Abstract
Recent data have shown that the proliferation and differentiation of the cranial base growth plate (CBGP) chondrocytes are modulated by mechanical stresses. However, little is known about the expression of genes and matrix molecules in the CBGP during development or under mechanical stresses. The objective of the present study was to determine whether several cartilage- and bone-related molecules are expressed in the CBGP and whether their expression is modulated by cyclic loading. The CBGP of normal 8-day-old rats (n=8) were isolated immediately after death, followed by extraction of total RNA and reverse transcription/polymerase chain reaction (RT-PCR) analysis. All studied genes, including type II and X collagens, biglycan, versican, osteocalcin, osteopontin, and fetal liver kinase 1, were expressed in the CBGP with a reproducible absence of decorin mRNA. In age- and sex-matched rats (n=10), exogenous cyclic forces were applied to the maxilla at 500 mN and 4 Hz for 20 min/day over 2 days, followed by RNA isolation and RT-PCR analysis. This exogenous cyclic loading consistently induced the expression of the decorin gene, which was non-detectable, by the current RT-PCR approach, in control neonatal CBGPs without loading. Immunolocalization of several of the above-studied gene products demonstrated their remarkable site-specific expression. Decorin proteoglycan was primarily expressed in the perichondrium instead of various cartilage growth zones, especially upon mechanical loading. These findings serve as baseline data for the expression of several genes and gene products in the neonatal CBGP. Mechanical modulation of decorin expression is consistent with recent reports of its susceptibility to mechanical loading in several connective tissues.
Keywords: Growth plate, Cartilage, Chondrocyte, Decorin, Bone, Rat (Sprague Dawley)
Introduction
Longitudinal growth can take place because of the presence of growth plates in the appendicular skeleton. During early postnatal development, the secondary ossification center separates articular cartilage and growth plate cartilage (Rivas and Shapiro 2002). Although articular cartilage normally remains on the articulating end of long bones throughout life, growth plate cartilage is only present before the arrest of physical growth, which is indicated as the completion of stature increase in the appendicular skeleton (Rivas and Shapiro 2002; Martin and Buckwalter 2003). Our understanding of growth plates has come from developmental studies that describe the structure and biology of these plates at various levels of organization (Alini and Roughley 2001; van der Eerden et al. 2003; Mao and Nah 2004). Attention has only recently been paid to the role of mechanical stresses in the development of growth plates (Carter et al. 1998; Stevens et al. 1999). Several types of mechanical stresses, especially hydrostatic stress, have been proposed to modulate the development of growth plates (Carter et al. 1998; Stevens et al. 1999). Cyclic mechanical loading affects the metabolism of matrix macromolecules to the degree of microscopic lengthening or growth suppression of growth plates in appendicular and craniofacial skeletons (Ohashi et al. 2002; Stokes et al. 2002a; Wang and Mao 2002a,b; Robling et al. 2001). The proliferation and differentiation of growth plate chondrocytes are regulated by mechanical loading, especially those with rapid force oscillation over time (Stokes et al. 2002b; Wang and Mao 2002a,b; Robling et al. 2001). Type X collagen is upregulated by stretch-induced matrix deformation of chondrocytes (Wu and Chen 2000). Several genes are known to be expressed in growth plate and metaphyseal bone in the appendicular skeleton: decorin, aggrecan, biglycan, versican, osteopontin (OPN), osteocalcin (OC), osteonectin, bone sialoprotein, and alkaline phosphatase (Woodard et al. 1997; Alini and Roughley 2001). To our knowledge, however, there has been no report of gene and matrix expression in the cranial base growth plate (CBGP) during normal development.
