Abstract
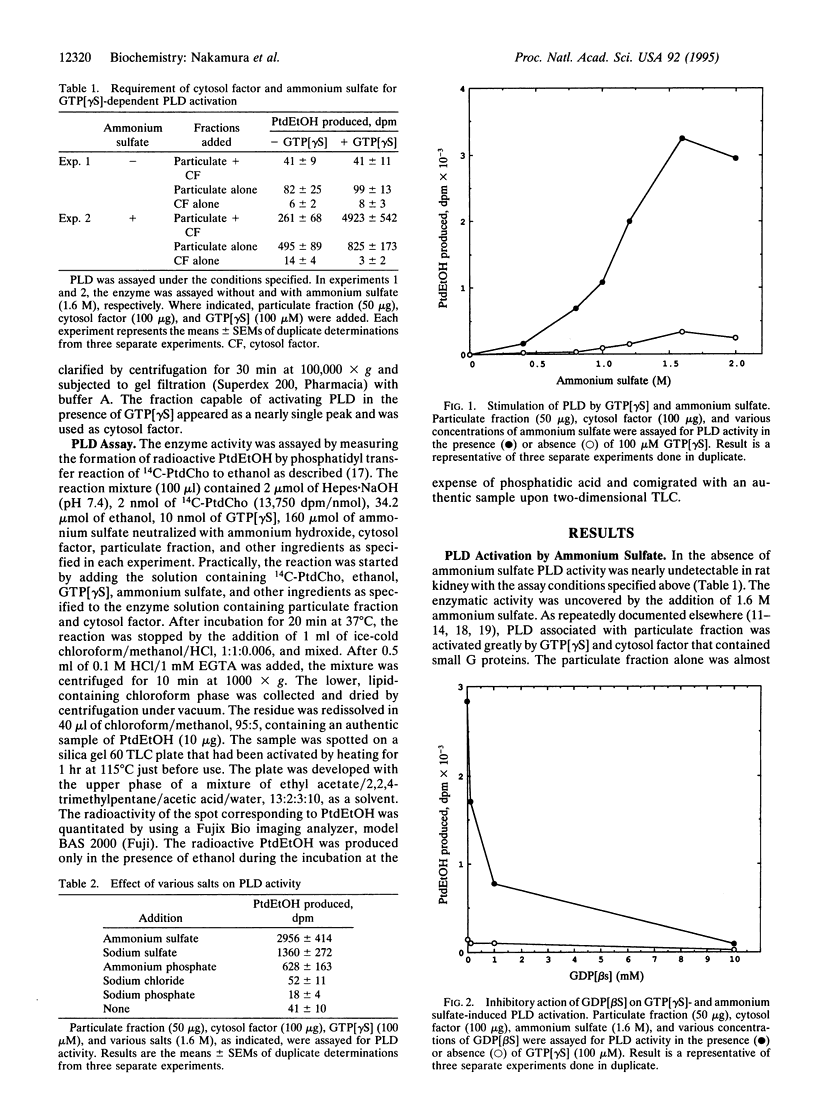
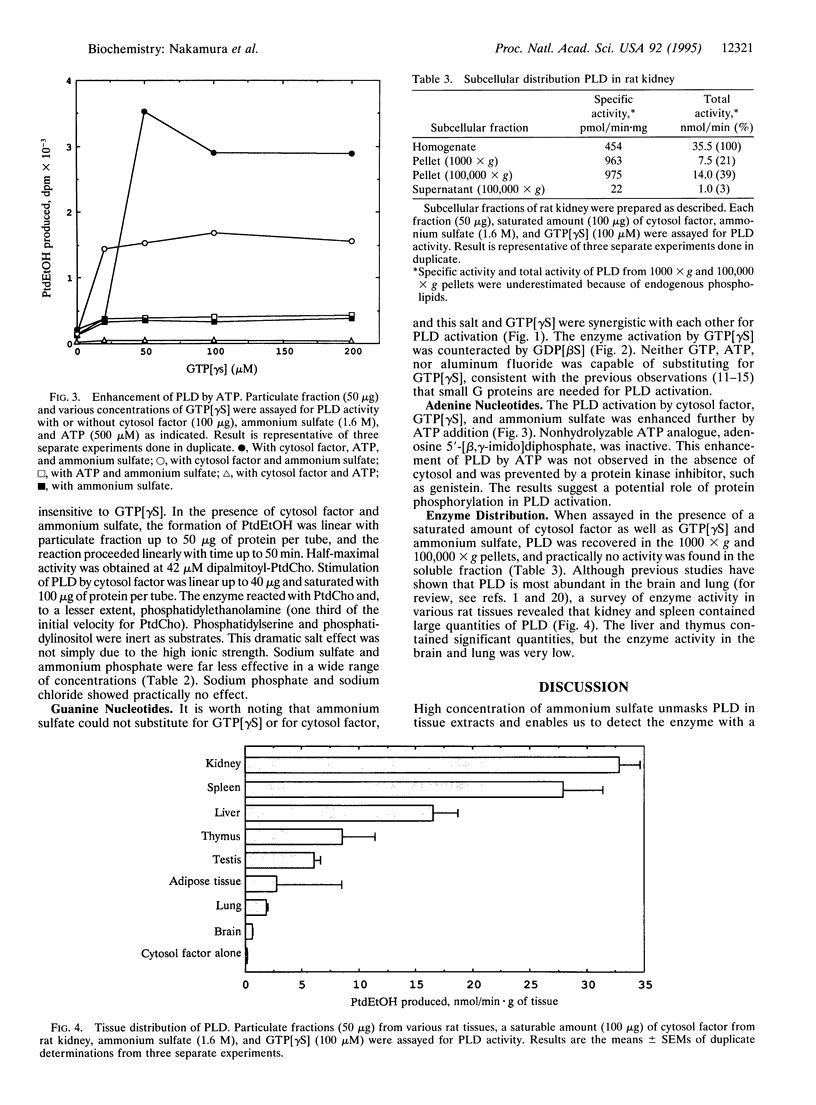
Phospholipase D (PLD) associated with the rat kidney membrane was activated by guanine 5'-[gamma-thio]triphosphate and a cytosol fraction that contained ADP-ribosylation factor. When assayed by measuring the phosphatidyl transfer reaction to ethanol with exogenously added radioactive phosphatidylcholine as substrate, the PLD required a high concentration (1.6 M) of ammonium sulfate to exhibit high enzymatic activity. Other salts examined were far less effective or practically inactive, and this dramatic action of ammonium sulfate is not simply due to such high ionic strength. Addition of ATP but not of nonhydrolyzable ATP analogue adenosine 5'-[beta, gamma-imido]diphosphate further enhanced the PLD activation approximately equal to 2- to 3-fold. This enhancement by ATP needed cytosol, implying a role of protein phosphorylation. A survey of PLD activity in rat tissues revealed that, unlike in previous observations reported thus far, PLD was most abundant in membrane fractions of kidney, spleen, and liver in this order, and the enzymatic activity in brain and lung was low.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthes J. C., Wang P., Siegel M. I., Egan R. W., Billah M. M. Granulocyte phospholipase D is activated by a guanine nucleotide dependent protein factor. Biochem Biophys Res Commun. 1991 Feb 28;175(1):236–243. doi: 10.1016/s0006-291x(05)81225-2. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarder M. R. A role for phospholipase D in control of mitogenesis. Trends Pharmacol Sci. 1994 Feb;15(2):57–62. doi: 10.1016/0165-6147(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Bowman E. P., Uhlinger D. J., Lambeth J. D. Neutrophil phospholipase D is activated by a membrane-associated Rho family small molecular weight GTP-binding protein. J Biol Chem. 1993 Oct 15;268(29):21509–21512. [PubMed] [Google Scholar]
