Abstract
Current treatments for diseases and trauma of dental, oral and craniofacial (DOC) structures rely on durable materials such as amalgam and synthetic materials, or autologous tissue grafts. A paradigm shift has taken place to utilize tissue engineering and drug delivery approaches towards the regeneration of these structures. Several prototypes of DOC structures have been regenerated such as temporomandibular joint (TMJ) condyle, cranial sutures, tooth structures and periodontium components. However, many challenges remain when taking in consideration the high demand for esthetics of DOC structures, the complex environment and yet minimal scar formation in the oral cavity, and the need for accommodating multiple tissue phenotypes. This review highlights recent advances in the regeneration of DOC structures, including the tooth, periodontium, TMJ, cranial sutures and implant dentistry, with specific emphasis on controlled release of signaling cues for stem cells, biomaterial matrices and scaffolds, and integrated tissue engineering approaches.
Keywords: Teeth, Dental pulp, Periodontium, Cranial sutures, Temporomandibular joint, Implant dentistry
1. Introduction
The complexity of dental, oral and craniofacial (DOC) structures presents special challenges for the regeneration of cartilage, bone, muscle, tendons, cranial sutures, temporomandibular joints (TMJ), salivary glands, periodontium and teeth (Fig. 1). Awide range of drug delivery matrices and scaffolds has been applied to accommodate the needs of regenerating DOC tissues. Cells that are responsible for tissue regeneration may be either delivered via biomaterial carriers or recruited in vivo by signaling molecules. Drug delivery by controlled release provides several critical advantages because bioactive factors are transported to the desirable milieu in biocompatible carriers, released in a controlled manner and may locally regulate multiple processes of cell chemotaxis, attachment, proliferation, differentiation, and morphogenesis. In the absence of drug delivery and controlled release, bioactive factors may undergo rapid diffusion and denature shortly after in vivo delivery, and often fail to induce the intended effects on target cells and tissues. Although control-released drugs or bioactive factors may also diffuse and denature, the continuous dosing provides sustained effects where subsequent doses act on cells that have already been sensitized by previous growth factor exposure.
Fig. 1.
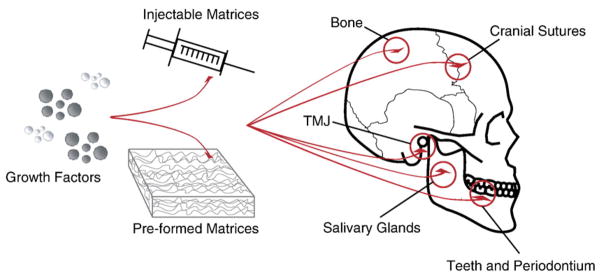
Drug delivery in dental, oral and craniofacial tissue engineering. Growth factors and other bioactive molecules may be delivered via in situ forming injectable matrices or pre-shaped implants for the regeneration of teeth, periodontium, cranial sutures, salivary glands, temporomandibular joints, and calvarial bone.
The auspice of regenerative medicine has rested on the delivery of stem cells towards the healing of diseased, traumatized or ageing tissues and organs. At this time, we believe that drug delivery is a highly effective adjunctive approach for cell based therapies. There is also the potential that controlled delivery of bioactive factors may circumvent the needs for cell delivery in certain defects, and may induce the homing of stem cells, progenitor cells and/or tissue-forming cells in traumatized or diseased tissues. The advantage of cell homing over ex vivo cell manipulation and delivery is the minimized laboratory and graft preparation as well as a commercially attractive potential for “off-the-shelf” products.
The nature of DOC structures determines that the regeneration process involves complex and composite tissues [1,2]. For example, the regeneration of the arthritic temporomandibular joint (TMJ) condyle involves at least two tissue phenotypes: cartilage and bone. Biological repair of dental caries frequently involves both enamel and dentin. When carious lesions infect the tooth pulp, healing involves the repair and regeneration of multiple cell types, such as neurons, odontoblasts, vascular cells and fibroblasts. Periodontal disease involves the periodontal ligament, cementum and alveolar bone. Salivary gland consists of ductal and accini cells interfacing with connective tissue cells in a vascularized environment. Accordingly, DOC regeneration frequently requires the manipulation of multiple tissue phenotypes [1,2]. Some of the features of DOC structures with regard to tissue engineering and tissue regeneration are as follows:
High demand for esthetics
Many tissues are highly vascularized
Often infectious environment of the oral cavity
Minimal scar formation in the oral cavity
Many DOC tissues are potential sources for stem cells
Multiple tissue phenotypes integrated or nearby
Demand for composite tissue engineering
Need to accommodate multiple tissue functions
Most tissues are superficial or readily exposed
Practiced by multiple medical and dental specialties
A number of tissue engineering projects geared towards DOC structures may serve as prototypes for tissue engineered structures elsewhere in the body. For example, the temporomandibular joint (TMJ) represents a prototype for the regeneration of other synovial joints, given its relatively smaller size in comparison with the hip or knee joints [2–4]. Success in regenerating a functional TMJ may be then scaled up to larger joints of the appendicular skeletal system. Similarly, many of the current tissue engineering techniques that target other regeneration systems may be applicable to DOC regeneration (Fig. 1). However, the scale or the depth of previous and existing work in the areas of dental tissue engineering is far inferior to what is needed for the regeneration of these structures. This review addresses the structural and functional requirements for DOC regeneration with special emphasis on matrices, scaffolding, drug delivery and biomaterial approaches.
2. Teeth and periodontium
Common conditions requiring dental treatment include caries, pulp and odontogenic infections, periodontal disease, tooth impactions, and malocclusion. Current clinical therapies for dental diseases are metallic or synthetic materials. Many of the dental diseases are unexplored fields in terms of biologically derived regeneration. For example, tooth decay, the second most prevalent disease in the United States, affects over 90% of the population. Tooth decay is initiated by acidogenic bacterial infection in plaque and can range from superficial enamel defects to widespread carious destruction. Partial or complete loss of tooth structure, may require restorations, tooth extraction, surgical titanium implants or tooth replacement using denture-based prostheses. Dental material in use broadly includes metal alloys, cements, acid-based polymer products, and synthetic materials such as composites resins. Also in use are known carcinogens such as mercury in amalgam and formocresol used in pulp therapy. Most restorative dental materials have a limited lifespan and may need to be repaired or replaced periodically. Tissue engineering may provide a viable alternative for the repair of tooth structures including the enamel, dentin, cementum and the tooth pulp. Fortunately, several groups have begun to explore approaches toward the ultimate regeneration of tooth as an organ [5–9]. Although the regeneration of an entire tooth as an organ is an admirable goal, it is also a long-term goal with numerous hurdles, not the least of which is that the eruption of native teeth is poorly understood. Even when a tooth is engineered in the alveolar bone, the eruption mechanism is likely a road block towards regenerating a functional and occluding tooth. On the other hand, if a tooth is regenerated ex vivo and ready for implantation, reconnection of apical vessels and nerves would be a critical challenge. Although it is expected that a great deal of biology is learned towards whole tooth engineering, it is predictable that the regeneration of separate tooth structures would be more fruitful at this time.
The anatomy of DOC tissues demonstrates distinct yet integrated structures. Externally, enamel coats the tooth, providing a compact and hard surface. During tooth development, enamel matrix is produced by the ameloblasts that derive from neural crest cells that migrate to form the oral ectoderm. Following enamel deposition, ameloblasts cease to exist in post-natal tissues. This is in contrast to the existence of adult or somatic stem cells in post-natal mammals such as mesenchymal stem cells [4,10–12]. To date, there is little experimental evidence that ameoloblasts can be isolated from one of the adult stem cell lineages. On the engineering side, enamel is a generously designed structure and is the hardest mineralized tissue in the body. The elastic modulus of enamel is approximately 12.2×106 psi with some spatial variability between apical and lateral aspects [13]. Without surprise, strong glass-, ceramic-or metal-based substitutes have been used as enamel replacements such as amalgam and composites.
A second dental cell type is the odontoblast that is responsible for the formation of dentin that underlies the enamel and is the bulk of the tooth. Odontoblasts derive from neural crest derived mesenchymal cells. Similar to the enamel, the mechanical properties of dentin are strenuous and require adequate substitutes and fixation [14]. In the context of tissue engineering, scaffolds and matrices for enamel and dentin must withstand the minimal forces necessary during mastication and occlusion shortly after reconstruction. Cementum coats the dentin in the root portion of the tooth and is formed by cementoblasts that also derive from neural crest derived mesenchymal cells. Cementum is considered a part of the periodontium and attaches to the alveolar bone via collagen fibers. Cementum is typically thinner, softer and more permeable than enamel, leading to greater susceptibility to abrasion upon root exposure to the oral environment. In contrast to the overlaying hard tissues of dentin, enamel and cementum, the dental pulp is soft tissue that resides in the central core of the tooth. The pulp, also partially originating from neural crest derived mesenchymal cells, has a rich vascular and nerve supply.
Erupted teeth are supported in bony sockets of the alveolar bone via fibers of the periodontal ligament (PDL). The PDL, similar in function to ligaments and tendons in the musculoskeletal system such as ACL, transmits mechanical forces and acts as a cushion during mastication, although the PDL has different structural attributes. Each of the hard tissues of the tooth including the enamel, dentin and cementum is formed by apparently a homogeneous cell type. For example, the dentin is formed apparently by odontoblasts alone. In contrast, the PDL and the dental pulp each is formed by a number of diverse cell populations. The PDL is formed by the interactions of cementoblasts, fibroblasts, osteoblasts in addition to blood vessel borne cells, whereas the dental pulp is formed by odontoblasts, fibroblasts, vascular and lymphatic system cells, as well as neurons. Accordingly, the selection of matrices and scaffolds in dental tissue regeneration must reflect these specific conditions and cell lineages, in addition to functional and mechanical requirements.
The multifaceted structure of teeth requires multidirectional engineering perspectives which likely include composite materials that satisfy the numerous requirements for functionality. These perspectives must then converge to the formation of regenerative tissues suitable for rapid established functionality post-intervention. Accordingly, previous efforts in DOC tissue engineering have encompassed a wide range of biomaterials, including biopolymers, ceramics, and metals. Additionally, the stimulatory, scaffolding, or structural functions of biomaterials can be improved by the synchronous activity of bioactive molecules. Drug delivery technology has been described extensively in tissue engineering procedures and is directly applicable in dental regeneration. Controlled delivery of growth factors have been demonstrated to promote alveolar bone regeneration, periodontal ligament formation, as well as pulp cell activation as described in the following paragraphs.
2.1. Molecular fingerprints during tooth development and regeneration
Similarly to hair and glands, teeth develop from ectoderm in vertebrate embryos with critical roles played by interactions between the ectoderm and the underlying mesenchyme [15]. Each step during tooth morphogenesis, ectoderm thickening, followed by the bud, cap, bell and late bell stages, hosts sequential and reciprocal molecular events that regulate the initiation, morphogenesis, differentiation and mineralization, as well as root formation and eruption of the tooth. Most of the signaling molecules regulating ectoderm and mesenchyme interactions during tooth development belong to the transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), Hedgehog, and Wnt families [15].
