pattern of α-syn accumulation under the PDGF promoter simulates some aspects of DLB. In contrast, in the mThy1-α-syn tg mice α-syn accumulation simulates aspects of PD [61]. Interestingly, AFF 1-induced antibodies were able to reduce the levels of α-syn oligomers in both models, without affecting monomers or total α-syn levels. A possible explanation to this observation is the fraction of α-syn oligomers relative to monomers is quantitatively small; therefore a significant reduction in oligomers may not translate in a significant reduction in total α-syn. It is now widely accepted that the oligomeric form of α-syn is the toxic species [15,37,83], and that α-syn oligomers can be released to the extracellular environment and propagate to neighboring cells [3,17,39]. Therefore, the fact that AFF 1-induced antibodies target specifically oligomeric α-syn suggests that this peptide might be of use for the treatment of not only PD, but of other synucleinopathies as well. Finally, in tg mice that overexpress human α-syn, this protein not only accumulates in CNS, but it is also robustly expressed in axonal fibers, in occasional cell bodies of the enteric nervous system, and in the heart [22]. Future studies will clarify if vaccination with AFFITOPEs® is also able to reduce peripheral accumulation of α-syn.
Our results suggest that the mechanism for reduction of α-syn oligomers after vaccination involves antigen-antibody internalization and microglia activation, leading to a reduction in toxic α-syn species and consequent amelioration of the pathology. Interestingly, together with the reduction in oligomeric α-syn, AFF 1-induced antibodies reduced neuroinflammation in the α-syn tg models. Inflammation plays an important role in the progression of neurodegenerative diseases, and reduction of neuroinflammation results in behavioral improvement [1,20]. In this sense, regulation of microglia is critical for controlling inflammation by releasing factors that induce reactivity of glial cells [32,73]. Analysis of cerebral cytokine expression following AFF 1 immunization revealed an upregulation of anti-inflammatory cytokines, such as IL-1Ra and IL-2. Interestingly, AFF 1 immunization also resulted in increased SDF-1 levels, a cytokine expressed by astrocytes in CNS [8] known to regulate expression and cleavage of fractalkine (CX3CL1) in cortical neurons [13]. Fractalkine exists in both membrane-bound and soluble forms [9,57]. Soluble fractalkine mediates chemotaxis of immune cells whilst membrane-bound fractalkine acts as an adhesion molecule, mediating leukocyte capture and infiltration [59]. Fractalkine and its receptor CX3CR1 have been proposed to mediate neuron-microglial communication in the CNS, due to their expression profile: Fractalkine is expressed by neurons and its receptor is expressed by microglia [23,26,54]. In a model of PD, the lack of CX3CR1 results in exacerbation of neuronal cell death associated with increased production and release of cytokines such as IL-1β by microglia [10]. We observed an increase in neuronal (membrane-bound) fractalkine levels in response to AFF 1 immunization, an increase that is concurrent to reduced microgliosis. The protein levels of the fractalkine receptor CX3CR1 were not affected. Therefore, these results show that immunization with AFF 1 did not only fail to induce α-syn-specific T-cell responses (autoimmunity), but it also reduced neuroinflammation and led to an up-regulation of fractalkine and other anti-inflammatory cytokines/chemokines. In Figure 11 we present a hypothetical scenario of cross-talk between neuron and glial cells which would result in reduced neuroinflammation after immunization with AFFITOPE® vaccines. Interestingly, in addition to the effects on fractalkine, AFF 1 immunization also increased levels of anti-inflammatory cytokines such as IL-1Ra, IL-2 and IL-27, and a reduction in iNOS levels, which suggests an effect of immunization in cytokine-producing cells, such as astrocytes [18] and microglia [70]. However the precise mechanistic role of fractalkine and other cytokines in the anti-inflammatory effects of AFF 1-induced antibodies remains to be studied.