- Brown H. A., Gutowski S., Moomaw C. R., Slaughter C., Sternweis P. C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993 Dec 17;75(6):1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Chalifa V., Möhn H., Liscovitch M. A neutral phospholipase D activity from rat brain synaptic plasma membranes. Identification and partial characterization. J Biol Chem. 1990 Oct 15;265(29):17512–17519. [PubMed] [Google Scholar]
- Cockcroft S. G-protein-regulated phospholipases C, D and A2-mediated signalling in neutrophils. Biochim Biophys Acta. 1992 Aug 14;1113(2):135–160. [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994 Jan 28;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Geny B., Paris S., Dubois T., Franco M., Lukowski S., Chardin P., Russo Marie F. A soluble protein negatively regulates phospholipase D activity. Partial purification and characterization. Eur J Biochem. 1995 Jul 1;231(1):31–39. [PubMed] [Google Scholar]
- Inoue H., Shimooku K., Akisue T., Nakamura S. Potential role of protein phosphorylation in GTP-gamma-S-dependent activation of phospholipase D. Biochem Biophys Res Commun. 1995 May 16;210(2):542–548. doi: 10.1006/bbrc.1995.1694. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Kanfer J. N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem. 1987 May;48(5):1597–1603. doi: 10.1111/j.1471-4159.1987.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Cantley L. C. Lipid second messengers. Cell. 1994 May 6;77(3):329–334. doi: 10.1016/0092-8674(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Malcolm K. C., Ross A. H., Qiu R. G., Symons M., Exton J. H. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem. 1994 Oct 21;269(42):25951–25954. [PubMed] [Google Scholar]
- Massenburg D., Han J. S., Liyanage M., Patton W. A., Rhee S. G., Moss J., Vaughan M. Activation of rat brain phospholipase D by ADP-ribosylation factors 1,5, and 6: separation of ADP-ribosylation factor-dependent and oleate-dependent enzymes. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11718–11722. doi: 10.1073/pnas.91.24.11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A., De Graan P. N., Gispen W. H., Wirtz K. W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992 Apr 15;267(11):7207–7210. [PubMed] [Google Scholar]
