Abstract
Vascularization is critical to the survival of engineered tissues. This study combined biophysical and bioactive approaches to induce neovascularization in vivo. Further, we tested the effects of engineered vascularization on adipose tissue grafts. Hydrogel cylinders were fabricated from poly(ethylene glycol) diacrylate (PEG) in four configurations: PEG alone, PEG with basic fibroblast growth factor (bFGF), microchanneled PEG, or both bFGF-adsorbed and microchanneled PEG. In vivo implantation revealed no neovascularization in PEG, but substantial angiogenesis in bFGF-adsorbed and/or microchanneled PEG. The infiltrating host tissue consisted of erythrocyte-filled blood vessels lined by endothelial cells, and immunolocalized to vascular endothelial growth factor (VEGF). Human mesenchymal stem cells were differentiated into adipogenic cells, and encapsulated in PEG with both microchanneled and adsorbed bFGF. Upon in vivo implantation subcutaneously in immunodeficient mice, oil red O positive adipose tissue was present and interspersed with interstitial fibrous (IF) capsules. VEGF was immunolocalized in the IF capsules surrounding the engineered adipose tissue. These findings suggest that bioactive cues and/or microchannels promote the genesis of vascularized tissue phenotypes such as the tested adipose tissue grafts. Especially, engineered microchannels may provide a generic approach for modifying existing biomaterials by providing conduits for vascularization and/or diffusion.
INTRODUCTION
Adipogenesis is a complex and yet often understudied biological process. During development, adipocytes differentiate from the mesenchymal lineage, the same progenitors from which osteoblasts, chondrocytes, myoblasts, and fibroblasts are derived.1-5 Adipocytes synthesize lipid vacuoles that are stored intracellularly, providing adipose tissue with unique properties and texture for a variety of physiological functions.6 Across mammalian species, adipose tissue serves to retain body heat and is important for fatty acid metabolism. Soft tissue defects are prevalent in chronic diseases, tumor removal, trauma, and congenital anomalies. For example, breast and facial cancers are life threatening, and once resected, leave patients with soft tissue defects and disfiguration. Mastectomy and tumor resection surgeries are examples of nonelective surgical procedures that subsequently mandate the replacement of lost soft tissue, and the restoration of the physical shape and physiological function.7-9 Trauma occurs in burns, war, or peacetime accidents, leading to soft tissue injuries in addition to skeletal fractures.10-12 In congenital anomalies such as hemifacial microsomia (one half of the face underdeveloped relative to the other half), a considerable amount of soft tissue is missing and needs to be reconstructed.13 In the majority of soft tissue defects, the bulk of the missing soft tissue is subcutaneous adipose tissue.14-17 Currently, autologous soft tissue grafts and synthetic materials are predominant clinical practice. Autologous grafts are immune compatible, but necessitate donor-site trauma and morbidity. Following mastectomy, autologous soft tissue grafts are prepared from the patient’s abdomen or back for the reconstruction of the lost breast tissue. Another drawback of autologous tissue grafting is postsurgical volume reduction, as high as 70%.18-24 Volume reduction may be due to the apoptosis of the transplanted mature adipocytes, their low tolerance to ischemia, and/or slow revascularization rate.21,25-28 Synthetic materials that have been utilized for soft tissue reconstruction include silicone gel implants. Despite various levels of reported clinical success, synthetic implants can fail unpredictably and with severe clinical consequences as exemplified by the reported negative sequelae of silicone breast implants such as leakage, foreign material reaction, capsular contraction, extrusion, and dislocation.29-31
Tissue engineering approaches have been utilized toward adipose tissue regeneration. Preadipocytes are considered advantageous in comparison to mature adipocytes because preadipocytes can proliferate to a certain extent ex vivo.26,32-34 Several preadipocyte cell lines, such as 3T3-L1 and 3T3-F442A, have been used in engineering adipose tissue in biomaterials such as polyglycolic acid (PGA) meshes.35-37 These adipogenic cell lines, despite their considerable value for in vitro studies, are immortalized and thus not as valuable as primary adipogenic cells for in vivo adipose tissue engineering. Primary preadipocytes seeded in porous polylactic acid (PLA) scaffolds generate adipose tissue upon in vivo implantation.26 Peptide-linked alginate implants support the adhesion and proliferation of sheep preadipocytes, and adipose tissue formation.38 Exposure of human and murine preadipocytes to basic fibroblast growth factor (bFGF) promotes adipogenic differentiation and adipose tissue formation in collagen or Matrigel scaffolds.33,39,40 However, the yield of preadipocytes is often low, likely due to cell damage during aspiration and/or liposuction.21,32 At this time, it is uncertain whether preadipocytes can be expanded to sufficient numbers in vitro for the healing of large soft tissue defects. We recently demonstrated that adipogenic cells derived from human mesenchymal stem cells (MSCs) can be encapsulated in native or synthetic polymers, such as collagen or poly(ethylene glycol) diacrylate (PEG), toward the in vitro and in vivo regeneration of adipose tissue.41-44 The phenotypic differentiation of hMSC-derived adipocytes is maintained after subcutaneous implantation in vivo in the dorsum of immunodeficient mice, and resulted in engineered adipose tissue that consisted of oil red O positive lipid vacuoles and expressed an adipogenic transcriptional factor, PPAR-γ2.41-44 In comparison with end-stage adipocytes with a limited capacity for self-replication,26,32 MSCs are capable of self-replenishing and generating a continuous supply of adipogenic cells.1,3,4,41,43,44
A critical and unmet challenge in adipose tissue engineering is suboptimal angiogenesis. Neovascularization becomes increasingly crucial as engineered adipose tissue is scaled up for clinical applications. A preadipocyte cell line, 3T3-F442A cells, has been injected subcutaneously in nude mice to generate fat pads.37 Neovascularization in the fat pad is derived from the host mice and not induced by the delivered 3T3-F442A cells.37 However, previous work has rarely addressed the engineering of angiogenesis in large adipose tissue grafts. Especially lacking is to engineer angiogenesis-promoting features such as physical channels in biomaterials capable of serving as conduits for neovascularization in adipose tissue grafts. We report below that engineered microchannels and/or delivered bFGF promote in vivo angiogenesis and host tissue ingrowth, providing the basis for inducing neovascularization in adipose tissue grafts. Human MSCs were differentiated into adipogenic cells and encapsulated in PEG hydrogel. Upon in vivo implantation subcutaneously in the dorsum of immunodeficient mice, oil red O positive adipose tissue was present and interspersed with interstitial fibrous (IF) capsules. Vascular endothelial growth factor (VEGF) was immunolocalized to IF capsules interposing the engineered adipose tissue. Taken together, these data demonstrate a potential for engineering vascularized adipose tissue grafts with biophysical design and/or the delivery of bioactive cues.
