Abstract
Human mesenchymal stem cells (hMSC) have been shown to differentiate into chondrocytes and form cartilage-like tissues when cultured with TGFβ3 at 10ng/ml. Previous attempts to engineer cartilage using hMSC have depended on in vitro pre-differentiation in order to form chondrogenic engineered constructs. Such techniques greatly increase the time of implant fabrication and suffer from loss of phenotype upon withdrawal from chondrogenic medium. The present study investigates a tissue engineered construct that includes sustained delivery of TGFβ3 and induces differentiation of hMSC into chondrocytes in situ using injectable thermosensitive gels.
I. INTRODUCTION
Skeletal motion is made possible by synovial joints, which include distinct yet integrated bone and cartilage tissues. Cartilage tissues provide a sliding surface during joint movement with minimal friction and are the main mediators of shock absorption, withstanding mechanical loading up to several times body weight. Debilitating disorders such as rheumatoid arthritis (RA), osteoarthritis (OA), and trauma result in deterioration of cartilage and osseous structures of the synovial joint [1]–[3]. Remarkably, everyone aged 65 or above has some level of OA which may or may not result in pain [1], [4]. Together with OA and trauma, RA is the major cause of physical disabilities resulting in $254 billion impact in the U.S. economy per year.
Current procedures in orthopedic medicine such as chondrocyte transfer, cartilage plug transplantation, and total joint replacement have demonstrated many hindrances such as donor site morbidity, limited tissue supply, immunorejection, potential transmission of pathogens, implant loosening, wear and tear [2], [5]–[7]. Over 400,000 total joint replacements are performed in the U.S. each year due to arthritis, trauma, and congenital anomalies and rely on metallic and synthetic materials, do not remodel with native structures, and may fail. In lieu of the mayoral traumatic events during total hip or knee replacements, less invasive techniques have been developed for the treatment of cartilage disorders. Arthroscopic treatments have shown promising results allowing for out-patient procedures. However, few current techniques focus on the regeneration of debilitated cartilage. Tissue engineering has great potential for cartilage repair, as it offers biomaterial, molecular and cellular tools. Injectable biomaterials such as thermosensitive chitosan gels, which closely resemble the cartilage extracellular matrix, may greatly benefit out-patient procedures as they are suitable carriers for autologous cells and bioactive molecules and promote differentiation. Moreover, TGFβ3, one of the most potent chondrogenic growth factors, if delivered continuously at adequate concentrations, may induce in situ chondrogenesis in failing articular cartilage.
Other bioactive molecules such as basic fibroblast growth factor (bFGF), have been studied in the context of cartilage repair. Intraarticular injections of bFGF contained in gelatin hydrogel microspheres suppressed the progression of OA in the rabbit model [8]. Such techniques suggest the feasibility of intraarticular injections as minimally invasive techniques for chondrogenic regeneration. Caveats of previous studies however, may include the absence of suitable matrices for cartilage growth and short bioactivity of growth factors due to inadequate delivery system. The present study, evaluates the combination of a chondrogenic matrix (chitosan gel) with controlled delivery of chondrogenic molecules (TGFβ3 in PLGA microspheres) and appropriate autologous cells (bone marrow derived stem cells), resulting in a procedure that includes active chondrogenic stimuli requiring little time and preparation, applicable in the operating room.
Pre-differentiation of stem cells into chondrocytes increases ex vivo manipulation time and may lead to loss of chondrogenic phenotype upon withdrawal from growth factor based medium. The present chitosan based construct provides a means for chondrogenic differentiation of stem cells after implantation. Chitosan hydrogels share characteristics with varios GAGs and hyaluronic acid in cartilage and support stem cell encapsulation and entrapment of chondrogenic matrix molecules such as aggrecan [9]–[11]. In order to induce chondrogenesis, TGFβ3 encapsulated in PLGA microspheres may be incorporated in chitosan gels, remain bioactive long-term, and is released in a controlled fashion. The objective of the present study was to promote in situ chondrogenesis by the delivered untreated stem cells after injection of cell/growth factor/chitosan solution into a culture well and subsequent gelation. The tissue engineered construct is in liquid injectable form at room temperature allowing manipulation, and becomes gelled at 37°C (body temperature). Such process circumvents the necessity for stem cell chondrogenic differentiation in vitro by inducing differentiation of stem cells via sustained release of bioactive chondrogenic factor long-term in situ and promotes cartilage repair requiring minimal cell manipulation (Fig. 1).