The sequential morphogenesis steps are characterized by the formation of signaling centers in the epithelium. Initially, the dental placodes are found during budding, and are characterized by the expression of activin, FGF and BMP-4 (bone morphogenetic protein-4). Placode development is further regulated by Wnts and ectodysplasin. Budding begins along with condensation of mesenchymal stem cells. At this point, the transcription factors Runx2 and Fgf3 regulate epithelial morphogenesis during the bud to cap transition. BMP-4 is also involved in enamel knot formation at the tip of the bud, by inducing expression of p21, resulting in knot cells leaving the cell cycle. The enamel knot expresses many other signaling molecules such as Shh, Bmp-2, Bmp-4, and Bmp-7, Fgf-3, Fgf-4, Fgf-9 and Fgf-20, and Wnt-3, Wnt 10a, and Wnt-10b [15]. SHH signaling is also crucial for the development of epithelial cervical loops flanking the enamel knots. Patterning of the tooth crown is regulated by the secondary enamel knots, which conserve the molecular expression signature and determine the sites for epithelial sheet folds and cusp development. The exact recapitulation of the molecular fingerprints during tooth formation may not be necessary in the process of engineering tooth structures. It is prudent however, to consider the spatial and temporal activity of cytokines and morphogens in order to establish optimal signaling cascades using drug delivery technologies. The delivery of commonly used growth factors such as FGF, BMP and TGF-β are discussed below in the context of regeneration of the dental pulp, as well as dentin and periodontal structures. A summary of drug delivery approaches for DOC regeneration is highlighted in Table 1.
Table 1.
Summary of delivery systems for growth factors involved in dentin, pulp, periodontium, cranial sutures and temporomandibular joint regeneration
| Tissue type | Growth factor | Delivery vehicle | References |
|---|---|---|---|
| Dentin and pulp | TGF-β1,2 and 3 | Agarose beads | [57] |
| BMP-7 | Agarose beads | [58] | |
| BMP-2/BMP-4 | Collagen sponge | [36] | |
| TGF-β1 | Alginate gels | [60] | |
| Periodontium | FGF-2 | Gelatinous carrier | [105] |
| FGF-2 | Sandwich collagen Membrane | [180] | |
| IGF-1 | Dextran-co-gelatin hydrogel microspheres | [108] | |
| BMP-12 | Collagen sponge | [111] | |
| BMP-2 | Slow dissolving collagen membranes | [116,117] | |
| PDGF-BB | βTCP | [53] | |
| Cranial sutures | TGF-β3 | Collagen gel | [181] |
| TGF-β3 | PLGA microspheres | [182] | |
| Temporomandibular joint | BMP-2 | Collagen carrier | [135] |
| BMP-2 | PLA/PGA copolymer-gelatin sponge complex | [143] | |
| TGF-β3 | Thermo-reversible hydrogel/hyaluronic acid | [146] | |
| TGF-β1 | Oligo(poly(ethylene glycol) fumarate) (OPF) | [148] |
2.2. Dental repair by delivery of matrices and growth factors
The dental pulp is a well vascularized and innervated connective tissue that shows some potential for healing in response to various stimuli and injury [16]. Typically, following pulpal exposure, pulp capping is a common procedure that induces dentine bridge formation and has proven to be an efficient model for repair and wound healing [17–20]. In order to maintain tooth structural integrity after trauma or corrective procedures, calcium hydroxide is sometimes used as a direct capping agent for the pulp [21]. Calcium hydroxide may contribute to reactionary dentin formation as a cavity liner as well as contributing to repair of pulp exposures [22]. However, endodontic treatment post-calcium hydroxide capping may become difficult when formation of necrotic and acute or chronic inflammatory zones are observed [23–26]. The high alkaline pH that calcium hydroxide promotes at the surface of the exposed pulp causes a controlled burn and subsequent scar, below which reparative cells are recruited in the central part of the pulp [21]. Given the replication potential of such reparative cells, it is unlikely that these cells are mature odontoblasts, since these typically are terminally differentiated. Consequently, there is the possibility for the existence of “dental pulp stem cells”, which have high proliferation and differentiation potential. These cells aggregate and proliferate adjacent to the wounded area and initiate repair forming osteodentin [21]. Further characterization of pulp stem cells indicate some similarities to bone marrow derived progenitor cells, such as extended self-renewal capabilities as well as multiple lineage differentiation [16,27–30]. Previous studies have demonstrated the differentiation potential of pulp derived cells into many lineage specific cells including dentin and cementum producing cells such as odontoblasts and cementoblasts [31–35]. TGFβs such as BMPs induce differentiation of pulp cells into odontoblasts [36–38]. Human dental pulp cells (hDPCs) may be cultured in various surfaces in order to regulate their differentiation. For instance, hDPCs cultured on dentin surfaces assume odontoblast-like morphologies with cytoplasmic processes that extend the dentinal tubule [33]. The high expandability potential of DPCs was demonstrated in comparable levels to immortal cell lines (NIH 3T3). Additionally, DPCs may be differentiated into mineral nodule forming cells with exposure to dexamethasone and 1,25-dihydroxyvitamin D3.
Alternatively, hyaluronic acid (HA) has been proposed as a substitute to calcium hydroxide, based on previous data demonstrating the stimulatory effects of high molecular weight HA on early healing of rat long bones in addition to circumventing the necrotic and inflammatory shortcomings of calcium hydroxide [39]. Results indicate that capping pulp exposures of rat molars with HA promotes the formation of reparative dentine that extended throughout the pulp chamber after 30–60 days, following differentiation of fibroblastic and odontoblast-like cells and globular calcified nodules formation at 1 week as well as the formation of a layer of reparative dentine by odontoblast-like cells over the dentine walls at 2 weeks [39]. Such findings indicating alternatives for pulp capping may open possibilities for regenerative drug delivery. Hyaluronic acid has been previously tested as a delivery scaffold for BMP-2 [40]. The release profile of BMP-2 from hyaluronic acid scaffolds shows gradual sustained release up to 28 days (~32% of total encapsulated BMP-2) when compared to fast release collagen gels (100% after 14 days). BMP-2 was released in bioactive form from hyaluronic acid as shown by alkaline phosphatase (ALP) activity induction of a pluripotent cell line indicating osteogenic differentiation. Hyaluronic acid is a versatile biocompatible and bioresorbable material, which is enzymatically degraded. Multiple growth factors may also be loaded concurrently in HA scaffolds such as BMP-2, IGF-1 (insulin-like growth factor-1), and TGF-β2 [41]. Ten days post-subcutaneous implantation of HA in rats, the newly formed tissue was primarily cartilage in BMP-2/IGF-1 loaded groups and mostly bone in the BMP-2/TGF-β2 groups.
Several studies implicate platelet-derived growth factor (PDGF) during the development of the ectomesenchyme and the differentiation of dental pulp cells [42–46]. Furthermore, PDGF is involved during growth of the pulpal mesenchyme in developing mouse molars. Given its important roles during tooth development and repair, PDGF delivery in dental systems has been explored. Matrices used in PDGF delivery for the regeneration of several other organ systems have been described including self-assembled nanofibers [47], chitosan-poly(L-lactide) (PLLA) composite matrices and chitosan coated on PLLA matrices [48], porous chondroitin-4-sulfate (CS)-chitosan sponge [49], ethylene vinyl acetate copolymer (EVAc) coated stainless-steel Kirschner wire (K-wire) [50], as well as PLGA microspheres [51].
In the context of DOC tissue engineering, the three PDGF dimers, PDGF AA, AB, and BB were cultured with dental pulp cells from rat incisors resulting in differential regulation. All three dimers suppressed alkaline phosphatase (ALP) activity, osteocalcin expression, calcium content, as well as the formation of dentin-like nodules. However, PDGF AB and BB stimulated expression of dentin sialoprotein (DSP) despite the lack of dentin-like nodules, whereas PDGF AA inhibited DSP. These diverse effects must be considered during odontoblast differentiation from pulp cells. Continuous exposure of genetically delivered PDGF-A has inhibitory effects on cementogenesis of immortalized cementoblasts implanted subcutaneously in severe combined immunodeficient (SCID) mice via upregulation of osteopontin and enhancement of multinucleated giant cells (MNGCs) [52]. In contrast, delivery of an antagonist to PDGF signaling inhibited mineralization of tissue-engineered cementum possibly due to the downregulation of bone sialoprotein and osteocalcin as well as stimulation of MNGCs. These results indicate that although PDGF-A delays mineral formation by cementoblasts, it may still be critically required for mineral neogenesis. Thus, controlled delivery for specific PDGF dimers will likely advance our knowledge of the temporal requirements for each molecule and lead to the optimization of their pattern of expression in engineered tissues. Accordingly, PDGF-BB has been delivered to patients with severe periodontal osseous defects during reconstructive procedures using β-tricalcium phosphate (βTCP) constructs. Administration of recombinant human PDGF-BB demonstrated pronounced increased levels of vascular endothelial growth factor (VEGF) as well as bone turnover markers in the wound fluid at 3 weeks [53]. Although diffusive delivery of soaked PDGF in βTCP resulted in promising regenerative responses, more complex delivery systems are likely necessary for more rapid, improved and less inflammatory repair. Ceramic based materials such as tricalcium phosphate (TCP) are useful for bone tissue engineering, including alveolar bone, due to their structural similarity to the inorganic portion of bone. TCP is protein free and does not induce immunologic activity. In conjunction to 3-D printing technology, customized TCP constructs may be fabricated to have the pre-designed shape of osteogenic defects, and degrade by the action of osteoclasts or via chemical dissolution by interstitial fluids, thus desirable during natural remodeling of bone [54].
2.3. Advanced delivery systems — Multiple factor and dose dependence
The need for complex drug delivery systems has been previously addressed; however still remains in its infancy. For instance, the complex delivery of PDGF and VEGF has been implicated in the formation of angiogenic networks for vascular tissue engineering [55]. Drug delivery studies typically rely on the delivery of single factors, which may not suffice signaling requirements, given that the development of tissues and organs is typically driven by the action of multiple growth factors [55]. Here, two molecules involved during angiogenesis, VEGF and PDGF, were delivered with distinct release kinetics from composite biomaterials. VEGF is an early required molecule during endothelial cell differentiation, whereas PDGF influence is expected later during maturation of blood vessels, by recruiting mural cells including smooth muscle cells. Consequently, their distinct temporal dependence during angiogenesis call for complex drug delivery systems with distinct release profiles from a single composite engineered construct. In order to address this need, VEGF was simply mixed with particulate PLG for rapid release, and PDGF-BB was encapsulated in PLG microspheres for prolonged sustained release. The differential release profile of multiple bioactive molecules is an important concept in dental drug delivery, not only in angiogenesis but also for the orchestrated morphogenesis of DOC structures.