MATERIALS AND METHODS
The present animal protocol was approved by the institutional animal care and use committee. All numerical data were treated with analysis of variance (ANOVA) and Bonferroni tests to determine any significant differences among and between groups at an α level of 0.05.
Cell-free PEG hydrogel preparation with microchannels and/or bFGF
PEG (MW 3400; Nektar, Huntsville, AL) was dissolved in phosphate-buffered saline (PBS) (6.6% w/v) supplemented with 133 units/mL penicillin and 133 mg/mL streptomycin (Invitrogen, Carlsbad, CA). A photoinitiator, 2-hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (Ciba, Tarrytown, NY), was added at a concentration of 50 mg/mL. The resulting PEG cylinders were photopolymerized with ultraviolet (UV) light at 365 nm for 5 min (Glos-Mark, Upper Saddle River, NJ). A total of four PEG hydrogel configurations were fabricated: (1) PEG hydrogel alone, (2) a total of three microchannels (diameter: 1 mm) (Fig. 1A), (3) 0.5 μg/μL bFGF adsorbed in PEG hydrogel without microchannels (Fig. 1B), and (4) a combination of 0.5 μg/μL bFGF and microchannels in PEG hydrogel (Fig. 1C). The rationale for selecting bFGF over VEGF is primarily because that bFGF promotes both angiogenesis and adipogenesis.33,39,40 In addition, bFGF and VEGF promote different phases of engineered angiogenesis.45
FIG. 1.
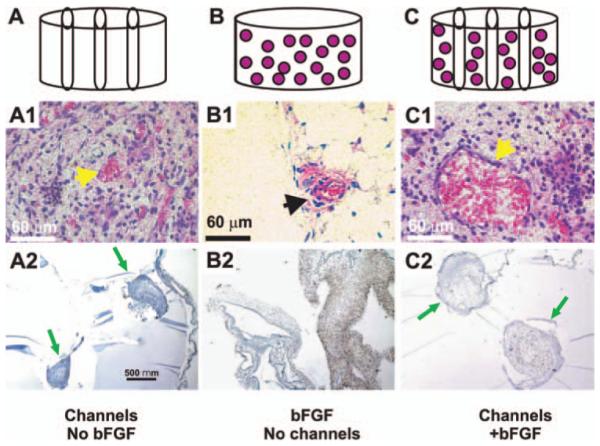
In vivo implantation of PEG hydrogel. PEG hydrogel was fabricated in four configurations: PEG alone, PEG with bFGF, microchanneled PEG, or both bFGF-adsorbed and microchanneled PEG. (A) PEG hydrogel molded into 6×4 mm (diameter×height) cylinder (without either bFGF or microchannels). (B) PEG hydrogel with three microchannels. (C) PEG hydrogel cylinder with microchannels and adsorbed with both 0.5 μg/μL bFGF and three microchannels. Following in vivo implantation subcutaneously in the dorsum of immunodeficient mice, the harvested PEG hydrogel samples showed distinct histological features. (A1) PEG hydrogel with microchannels but without bFGF showed host tissue infiltration primarily in the lumen of microchannels, and scarcely in the rest of PEG hydrogel. The infiltrating host tissue includes erythrocyte-filled blood vessels that are lined by endothelial cells (arrow). (A2) VEGF was immunolocalized only to host-derived tissue within the lumen of microchannels, indicating the vascular nature of the infiltrating host tissue. Arrows point to microchannels and the infiltrating host tissue. (B1) PEG hydrogel with bFGF but without microchannels showed apparently random and isolated islands of infiltrating host tissue (arrow). There is random infiltration of host tissue into bFGF-adsorbed PEG hydrogel. The infiltrating host tissue includes vascular structures with erythrocyte-filled blood vessels that are lined by endothelial cells (arrow). (B2) VEGF was immunolocalized to host-derived tissue within PEG hydrogel (without microchannels). (C1) PEG hydrogel with both microchannels and bFGF showed host tissue infiltration only in the lumen of microchannels, but scarcely in the rest of the PEG hydrogel. The infiltrating host tissue includes vascular structures with erythrocyte-filled blood vessels that are lined by endothelial cells (arrow). (C2) VEGF was immunolocalized only to host-derived tissue within the lumen of microchannels. Since no cells were delivered in any of the PEG hydrogel samples in this experiment, tissue infiltration following in vivo implantation must be derived from the host. Arrows point to microchannels. Color images available online at www.liebertpub.com/ten.
In vivo implantation of cell-free PEG hydrogel with microchannels and/or bFGF
Male severe combined immune deficiency (SCID) mice (strain C.B17; 4–5 weeks old) were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (4 mg/kg). The mouse dorsum was clipped of hair and placed in a prone position, followed by disinfection with 10% povidone iodine and 70% alcohol. A 1-cm-long linear cut was made along the upper midsagittal line of the dorsum, followed by blunt dissection to create subcutaneous pouches. Each SCID mouse received three PEG hydrogel implants: PEG with microchannels but without bFGF, bFGF-adsorbed PEG without microchannels, and PEG with both bFGF and microchannels. The implantation of PEG hydrogel samples with mixed conditions is advantageous by ensuring that various experimental parameters are tested in the same animal, and is based on our previous observation of a lack of significant cross influences among various experimental groups implanted in the same animal.42,46,47 The incision was closed with absorbable plain gut 4-0 sutures. All PEG hydrogel cylinders were implanted in vivo for 4 weeks.