The CBGP is located between the sphenoid and occipital bones and is the only growth plate in the cranial base that remains anabolically active up to adolescent age in humans (Mao and Nah 2004). As such, the CBGP or the spheno-occipital synchondrosis is primarily responsible for the growth of the cranial base. Lengthening of the rest of the craniofacial skeleton, such as cranial vault bones and facial bones, is facilitated by cranial sutures (Enlow 2000; Mao 2002). Because of the complexity and multiple articulations among craniofacial bones, the CBGP is also responsible for the positioning of all facial skeletal structures, such as the maxilla and mandible (Baume 1956; Friede et al. 1990; for a review, see Mao and Nah 2004). Premature arrest of the CBGP leads to visible craniofacial anomalies (Hoyte 1991; Rosenberg et al. 1997). Despite its importance in craniofacial development, little is known about the biology of the CBGP beyond its histological structure. Microscopically, the CBGP consists of the equivalent of two apparently typical appendicular growth plates merged at their reserve zones and exhibits bidirectional growth during endochondral ossification (Mao and Nah 2004). Short doses of cyclic mechanical forces induce anabolic responses of the CBGP observed as histomorphometric changes in growth plate width and area (Wang and Mao 2002a,b). Bromodeoxyuridine-labeled chondrocytes increase their proliferation rates upon cyclic loading, similar to observations in appendicular growth plates (Farnum et al. 2000; Stokes et al. 2002a; Farnum et al. 2003; Wang and Mao 2002b). However, little is known about the gene and matrix expression in the CBGP under mechanical loading. The mechanical properties of the CBGP have recently been investigated (Allen and Mao 2004; Radhakrishnan et al. 2004) and are comparable with those of appendicular growth plates (Cohen et al. 1998), indicating the capacity of the CBGP to withstand mechanical stresses. The objective of the present study has been to determine the expression of several genes and gene products in the CBGP during early postnatal development and upon known doses of mechanical stresses.
Materials and methods
Animal model and tissue harvest
Eighteen 8-day-old postnatal (P8) normal male Sprague-Dawley rats were randomly allocated to the control group (n=8) or the mechanical loading group (n=10). Rats at P8 were used because we were interested in the expression of genes in the CBGP at an early postnatal age. The slightly greater sample size for the loaded group (10) than the control group (8) was attributable to the slightly higher intrinsic variance associated with experimental manipulation. The normal control rats were housed for two consecutive days and then killed by pentobarbital overdose (100 mg/kg). This animal research was approved by the Institutional Animal Care Committee.
Reverse transcription/polymerase chain reaction and gel electrophoresis
The entire CBGP with a small amount of adjacent subchondral bone was harvested under aseptic and RNA-free conditions. Total RNA was extracted separately from each of the harvested CBGP samples with TRIzol (Life Technologies, Gaithersburg, Md.) according to the manufacturer’s specifications. The RNA concentration was quantified by measuring spectrophotometric light absorbance at a wavelength of 260 nm. Reverse transcription/polymerase chain reaction (RT-PCR; RNA PCR core kit, Applied Biosystems) was performed to identify mRNA expression of type II and X collagens, decorin, biglycan, versican, OC, OPN, and fetal liver kinase 1 (Flk-1; Table 1). We used MuLV reverse transcriptase for RT and Taq polymerase for DNA polymerization. The mRNAs of decorin, biglycan, versican, type II collagen, and type X collagen were selected as in our previous study (Wang and Mao 2002a,b). The oligonucleotide primers were deduced from published gene sequences (Table 1). DNA amplification was performed in the presence of 1 mM MgCl2 with an initial denaturation temperature of 94°C for 1 min for biglycan, decorin, OPN, and Flk-1, for 2 min for type II and X collagens, and for 5 min for versican, or 95°C for 15 s for OC, followed by 30 cycles of denaturation at 94°C (1 min; all genes), annealing at 50°C (Flk-1), 53°C (versican), 54°C (type X collagen, 56°C (OC, type II collagen), 57°C (OPN) for 1 min, or 55°C (biglycan, decorin) for 2 min, and extension at 72°C for 3 min. The amplified mRNA products were separated by electrophoresis in 1.5% agarose gels and photographed under UV light in the presence of ethidium bromide (FisherBiotech, Pittsburgh, Pa.). RT-PCR and gel electrophoresis were performed in triplicates for all specimens. The intensity of gel bands was normalized against the band for D-glyceraldehyde-3-phosphate dehydrogense (GAPDH; a housekeeping gene) by computerized image analysis.
Table 1.