Applications for multiple signaling are illustrated by the differentiation of dental papillae cells using combinations of TGFβ1 and BMP-2 molecules [56]. TGFβ1, BMP-2 and the ethylenediaminetetraacetic acid (EDTA)-soluble fraction of dentin proteins from rabbit incisor teeth stimulated odontoblast differentiation of day-17 first lower mouse molar dental papillae cells. Multiple TGF-β isoforms have also been delivered concurrently using agarose beads as delivery vehicles. Growth factor loaded beads were placed over odontoblast cultures from rat incisor teeth and stimulated predentine secretion [57,58]. Expression of the TGFβ isoforms TGFβ1, TGFβ2 and TGFβ3, result in the sequestration of odontoblasts in the dentin matrix. Although TGFβ2 showed minimal effects, mitogenic effects on the cells of the subodontoblast layer were observed in proximity to the sites of TGFβ3 application. Additionally, tertiary dentine formation typically follows administration of BMP-7 when applied to dentine slices from monkey teeth [58].
In addition to complex delivery systems, growth factor release must account for the probable dose dependence of various cell types. For instance, 2 μg of BMP-2 delivered in collagen sponges to amputated pulp in dogs show mineralized osteodentine-like tissue containing embedded osteodentinocytes 70 days after implantation with only some unmineralized fibrous tissue and pulp-like loose connective tissue [36]. However, when lower doses (660 or 220 ng) of BMP-2 are applied, only unmineralized fibrous and pulp tissues were observed. Furthermore, little pulp tissue proliferation was observed with the administration of TGF-β1, suggesting a possible inhibitory effect in pulp regeneration. Nevertheless, it is imperative to also note that when TGF-β1 is implanted short term at mechanically exposed pulps of dog molar and canine teeth, differentiation of odontoblast-like cells and stimulation of primary odontoblasts is observed [59].
Given the apparent role of TGF-β1 in pulp cell regulation, alginate gels have been used as delivery matrices in the dentin-pulp complex [60]. Alginates provide extracellular matrices with hydration properties that facilitate cellular wound healing activities. Alginate gels were fabricated using calcium chloride TGF-β1 solutions. TGF-β1 loaded gels were cultured on the pulpal cut surface of bisected human tooth slices showing two different forms of repair responses, reactionary dentinogenesis and reparative dentinogenesis. The former was represented by upregulation of matrix secretion by preexisting odontoblasts, and the latter induced de novo dentinogenesis along the cut pulpal surface. Moreover, a delivered TGF-β1 dose response was observed revealing the intermediate dose of 50 ng as most efficient, yielding the largest predentin width. Similarly to other growth factors, TGF-β1 has been delivered using many biocompatible materials, including chitosan/collagen scaffolds [61], chitosan microspheres [62], poly(D,L-lactic-co-glycolic acid) (PLGA) microspheres [63], tri-co-polymer scaffold with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) [64], injectable oligo(poly(ethylene glycol) fumarate) (OPF) and gelatin microparticles [65].
2.4. Cell–scaffold interactions
The choice of scaffolding material plays pivotal role in dental structure morphogenesis. For instance, dental pulp cells grown in collagenous, ceramic or metal (titanium) matrices, may develop differential expression of dental matrix markers such as dentin sialophosphoprotein (DSPP) [31]. These cells initially express STRO-1, a typical marker for multipotential cells such as bone marrow derived mesenchymal stem cells. Although many similarities were observed between the biomaterials used, after implantation, limited mineralization was found only in the ceramic scaffold. It is postulated that in contrast with other studies, the open porosity of the ceramic HA/TCP sintered construct could have induced a proliferative response resulting in a DSPP positive yet connective tissue-like structure.
Other extracellular matrix (ECM) components such as fibronectin have been shown to mediate the binding of signaling molecules and regulate cell attachment, which aid in regulating the interactions between cells and ECM resulting in the reorganization of preodontoblasts cytoskeleton during pulpal wound healing [66,67]. Additionally, collagens also regulate preodontoblast rearrangement and aid in the attachment of new odontoblasts to pulp tissue [68]. Bone sialoprotein when used for the restoration of pulp defects appears to stimulate the differentiation of cells which secrete a homogeneous dentin-like deposit and organized extracellular matrix more efficiently than other capping materials such as calcium hydroxide, inducing the development of thick reparative dentinal tissue in the pulp [69]. Thus, the choice of drug delivery scaffolds and matrices must take into account the individual contribution of the biomaterials themselves during the regeneration process.
Recently, synthetic materials have been fabricated into nanometer scale structures in attempts to simulate the matrix environment in which seeded cells can be accommodated to proliferate and differentiate towards desired lineages [70–73]. Nanofibers formed by electrospinning have been shown to mimic the ECM environment to various degrees when cultured with several cell types [74–78]. Poly (D,L-lactide-co-glycolide) (PLGA) has been approved for several biomedical applications in humans and is widely used as scaffold materials in tissue engineering [79–81]. The porosity and tensile properties of electrospun PLGA nanofibers have been characterized [82]. Osteoblast adhesion has been shown to occur when seeded in PLGA nanofibers [83]. Poly(ε-caprolactone) (PLC) nanofibers accommodated the differentiation of hMSCs into adipogenic, osteogenic and chondrogenic cells [84]. We have recently demonstrated that human mesenchymal stem cells can proliferate and maintain differentiation characteristics towards osteoblasts and chondrocytes when seeded in PLGA nanofibers fabricated by electrospinning (Fig. 2C). PLGA of 85:15 ratio was electrospun into non-woven fibers with an average diameter of 760±210 nm. The average Young’s modulus of electrospun PLGA nanofibers was 42±26 kPa, per nanoindentation with atomic force microscopy. Human MSCs (hMSCs) were seeded at a density of 2×106 cells/mL in PLGA nanofiber sheets. After 2 week culture on PLGA nanofiber scaffold, hMSCs remained as precursors upon immunoblotting with hKL12 antibody. The overwhelming majority of hMSCs was viable and proliferating in PLGA nanofiber scaffolds up to the tested 14 days, as assayed by live/dead tests, DNA content and BrdU. In a separate experiment, hMSCs seeded in PLGA nanofiber scaffolds were differentiated into chondrogenic (hMSC-Ch) and osteogenic (hMSC-Ob) cells. SEM taken up to 7 days after cell seeding revealed that hMSCs, hMSC-Ob and hMSC-Ch apparently attached to PLGA nanofibers. Histological assays revealed that hMSCs continuously differentiated into chondrogenic cells and osteogenic cells after 2 week incubation on PLGA nanofibers. Taken together, these data represent an original investigation of continuous differentiation of hMSCs into chondrogenic and osteogenic cells in PLGA nanofiber scaffolds.
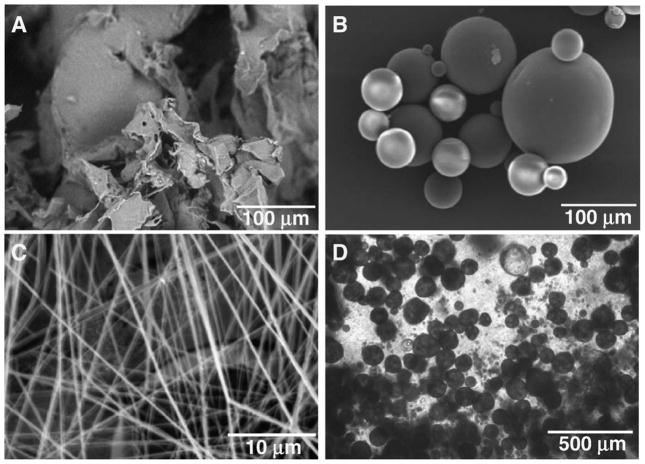
Fig. 2.
Various forms of poly-lactic-co-glycolic acid (PLGA) based matrices and scaffolds for dental, oral and craniofacial tissue engineering. A. Porous PLGA sponge fabricated using salt-leaching techniques. B. PLGA microspheres encapsulating growth factors showing smooth spherical surface and wide range of diameters. C. PLGA nanofibers fabricated using electrospining techniques. D. PLGA microspheres in chitosan-based gels for advanced controlled delivery and cell interaction. A, B, C: scanning electron microscopy (SEM). D: phase contrast image.
2.5. Periodontal regeneration
The periodontium consists of cementum, periodontal ligament (PDL), and alveolar bone and serves to transmit mechanical forces and as supporting structure and anchorage for the teeth into the mandible and maxilla. Periodontal disease is characterized by inflammation of periodontal tissues, eventually leading to degeneration of the periodontium [85]. If periodontal disease is left untreated, tooth loss can occur [86]. Periodontal tissue engineering should address the restoration of the PDL along with its orientation and fibrous infiltration into exposed tooth roots and surrounding bone, as well as regeneration of cementum by cementoblasts adjacent to the root, and restoration of the alveolar bone [87]. Previous matrices utilized in regeneration of periodontal structures include bone autografts, allografts, cell occlusive barrier membranes, bioglass, beta-tricalcium phosphate (βTCP), polytetrafluorethylene, poly lactic-co-glycolic acid (PLGA), collagen membranes, and enamel matrix derivatives [88–96]. Undoubtedly, the incorporation of bioactive molecules in successful matrix materials may enhance their varied regenerative capacities. Thus, the effects of many growth factors on periodontal tissue cells must be evaluated for their implication in periodontal tissue engineering using drug delivery technologies.
When choosing a scaffold matrix material for periodontal regeneration and drug delivery, careful consideration of the natural ECM in the periodontal connective tissues may elucidate some candidates. Collagens, proteoglycans, as well as non-collagenous glycoproteins are typically found in the periodontium, and some have been reviewed in the perspective of dental regeneration. For instance, powerful morphogens such as BMP-2 bind to heparin, heparin sulfate, types I and IV collagens, type II procollagen, fibrillins, proteoglycans, and noggin, chordin, and DAN decreasing the rate of proteolysis [97,98]. In the context of mineralized tissue regeneration such as alveolar bone and cementum, hydroxiapatite is a natural contender for a delivery vehicle due to its affinity for proteins, and in fact, when compared to collagens, TCP, glass beads, polymethylmethacrylate, and a bone cement, hydroxyapatite was the most suitable for bone induction along with insoluble collagens.
The initiation of adequate periodontal regeneration may be assisted by an array of growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-βs (TGFβs) and insulin-like growth factor-1 (IGF-1) [86,99,100]. PDGF receptors α and β are expressed in regenerating periodontal soft and hard tissues [101]. PDGF may be used as a mitogen for fibroblasts and progenitors during periodontal regeneration and has been shown to promote matrix synthesis and cell attachment to tooth dentinal surfaces [86,102–104]. Subsequently, fibroblast growth factor-2 (FGF2) and TGFβ contribute to the further proliferation of local cells. Previous reports on the effects of basic fibroblast growth factor on human gingival epithelial cells as well as during wound healing and periodontal regeneration of alveolar bone defects, promoted supplementary work experiments for FGF-2 delivery in periodontal repair. FGF-2 delivered topically with gelatinous carriers in furcation defects in dogs induced increased new bone formation rate, new trabecular bone formation rate, as well as new cementum formation rate [105–107]. Additionally, IGF-1 has been previously demonstrated to improve periodontal regeneration using controlled delivery approaches. IGF-1 was encapsulated in dextran-co-gelatin hydrogel microspheres by polyionic complexation and delivered to Class III furcation defects in molars of dogs for controlled release. Adequate width of regenerative PDL, regular Sharpey’s fibers, and alveolar bone reconstruction was observed after 4–8 weeks [108].