Harvest of PEG hydrogel samples, histology, and immunohistochemistry
Four weeks following subcutaneous implantation in the dorsum of SCID mice, PEG hydrogel samples of all groups were harvested. Following carbon dioxide (CO2) asphyxiation, an incision was made aseptically in the dorsum of the SCID mouse. Upon careful removal of the surrounding fibrous capsule, PEG hydrogel cylinders were isolated from the host, rinsed with PBS, and fixed in 10% formalin for 24 h. The harvested samples were then embedded in paraffin and sectioned in the transverse plane (transverse to microchannels, c.f., Fig. 1A) at 5 μm thickness. Paraffin sections were stained with hematoxylin and eosin. Sequential adjacent sections were prepared for immunohistochemistry. Sections were deparaffinized, washed in PBS, and digested for 30 min at room temperature with bovine testicular hyaluronidase (1600 U/mL) in sodium acetate buffer at pH 5.5 with 150 mM sodium chloride. All immunohistochemistry procedures followed our previous methods.42,46,47 Briefly, sections were treated with 5% bovine serum albumin for 20 min at room temperature to block nonspecific reactions. The following antibodies were used: anti-VEGF (ABcam, Cambridge, MA), and biotin-labeled lectin from tritium vulgaris (wheat germ agglutinin) (WGA) with or without its inhibitor, acetylneuraminic acid (Sigma, St. Louis, MO). WGA binds to carbohydrate groups of vascular endothelial cells rich in α-D-GlcNAc and NeuAc.48,49 After overnight incubation with primary antibodies in a humidity chamber, sections were rinsed with PBS and incubated with IgG anti-mouse secondary antibody (1:500; Antibodies, Davis, CA) for 30 min. Sections were then incubated with streptavidin-horseradish peroxidase (HRP) conjugate for 30 min in humidity chamber. After washing in PBS, the double-linking procedure with the secondary antibody was repeated. Slides were developed with diaminobenzadine solution and counter-stained with Mayer’s hematoxylin for 3 to 5 min. Counter-stained slides were dehydrated in graded ethanol and cleared in xylene. The same procedures were performed for negative controls except for the omission of the primary antibodies.
Isolation and culture expansion of human bone marrow–derived MSCs (hMSCs)
Human MSCs were isolated from fresh bone marrow samples of two anonymous adult donors (AllCells, Berkeley, CA), per our previous methods.3,50-52 After transferring bone marrow sample to a 50 mL tube, a total of 750 μL of RosetteSep was added (StemCell Technologies, Vancouver, Canada) and incubated for 20 min at room temperature. Then 15 mL of PBS in 2% fetal bovine serum (FBS) and 1 mM ethylenediaminetetraacetic acid (EDTA) solution was added to the bone marrow sample to a total volume of approximately 30 mL. The bone marrow sample was then layered on 15 mL of Ficoll-Paque (StemCell Technologies) and centrifuged 25 min at 3000 g and room temperature. The entire layer of enriched cells was removed from Ficoll-Paque interface. The cocktail was centrifuged at 1000 rpm for 10 min. The solution was aspirated into 500 μL Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO) with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) and 1% antibiotic-antimycotic (Gibco, Carlsbad, CA), referred to as basal medium there-after. The isolated mononuclear cells were counted, plated at approximately 0.5–1×106 cells per 100-mm Petri dish, and incubated in basal medium at 37°C and 5% CO2. After 24 h, nonadherent cells were discarded, whereas adherent mononuclear cells were washed twice with PBS and incubated for 12 days with fresh medium change every other day.4 Upon 90% confluence, cells were removed from the plates using 0.25% trypsin and 1 mM EDTA for 5 min at 37°C, counted, and re-plated in 100-mm Petri dishes, referred to as passage 1 cells.
Differentiation of human MSCs into adipogenic cells
Second- and third-passage hMSCs were induced to differentiate into adipogenic cells by exposure to adipogenic medium consisting of basal medium supplemented with 0.5 μM dexamethasone, 0.5 μM isobutylmethylxanthine, and 50 μM indomethacin, per our prior methods.3,41,43,44 A subpopulation of hMSCs was continuously cultured in basal medium also in 95% air and 5% CO2 at 37°C with medium changes every other day. Oil red O staining (Sigma-Aldrich) was used to verify adipogenesis (lipid formation). For in vitro assessment of adipogenic differentiation, hMSCs were treated with adipogenic medium for up to 5 weeks. Monolayer cultured hMSCs with or without adipogenic differentiation were fixed in 10% formalin and subjected to oil red O staining. Glycerol content was quantified by enzyme-linked immunosorbent assay (ELISA) per our previous methods and per manufacturer’s protocol (Sigma FG0100).42,46,47,52 Glycerol content was calculated per DNA content (ng of DNA/mg dw of the hydrogel) using Hoechst 33258 per our previous methods.52 The rationale for quantifying glycerol content is that glycerol is synthesized by mature adipocytes and therefore a valid indication of adipogenesis from stem cells.