Fig. 1. Schematic diagram of conventional and present approaches for the fabrication of chondrogenic injectable tissue engineered constructs.
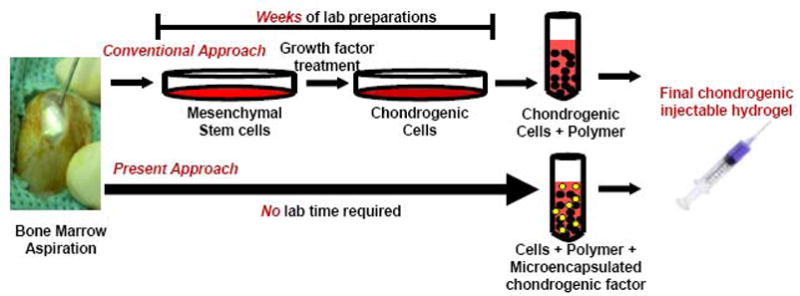
Bone marrow may be aspirated from the marrow cavity of bones, such as the tibia and iliac crest. In the conventional approach for engineering cartilage, tissue engineers isolate mesenchymal progenitors from the bone marrow using negative selection techniques. Mesenchymal stem cells are then plated, culture expanded and treated with growth factors to induce chondrogenic differentiation. This laborious process may take up to several weeks of laboratory manipulation. In the present approach, bone marrow progenitors may be directly added to a chondrogenic gel material that includes controlled delivery of chonrogenic factors such as TGFβ3 that promote chondrogenesis in situ or in vivo, circumventing laboratory cell manipulation and differentiation.
II. MATERIALS AND METHODS
Thermosensitive Chitosan Gel Fabrication
Chitosan (source: crustacean chitin; ICN Biomedicals Inc., Aurora, Ohio) was dissolved in 1N HCl for 24hrs and autoclaved at 121°C for 10 mins. Filter sterile 55% glycerol 2-phosphate disodium salt hydrate (GP) (FW:216.04; Sigma, St. Louis, MO) was added to chitosan solution on ice bath to form the thermosensitive gel (2% chitosan, 8.2% GP). This solution is in liquid form at RT and gels at 37°C (Fig 2). Previous studies on the optimization of sterilization procedures of chitosan revealed that autoclave for 10 minutes at 121°C maintains functionality of fabricated gels [12]. Sterilization of each solution was performed separately since temperature rise would gel the mixed solution.
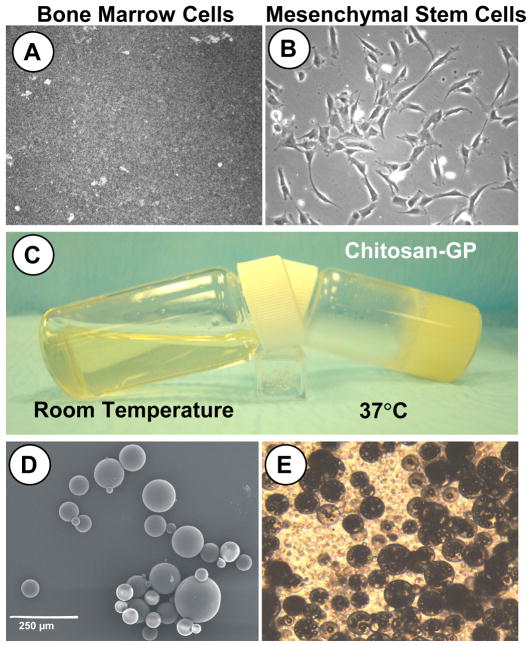
Fig. 2. Chondrogenic tissue engineered construct preparation.
A. Freshly harvested bone marrow plated in culture dish. B. Mesenchymal stem cells adhered to plastic culture dish for expansion in basic culture medium (no chondrogenic factors added). C. Chitosan-GP in liquid form at room temperature and Chitosan-GP in gel form after 30 minutes at 37°C (body temperature). D. SEM of PLGA microspheres encapsulating TGFβ3. E. Light microscopy image of Chitosan-GP gel mixed with hMSCs and TGFβ3 encapsulating PLGA microspheres (final injectable solution).