During alveolar bone regeneration, bone morphogenetic proteins (BMPs) are expressed and may be responsible for the osteogenic differentiation of migrated progenitors [109]. BMPs are typically effective osteogenic molecules that may induce ectopic bone formation [110]. Previous studies have demonstrated that BMP-12 loaded in collagen sponges improved periodontal ligament regeneration [111]. Although BMP-2 was more efficient for bone regeneration, application of BMP-12 showed functionally oriented PDL bridging new bone and cementum. Additionally BMP-7, has been shown to induce bone regeneration adjacent to endosseous dental implants, as well as maxillary sinus floor augmentation [112–114]. Interestingly, co-application of BMP-2 and BMP-7 for the regeneration of furcation defects in the mandibular molars of baboons, does not enhance alveolar regeneration when compared to the morphogens administered alone [115]. BMP-2 alone showed greater mineral bone and osteoid formation and remodeling, whereas administration of BMP-7 alone yielded significant cementogenesis. Delivery of BMP-2 from slow dissolving collagen membranes demonstrate improved cementogenesis over faster collagen gel delivery [116,117]. The slowed diffusion rate of the morphogen may play a role in localizing and maximizing the effects of released BMP-2, resulting in more adequate cementum formation.
3. Cranial sutures
Cranial sutures are the soft tissue interface between mineralized calvarial bones and are the immobile joints of the skull. The suture proper and sutural margins consist of multiple cell lineages, which may include fibroblast-like cells and matrices sandwiched between osteoblast-lined bone formation fronts [118,119]. During skull development, calvarial growth occurs at the cranial sutures, however they are kept “open” and unossified for continuous growth. At skeletal maturity, these joints fuse and osteogenic growth ceases. The intracranial layer that overlies the brain, the dura mater, serves as the main regulator of cranial suture function [120,121]. Cranial sutures express very intricate patterns of molecules in a highly regulated temporospatial manner. During suture patency, cranial sutures express high levels of TGF-β3, especially along the edges of the bone fronts [122]. As the skull reaches maturity, TGF-β3 expression is decreased, and TGF-β2 becomes highly expressed along the osteogenic fronts. When this signaling balance becomes chronologically erroneous, premature osseous fusion may occur, causing a condition called craniosynostosis. One in every ~2500 human newborns suffers from craniosynostosis. Affected children may develop visible craniofacial disfigurations, increased intracranial pressure and severe neurological disorders such as mental retardation, blindness and seizure. Our understanding of the normal molecular events during cranial morphogenesis however, may allow the regulation of sutural function by controlled delivery of TGF-βs. As described for various molecules involved in dental regeneration, controlled release of cytokines involved in craniofacial development include lipid nanoparticles, chitosan or gelatin-based particles, collagen, ceramics, and porous glass [123]. Given the long-term need for cranial suture regulation throughout the development of the skull in early childhood, poly-lactic-co-glycolic acid (PLGA) microspheres offer great advantage over rapid release systems, due to their potential for prolonged release as well as injectable dimensions.
Synthetic polyesters such as PLGA and polycaprolactone (PCL) have been extensively used in several tissue engineering models including craniofacial repair [124–127]. PLGA is a prime candidate for use in regenerative medicine and dentistry due to its biocompatibility, controlled structural and mechanical properties, tailored degradation rates, and the potential as growth factor delivery vehicles. Poly-lactic acid (PLA), poly-glycolic (PGA), and their copolymers (PLGA), are principal tools for scaffold biomaterial fabrication and drug delivery. PLGA may be fabricated in various forms, including sheets, blocks, microspheres, and nanofibers with designed architecture and degradation rates (Fig. 2). PLGA is degraded by hydrolysis and its byproducts are safely eliminated in the body by the Kreb’s cycle, causing minimal inflammatory responses. PLGA is also combined with other polymers to form composite materials of optimized properties, such as polyethylene glycol (PEG) — PLGA composites. Composite polymers may improve mechanical strength, further control degradation rates, and stimulate cell growth. Many growth factors and hormones such as BMPs, TGFβs, FGFs, and insulin have been encapsulated in PLGA microspheres for controlled delivery [123]. The work of the authors have involved the microencapsulation and sustained release of TGF-β3 from PLGA micro-spheres, as well as microspheres embedded in thermosensitive hydrogels (Fig. 2B, D). Sustained release of TGF-β3 was observed up to 36 days, at which point approximately 35% of encapsulated growth factor had been released in a controlled fashion [123]. The fabricated controlled delivery system for TGF-β3 was implemented in a tissue engineered cranial suture rodent model, similar to the corrective surgical procedure in human craniosynostosis patients. Here, the osteogenically fused posterior interfrontal suture of rats was surgically removed to release any bony contact between the frontal bones creating a gap of empirical size between them, simulating the gaps created in craniosynostosis procedures. However, in the tissue engineering rat model, instead of leaving an empty gap as in present conventional approaches, an implant designed for preventing re-ossification was locally delivered. The implant consisted of autologous bone marrow derived stem cells and TGF-β3 encapsulated in PLGA microspheres in a pre-sized collagen sponge carrier (Fig. 3A). Results indicate a decrease in bone deposition and the formation of a bone–fibrous tissue–bone interface analogous to normal cranial sutures after implantation of TGF-β3 loaded constructs (Fig. 3C). Placebo treated cranial defects resulted in progressive bone healing after 4 weeks (Fig. 3B). Although this approach may need further investigations to achieve more controlled regulation of new tissue formation, it is the first attempt to develop technologies for a tissue-engineered implant in craniosynostosis treatments. The continuous release of TGF-β3 may replicate the correct signaling pathways that occur during normal cranial morphogenesis offsetting the molecular mishaps of craniosynostosis (e.g. untimely downregulation of TGF-β3 and upregulation of TGF-β2). These findings demonstrate a regenerated cranial suture from autologous stem cells, and replacement of a synostosed cranial suture in an in vivo model. A biologically derived cranial suture may prove to reduce surgical trauma from craniotomy in craniosynostosis patients to localized osteotomy. Additionally, a biologically derived cranial suture may accommodate subsequent craniofacial growth, in contrast to clinically observed secondary synostosis after the resection of synostosed cranial sutures in some patients.
Fig. 3.
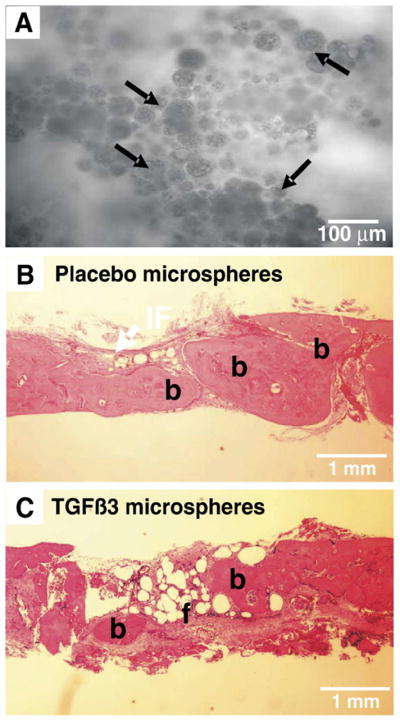
Engineered cranial suture implants. A. TGF-β3 loaded poly-lactic-co-glycolic acid (PLGA) microspheres (arrows) in gelatin sponge as a pre-sized engineered implant. B. Osteogenic healing of osteotomized region of the fused rat cranial suture after implantation of placebo loaded collagen sponge implants (4 weeks). C. Formation of a bone–fibrous tissue–bone interface and regulation of osteogenesis in the osteotomized region of the fused rat cranial suture after implantation of TGF-β3 treated engineered cranial suture implants (4 weeks). H&E stain. b: bone, f: fibrous tissue.
4. Temporomandibular joints (TMJ)
Disorders of the temporomandibular joint (TMJ) affect over 10 million Americans by some estimates (NIH, 2006), with approximately 3–4% of the population seeking treatment [128]. The TMJ connects the mandible to the temporal bone of the skull. As a diarthrotic joint, layers of cartilage are present on both the condyle of the mandible and the glenoid fossa and articular eminence of the temporal bone [128]. The TMJ disc is a fibrocartilaginous disc that sits between the two cartilage layers, connected to the joint capsule around its periphery near its attachment to the condylar neck [128].
Many skeletal and cartilage factors have been implicated in the growth of the TMJ cartilage layers and fibrocartilage disk. Effects on cells isolated from the TMJ disk have been documented for PDGF, IGF-1, bFGF, and TGFβ1 [128–133]. Each growth factor displayed distinctive effects on cellular proliferation and extracellular matrix production, suggesting that a combination cocktail of growth factors would be more beneficial [129]. In mouse explants of the mandibular condyle, the growth factors TGFβ1, IGF-1, and growth hormone stimulated proteoglycan synthesis in cartilage layers, while interleukin-1α (IL-1 α) exerted an inhibitory effect [134]. Using a collagen carrier, BMP-2 was demonstrated to increase cartilage regeneration in internally deranged rabbit TMJ [135].
Efforts are currently underway in tissue engineering the different structures of the TMJ. Toward engineering a TMJ disc, TMJ disc cells were isolated and the growth factors necessary to maintain the cells in vitro were established. The TMJ disc cells were seeded onto non-woven poly-glycolic acid (PGA) scaffolds and could be cultured up to 6 weeks [136]. Use of the growth factors bFGF, IGF-1, and TGFβ1 either alone [136] or in combination [129] modulated cellular proliferation and matrix biosynthesis. The use of a rotating bioreactor for TMJ disc culture was also explored, with no clear beneficial results [133]. In these studies, the need for high levels of growth factors (100 ng/mL for IGF-1 and bFGF and 30 ng/mL TGFβ1) suggests that a controlled drug delivery system may potentially be advantageous, especially for maintenance of the disc when implanted in vivo. Tissue engineering of the condyle portion of the TMJ requires the fabrication of an osteochondral construct containing both a cartilage layer and underlying subchondral bone. Toward this end, the large amount of work into osteochondral constructs for appendicular skeletal joints can be directly applied to the articular condyle of the TMJ [137–142]. Specifically for the TMJ, polylactic/polyglycolic copolymer was used in a gelatin sponge complex (PGS) as a carrier for BMP-2 and demonstrated increased bone and cartilage-like tissue regeneration in rabbit after condylectomy [143]. Interestingly, no significant increase in growth was realized with addition of BMP-2, attributed to the osteoinductive capabilities of the PGS scaffold. Computational design and solid freeform fabrication allows layer-by-layer fabrication of complex scaffolds with defined architecture, and can even be matched to patient-specific computed tomography (CT) or magnetic resonance imaging (MRI) scans. This technology allows production of biphasic osteochondral constructs in which the subchondral portion incorporates internal architecture and biological factors ideal for bone ingrowth and the upper layer incorporates cartilage-specific parameters [144]. Non-invasive injectable systems for drug and/or cell delivery hold promise for treatment of cartilage defects, and have been accomplished using PLGA microspheres [145], thermo-reversible hydrogels [146,147], oligo(poly(ethylene glycol) fumarate) (OPF) [65,148], and alginate [149]. Similar approaches to TMJ repair may prove beneficial to regeneration of cartilage without surgery.