Encapsulation of hMSC-derived adipogenic cells in PEG hydrogel and in vivo implantation
Aqueous PEG was dissolved in sterile PBS supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco) to a final solution of 10% w/v. The photoinitiator, 2-hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (Ciba), was added to the PEG solution. After 1 week of adipogenic differentiation or culture in basal medium, hMSCs or hMSC-derived adipogenic cells were removed from the culture plates with 0.25% trypsin and 1 mM EDTA for 5 min at 37°C, counted, and resuspended separately in PEG polymer/photoinitiator solutions at a density of 3×106 cells/mL. An aliquot of 75 μL cell/polymer/photoinitiator suspension was loaded into sterilized plastic caps of 0.075 mL microcentrifuge tubes (6×4 mm, diameter×height) (Fisher Scientific, Hampton, NH), followed by photopolymerization with long-wave, 365-nm UV lamp (Glo-Mark) at an intensity of approximately 4 mW/cm2 for 5 min. The constructs were transferred to 12-well plates in corresponding media. A total of 0.5 μg/μL bFGF was adsorbed in PEG hydrogel prior to photopolymerization. The creation of three microchannels followed the approach as described above. Twelve weeks following subcutaneous implantation in the dorsum of immunodeficient mice (n = 5), PEG hydrogel samples of all groups were harvested. All tissue processing, histological, and immunohistochemical procedures were the same as described above.
RESULTS
Upon 4-week in vivo implantation in the dorsum of SCID mice, the implanted PEG hydrogel samples were well integrated with surrounding host subcutaneous tissue. It required effort to surgically isolate the implanted samples from the surrounding tissue and the removal of the fibrous tissue capsule. PEG hydrogel with microchannels but without bFGF demonstrated host tissue infiltration only in the lumen of microchannels, but not in the rest of PEG (Fig. 1A1). This is verified by immunolocalization of VEGF to host-derived tissue in the lumen of microchannels in PEG hydrogel, and the scarcity of host tissue infiltration in the rest of the PEG hydrogel (between microchannels) (Fig. 1A2), indicating that the infiltrated host tissue is vascularized. In contrast, PEG hydrogel adsorbed with bFGF but without microchannels demonstrated apparently random and isolated areas of host tissue infiltration, but nonetheless consisted of blood vessel–like structures as shown in Figure 1B1. Immunolocalization of VEGF again was positive in the infiltrating host tissue, indicating its vascular nature (Fig. 1B2). Interestingly, PEG hydrogel with both microchannels and bFGF demonstrated host tissue ingrowth only in microchannels, but scarcely in the rest of PEG hydrogel between microchannels (Fig. 1C1). The infiltrating host tissue was also immunolocalized positively to VEGF (Fig. 1C2). PEG hydrogel lacking both microchannels and bFGF showed no host tissue infiltration (data not shown), consistent with our previous reports showing a lack of host tissue infiltration into PEG hydrogel.41,43,44 Given that no cells are delivered in PEG hydrogel with or without bFGF and/or microchannels in this experiment, any and all infiltrating tissue into PEG hydrogel must be host derived.
Human MSCs were differentiated into adipogenic cells in vitro, as demonstrated in Figure 2, over the observed 35 days in ex vivo culture. In comparison with hMSCs without adipogenic differentiation (Fig. 2A–E), hMSC-derived adipogenic cells reacted positively to oil red O staining, and progressively so over the tested 35 days (Fig. 2F–J). This is consistent with our previous data showing the expression of PPAR-γ2 by hMSC-derived adipogenic cells following less than 2 weeks of treatment in adipogenic medium,41 and further indicates that hMSCs are capable of continuous adipogenic differentiation. The average glycerol content of hMSC-derived adipogenic cell samples was significantly higher than that of hMSCs (without adipogenic differentiation) at 28 and 35 days in culture, suggesting that hMSC-derived adipogenic cells gradually accumulate intracellular lipid vacuoles in vitro (Fig. 3). As we previously demonstrated, glycerol content is a reliable marker for distinguishing stem and progenitor cells from adipocytes, whereas of PPAR-γ2 is a critical transcriptional factor in the process of engineered adipogenic differentiation.4,41,52
FIG. 2.

Adipogenic differentiation of human mesenchymal stem cells (hMSCs) in oil red O staining for lipid vacuoles. Top row (A–E): hMSCs without adipogenic differentiation over 35 days. Bottom row (F–J): hMSCs in adipogenic induction medium over 35 days. Oil red O staining is negative for hMSCs during 35 days of culture. In contrast, hMSCs treated in adipogenic medium showed gradually intense oil red O staining over the observed 35 days. Color images available online at www.liebertpub.com/ten.
FIG. 3.

Glycerol content of hMSCs and hMSC-derived adipogenic cells. Glycerol content was quantified by ELISA per our previous methods. Glycerol content of hMSC-derived adipogenic cells was significantly higher than hMSCs at days 28 and 35, suggesting that hMSC-derived adipogenic cells continue to mature into adipocytes with the capacity to synthesize glycerol.
In a parallel experiment to utilize the above-described model system of microchannels and bioactive factor in PEG hydrogel, we encapsulated hMSCs and hMSC-derived adipogenic cells separately in PEG hydrogel samples and attempted to determine whether the engineered microchannels and bFGF promoted vascularized adipogenesis. Following 12-week in vivo implantation subcutaneously in the dorsum of immunodeficient mice, the implanted PEG hydrogel samples with microchannels and bFGF were well integrated with surrounding host subcutaneous tissue. As in our previous studies,42-44 PEG hydrogel was not permissive to host cell infiltration (Fig. 4A, A’). However, PEG hydrogel with engineered microchannels and adsorbed bFGF showed not only darker color, but also three red circles in the transverse plane (Fig. 4B, B’). Further, PEG hydrogel with both microchannels and bFGF, and seeded with hMSC-derived adipogenic cells showed not only darker color but also red circles (Fig. 4C, C’). Upon histological and immunohistochemical examination, PEG hydrogel encapsulating hMSC-derived adipogenic cells with built-in microchannels and bFGF showed islands of adipose tissue formation (Fig. 5A). The engineered adipose tissue was interposed by IF capsules (Fig. 5A). Multiple islands of the engineered adipose tissue were oil red O positive, shown as a representative in Figure 5B, suggesting the presence of engineered adipogenesis. Anti-VEGF antibody showed positive staining in the IF capsules (Fig. 5C), and anti-WGA lectin antibody was localized to the vicinity of engineered adipose tissue (Fig. 5D), suggesting that engineered neovascularization has promoted adipogenesis. In contrast, PEG hydrogel with adsorbed bFGF and microchannels but encapsulating hMSCs (without adipogenic differentiation) showed negative oil red O staining (data not shown).