Rat cartilage-related and bone-related genes and primer sequences
| Genes | Primer sequence | PCR size in bp (reference) |
|---|---|---|
| Biglycan | 5′-ACT TCA CCT TGG ATG ATG GGC TG-3′ | 325 (Pyke et al. 1997) |
| 5′-CCT TCT CAT GGA TCT TGG AGA TC-3′ | ||
| Type II collagen | 5′-GAA GCA CAT CTG GTT TGG AG-3′ | 448 (Handa et al. 2001) |
| 5′-TTG GGG TTG AGG GTT TTA CA-3′ | ||
| Type X collagen | 5′-ACA AAG AGC GGA CAG AGA CC-3′ | 442 (Handa et al. 2001) |
| 5′-AGA AGG ACG AGT GGA CAT AC-3′ | ||
| Decorin | 5′-TGA AGG ACT TGC ATA CCT TG-3′ | 771 (Yamada et al. 1999) |
| 5′-GTT ACT TGT AGT TCC CAA GT-3′ | ||
| Versican | 5′-GAC TAT GGC TGG CAC AA-3′ | 575 (Lemire et al. 1996) |
| 5′-GTC CTT TGG TAT GCA GA-3′ | ||
| Osteocalcin | 5′-CAG ACC TAG CAG ACA CCA TGA G -3′ | 416 (Lisignoli et al. 2001) |
| 5′-CGT CCA TAC TTT CGA GGC AG -3′ | ||
| Osteopontin | 5′-CTC GCG GTG AAA GTG GCT GA-3′ | 871 (Zohar et al. 1997) |
| 5′-GAC CTC AGA AGA TGA ACT CT-3′ | ||
| Fetal liver kinase 1 | 5′-GGG AAA GAC TAT GTT GGG -3′ | 490 (Yeh and Lee 1999) |
| 5′-ATC AAT CTT GAC CCC AGG-3′ |
Mechanical loading
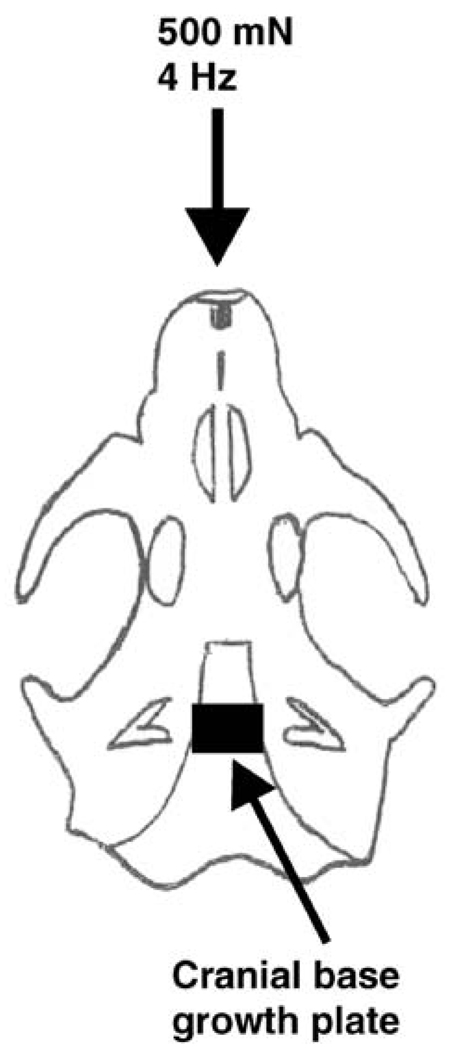
Cyclic mechanical loading was applied to 10 age- and sex-matched rats for 20 min/day over two consecutive days at 500 mN and 4 Hz via the rat maxilla. This cyclic loading parameter yielded a total of four cycles of mechanical stimulation per second. Under general anesthesia induced by an intramuscular injection of 100 mg/kg ketamine (100 mg/ml; Aveco, Fort Dodge, Iowa) and 5 mg/kg xylazine (20 mg/ml; Mobey, Shawnee, Kan.), the rats in the mechanical loading group were placed in a supine position with the premaxilla secured tightly to a custom-designed tissue loader, an electromechanical device capable of delivering cyclic forces under computer control (Almubarak et al. 2005; Collins et al. 2005). The rationale for the selection of the loading amplitude of 500 mN and frequency of 4 Hz was based on our previous studies in which gene expression changes were observed in cranial sutures (Almubarak et al. 2005; Collins et al. 2005). The direction of mechanical loading was along the mid-sagittal plane, whereas the point of application of the exogenous cyclic compressive forces was against the maxillary incisors, as illustrated in Fig. 1.
Fig. 1.