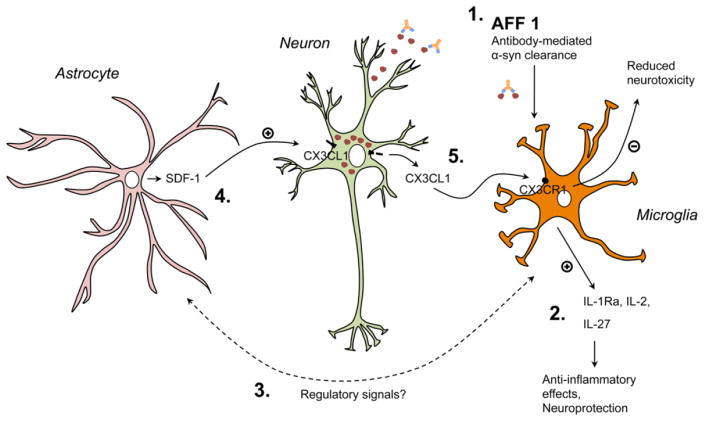
Fig. 11. Possible anti-inflammatory mechanisms elicited by immunization with AFF 1 in α-syn tg mice.
Microglial cells of the α-syn tg mice immunized with AFF 1 would clear out antibody-α-syn complexes, reducing its extracellular toxicity (1). Additionally, reduced levels of α-syn and regulatory signals would stimulate glial cells to increase the production of anti-inflammatory cytokines, such as IL-1Ra, IL-2, IL-27 (2) and SDF-1 (3). SDF-1 regulates fractalkine (CX3CL1) levels in neurons and induces its cleavage from the neuronal membrane (4). Soluble fractalkine would then interact with its receptor (CX3CR1) in microglial cells, inducing a reduction in microglial-dependent neurotoxicity (5). However, the precise molecular mechanisms by which immunization with AFF 1 would induce anti-inflammatory cytokine production remain to be studied. Red spheres, α-syn oligomers
Taken together, our results show that this new generation of vaccines for active immunization, modeled after the C-terminal region of α-syn, might represent a valuable alternative for the treatment and prevention of PD and related synucleinopathies and provide valuable information about the mechanisms by which immunotherapeutic treatment aids in these neurodegenerative disorders. Along these lines AFFITOPE® vaccines have been introduced to clinical testing with the vaccine candidate PD01A being currently assessed in a monocentric phase I study in early PD patients.
Supplementary Material
Acknowledgments
We thank Andrea Achleitner, Martina-Anna Gschirtz, Michael Hierzer, Beate Pilz, Martina Trefil and Christina Wöss for their contribution in conducting the experiments. This work was funded by the National Institutes of Health (NIH) grants NS044233, AG18440, NS047303, AG022074 and NS057096. In addition, funding was provided by Austrian Science promotion agency (FFG) grants 813335, 817969, 821453 and by the Michael J. Fox foundation for Parkinson’s research (MJFF) grant: AFFITOPE® based immunotherapeutic strategies for Parkinson’s disease.
Footnotes
Note: Markus Mandler and Elvira Valera are co-first authors
CONFLICT OF INTEREST
The authors Markus Mandler, Harald Weninger, Radmila Santic, Stefanie Meindl, Benjamin Vigl, Oskar Smrzka and Achim Schneeberger are employees of AFFiRiS, the company that commercialize the AFFITOPEs® described in the manuscript. The author Frank Mattner is co-founder of AFFiRiS. The authors Elvira Valera, Edward Rockenstein, Christina Patrick, Anthony Adame and Eliezer Masliah declare that they have no conflict of interest.