FIG. 4.

In vivo implantation of bFGF and microchanneled PEG hydrogel loaded with adipogenic cells derived from hMSCs. Diagrams (top row) and corresponding representative photographs at the time of harvest of in vivo samples. (A) PEG hydrogel molded into 6×4 mm (width×height) cylinder (without either bFGF or microchannels). (B) PEG hydrogel cylinder loaded with both 0.5 μg/μL bFGF and three microchannels, but without the delivery of cells. (C) PEG hydrogel cylinder loaded with both 0.5 μg/μL bFGF and three microchannels, in addition to the encapsulation of adipogenic cells that have been derived from human mesenchymal stem cells at a cell seeding density of 3×106 cells/mL. Following in vivo implantation subcutaneously in the dorsum of immunodeficient mice, the harvested PEG hydrogel samples showed distinct histological features. (A’) PEG hydrogel cylinder without either microchannels or bFGF showed somewhat transparent appearance. (B’) PEG hydrogel cylinder with both bFGF and three microchannels, but without delivered cells, showed darker color and a total of three openings of microchannels (arrows) that are confirmed to be areas of host cell infiltration histologically (c.f., Fig. 1A1, 1A2). (C’) PEG hydrogel cylinder with both microchannels and bFGF in addition to encapsulated hMSC-derived adipogenic cells showed the opening of microchannels (red color and pointed with arrows) that are confirmed to be areas of host cell infiltration histologically in Figure 5. Color images available online at www.liebertpub.com/ten.
FIG. 5.
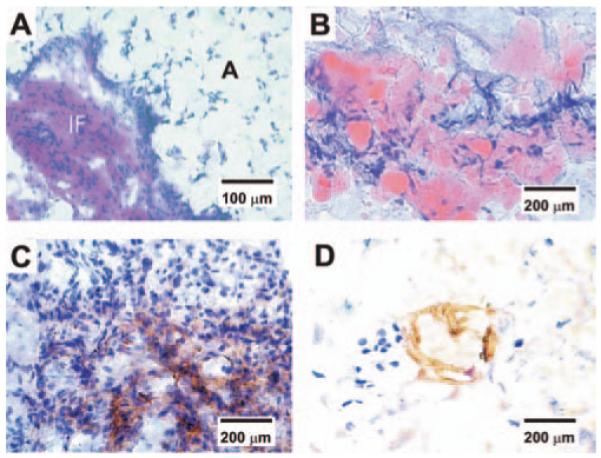
Histological and immunohistochemical characterization of vascularized adipose tissue from human mesenchymal stem cells. (A) Hematoxylin and eosin staining revealed IF tissue interposing between foam-like space labeled with A for adipose tissue. The presence of adipose tissue is confirmed in (B), showing substantial oil red O positive staining in PEG hydrogel encapsulating hMSC-derived adipogenic cells, in addition to bFGF and built-in microchannels. In contrast, there is no evidence of adipogenesis in PEG hydrogel with bFGF and built-in microchannels, but with hMSCs (without adipogenic differentiation). (C) Positive immunolocalization of VEGF antibody in the IF tissue interposing areas of adipogenesis, indicating the presence of vascular supply. (D) Positive immunolocalization of lectin WGA in the interstitial fibrous tissue interposing areas of adipogenesis, serving as further indication of the presence of vascular endothelial cells. Color images available online at www.liebertpub.com/ten.
DISCUSSION
The present data demonstrate not only a novel approach to induce host-derived vascularization in vivo, but also original findings that physical microchannels and/or a bioactive factor, bFGF, promotes vascularized adipogenesis in vivo from human MSCs. This study is a continuation of our ongoing work in the direction of engineering adipose tissue from MSCs.41,43,44,52 The critical difference of this study from our previous work is the addition of approaches that promote vascularization. Angiogenesis is necessary for the survival and maintenance of the majority of engineered tissues. In comparison with previous approaches in the attempt to vascularize engineered adipose tissue, the novelty of the present approach is in the creation of microchannels, which provide conduits for ingrowing tissue and vascular-like structures, and a demonstration that a combination of MSCs seeded in hydrogel with microchannels and bFGF generates vascularized adipose tissue grafts. In conjunction with positive staining by oil red O, a typical adipogenesis marker, we showed the vascular characteristics of the engineered adipose tissue by VEGF and WGA immunoblotting. Ongoing work is characterizing the vascularized adipogenic tissue using additional vascular endothelial markers such as PECAM, CD31, CD34, CD144, Flk-1, and von Willebrand factor. Additional attempt also focuses on the creation of increasingly finer microchannels so that vascularization and perfusion are further enabled in the PEG hydrogel and more in the vicinity of the seeded hMSC-adipogenic cells. A further alternative is to isolate and study the involvement of endothelial progenitor cells, for example, from bone marrow or peripheral blood, in engineered angiogenesis.