Representation of the rat cranial base growth plate (CBGP) showing the application of exogenous mechanical forces (cf. Almubarak et al. 2005; Collins et al. 2005). The direction of mechanical forces applied to the rat maxilla at 500 mN and 4 Hz for 20 min/day over two consecutive days is indicated (downward pointing arrow). The applied exogenous forces were transmitted as mechanical strain to bone adjacent to the CBGP (Oberheim and Mao 2002)
Histology and immunohistochemistry
The harvested control and mechanically loaded CBGP samples with subchondral bone on both the sphenoid and occipital sides were fixed in 10% paraformaldehyde, decalcified in 0.5 M EDTA solution, and paraffin-embedded by using standard histologic procedures. Sequential sections were stained with either hematoxylin and eosin (H&E) or safranin-O/fast green (Sigma, St. Louis, Mo.). Immunohistochemical procedures were similar to those described in detail elsewhere (Mao et al. 1998; Alhadlaq and Mao 2005; Sundaramurthy and Mao 2006). Serial consecutive sections adjacent to those used for histologic examination were deparaffinized, washed in phosphate-buffered saline (PBS), and digested for 30 min at room temperature with bovine testicular hyaluronidase (1,600 U/ml) in sodium acetate buffer (pH 5.5) with 150 mM NaCl. Sections were treated with 5% bovine serum albumin for 20 min at room temperature to block nonspecific reactions. The antibodies used were as follows: anti-decorin (LF-113, kindly provided by Dr. Larry W. Fisher), antibodies to type II and X collagens and antibodies to versican, OC, and OPN for immunohistochemical analysis were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, Iowa). Type X collagen was immunolocalized with monoclonal antibody X-AC9 (1:2). Versican was immunolocalized with monoclonal antibody 12C5. OPN was immunolocalized with monoclonal antibody MPIIIB10 (1:2). After overnight incubation with the primary antibody in a humidity chamber, sections were rinsed with PBS and incubated with biotinylated anti-mouse IgG secondary antibody (1:500; Antibodies, Davis, Calif.) for 30 min. Sections were then incubated with streptavidin/horseradish peroxidase conjugate for 30 min in a humidity chamber. After washes in PBS, the double-linking procedure with the secondary antibody was repeated. Slides were developed with diaminobenzidine solution and counterstained with Mayer’s hematoxylin for 3–5 min. Counterstained slides were dehydrated in graded ethanol and cleared in xylene. The same procedures were performed for negative controls, except for the omission of the primary antibodies.
Data analysis and statistics
The ratio of the GAPDH competimers to the target gene primers was used to compare the levels of expression between control and mechanically loaded samples. Upon confirmation of normal data distribution, Student’s t-test was employed to compare gene expression between control and mechanical loading groups. For skewed data distribution, the Mann-Whitney test was used. For all statistical analysis, P<0.05 was considered to be significant.
Results
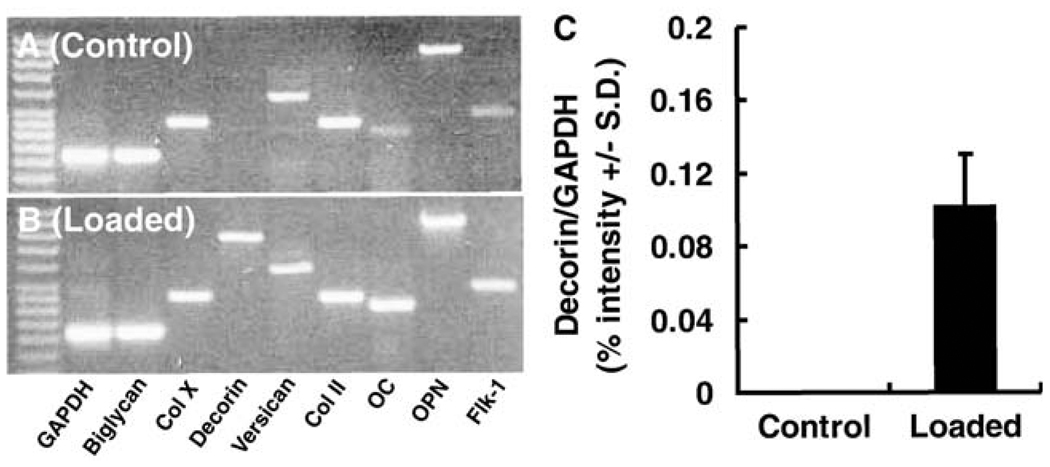
The representative mRNA expression of the studied genes in the control neonatal rat CBGP samples (without mechanical loading) is shown in Fig. 2a. Cartilage matrix-related genes, such as biglycan, type II and X collagens, and versican were expressed, but the decorin gene was non-detectable with the present RT-PCR approach (Fig. 2a); this was confirmed in three separate experiments in each rat. Bone matrix-related genes such as OC and OPN, together with an angiogenesis-related gene, Flk-1, were also expressed in controls (Fig. 2a).