The authors have demonstrated the feasibility of tissue engineering a synovial joint condyle in the shape of the TMJ using hydrogel matrix loaded with both cartilaginous and osseous components from a single population of rat bone marrow-derived mesenchymal stem cells (rMSCs). Rat MSCs were isolated from femur and tibia bone marrow of adult rats and exposed separately to either chondrogenic or osteogenic supplements [3,150]. Poly (ethylene glycol) diacrylate (PEGDA) was dissolved in PBS with a biocompatible ultraviolet photoinitiator [3,150]. MSC-derived chondrogenic and osteogenic cells were suspended in PEGDA polymer/photoinitator solution and loaded into a negative mold of a cadaver adult human mandibular condyle in two stratified and yet integrated layers. The photopolymerized osteochondral construct was removed from the mold and implanted in the dorsum of severe combined immunodeficient (SCID) mice (4–5 week old males). At both 4 and 8 weeks in vivo implantation, the tissue-engineered synovial joints retained the macroscopic shape and dimensions of the cell-hydrogel construct. Importantly, the constructs retained both chondrogenic and osseous layers, expressing cartilage-specific glycosaminoglycans (GAGs) in the upper chondrogenic layer and bony mineral nodules revealed by von Kossa staining in the lower osseous layer [3,150]. Using an identical approach but increasing the cell-seeding density 4-fold, further tissue maturation of both the chondrogenic and osseous layers as well as mutual infiltration forming a primitive osteochondral interface was achieved after 12-week subcutaneous implantation in SCID mice [4]. Improvements to the tissue-engineered TMJ condyle are warranted along multiple fronts including structural integrity, tissue maturation, mechanical strength, and host integration [151], and the aforementioned approaches of drug delivery from hydrogels will likely be critical in this process.
5. Drug delivery in implant dentistry
Despite the great advances in completely tissue engineered oral structures, the current gold standard for load bearing craniofacial replacements remains titanium implants. Since the initial studies establishing its use in dentistry [152], titanium’s strength, durability, and biocompatibility has led to innumerable applications, including single tooth replacement to complete bridges in the mandible and maxilla and even for craniofacial reconstructions. Titanium has high success rates of initial anchorage (over 90%) and has been shown to co-exist with host bone for the life of the patient [153], but failures still occur during both initial bone integration and long-term fixation.
Critical for initial and long-term efficacy of titanium implants is establishment of adequate bone fixation. This process resembles natural fracture repair and requires the cooperation of multiple cell and tissue types resulting in bone ingrowth into the irregular surfaces of the titanium and eventually direct contact between bone and titanium (osseointegration) [152,154]. During the initial inflammatory phase, disrupted bone matrix and inflammatory cells release over 100 biologically active substances, including the skeletal growth factors platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), fibroblast growth factor (FGF), and insulin-like growth factor (IGF) [155]. This initial phase is critical to set in motion the complex processes to follow, as the administration of anti-inflammatory drugs can significantly decrease bone ingrowth [156,157]. During the repair phase, proliferation and deposition of extracellular matrix by immature bone progenitors as well as establishment of a new vascular bed via angiogenesis is induced by growth factors such as TGFβ, bFGF, PDGF, and vascular endothelial growth factor (VEGF). The mineralization and maturation phase is the final phase of fracture repair in which differentiation factors such as TGFβ and bone morphogenetic protein 2 (BMP2) are elevated and critical extracellular matrix components such as osteocalcin and osteopontin begin to be expressed. The spatial and temporal profiles of numerous growth factors have been studied for fracture repair [158,159], and some of these growth factors such as TGFβ, PDGF, and BMP have been demonstrated in oral implant sites [160,161].
Because of its similarities to fracture repair, titanium dental implant osseointegration could potentially be improved by delivery of the same growth factors. Although not a defined cocktail, platelet rich plasma contains a multitude of growth factors expressed during the inflammation phase, and has been used to increase the volume of peri-implant bone in rat tibiae [162] and increase bone ingrowth when delivered to peri-implant defects via a demineralized freeze dried bone carrier [163]. In applications where there are existing gaps, such as craniofacial reconstruction or augmentation of bone at implant sites or peri-implant defects, increased regeneration of bone, often at comparable levels to bone grafts, has been accomplished with delivery of TGFβ and BMP-2 via various scaffolds including absorbable collagen sponge [164], 25% pluronic gel [165], refined collagen gelatin (Gelfoam) [166,167], titanium mesh [168,169], and beta tricalcium phosphate (β-TCP) [166]. However, minimizing gaps between implant and host bone is necessary, thus the application of bulky scaffolds is usually not possible and alternative delivery approaches are required. Adsorption of TGFβ and/or BMP to tricalcium phosphate or hydroxyapatite coatings on titanium implants leads to enhancement of bone ingrowth in gap models [170–173]. However, adsorbed growth factor in these systems is rapidly exhausted whereas levels of TGFβ and BMP-2 remain elevated throughout the fracture repair process [158]. More complex methods of growth factor adhesion to surface coatings have been explored, including fabrication of ultrahydrophillic titanium surfaces [174] and incorporation of BMP-2 directly into calcium phosphate coatings [175], showing promising results.
To mimic the temporal profile of growth factors, controlled release technology has been explored for titanium implants. Titanium and steel wires were coated with poly(D,L-lactide) incorporating TGFβ or IGF-1 and demonstrated continuous release of the growth factors up to 42 days in vitro and in vivo [176]. The authors have fabricated hollow titanium implants with 1 mm macropores; these hollow implants maintain the biomechanical strength required for oral load-bearing applications but provide space for tissue engineered scaffolds delivering bioactive molecules or cells (Fig. 4A). A controlled release system for TGFβ1 using PLGA microspheres has also been developed by the authors, as well as other groups [63], that releases the growth factor in a controlled fashion up to 4 weeks. With a diameter averaging 64 μm, the PLGA microspheres could be injected into a gelatin carrier (Gelfoam) and subsequently packed into the hollow core of the titanium implant. The hollow implants were placed unicortically into the humeri of adult New Zealand white rabbits and allowed to osseointegrate for 4 weeks. Incorporation of TGFβ1 in the hollow implant led to significantly increased osseointegration parameters such as bone-to-implant contact (BIC) and bone volume within the 1 mm macropores (Fig. 4B–E) as compared to placebo controls (Fig. 4F, G). Adsorption of TGFβ1 to gelatin sponge and controlled release via PLGA microspheres resulted in comparable outcomes, but 10× more TGFβ1 was required when delivered by gelatin sponge. The use of controlled release of growth factors by way of hollow implants is a novel approach to enhancing osseointegration and may be useful in implant dentistry, in which hollow implants have been in clinical use for years [177,178]. More advanced biomaterials, such as microporous titanium [179], may also potentially be used as high strength carriers.
Fig. 4.
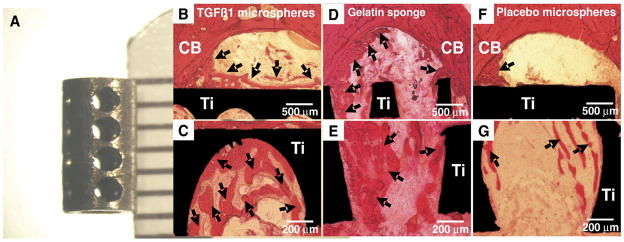
Controlled release of TGF-β1 from poly-lactic-co-glycolic acid (PLGA) microspheres increases bone ingrowth into hollow titanium implants. A. Hollow titanium implants with 1 mm macropores were custom fabricated. To compare outcome to a rapid release system, 1 μg of TGFβ1 was also adsorbed to gelatin sponge carrier (D and E). After 4 weeks of implantation in rabbit humeri, delivery of TGF-β1 led to increases in bone-to-implant contact (BIC) and bone volume within 1 mm macropores (BV/TV) (B–E) as compared to placebo control spheres (F and G). Approximately 10 times more TGFβ1 was required for rapid release from gelatin sponge (D and E) to obtain comparable results to controlled release using PLGA microspheres (B and C). H&E stain. CB: cortical bone, Ti: titanium implant.
6. Conclusions
Previous work has explored the application of biomaterial matrices and drug delivery in the regeneration and/or de novo formation of DOC structures. A wide range of biomaterials including polymeric hydrogels, porous scaffolds, nanofibers and microparticles have been utilized toward the regeneration of tooth, periodontal ligament, salivary gland, cranial sutures, and the temporomandibular joint (TMJ). Although the goal of eventually regenerating a whole tooth is admirable, whole tooth regeneration is likely one of the last technologies to be commercially realized. The more near-term technologies include the regeneration of individual tooth structures, osteochondral grafts for the TMJ, cranial sutures and bone regeneration for periodontal defects. Integrated approaches that capture the fundamental knowledge in multiple biology and engineering disciplines are not only desirable, but necessary for proper regeneration of DOC structures. Specific structural and mechanical characteristics as outlined in the Introduction of this review, need to be considered in the design of tissue engineering or tissue regeneration approaches. It is predictable that successful approaches need to exert not only more control at micro-scale and nano-scale levels, but also rigorously test increasingly refined engineering techniques in animal models towards the regeneration of DOC structures.
Acknowledgments
We are grateful to the members of the Tissue Engineering and Regenerative Medicine Laboratory (TERML) for their discussion. We thank Janina Acloque, Maryann Wanner and Richard Abbott for administrative support. Generous support from the National Institutes of Health is gratefully acknowledged, through NIH grants DE13964, DE15391 and EB02332 to J.J.M., for the effort spent on composing this manuscript along with some of the experimental data presented in this manuscript.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Matrices and Scaffolds for Drug Delivery in Tissue Engineering”.