In the present work, microchannels and bFGF have been shown to induce vascularization in PEG hydrogel with a molecular weight of 3400 and the level of cross-linking that otherwise does not permit host cell infiltration or hostderived vascularization upon in vivo implantation, as in previous work.41,43,44 Previous meritorious approaches have functionalized PEG with molecular motifs such as RGF peptides.53-56 Our approach of adsorption of bioactive cues and especially microchanneling in PEG hydrogel provides a parallel approach to Arg-Gly-Asp (RGD) tethering. The present approach of microchanneling with or without the adsorption of bioactive cues provides alternatives for inducing host cell infiltration. Creation of microchannels in Food and Drug Administration (FDA)-approved materials, such as PEG, has the potential for relatively expedited regulatory pathways. In addition to its angiogenic effects, bFGF has been found to promote adipogenesis,57-59 and our data are consistent with these previous findings. Also, we previously showed that adipogenesis could be induced in vivo in threedimensional hydrogel from human MSCs.41 MSCs may offer a unique advantage over adipocytes or preadipocytes in adipose tissue engineering in which MSCs are capable of continuously self-replicating and differentiating into adipogenic cells, thus potentially providing a continuous supply of adipose tissue–forming cells.1,3 Since our previous work,41 there have been additional reports of the use of MSCs toward adipose tissue engineering.43,44,57 Regardless of cell source being adipocytes, preadipocytes, or MSCs, cell density is likely a central issue and as a function of the regenerative outcome.60-62 In this study, 3 million MSC-derived adipogenic cells per mL were utilized and yielded in vivo adipogenesis. This represents a slight reduction from our previous work of 5 million cells per mL41 and was intended to determine whether in vivo adipogenesis can be accomplished with a smaller cell seeding density, which equates to shorter ex vivo cell expansion time and is desirable for translational approaches. However, there is likely a threshold cell seeding density below which adipogenesis is compromised. A factor that compensates for the present lower cell seeding density (3×106 cells/mL) is the probability of host cell infiltration via microchannels and for bFGF in PEG hydrogel. We are in the process of labeling cells that are to be delivered in vivo with a specific goal to determine the relative contribution to adipogenesis by delivered cells versus host cells. In the experiment to encapsulate hMSC-derived adipogenic cells in bFGF-adsorbed and microchanneled PEG hydrogel, there is a possibility that hMSCs or hMSC-derived adipogenic cells are also responsible for host-derived angiogenesis. This needs to be studied also by in vivo cell labeling and cell tracking.
The seeded cells in the present study likely represent a heterogeneous population of hMSCs and hMSC-derived adipogenic cells. It is probable that the in vivo harvested samples of the engineered adipose tissue also contain hMSCs, hMSC-derived adipogenic cells, or preadipocyte, in addition to mature adipocytes. Our in vitro data of continuing adipogenic differentiation of hMSCs provide some support of this assertion. Whereas some of the hMSC-derived adipogenic cells are end-stage adipocytes with accumulating oil red O positive intracellular lipid vacuoles, others are adipogenic cells and/or hMSCs with the capacity for proliferation.1,3,43 Continuous proliferation of hMSCs and hMSC-derived adipogenic cells likely replenishes the supply of adipose tissue–forming cells. However, terminally differentiated adipocytes are not anticipated to further proliferate.28 Also in this study, histological sections reveal the presence of fibroblast-like cells in the IF tissue that interposes the oil red O positive adipose tissue, which is characteristic of the native adipose tissue.
A critical aspect of reconstructive and plastic surgeries is the maintenance of shape and dimensions. Hypothetically, a successfully tissue-engineered kidney from stem cells, when realized, does not necessarily need to have the precise shape as the patient’s original kidney so long as the engineered kidney fulfills physiological functions in vivo. In contrast, soft tissue reconstruction or augmentation must maintain the desired shape and dimensions, in addition to appropriate physiological functions. Current soft tissue reconstruction procedures suffer from postoperative volume reduction, as severe as up to 70% over time.15,21,26 Volume maintenance of alginate gel constructs seeded with preadipocytes is between 19% and 88% after 8 weeks of subcutaneous implantation in rats.63 As quantified in our previous work,42-44,46,47 volume retention of the engineered adipose tissue grafts in this work is virtually 100%. The maintenance of predefined shape and dimensions can be attributed to at least the following two factors. First, the slow degradation rate of PEG hydrogel must have contributed to dimension maintenance. Second, hMSC-derived adipogenic cells encapsulated in PEG hydrogel in this study are likely still heterogeneous, containing a percentage of hMSCs and potentially other cells. Since PEG hydrogel implants with bFGF and/or seeded with cells were implanted in immunodeficient animals, it is unknown whether shape maintenance is facilitated by presumed attenuation of inflammatory response of the host. This needs to be studied in nonimmunodeficient animal models. The encapsulated hMSCs may undergo adipogenic differentiation in vivo or remain MSCs. Either way, the native adipogenesis process is simulated in the sense that whereas the majority of the cells in adipose tissue are adipocytes at various stages of maturation, there are fibroblasts and adipose progenitor cells in the interstitial tissue.6 A recent in vitro study showed that insertion of laminin-binding or other peptides in PEG hydrogel has effects on preadipocyte adhesion and proliferation.53 It would be of interest to study whether insertion of peptides affects the adhesion and proliferation of MSCs.
The regeneration of soft tissue defects resulting from trauma, congenital anomalies, chronic diseases, and tumor surgery relies primarily on adipose tissue, in addition to fibroblastic capsules and vascularized structures that provide conduits for nutrient supply and perfusion. Taken together, the present study represents a critical step forward from our previous work and demonstrates that adipose tissue with predefined shape and dimensions can be engineered from adult human stem cells with host-derived vascularization.
ACKNOWLEDGMENTS
We are grateful to Drs. Christine Rodhe and June Wu, Department of Plastic Surgery, Columbia University, for their clinical comments on the manuscript. Ms. Sarah Kennedy is acknowledged for technical assistance. We thank five anonymous reviewers, whose insightful comments have substantially helped improve the quality of the manuscript. We thank Sarah Kennedy and Bhranti Shah for editorial assistance. This research was supported by NIH grant EB006261 to J.J.M.