Fig. 2.
mRNA expression of cartilage- and bone-related genes in CBGP at postnatal day 8. a Representative gel image showing the expression of biglycan, type X collagen (Col X), versican, type II collagen (Col II), osteocalcin (OC), osteopontin (OPN), and fetal liver kinase 1 (Flk-1; an angiogenesis-related gene). Note the absence of decorin gene expression in control CBGP samples without mechanical loading. b Representative gel image showing the expression of the decorin gene (non-detectable in a), together with the expression of biglycan, type X collagen (Col X), versican, type II collagen (Col II), OC, OPN, and Flk-1. c Semiquantitative analysis of luminosity of the average intensity of gel bands of decorin gene expression (ratio to GAPDH expression) in control (n=8) and mechanically loaded (n=10) samples showing the average intensity of decorin expression in mechanically loaded samples (0.10±0.03), in contrast to the lack of decorin expression in age- and sex-matched control samples
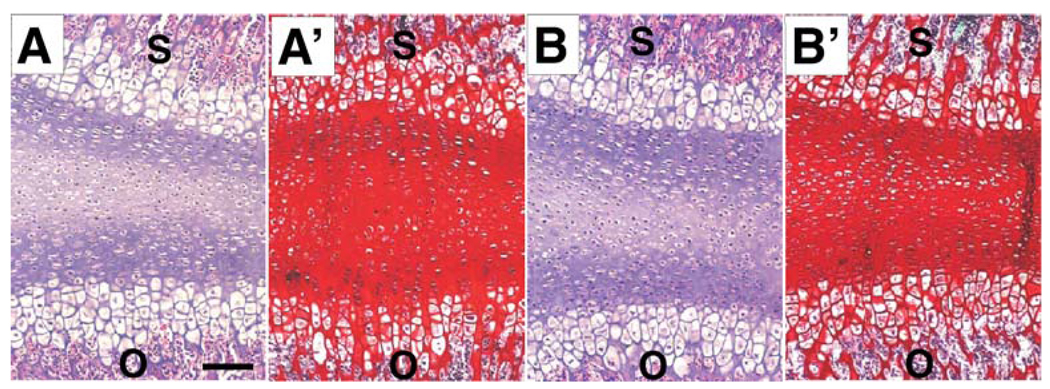
Immunohistochemical data demonstrated the location of some of these gene products in the CBGP. In comparison with an absence of decorin mRNA expression in the control CBGPs, decorin mRNA was expressed in the age- and sex-matched samples upon application of short doses of cyclic loading at 500 mN and 4 Hz for 20 min/day over two consecutive days, as shown in Fig. 2b. Semi-quantitative luminosity analysis of gel bands revealed that the average intensity of decorin mRNA expression in mechanically loaded samples was 0.10±0.03 (n=10), in contrast to a lack of decorin mRNA expression in the age- and sex-matched control samples (n=8; Fig. 2c). Other genes that were expressed in control samples including biglycan, type II and X collagens, versican, OC, OPN, and Flk-1 were also expressed in the mechanically loaded CBGP samples as exemplified in Fig. 2b. Representative microscopic sections of control and mechanically loaded CBGP samples stained with H&E each revealed the equivalent of two apparently typical appendicular growth plates with their reserve zones merged in the center (Fig. 3a,b). No substantial changes in growth plate structure were detected between the control samples (Fig. 3a) and mechanically loaded samples (Fig. 3b), probably because of the short doses of mechanical loading (20 min/days over 2 days). Safranin O and fast green counterstaining demonstrated abundant chondroitin sulfate and keratan sulfate proteoglycans in the CBGP in both control and mechanically loaded CBGP samples (Fig. 3a’,b’).
Fig. 3.