- Nakamura S., Nishizuka Y. Lipid mediators and protein kinase C activation for the intracellular signaling network. J Biochem. 1994 Jun;115(6):1029–1034. doi: 10.1093/oxfordjournals.jbchem.a124451. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995 Apr;9(7):484–496. [PubMed] [Google Scholar]
- Okamura S., Yamashita S. Purification and characterization of phosphatidylcholine phospholipase D from pig lung. J Biol Chem. 1994 Dec 9;269(49):31207–31213. [PubMed] [Google Scholar]
- Olson S. C., Bowman E. P., Lambeth J. D. Phospholipase D activation in a cell-free system from human neutrophils by phorbol 12-myristate 13-acetate and guanosine 5'-O-(3-thiotriphosphate). Activation is calcium dependent and requires protein factors in both the plasma membrane and cytosol. J Biol Chem. 1991 Sep 15;266(26):17236–17242. [PubMed] [Google Scholar]
- Singer W. D., Brown H. A., Bokoch G. M., Sternweis P. C. Resolved phospholipase D activity is modulated by cytosolic factors other than Arf. J Biol Chem. 1995 Jun 23;270(25):14944–14950. doi: 10.1074/jbc.270.25.14944. [DOI] [PubMed] [Google Scholar]
- Stasek J. E., Jr, Natarajan V., Garcia J. G. Phosphatidic acid directly activates endothelial cell protein kinase C. Biochem Biophys Res Commun. 1993 Feb 26;191(1):134–141. doi: 10.1006/bbrc.1993.1194. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- el-Moatassim C., Dubyak G. R. A novel pathway for the activation of phospholipase D by P2z purinergic receptors in BAC1.2F5 macrophages. J Biol Chem. 1992 Nov 25;267(33):23664–23673. [PubMed] [Google Scholar]
- el-Moatassim C., Dubyak G. R. Dissociation of the pore-forming and phospholipase D activities stimulated via P2z purinergic receptors in BAC1.2F5 macrophages. Product inhibition of phospholipase D enzyme activity. J Biol Chem. 1993 Jul 25;268(21):15571–15578. [PubMed] [Google Scholar]