REFERENCES
- 1.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs) J Bone Miner Res. 2004;19:256–264. doi: 10.1359/JBMR.0301220. [DOI] [PubMed] [Google Scholar]
- 6.Feve B. Adipogenesis: cellular and molecular aspects. Best Pract Res Clin Endocrinol Metab. 2005;19:483–499. doi: 10.1016/j.beem.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Yaremchuk MJ. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111:1818–1827. doi: 10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- 8.Coppit GL, Lin DT, Burkey BB. Current concepts in lip reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2004;12:281–287. doi: 10.1097/01.moo.0000130574.03032.e2. [DOI] [PubMed] [Google Scholar]
- 9.Vural E. Surgical reconstruction in patients with cancer of the head and neck. Curr Oncol Rep. 2004;6:133–140. doi: 10.1007/s11912-004-0025-2. [DOI] [PubMed] [Google Scholar]
- 10.Thaller SR, Zarem HA, Kawamoto HK. Surgical correction of late sequelae from facial bone fractures. Am J Surg. 1987;154:149–153. doi: 10.1016/0002-9610(87)90306-0. [DOI] [PubMed] [Google Scholar]
- 11.Roncevic R, Roncevic D. Extensive, traumatic fractures of the orbit in war and peace time. J Craniofac Surg. 1999;10:284–300. [PubMed] [Google Scholar]
- 12.Peterson SL, Moore EE. The integral role of the plastic surgeon at a level I trauma center. Plast Reconstr Surg. 2003;112:1371–1375. doi: 10.1097/01.PRS.0000082815.79881.51. discussion 1377–1378. [DOI] [PubMed] [Google Scholar]
- 13.Kaban LB, Padwa BL, Mulliken JB. Surgical correction of mandibular hypoplasia in hemifacial microsomia: the case for treatment in early childhood. J Oral Maxillofac Surg. 1998;56:628–638. doi: 10.1016/s0278-2391(98)90465-7. [DOI] [PubMed] [Google Scholar]
- 14.Monahan R, Seder K, Patel P, Alder M, Grud S, O’Gara M. Hemifacial microsomia. Etiology, diagnosis and treatment. J Am Dent Assoc. 2001;132:1402–1408. doi: 10.14219/jada.archive.2001.0055. [DOI] [PubMed] [Google Scholar]
- 15.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 16.Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587–603. viii. [PubMed] [Google Scholar]
- 17.Brey EM, Patrick CW., Jr. Tissue engineering applied to reconstructive surgery. IEEE Eng Med Biol Mag. 2000;19:122–125. doi: 10.1109/51.870241. [DOI] [PubMed] [Google Scholar]
- 18.Billings E, Jr., May JW., Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Butler PE, Lee WP, Sims CD, Randolph MA, Vacanti CA, Yaremchuk MJ. Cell transplantation from limb allografts. Plast Reconstr Surg. 1998;102:161–168. doi: 10.1097/00006534-199807000-00025. discussion 169-170. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KY, Halberstadt CR, Holder WD, Mooney DJ. Breast Reconstruction. In: Lanza RP, Langer R, Vacanti JP, editors. Principles of Tissue Engineering. Academic Press; Sand Diego: 2000. pp. 409–423. [Google Scholar]
- 22.Disa JJ, Liew S, Cordeiro PG. Soft-tissue reconstruction of the face using the folded/multiple skin island radial forearm free flap. Ann Plast Surg. 2001;47:612–619. doi: 10.1097/00000637-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Hart D. Overcoming complications of breast implants. Plast Surg Nurs. 2003;23:55–63. doi: 10.1097/00006527-200323020-00005. [DOI] [PubMed] [Google Scholar]
- 24.Smahel J. Experimental implantation of adipose tissue fragments. Br J Plast Surg. 1989;42:207–211. doi: 10.1016/0007-1226(89)90205-1. [DOI] [PubMed] [Google Scholar]
- 25.Smahel J. Adipose tissue in plastic surgery. Ann Plast Surg. 1986;16:444–453. [PubMed] [Google Scholar]
- 26.Patrick CW., Jr. Adipose tissue engineering: the future of breast and soft tissue reconstruction following tumor resection. Semin Surg Oncol. 2000;19:302–311. doi: 10.1002/1098-2388(200010/11)19:3<302::aid-ssu12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Galdino GM, Nahabedian M, Chiaramonte M, Geng JZ, Klatsky S, Manson P. Clinical applications of three-dimensional photography in breast surgery. Plast Reconstr Surg. 2002;110:58–70. doi: 10.1097/00006534-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Kononas TC, Bucky LP, Hurley C, May JW., Jr. The fate of suctioned and surgically removed fat after reimplantation for soft-tissue augmentation: a volumetric and histologic study in the rabbit. Plast Reconstr Surg. 1993;91:763–768. doi: 10.1097/00006534-199304001-00001. [DOI] [PubMed] [Google Scholar]
- 29.Ojo-Amaize EA, Conte V, Lin HC, Brucker RF, Agopian MS, Peter JB. Silicone-specific blood lymphocyte response in women with silicone breast implants. Clin Diagn Lab Immunol. 1994;1:689–695. doi: 10.1128/cdli.1.6.689-695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzin WE, Feng LJ, Abbuhl M, Klein MA. Phenotype of lymphocytes associated with the inflammatory reaction to silicone gel breast implants. Clin Diagn Lab Immunol. 1996;3:156–161. doi: 10.1128/cdli.3.2.156-161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naim JO, Satoh M, Buehner NA, Ippolito KM, Yoshida H, Nusz D, Kurtelawicz L, Cramer SF, Reeves WH. Induction of hypergammaglobulinemia and microphage activation by silicone gels and oils in female ASW mice. Clin Diagn Lab Immunol. 2000;7:366–370. doi: 10.1128/cdli.7.3.366-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Heimburg D, Kuberka M, Rendchen R, Hemmrich K, Rau G, Pallua N. Preadipocyte-loaded collagen scaffolds with enlarged pore size for improved soft tissue engineering. Int J Artif Organs. 2003;26:1064–1076. doi: 10.1177/039139880302601204. [DOI] [PubMed] [Google Scholar]
- 33.Kelly JL, Findlay MW, Knight KR, Penington A, Thompson EW, Messina A, Morrison WA. Contact with existing adipose tissue is inductive for adipogenesis in matrigel. Tissue Eng. 2006;12:2041–2047. doi: 10.1089/ten.2006.12.2041. [DOI] [PubMed] [Google Scholar]