Histological examination of CBGP samples with and without mechanical loading. Representative H&E image of a control CBGP (a) and a mechanically loaded CBGP (b) showing the equivalent of two appendicular growth plates with their reserve zones merged together. The sphenoid (s) and occipital (o) bones are labeled for orientation. Representative safranin O and fast green images show abundant chondroitin sulfate and keratan sulfate proteoglycans in the CBGP in a control CBGP sample (a’) and a mechanically loaded CBGP sample (b’). Bar 50 µm
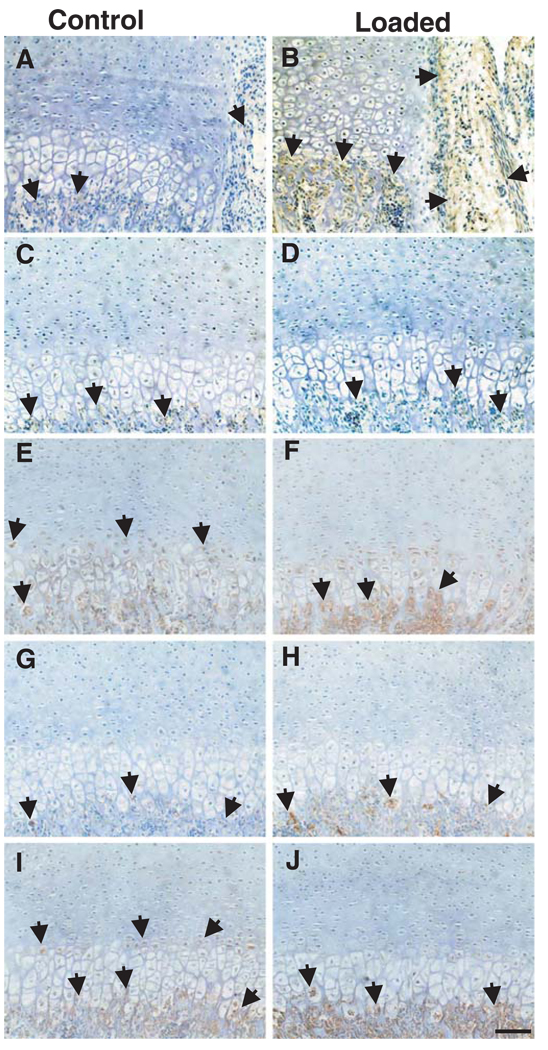
Immunolocalization of several gene products in the CBGP and subchondral bone with and without mechanical loading is shown in Fig. 4. Decorin expression upon mechanical loading was marked in the perichondrium and subchondral bone adjacent to perichondrium (arrows in Fig. 4b), in comparison to the control sample (arrows in Fig. 4a). However, little decorin expression was present in various zones of the growth plate or subchondral bone in areas away from the perichondrium in either the control or mechanically loaded CBGP sample (arrows in Fig. 4c,d, respectively). Type X collagen, a typical chondrocyte hypertrophy marker, was expressed primarily in the hypertrophic zones of both control and mechanically loaded CBGP samples (arrows in Fig. 4e,f). Versican was minimally present in the subchondral bone of the control sample (Fig. 4g) but was abundantly expressed in the subchondral bone of the mechanically loaded sample (Fig. 4h). OPN, a typical osteogenesis marker, was immunolocalized primarily in the subchondral bone in both control and mechanically loaded CBGP samples (arrows in Fig. 4i,j, respectively).
Fig. 4.