References
- 1.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129 (2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amschl D, Neddens J, Havas D, Flunkert S, Rabl R, Romer H, Rockenstein E, Masliah E, Windisch M, Hutter-Paier B. Time course and progression of wild type alpha-synuclein accumulation in a transgenic mouse model. BMC Neurosci. 2013;14:6. doi: 10.1186/1471-2202-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angot E, Brundin P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S143–147. doi: 10.1016/S1353-8020(09)70802-8. [DOI] [PubMed] [Google Scholar]
- 4.Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008;33 (12):2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 5.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27 (34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach P, Tschäpe JA, Kopietz F, Braun G, Baade JK, Wiederhold KH, Staufenbiel M, Prinz M, Deller T, Kalinke U, Buchholz CJ, Müller UC. Vaccination with Abeta-displaying virus-like particles reduces soluble and insoluble cerebral Abeta and lowers plaque burden in APP transgenic mice. J Immunol. 2009;182 (12):7613–7624. doi: 10.4049/jimmunol.0803366. [DOI] [PubMed] [Google Scholar]
- 7.Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E, Lee SJ. Antibody-Aided Clearance of Extracellular α-Synuclein Prevents Cell-to-Cell Aggregate Transmission. J Neurosci. 2012;32 (39):13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 1999;73 (6):2348–2357. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- 9.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385 (6617):640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 10.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9 (7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 11.Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9 (2):297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proceedings of the National Academy of Sciences of the United States of America. 2000;97 (2):571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook A, Hippensteel R, Shimizu S, Nicolai J, Fatatis A, Meucci O. Interactions between chemokines: regulation of fractalkine/CX3CL1 homeostasis by SDF/CXCL12 in cortical neurons. J Biol Chem. 2010;285 (14):10563–10571. doi: 10.1074/jbc.M109.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE. 2010;5 (2):e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27 (34):9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73 (4):685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106 (31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eizenberg O, Faber-Elman A, Lotan M, Schwartz M. Interleukin-2 transcripts in human and rodent brains: possible expression by astrocytes. J Neurochem. 1995;64 (5):1928–1936. doi: 10.1046/j.1471-4159.1995.64051928.x. [DOI] [PubMed] [Google Scholar]
- 19.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24 (42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Games D, Seubert P, Rockenstein E, Patrick C, Trejo M, Ubhi K, Ettle B, Ghassemiam M, Barbour R, Schenk D, Nuber S, Masliah E. Axonopathy in an alpha-synuclein transgenic model of Lewy body disease is associated with extensive accumulation of C-terminal-truncated alpha-synuclein. Am J Pathol. 2013;182 (3):940–953. doi: 10.1016/j.ajpath.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O. alpha-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis. 2012;47 (2):258–267. doi: 10.1016/j.nbd.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95 (18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10 (4):717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto M, Masliah E. Alpha-synuclein in Lewy body disease and Alzheimer’s disease. Brain Pathol. 1999;9 (4):707–720. doi: 10.1111/j.1750-3639.1999.tb00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37 (4):314–327. [PubMed] [Google Scholar]
- 27.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14 (2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 28.Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski JQ, Lee VM. Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol. 1996;148 (5):1517–1529. [PMC free article] [PubMed] [Google Scholar]
- 29.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287 (23):19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler G, Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. European journal of immunology. 1976;6 (7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 31.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27 (6):1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19 (8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 33.Lalonde R, Qian S. Exploratory activity, motor coordination, and spatial learning in Mchr1 knockout mice. Behav Brain Res. 2007;178 (2):293–304. doi: 10.1016/j.bbr.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Lalonde R, Strazielle C. Exploratory activity and motor coordination in old versus middle-aged C57BL/6J mice. Archives of gerontology and geriatrics. 2009;49 (1):39–42. doi: 10.1016/j.archger.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Lansbury PT., Jr Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proceedings of the National Academy of Sciences of the United States of America. 1999;96 (7):3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14 (1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322 (5):1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372 (3):423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285 (12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SJ, Lim HS, Masliah E, Lee HJ. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res. 2011;70 (4):339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7 (2):e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luthi-Carter R. Progress towards a vaccine for Huntington’s disease. Mol Ther. 2003;7 (5 Pt 1):569–570. doi: 10.1016/s1525-0016(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 43.Mandler M, Rockenstein E, Ubhi K, Hansen L, Adame A, Michael S, Galasko D, Santic R, Mattner F, Masliah E. Detection of peri-synaptic amyloid-β pyroglutamate aggregates in early stages of Alzheimer’s disease and in AβPP transgenic mice using a novel monoclonal antibody. J Alzheimers Dis. 2012;28 (4):783–794. doi: 10.3233/JAD-2011-111208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46 (6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6 (4):e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287 (5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 47.Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98 (21):12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKeith IG. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurologic clinics. 2000;18 (4):865–902. doi: 10.1016/s0733-8619(05)70230-9. [DOI] [PubMed] [Google Scholar]