References
- 1.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 2.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–956. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 4.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–944. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 5.Yelick PC, Vacanti JP. Bioengineered teeth from tooth bud cells. Dent Clin North Am. 2006;50(2) doi: 10.1016/j.cden.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe PT, Young CS. Test-tube teeth. Sci Am. 2005;293(2) doi: 10.1038/scientificamerican0805-34. [DOI] [PubMed] [Google Scholar]
- 7.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81(10) doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 8.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S, Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE. 2006;1:1920. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28(4) doi: 10.1016/j.biomaterials.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 10.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 11.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 12.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig RG, Peyton FA, Johnson DW. Compressive properties of enamel, dental cements, and gold. J Dent Res. 1961;40(5):936–945. [Google Scholar]
- 14.Craig RG, Peyton FA. Elastic and mechanical properties of human dentin. J Dent Res. 1958;37:710–718. doi: 10.1177/00220345580370041801. [DOI] [PubMed] [Google Scholar]
- 15.Thesleff I. Developmental biology and building a tooth. Quintessence Int. 2003;34:613–620. [PubMed] [Google Scholar]
- 16.Liu H, Gronthos S, Shi S. Dental pulp stem cells. Methods Enzymol. 2006;419:99–113. doi: 10.1016/S0076-6879(06)19005-9. [DOI] [PubMed] [Google Scholar]
- 17.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 18.Rowe AH. Root canal therapy. Br Dent J. 1967;123:543. [PubMed] [Google Scholar]
- 19.Cox CF, Bergenholtz G. Healing sequence in capped inflamed dental pulps of Rhesus monkeys (Macaca mulatta) Int Endod J. 1986;19:113–120. doi: 10.1111/j.1365-2591.1986.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 20.Cvek M, Granath L, Cleaton-Jones P, Austin J. Hard tissue barrier formation in pulpotomized monkey teeth capped with cyanoacrylate or calcium hydroxide for 10 and 60 minutes. J Dent Res. 1987;66:1166–1174. doi: 10.1177/00220345870660061501. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg M, Lacerda-Pinheiro S, Jegat N, Six N, Septier D, Priam F, Bonnefoix M, Tompkins K, Chardin H, Denbesten P, Veis A, Poliard A. The impact of bioactive molecules to stimulate tooth repair and regeneration as part of restorative dentistry. Dent Clin North Am. 2006;50:277–298. x. doi: 10.1016/j.cden.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Bergenholtz G. Advances since the paper by Zander and Glass (1949) on the pursuit of healing methods for pulpal exposures: historical perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endo. 2005;100:S102–S108. doi: 10.1016/j.tripleo.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Harrop TJ, Mackay B. Electron microscopic observations on healing in dental pulp in the rat. Arch Oral Biol. 1968;13:365–385. doi: 10.1016/0003-9969(68)90165-9. [DOI] [PubMed] [Google Scholar]
- 24.Horsted P, El AK, Langeland K. Capping of monkey pulps with Dycal and a Ca-eugenol cement. Oral Surg Oral Med Oral Pathol. 1981;52:531–553. doi: 10.1016/0030-4220(81)90366-2. [DOI] [PubMed] [Google Scholar]
- 25.Jaber L, Mascres C, Donohue WB. Electron microscope characteristics of dentin repair after hydroxylapatite direct pulp capping in rats. J Oral Pathol Med. 1991;20:502–508. doi: 10.1111/j.1600-0714.1991.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 26.Mjor IA, Dahl E, Cox CF. Healing of pulp exposures: an ultrastructural study. J Oral Pathol Med. 1991;20:496–501. doi: 10.1111/j.1600-0714.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 27.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, Denbesten P, Robey G, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 29.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 30.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Frank W, van Kuppevelt XTH, Daamen WF, Bian Z, Jansen JA. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials. 2006;27:5658–5668. doi: 10.1016/j.biomaterials.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. doi: 10.1089/ten.2006.12.2813. (in press) (Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225–236. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 34.Yokose S, Kadokura H, Tajima Y, Fujieda K, Katayama I, Matsuoka T, Katayama T. Establishment and characterization of a culture system for enzymatically released rat dental pulp cells. Calcif Tissue Int. 2000;66:139–144. doi: 10.1007/s002230010028. [DOI] [PubMed] [Google Scholar]
- 35.Yokose S, Kadokura H, Tajima N, Hasegawa A, Sakagami H, Fujieda K, Katayama T. Platelet-derived growth factor exerts disparate effects on odontoblast differentiation depending on the dimers in rat dental pulp cells. Cell Tissue Res. 2004;315:375–384. doi: 10.1007/s00441-003-0839-5. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and-4 with collagen matrix. Arch Oral Biol. 1994;39:1085–1089. doi: 10.1016/0003-9969(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Ogawa M, Hata Y, Bessho K. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205–208. doi: 10.1097/00004770-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki T, Kawamata-Kido H. Providing an environment for reparative dentine induction in amputated rat molar pulp by high molecular-weight hyaluronic acid. Arch Oral Biol. 1995;40:209–219. doi: 10.1016/0003-9969(95)98810-l. [DOI] [PubMed] [Google Scholar]
- 40.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59:573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 41.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima M. The effects of growth factors on DNA synthesis, proteoglycan synthesis and alkaline phosphatase activity in bovine dental pulp cells. Arch Oral Biol. 1992;37:231–236. doi: 10.1016/0003-9969(92)90093-n. [DOI] [PubMed] [Google Scholar]
- 43.Rutherford RB, TrailSmith MD, Ryan ME, Charette MF. Synergistic effects of dexamethasone on platelet-derived growth factor mitogenesis in vitro. Arch Oral Biol. 1992;37:139–145. doi: 10.1016/0003-9969(92)90009-w. [DOI] [PubMed] [Google Scholar]
- 44.Hu JC, Zhang C, Slavkin HC. The role of platelet-derived growth factor in the development of mouse molars. Int J Dev Biol. 1995;39:939–945. [PubMed] [Google Scholar]
- 45.Chai Y, Bringas P, Jr, Mogharei A, Shuler CF, Slavkin HC. PDGF-A and PDGFR-alpha regulate tooth formation via autocrine mechanism during mandibular morphogenesis in vitro. Dev Dyn. 1998;213:500–511. doi: 10.1002/(SICI)1097-0177(199812)213:4<500::AID-AJA14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 46.Denholm IA, Moule AJ, Bartold PM. The behaviour and proliferation of human dental pulp cell strains in vitro, and their response to the application of platelet-derived growth factor-BB and insulin-like growth factor-1. Int Endod J. 1998;31:251–258. doi: 10.1046/j.1365-2591.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Nam SH, Im SY, Park YJ, Lee YM, Seol YJ, Chung CP, Lee SJ. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J Control Release. 2002;78:187–197. doi: 10.1016/s0168-3659(01)00498-9. [DOI] [PubMed] [Google Scholar]
- 49.Park YJ, Lee YM, Park SN, Sheen SY, Chung CP, Lee SJ. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials. 2000;21:153–159. doi: 10.1016/s0142-9612(99)00143-x. [DOI] [PubMed] [Google Scholar]
- 50.Walsh WR, Kim HD, Jong YS, Valentini RF. Controlled release of platelet-derived growth factor using ethylene vinyl acetate copolymer (EVAc) coated on stainless-steel wires. Biomaterials. 1995;16:1319–1325. doi: 10.1016/0142-9612(95)91047-3. [DOI] [PubMed] [Google Scholar]
- 51.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anusaksathien O, Jin Q, Zhao M, Somerman MJ, Giannobile WV. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J Periodontol. 2004;75:429–440. doi: 10.1902/jop.2004.75.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooke JW, Sarment DP, Whitesman LA, Miller SE, Jin Q, Lynch SE, Giannobile WV. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441–1450. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zerbo IR, Bronckers AL, Gde L, Burger EH. Localisation of osteogenic and osteoclastic cells in porous beta-tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials. 2005;26:1445–1451. doi: 10.1016/j.biomaterials.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 56.Begue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, Lesot H. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992;36:491–503. [PubMed] [Google Scholar]
- 57.Sloan AJ, Smith AJ. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1–3 in vitro. Arch Oral Biol. 1999;44:149–156. doi: 10.1016/s0003-9969(98)00106-x. [DOI] [PubMed] [Google Scholar]
- 58.Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000;45:173–177. doi: 10.1016/s0003-9969(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 59.Tziafas D, Alvanou A, Papadimitriou S, Gasic J, Komnenou A. Effects of recombinant basic fibroblast growth factor, insulin-like growth factor-II and transforming growth factor-beta 1 on dog dental pulp cells in vivo. Arch Oral Biol. 1998;43:431–444. doi: 10.1016/s0003-9969(98)00026-0. [DOI] [PubMed] [Google Scholar]
- 60.Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res. 2002;43:387–390. doi: 10.1080/03008200290000574. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Cheng X, Wang J, Wang Y, Shi B, Huang C, Yang X, Liu T. Novel chitosan/collagen scaffold containing transforming growth factor-beta1 DNA for periodontal tissue engineering. Biochem Biophys Res Commun. 2006;344:362–369. doi: 10.1016/j.bbrc.2006.03.106. [DOI] [PubMed] [Google Scholar]
- 62.Lee JE, Kim SE, Kwon IC, Ahn HJ, Cho H, Lee SH, Kim HJ, Seong SC, Lee MC. Effects of a chitosan scaffold containing TGF-beta1 encapsulated chitosan microspheres on in vitro chondrocyte culture. Artif Organs. 2004;28:829–839. doi: 10.1111/j.1525-1594.2004.00020.x. [DOI] [PubMed] [Google Scholar]
- 63.Lu L, Stamatas GN, Mikos AG. Controlled release of transforming growth factor beta1 from biodegradable polymer microparticles. J Biomed Mater Res. 2000;50(3):1905. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 64.Chou CH, Cheng WT, Lin CC, Chang CH, Tsai CC, Lin FH. TGF-beta1 immobilized tri-co-polymer for articular cartilage tissue engineering. J Biomed Mater Res, B Appl Biomater. 2006;77:338–348. doi: 10.1002/jbm.b.30432. [DOI] [PubMed] [Google Scholar]
- 65.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 66.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 67.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 68.Kitasako Y, Shibata S, Cox CF, Tagami J. Location, arrangement and possible function of interodontoblastic collagen fibres in association with calcium hydroxide-induced hard tissue bridges. Int Endod J. 2002;35:996–1004. doi: 10.1046/j.1365-2591.2002.00606.x. [DOI] [PubMed] [Google Scholar]
- 69.Decup F, Six N, Palmier B, Buch D, Lasfargues JJ, Salih E, Goldberg M. Bone sialoprotein-induced reparative dentinogenesis in the pulp of rat’s molar. Clin Oral Investig. 2000;4:110–119. doi: 10.1007/s007840050126. [DOI] [PubMed] [Google Scholar]
- 70.Baumgartner PK. Electrostatic spinning of acrylic microfibers. J Colloid Interface Sci. 1971;36:71–79. [Google Scholar]