Fabrication of PLGA Microspheres Encapsulating TGFβ3
Microspheres of poly(DL-lactic-co-glycolic acid) (PLGA; Sigma, St. Louis, MO) of 50:50 PLA:PGA ratio (Sigma, St. Louis, MO) were prepared using double emulsion technique ((water-in-oil)-in-water) (Fig. 2D, 2E). A total of 250 mg PLGA was dissolved into 1 ml dichloromethane. Recombinant human TGFβ3 with molecular weight of 25kDa (R&D Systems, Minneapolis, MN) was diluted in 50 μl of reconstituting solution per manufacturer protocol and added to the PLGA solution, forming a mixture (primary emulsion) that was emulsified for 1 min (water-in-oil). The primary emulsion was then added to 2 ml of 1% polyvinyl alcohol (PVA, MW 30,000–70,000), followed by 1 min mixing ((water-in-oil)-in-water). Upon adding 100 ml 0.1% PVA solution, the mixture was stirred for 1 min. A total of 100 ml of 2% isopropanol was added to the final emulsion and continuously stirred for 2 hrs under chemical hood to remove the solvent. PLGA microspheres containing TGFβ3 were isolated using filtration (2 μm filter) and washed with distilled water. Microspheres were frozen in liquid nitrogen for 30 min and lyophilized for 48 hrs. Freeze-dried PLGA microspheres were stored at −20°C prior to use.
Tissue Engineered Construct Fabrication
Human mesenchymal stem cells (hMSCs) were seeded in thermosensitive chitosan gels at 5×106 cells/ml at room temperature for all experimental groups. PLGA microspheres encapsulating TGFβ3 were added to cell/chitosan solution to sustain continuous release of approximately 10ng/ml TGFβ3. Cell/Chitosan/Microspheres construct was injected into the wells of a 96-well plate at room temperature and allowed to gel at 37°C in incubator.
III. RESULTS
Release of TGFβ3 from PLGA Microspheres Within Tissue Engineered Constructs
TGFβ3 was released in a sustained fashion up to 4 weeks from PLGA microspheres embedded in chitosan-GP thermosensitive gels. The typical initial burst release over the first few days (4–7 days) was not observed for the case of chitosan-GP embedded PLGA microspheres (Fig. 3). The R2 value representing linear correlation in the release curve was 0.99, which characterizes high linearity.
Fig. 3. TGFβ3 Release Profile from PLGA Microspheres in Chitosan-GP gel.
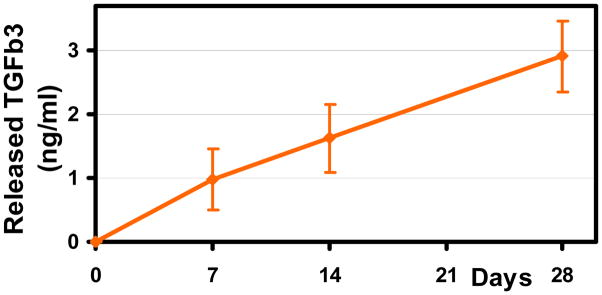
Microencapsulated TGFβ3 was released up to 28 days from PLGA microspheres (10mg) in Chitosan-GP gel in a sustained fashion. Typical initial burst release of encapsulated growth factor was attenuated by chitosan gel resulting in linear release.
In Situ Chondrogenesis of hMSCs in Thermosensitive Chitosan-GP Gels by Controlled Delivery of TGFβ3
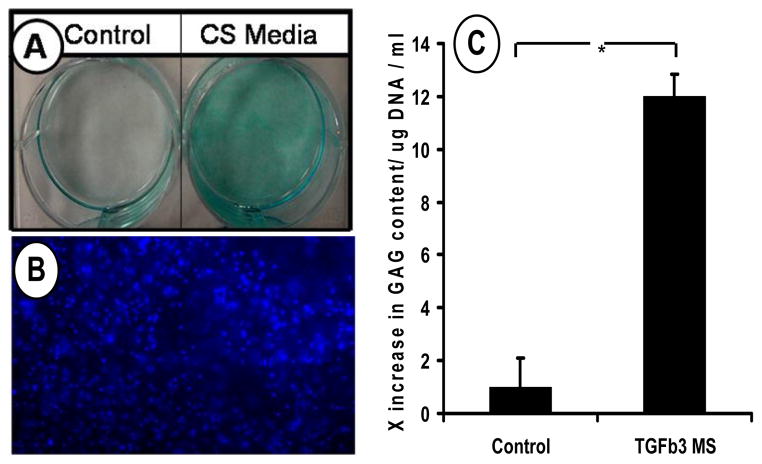
Human mesenchymal stem cells (hMSCs) were isolated from the bone marrow of healthy donors by negative selection and culture plated for expansion using growth medium. Without any prior chondrogenic treatment, hMSCs seeded in chitosan-GP gels that included controlled delivery of TGFβ3 by PLGA microspheres exhibited chondrogenic differentiation and synthesis of cartilage-like extracellular matrix molecules such as glycosaminoglycans (GAG) after 10 days of culture (Fig. 4C). Controls, which included hMSCs seeded at the same density in chitosan-GP gels, without TGFβ3, had significantly lower GAG content. The observed increase in GAG production by hMSCs cultured with controlled delivery of TGFβ3 within the chondrogenic injectable constructs was approximately 12-fold. Cell seeding was confirmed by DAPI nuclear staining of gels (Fig. 4B). These findings suggest that the laborious pre-differentiation of bone marrow derived stem cells that may require many weeks of cell manipulations and growth factor treatment, may be circumvented by the present approach.