- 49.Menéndez-González M, Pérez-Piñera P, Martínez-Rivera M, Muñiz AL, Vega JA. Immunotherapy for Alzheimer’s disease: rational basis in ongoing clinical trials. Curr Pharm Des. 2011;17 (5):508–520. doi: 10.2174/138161211795164112. [DOI] [PubMed] [Google Scholar]
- 50.Metz GA, Schwab ME. Behavioral characterization in a comprehensive mouse test battery reveals motor and sensory impairments in growth-associated protein-43 null mutant mice. Neuroscience. 2004;129 (3):563–574. doi: 10.1016/j.neuroscience.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 51.Miller TW, Shirley TL, Wolfgang WJ, Kang X, Messer A. DNA vaccination against mutant huntingtin ameliorates the HDR6/2 diabetic phenotype. Mol Ther. 2003;7 (5 Pt 1):572–579. doi: 10.1016/s1525-0016(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 52.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408 (6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 53.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20 (9):3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429 (2):167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 55.Okuno T, Nakatsuji Y, Kumanogoh A, Moriya M, Ichinose H, Sumi H, Fujimura H, Kikutani H, Sakoda S. Loss of dopaminergic neurons by the induction of inducible nitric oxide synthase and cyclooxygenase-2 via CD 40: relevance to Parkinson’s disease. J Neurosci Res. 2005;81 (6):874–882. doi: 10.1002/jnr.20599. [DOI] [PubMed] [Google Scholar]
- 56.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proceedings of the National Academy of Sciences of the United States of America. 2009;106 (31):12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387 (6633):611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 58.Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007;282 (8):5862–5870. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- 59.Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31 (5):711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rockenstein E, Crews L, Masliah E. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv Drug Deliv Rev. 2007;59 (11):1093–1102. doi: 10.1016/j.addr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68 (5):568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 62.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and Pathology of Synucleinopathies and Tauopathies Related to Parkinsonism. JAMA neurology. 2013:1–7. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400 (6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 64.Schneeberger A, Mandler M, Mattner F, Schmidt W. AFFITOME® technology in neurodegenerative diseases: the doubling advantage. Hum Vaccin. 2010;6 (11):948–952. doi: 10.4161/hv.6.11.13217. [DOI] [PubMed] [Google Scholar]
- 65.Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S11–13. doi: 10.1016/S1353-8020(11)70006-2. [DOI] [PubMed] [Google Scholar]
- 66.Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30 (24):8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sigurdsson EM, Brown DR, Daniels M, Kascsak RJ, Kascsak R, Carp R, Meeker HC, Frangione B, Wisniewski T. Immunization delays the onset of prion disease in mice. Am J Pathol. 2002;161 (1):13–17. doi: 10.1016/S0002-9440(10)64151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunologic research. 1999;20 (2):147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 69.Solomon B. Active immunization against Alzheimer’s beta-amyloid peptide using phage display technology. Vaccine. 2007;25 (16):3053–3056. doi: 10.1016/j.vaccine.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 70.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040 (1–2):202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 71.Spencer B, Michael S, Shen J, Kosberg K, Rockenstein E, Patrick C, Adame A, Masliah E. Lentivirus Mediated Delivery of Neurosin Promotes Clearance of Wild-type α-Synuclein and Reduces the Pathology in an α-Synuclein Model of LBD. Molecular therapy : the journal of the American Society of Gene Therapy. 2012 doi: 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29 (43):13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1 (1):14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trojanowski JQ, Lee VM. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch Neurol. 1998;55 (2):151–152. doi: 10.1001/archneur.55.2.151. [DOI] [PubMed] [Google Scholar]
- 75.Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, Sergeant N, Schraen-Maschke S, Blum D, Buee L. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9 (4):397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsigelny IF, Bar-On P, Sharikov Y, Crews L, Hashimoto M, Miller MA, Keller SH, Platoshyn O, Yuan JX, Masliah E. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. The FEBS journal. 2007;274 (7):1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- 77.Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V, Winkler J, Masliah E. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy. Exp Neurol. 2012;234 (2):405–416. doi: 10.1016/j.expneurol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30 (18):6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: Focus on α-synucleinopathies. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20 (16):6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35 (43):13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 82.Wilcock DM, Colton CA. Anti-amyloid-beta immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J Alzheimers Dis. 2008;15 (4):555–569. doi: 10.3233/jad-2008-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108 (10):4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.