- 71.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6(5) doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 72.Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, Choi SC, Park WH, Min BM. Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 2006;27(21) doi: 10.1016/j.biomaterials.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91(3):1905. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 74.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27(4) doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 75.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11(1–2) doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 76.Schindler M, Ahmed I, Kamal J, Nur E, Kamal A, Grafe TH, Young Chung H, Meiners S. A synthetic nanofibrillar matrix promotes in vivo-like organization and morphogenesis for cells in culture. Biomaterials. 2005;26(28) doi: 10.1016/j.biomaterials.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25(10) doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 78.Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10(7–8) doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 79.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55(2) doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 80.Behravesh E, Yasko AW, Engel PS, Mikos AG. Synthetic biodegradable polymers for orthopaedic applications. Clin Orthop Relat Res. 1999;367(Suppl) doi: 10.1097/00003086-199910001-00012. [DOI] [PubMed] [Google Scholar]
- 81.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2) doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 82.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):1915. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 83.Price RL, Ellison K, Haberstroh KM, Webster TJ. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J Biomed Mater Res A. 2004;70(1):1901. doi: 10.1002/jbm.a.30073. [DOI] [PubMed] [Google Scholar]
- 84.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26(25) doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Kocher T, Konig J, Dzierzon U, Sawaf H, Plagmann HC. Disease progression in periodontally treated and untreated patients—a retrospective study. J Clin Periodontol. 2000;27:866–872. doi: 10.1034/j.1600-051x.2000.027011866.x. [DOI] [PubMed] [Google Scholar]
- 86.Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50(ix):245–263. doi: 10.1016/j.cden.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 88.Kimble KM, Eber RM, Soehren S, Shyr Y, Wang HL. Treatment of gingival recession using a collagen membrane with or without the use of demineralized freeze-dried bone allograft for space maintenance. J Periodontol. 2004;75:210–220. doi: 10.1902/jop.2004.75.2.210. [DOI] [PubMed] [Google Scholar]
- 89.Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restor Dent. 2000;20:19–29. [PubMed] [Google Scholar]
- 90.Nevins ML, Camelo M, Nevins M, King CJ, Oringer RJ, Schenk RK, Fiorellini JP. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int J Periodontics Restor Dent. 2000;20:458–467. [PubMed] [Google Scholar]
- 91.Scher EL, Day RB, Speight PM. New bone formation after a sinus lift procedure using demineralized freeze-dried bone and tricalcium phosphate. Implant Dent. 1999;8:49–53. doi: 10.1097/00008505-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 92.Sculean A, Pietruska M, Schwarz F, Willershausen B, Arweiler NB, Auschill TM. Healing of human intrabony defects following regenerative periodontal therapy with an enamel matrix protein derivative alone or combined with a bioactive glass. A controlled clinical study. J Clin Periodontol. 2005;32:111–117. doi: 10.1111/j.1600-051X.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 93.Trombelli L, Heitz-Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002;29(Suppl 3):117–135. doi: 10.1034/j.1600-051x.29.s3.7.x. [DOI] [PubMed] [Google Scholar]
- 94.Moses O, Pitaru S, Artzi Z, Nemcovsky CE. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005;16:210–219. doi: 10.1111/j.1600-0501.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 95.Stavropoulos A, Sculean A, Karring T. GTR treatment of intrabony defects with PLA/PGA copolymer or collagen bioresorbable membranes in combination with deproteinized bovine bone (Bio-Oss) Clin Oral Investig. 2004;8:226–232. doi: 10.1007/s00784-004-0277-0. [DOI] [PubMed] [Google Scholar]
- 96.Rosing CK, Aass AM, Mavropoulos A, Gjermo P. Clinical and radiographic effects of enamel matrix derivative in the treatment of intrabony periodontal defects: a 12-month longitudinal placebo-controlled clinical trial in adult periodontitis patients. J Periodontol. 2005;76:129–133. doi: 10.1902/jop.2005.76.1.129. [DOI] [PubMed] [Google Scholar]
- 97.Reddi AH. Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: noggin, chordin and DAN. Arthritis Res. 2001;3:1–5. doi: 10.1186/ar133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paralkar VM, Nandedkar AK, Pointer RH, Kleinman HK, Reddi AH. Interaction of osteogenin, a heparin binding bone morphogenetic protein, with type IV collagen. J Biol Chem. 1990;265:17281–17284. [PubMed] [Google Scholar]
- 99.Tozum TF, Demiralp B. Platelet-rich plasma: a promising innovation in dentistry. J Can Dent Assoc. 2003;69:664. [PubMed] [Google Scholar]
- 100.Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74:849–857. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 101.Parkar MH, Kuru L, Giouzeli M, Olsen I. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Arch Oral Biol. 2001;46:275–284. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 102.Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64:142–148. doi: 10.1902/jop.1993.64.2.142. [DOI] [PubMed] [Google Scholar]
- 103.Haase HR, Clarkson RW, Waters MJ, Bartold PM. Growth factor modulation of mitogenic responses and proteoglycan synthesis by human periodontal fibroblasts. J Cell Physiol. 1998;174:353–361. doi: 10.1002/(SICI)1097-4652(199803)174:3<353::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 104.Marcopoulou CE, Vavouraki HN, Dereka XE, Vrotsos IA. Proliferative effect of growth factors TGF-beta1, PDGF-BB and rhBMP-2 on human gingival fibroblasts and periodontal ligament cells. J Int Acad Periodontol. 2003;5:63–70. [PubMed] [Google Scholar]
- 105.Murakami S, Takayama S, Kitamura M, Shimabukuro Y, Yanagi K, Ikezawa K, Saho T, Nozaki T, Okada H. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res. 2003;38:97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 106.Sae-Lim V, Ong WY, Li Z, Neo J. The effect of basic fibroblast growth factor on delayed-replanted monkey teeth. J Periodontol. 2004;75:1570–1578. doi: 10.1902/jop.2004.75.12.1570. [DOI] [PubMed] [Google Scholar]
- 107.Takayama S, Yoshida J, Hirano H, Okada H, Murakami S. Effects of basic fibroblast growth factor on human gingival epithelial cells. J Periodontol. 2002;73:1467–1473. doi: 10.1902/jop.2002.73.12.1467. [DOI] [PubMed] [Google Scholar]
- 108.Chen FM, Zhao YM, Wu H, Deng ZH, Wang QT, Zhou W, Liu Q, Dong GY, Li K, Wu ZF, Jin Y. Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J Control Release. 2006;114:209–222. doi: 10.1016/j.jconrel.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 109.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 110.Urist MR. Bone: formation by autoinduction. Science. 1965;150(698):1912. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 111.Wikesjo UM, Sorensen RG, Kinoshita A, Jian LX, Wozney JM. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 2004;31:662–670. doi: 10.1111/j.1600-051X.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 112.Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TC. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontol. 1998;69:129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 113.Rutherford RB, Sampath TK, Rueger DC, Taylor TD. Use of bovine osteogenic protein to promote rapid osseointegration of endosseous dental implants. Int J Oral Maxillofac Implants. 1992;7:297–301. [PubMed] [Google Scholar]
- 114.van den Bergh JP, ten Bruggenkate CM, Groeneveld HH, Burger EH, Tuinzing DB. Recombinant human bone morphogenetic protein-7 in maxillary sinus floor elevation surgery in 3 patients compared to autogenous bone grafts. A clinical pilot study. J Clin Periodontol. 2000;27:627–636. doi: 10.1034/j.1600-051x.2000.027009627.x. [DOI] [PubMed] [Google Scholar]
- 115.Ripamonti U, Crooks J, Petit JC, Rueger DC. Periodontal tissue regeneration by combined applications of recombinant human osteogenic protein-1 and bone morphogenetic protein-2. A pilot study in Chacma baboons (Papio ursinus) Eur J Oral Sci. 2001;109:241–248. doi: 10.1034/j.1600-0722.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- 116.King GN, King N, Hughes FJ. The effect of root surface demineralization on bone morphogenetic protein-2-induced healing of rat periodontal fenestration defects. J Periodontol. 1998;69:561–570. doi: 10.1902/jop.1998.69.5.561. [DOI] [PubMed] [Google Scholar]
- 117.King GN, King N, Hughes FJ. Effect of two delivery systems for recombinant human bone morphogenetic protein-2 on periodontal regeneration in vivo. J Periodontal Res. 1998;33:226–236. doi: 10.1111/j.1600-0765.1998.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 118.Pritchard JJ, Scott JH, Girgis FG. The structure and development of cranial and facial sutures. J Anat. 1956;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- 119.Opperman LA, Adab K, Gakunga PT. Transforming growth factor-beta 2 and TGF-beta 3 regulate fetal rat cranial suture morphogenesis by regulating rates of cell proliferation and apoptosis. Dev Dyn. 2000;219:237–247. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1044>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 120.Opperman LA, Chhabra A, Nolen AA, Bao Y, Ogle RC. Dura mater maintains rat cranial sutures in vitro by regulating suture cell proliferation and collagen production. J Craniofac Genet Dev Biol. 1998;18:150–158. [PubMed] [Google Scholar]
- 121.Mooney MP, Burrows AM, Smith TD, Losken HW, Opperman LA, Dechant J, Kreithen AM, Kapucu R, Cooper GM, Ogle RC, Siegel MI. Correction of coronal suture synostosis using suture and dura mater allografts in rabbits with familial craniosynostosis. Cleft Palate-Craniofac J. 2001;38:206–225. doi: 10.1597/1545-1569_2001_038_0206_cocssu_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 122.Longaker MT. Role of TGF-beta signaling in the regulation of programmed cranial suture fusion. J Craniofac Surg. 2001;12:389–390. doi: 10.1097/00001665-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 123.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–546. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 125.Isogai N, Landis W, Kim TH, Gerstenfeld LC, Upton J, Vacanti JP. Formation of phalanges and small joints by tissue-engineering. J Bone Joint Surg Am. 1999;81:306–316. doi: 10.2106/00004623-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 126.Landes CA, Ballon A, Roth C. In-patient versus in vitro degradation of P(L/DL)LA and PLGA. J Biomed Mater Res, B Appl Biomater. 2006;76:403–411. doi: 10.1002/jbm.b.30388. [DOI] [PubMed] [Google Scholar]
- 127.Landes CA, Ballon A, Roth C. Maxillary and mandibular osteosyntheses with PLGA and P(L/DL)LA implants: a 5-year inpatient biocompatibility and degradation experience. Plast Reconstr Surg. 2006;117:2347–2360. doi: 10.1097/01.prs.0000218787.49887.73. [DOI] [PubMed] [Google Scholar]
- 128.Detamore MS, Athanasiou KA. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;6 doi: 10.1089/10763270360727991. (Ref Type: Map) [DOI] [PubMed] [Google Scholar]