- 34.Hemmrich K, von Heimburg D, Cierpka K, Haydarlioglu S, Pallua N. Optimization of the differentiation of human preadipocytes in vitro. Differentiation. 2005;73:28–35. doi: 10.1111/j.1432-0436.2005.07301003.x. [DOI] [PubMed] [Google Scholar]
- 35.Fischbach C, Spruss T, Weiser B, Neubauer M, Becker C, Hacker M, Gopferich A, Blunk T. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54–64. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Fischbach C, Seufert J, Staiger H, Hacker M, Neubauer M, Gopferich A, Blunk T. Three-dimensional in vitro model of adipogenesis: comparison of culture conditions. Tissue Eng. 2004;10:215–229. doi: 10.1089/107632704322791862. [DOI] [PubMed] [Google Scholar]
- 37.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 38.Halberstadt C, Austin C, Rowley J, Culberson C, Loebsack A, Wyatt S, Coleman S, Blacksten L, Burg K, Mooney D, Holder W., Jr. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Eng. 2002;8:309–319. doi: 10.1089/107632702753725067. [DOI] [PubMed] [Google Scholar]
- 39.Toriyama K, Kawaguchi N, Kitoh J, Tajima R, Inou K, Kitagawa Y, Torii S. Endogenous adipocyte precursor cells for regenerative soft-tissue engineering. Tissue Eng. 2002;8:157–165. doi: 10.1089/107632702753503144. [DOI] [PubMed] [Google Scholar]
- 40.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 41.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556–566. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 42.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–944. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 43.Stosich MS, Mao JJ. Stem cell-based soft tissue grafts for plastic and reconstructive surgeries. Semin Plast Surg. 2005;19:251–260. [Google Scholar]
- 44.Stosich MS, Mao JJ. Adipose tissue engineering from human mesenchymal stem cells: clinical implication in plastic and soft tissue reconstructive surgeries. Plast Reconstr Surg. 2007;119:71–83. doi: 10.1097/01.prs.0000244840.80661.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 46.Mao JJ, Rahemtulla F, Scott PG. Proteoglycan expression in the rat temporomandibular joint in response to unilateral bite raise. J Dent Res. 1998;77:1520–1528. doi: 10.1177/00220345980770070701. [DOI] [PubMed] [Google Scholar]
- 47.Sundaramurthy S, Mao JJ. Modulation of endochondral development of the distal femoral condyle by mechanical loading. J Orthop Res. 2006;24:229–241. doi: 10.1002/jor.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jinga VV, Gafencu A, Antohe F, Constantinescu E, Heltianu C, Raicu M, Manolescu I, Hunziker W, Simionescu M. Establishment of a pure vascular endothelial cell line from human placenta. Placenta. 2000;21:325–336. doi: 10.1053/plac.1999.0492. [DOI] [PubMed] [Google Scholar]
- 49.Izumi K, Feinberg SE, Terashi H, Marcelo CL. Evaluation of transplanted tissue-engineered oral mucosa equivalents in severe combined immunodeficient mice. Tissue Eng. 2003;9:163–174. doi: 10.1089/107632703762687645. [DOI] [PubMed] [Google Scholar]
- 50.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–546. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yourek G, Alhadlaq A, Ratel R, McCormick S, Reilly GC, Mao JJ. Nanophysical properties of living cells: the cytoskeleton. In: Dutta M, Stroscio M, editors. Biological Nanostructures and Applications of Nanostructures in Biology: Electrical, Mechanical, and Optical Properties. Kluwer Academic Publishing; New York: 2004. pp. 69–97. [Google Scholar]
- 52.Hong L, Peptan I, Clark P, Mao JJ. Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Ann Biomed Eng. 2005;33:511–517. doi: 10.1007/s10439-005-2510-7. [DOI] [PubMed] [Google Scholar]
- 53.Patel PN, Gobin AS, West JL, Patrick CW., Jr. Poly(ethylene glycol) hydrogel system supports preadipocyte viability, adhesion, and proliferation. Tissue Eng. 2005;11:1498–1505. doi: 10.1089/ten.2005.11.1498. [DOI] [PubMed] [Google Scholar]
- 54.Knerr R, Weiser B, Drotleff S, Steinem C, Gopferich A. Measuring cell adhesion on RGD-modified, self-assembled PEG monolayers using the quartz crystal microbalance technique. Macromol Biosci. 2006;20:827–838. doi: 10.1002/mabi.200600106. [DOI] [PubMed] [Google Scholar]
- 55.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photo-polymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12:1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 57.Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, Goepferich A, Blunk T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005;11:1840–1851. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 58.Cho SW, Kim I, Kim SH, Rhie JW, Choi CY, Kim BS. Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochem Biophys Res Commun. 2006;345:588–594. doi: 10.1016/j.bbrc.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 59.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12:1475–1487. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 60.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 61.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Troken AJ, Marion NM, Hollister SJ, Mao JJ. Cell density in synovial joint tissue engineering. In: Special Issue on Articular Cartilage Tissue Engineering. J Eng Med. 2007;221:429–440. doi: 10.1243/09544119JEIM288. [DOI] [PubMed] [Google Scholar]
- 63.Marler JJ, Guha A, Rowley J, Koka R, Mooney D, Upton J, Vacanti JP. Soft-tissue augmentation with injectable alginate and syngeneic fibroblasts. Plast Reconstr Surg. 2000;105:2049–2058. doi: 10.1097/00006534-200005000-00020. [DOI] [PubMed] [Google Scholar]