Fig. 4. Chondrogenesis of bone marrow derived human mesenchymal stem cells (hMSC) in tissue engineered constructs.
A. Chondrogenic differentiation of hMSC in monolayer culture supplemented with 10ng/ml TGFβ3 demonstrating chondrogenic potential of hMSC (CS=Chondrogenic Supplements). B. DAPI staining of tissue engineered construct seeded with hMSC after 14days of culture. C. Glycosaminoglycan (GAG) of hMSC cultured in thermosensitive chitosan with or without TGFβ3 loaded PLGA microspheres after 14 days. TGFβ3 from PLGA microspheres induced chondrogenesis in engineered constructs indicated by increased GAG content.
The chondrogenic effects of TGFβ3 on hMSCs was verified using a monolayer culture system, where hMSCs were plated in 6-well plates at high densities (2.5×106 cells/ml) and cultured under chondrogenic supplements including 10ng/ml of TGFβ3. Human MSCs differentiated into chondrocyte-like cells that produced cartilage-like matrix after 14 days in culture as suggested by alcian blue staining of sulfated glycosaminoglycans, a typical cartilage ECM molecule (Fig. 4A).
IV. CONCLUSION
In situ chondrogenesis by controlled delivery of TGFβ3 may significantly decrease laboratory fabrication time of chondrogenic constructs for the treatment of cartilage degeneration. Severe cases of cartilage degradation as in osteoarthritis cause debilitating pain, thus, prompt treatment is necessary. The development of a controlled release engineered construct that enables post-implantation chondrogenesis may result in “off the shelf” technology ready for operating room use. Bone marrow progenitors may be readily isolated from patients and instantly mixed with the injectable controlled delivery gel system proposed in the present study. The autologous implant may then be injected back into the donor patient for cartilage repair requiring minimal laboratory manipulation.
Acknowledgments
Supported by NIH grants DE13964, DE15391 and EB02332 to J.J.M.
References
- 1.Hayes DW, Jr, Brower RL, John KJ. Articular cartilage. Anatomy, injury, and repair. Clin Podiatr Med Surg. 2001;18:35–53. [PubMed] [Google Scholar]
- 2.Buckwalter JA. Articular cartilage injuries. Clin Orthop. 2002;402:21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61:84–86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon L, Warwick DJ, Nayagam S. Apley’s System of Orthopaedics and Fractures. Oxford Univ. Press; New York: 2001. [Google Scholar]
- 5.Hangody L, Feczko P, Bartha L, Bodo G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop. 2001;S391:S328–336. doi: 10.1097/00003086-200110001-00030. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop. 2001;393:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad CS, Guiney WB, Drinkwater CJ. Evaluation of donor site intrinsic healing response in autologous osteochondral grafting of the knee. Arthroscopy. 2002;18:95–98. doi: 10.1053/jars.2002.25967. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Takahashi KA, Arai Y, Tonomura H, Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y, Kubo T. The therapeutic effects of basic fibroblast growth factor contained in gelatin hydrogel microspheres on experimental osteoarthritis in the rabbit knee. Arthritis Rheum. 2006 Jan;54(1):264–70. doi: 10.1002/art.21561. [DOI] [PubMed] [Google Scholar]
- 9.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 10.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 11.Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000 doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Jarry C, Chaput C, Chenite A, Renaud MA, Buschmann M, Leroux JCW. Effects of steam sterilization on thermogelling chitosan-based gels. J Biomed Mater Res. 2001;58(1):127–35. doi: 10.1002/1097-4636(2001)58:1<127::aid-jbm190>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]