- 129.Almarza AJ, Athanasiou KA. Evaluation of three growth factors in combinations of two for temporomandibular joint disc tissue engineering. Arch Oral Biol. 2006;51 doi: 10.1016/j.archoralbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 130.Hanaoka K, Tanaka E, Takata T, Miyauchi M, Aoyama J, Kawai N, Dalla-Bona DA, Yamano E, Tanne K. Platelet-derived growth factor enhances proliferation and matrix synthesis of temporomandibular joint disc-derived cells. Angle Orthod. 2006;76(3) doi: 10.1043/0003-3219(2006)076[0486:PGFEPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 131.Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol. 2004;49(7) doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 132.Tanimoto K, Suzuki A, Ohno S, Honda K, Tanaka N, Doi T, Yoneno K, Ohno-Nakahara M, Nakatani Y, Ueki M, Tanne K. Effects of TGF-beta on hyaluronan anabolism in fibroblasts derived from the synovial membrane of the rabbit temporomandibular joint. J Dent Res. 2004;83(1) doi: 10.1177/154405910408300108. [DOI] [PubMed] [Google Scholar]
- 133.Detamore MS, Athanasiou KA. Use of a rotating bioreactor toward tissue engineering the temporomandibular joint disc. Tissue Eng. 2005;11(7–8) doi: 10.1089/ten.2005.11.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blumenfeld I, Gaspar R, Laufer D, Livne E. Enhancement of toluidine blue staining by transforming growth factor-beta, insulin-like growth factor and growth hormone in the temporomandibular joint of aged mice. Cells Tissues Organs. 2000;167(2–3) doi: 10.1159/000016775. [DOI] [PubMed] [Google Scholar]
- 135.Suzuki T, Bessho K, Fujimura K, Okubo Y, Segami N, Iizuka T. Regeneration of defects in the articular cartilage in rabbit temporomandibular joints by bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2002;40(3) doi: 10.1054/bjom.2001.0720. [DOI] [PubMed] [Google Scholar]
- 136.Detamore MS, Athanasiou KA. Evaluation of three growth factors for TMJ disc tissue engineering. Ann Biomed Eng. 2005;33(3) doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kreklau B, Sittinger M, Mensing MB, Voigt C, Berger G, Burmester GR, Rahmanzadeh R, Gross U. Tissue engineering of biphasic joint cartilage transplants. Biomaterials. 1999;20(18) doi: 10.1016/s0142-9612(99)00061-7. [DOI] [PubMed] [Google Scholar]
- 138.Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed L. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46(9) doi: 10.1002/art.10493. [DOI] [PubMed] [Google Scholar]
- 139.Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7(4) doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 140.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87(5) doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 141.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11(5–6) doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 142.Solchaga LA, Temenoff JS, Gao J, Mikos AG, Caplan AI, Goldberg VM. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthr Cartil. 2005;13(4) doi: 10.1016/j.joca.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 143.Ueki K, Takazakura D, Marukawa K, Shimada M, Nakagawa K, Takatsuka S, Yamamoto E. The use of polylactic acid/polyglycolic acid copolymer and gelatin sponge complex containing human recombinant bone morphogenetic protein-2 following condylectomy in rabbits. J Craniomaxillofac Surg. 2003;31(2) doi: 10.1016/s1010-5182(02)00187-7. [DOI] [PubMed] [Google Scholar]
- 144.Schek RM, Taboas JM, Hollister SJ, Krebsbach PH. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res. 2005;8(4) doi: 10.1111/j.1601-6343.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 145.Kang SW, Yoon JR, Lee JS, Kim HJ, Lim HW, Lim HC, Park JH, Kim BS. The use of poly(lactic-co-glycolic acid) microspheres as injectable cell carriers for cartilage regeneration in rabbit knees. J Biomater Sci, Polym Ed. 2006;17(8) doi: 10.1163/156856206777996862. [DOI] [PubMed] [Google Scholar]
- 146.Na K, Kim S, Woo DG, Sun BK, Yang HN, Chung HM, Park KH. Synergistic effect of TGFbeta-3 on chondrogenic differentiation of rabbit chondrocytes in thermo-reversible hydrogel constructs blended with hyaluronic acid by in vivo test. J Biotechnol. 2006:1912. doi: 10.1016/j.jbiotec.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 147.Fisher JP, Jo S, Mikos AG, Reddi AH. Thermoreversible hydrogel scaffolds for articular cartilage engineering. J Biomed Mater Res A. 2004;71(2):1901. doi: 10.1002/jbm.a.30148. [DOI] [PubMed] [Google Scholar]
- 148.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75(1):1901. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 149.Paige KT, Cima LG, Yaremchuk MJ, Vacanti JP, Vacanti CA. Injectable cartilage. Plast Reconstr Surg. 1995;96(6) doi: 10.1097/00006534-199511000-00024. [DOI] [PubMed] [Google Scholar]
- 150.Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A, Mao JJ. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 151.Mao JJ. Stem-cell-driven regeneration of synovial joints. Biol Cell. 2005;97:289–301. doi: 10.1042/BC20040100. [DOI] [PubMed] [Google Scholar]
- 152.Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2) doi: 10.3109/02844316909036699. [DOI] [PubMed] [Google Scholar]
- 153.Ashley ET, Covington LL, Bishop BG, Breault LG. Ailing and failing endosseous dental implants: a literature review. J Contemp Dent Pract. 2003;4(2):1915. [PubMed] [Google Scholar]
- 154.Kienapfel H, Sprey C, Wilke A, Griss P. Implant fixation by bone ingrowth. J Arthroplast. 1999;14(3) doi: 10.1016/s0883-5403(99)90063-3. [DOI] [PubMed] [Google Scholar]
- 155.Probst A, Spiegel HU. Cellular mechanisms of bone repair. J Invest Surg. 1997;10(3) doi: 10.3109/08941939709032137. [DOI] [PubMed] [Google Scholar]
- 156.Cook SD, Salkeld SL, Rueger DC. Evaluation of recombinant human osteogenic protein-1 (rhOP-1) placed with dental implants in fresh extraction sites. J Oral Implantol. 1995;21(4) [PubMed] [Google Scholar]
- 157.Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, Fox N, Genovese M, Regula D, Smith RL. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20(6) doi: 10.1016/S0736-0266(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 158.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):1901. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 159.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 160.Schierano G, Canuto RA, Navone R, Peirone B, Martinasso G, Pagano M, Maggiora M, Manzella C, Easton M, Davit A, Trombetta A, Amedeo S, Biolatti B, Carossa S, Preti G. Biological factors involved in the osseointegration of oral titanium implants with different surfaces: a pilot study in minipigs. J Periodontol. 2005;76(10) doi: 10.1902/jop.2005.76.10.1710. [DOI] [PubMed] [Google Scholar]
- 161.Damrongsri D, Geva S, Salvi GE, Cooper LF, Limwongse V, Offenbacher S. Effects of Delta12-prostaglandin J2 on bone regeneration and growth factor expression in rats. Clin Oral Implants Res. 2006;17(1) doi: 10.1111/j.1600-0501.2005.01181.x. [DOI] [PubMed] [Google Scholar]
- 162.Fontana S, Olmedo DG, Linares JA, Guglielmotti MB, Crosa ME. Effect of platelet-rich plasma on the peri-implant bone response: an experimental study. Implant Dent. 2004;13(1) doi: 10.1097/01.id.0000116455.68968.29. [DOI] [PubMed] [Google Scholar]
- 163.Sanchez AR, Sheridan PJ, Eckert SE, Weaver AL. Regenerative potential of platelet-rich plasma added to xenogenic bone grafts in peri-implant defects: a histomorphometric analysis in dogs. J Periodontol. 2005;76(10) doi: 10.1902/jop.2005.76.10.1637. [DOI] [PubMed] [Google Scholar]
- 164.Cochran DL, Schenk R, Buser D, Wozney JM, Jones AA. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J Periodontol. 1999;70(2) doi: 10.1902/jop.1999.70.2.139. [DOI] [PubMed] [Google Scholar]
- 165.Clokie CM, Bell RC. Recombinant human transforming growth factor beta-1 and its effects on osseointegration. J Craniofac Surg. 2003;14(3) doi: 10.1097/00001665-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 166.Clarke SA, Brooks RA, Lee PT, Rushton N. Bone growth into a ceramic-filled defect around an implant. The response to transforming growth factor beta1. J Bone Joint Surg Br. 2004;86(1) (Ref Type: Map) [PubMed] [Google Scholar]
- 167.Yamamoto M, Tabata Y, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Bone regeneration by transforming growth factor beta1 released from a biodegradable hydrogel. J Control Release. 2000;64(1–3):1914. doi: 10.1016/s0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 168.Vehof JW, Haus MT, de Ruijter AE, Spauwen PH, Jansen JA. Bone formation in transforming growth factor beta-I-loaded titanium fiber mesh implants. Clin Oral Implants Res. 2002;13(1) doi: 10.1034/j.1600-0501.2002.130112.x. [DOI] [PubMed] [Google Scholar]
- 169.Jansen JA, Vehof JW, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H, Hedberg EL, Mikos AG. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101:127–136. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 170.Sumner DR, Turner TM, Purchio AF, Gombotz WR, Urban RM, Galante JO. Enhancement of bone ingrowth by transforming growth factor-beta. J Bone Joint Surg Am. 1995;77(8) doi: 10.2106/00004623-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 171.Lind M. Growth factor stimulation of bone healing. Effects on osteoblasts, osteomies, and implants fixation. Acta Orthop Scand, Suppl. 1998;283 [PubMed] [Google Scholar]
- 172.Sumner DR, Turner TM, Urban RM, Virdi AS, Inoue N. Additive enhancement of implant fixation following combined treatment with rhTGF-beta2 and rhBMP-2 in a canine model. J Bone Joint Surg Am. 2006;88(4) doi: 10.2106/JBJS.E.00846. [DOI] [PubMed] [Google Scholar]
- 173.Zhang R, An Y, Toth CA, Draughn RA, Dimaano NM, Hawkins MV. Osteogenic protein-1 enhances osseointegration of titanium implants coated with peri-apatite in rabbit femoral defect. J Biomed Mater Res, B Appl Biomater. 2004;71(2):1915. doi: 10.1002/jbm.b.30110. [DOI] [PubMed] [Google Scholar]
- 174.Becker J, Kirsch A, Schwarz F, Chatzinikolaidou M, Rothamel D, Lekovic V, Laub M, Jennissen HP. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhBMP-2). A pilot study in dogs. Clin Oral Investig. 2006;10(3) doi: 10.1007/s00784-006-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y, Hunziker EB, Layrolle P, De Bruijn JD, de Groot K. Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 2004;10(1–2) doi: 10.1089/107632704322791745. [DOI] [PubMed] [Google Scholar]
- 176.Schmidmaier G, Wildemann B, Stemberger A, Haas NP, Raschke M. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J Biomed Mater Res. 2001;58(4) doi: 10.1002/jbm.1040. [DOI] [PubMed] [Google Scholar]
- 177.Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, Belser UC, Lang NP. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997;8(3) doi: 10.1034/j.1600-0501.1997.080302.x. [DOI] [PubMed] [Google Scholar]
- 178.Merickse-Stern R, Aerni D, Geering AH, Buser D. Long-term evaluation of non-submerged hollow cylinder implants. Clinical and radiographic results. Clin Oral Implants Res. 2001;12(3) doi: 10.1034/j.1600-0501.2001.012003252.x. [DOI] [PubMed] [Google Scholar]
- 179.Thelen S, Barthelat F, Brinson LC. Mechanics considerations for microporous titanium as an orthopedic implant material. J Biomed Mater Res A. 2004;69(4):1915. doi: 10.1002/jbm.a.20100. [DOI] [PubMed] [Google Scholar]
- 180.Nakahara T, Nakamura T, Kobayashi E, Inoue M, Shigeno K, Tabata Y, Eto K, Shimizu Y. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003;9:153–162. doi: 10.1089/107632703762687636. [DOI] [PubMed] [Google Scholar]
- 181.Opperman LA, Moursi AM, Sayne JR, Wintergerst AM. transforming growth factor-beta 3(Tgf-beta3) in a collagen gel delays fusion of the rat posterior interfrontal suture in vivo. Anat Rec. 2002;267:120–130. doi: 10.1002/ar.10094. [DOI] [PubMed] [Google Scholar]
- 182.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12(3) doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]