Immunolocalization of selected bone- and cartilage-related markers in the CBGP. a Representative minimum immunolocalization of decorin, with expression in the subchondral bone and perichondrium of a control CBGP sample (arrows). b In contrast, decorin expression was marked in the perichondrium and subchondral bone adjacent to perichondrium of a mechanically loaded CBGP sample (arrows). c, d Little decorin expression was present in either the growth plate or subchondral bone in areas away from the perichondrium in either control and mechanically loaded CBGP samples (arrows in c, d, respectively). e Immunolocalization of type X collagen, a typical chondrocyte hypertrophy marker, primarily in the hypertrophic zones of a control CBGP sample (arrows). f Expression of type X collagen in the hypertrophic zones of a mechanically loaded CBGP sample (arrows). g, h Versican was immunolocalized in the subchondral bone of both control and mechanically loaded CBGP samples (arrows in g, h, respectively). i, j Immunolocalization of OPN, a typical osteogenesis marker, primarily in the subchondral bone in both control and mechanically loaded CBGP samples (arrows in i, j, respectively). Bar 50 µm
Discussion
The present work demonstrates that several typical cartilage- and bone-related genes and gene products are expressed in the neonatal CBGP: type II and X collagens, biglycan, versican, OC, and OPN, together with an angiogenesis-related gene, Flk-1. The most surprising findings are (1) the absence of the product of the decorin gene in the normal 8-day-old rat CBGP, and (2) the mechanically induced expression of the decorin gene upon short doses of cyclic forces. Decorin is a small proteoglycan present widely in the extracellular matrix of connective tissues. The absence of decorin gene expression in the normal rat CBGP at P8 may be related to several previous findings (see below). Decorin is probably a modulator of collagen fibrillogenesis (Mochida et al. 2003; Redaelli et al. 2003). In immature connective tissue structures, such as the 8-day-old growth plate cartilage of rat, decorin is considered to act as a linker between collagen fibrils in serial orientation (Redaelli et al. 2003).
The potential involvement of decorin in mechanotransduction is consistent with several previous findings. For instance, the breakdown of small collagen fibrils by mechanical stresses is accompanied by an increased expression of decorin gene and other cartilage-related genes (Wang and Sanders 2003). A catabolic fragment of decorin, named decorunt, lacks the binding affinity to type I collagen (Carrino et al. 2003). Tail tendons demonstrate larger and faster stress relaxation properties in decorin knockout mice (Elliott et al. 2003; Robinson et al. 2004). Annulus fibrosus cells encapsulated in alginate gel increase their decorin mRNA expression upon unconfined compression (Chen et al. 2004), although cells of the meniscus decrease their decorin mRNA expression upon both static and dynamic compressions (Upton et al. 2003). Decorin mRNA expression is higher in non-attached scars than in attached scars (Lo et al. 2003). Bronchial fibroblasts increase their decorin mRNA expression upon mechanical stretch (Ludwig et al. 2004). The immunolocalization of decorin in the present work demonstrates decorin expression markedly in the perichondrium and adjacent subchondral bone, instead of in various zones of growth plate cartilage. The significance of the distribution of decorin warrants further investigation concerning the speculative involvement of decorin in mechanotransduction. The recent demonstration of binding forces between decorin core protein and collagen fibrils in the range of 12.4×103 nN is another indication of the potential involvement of decorin in mechanically activated pathways (Vesentini et al. 2005). Mechanically induced structural changes in the extracellular matrix may influence chondrocyte behavior via trans-membrane coupling, e.g., integrin-mediated mechanotransduction pathways (Loeser 2002; Mobasheri et al. 2002; Millward-Sadler and Salter 2004).
The immunolocalization of several gene products reveals their characteristic distribution patterns. The expression of type X collagen, a typical chondrocyte hypertrophy marker, in hypertrophic and degenerative chondrocytes correlates with its mRNA expression in both control and mechanically loaded samples and is consistent with previous findings (Barry et al. 2001). In addition to type X collagen, other gene products such as versican and OPN primarily occur in the degenerating chondrocyte layer of the CBGP. Osteogenesis-related genes, such as OC, OPN, and the angiogenesis-related gene, Flk-1, are also expressed in the subchondral bone of the P8 cranial-base growth plate. The presence of OPN, OC, and Flk-1 indicate the commencement of active bone formation (Kobayashi et al. 1999). The qualitative increases in the expression of both the OPN and Flk-1 genes may have implications for future studies. OPN is an osteogenesis marker (Woodard et al. 1997), and its apparent increase is probably related to enhanced osteogenesis by mechanical loading (Morinobu et al. 2003). The qualitatively increased expression of Flk-1 is possibly related to the potential enhancement of angiogenesis in the perichondium and/or the subchondral bone. Since neither OPN nor Flk-1 have been shown to be expressed in the CBGP before, and since the presently reported increases are qualitative instead of quantitative, caution should be used in the interpretation of the mechanical enhancement of their expression.
The present experiments can be improved in a number of ways. First, it is unknown whether a catabolic fragment of decorin, decorunt, is expressed in the neonatal CBGP in the absence of decorin mRNA. A follow-up experiment may illustrate whether these two isoforms of decorin, decorunt and decorin, have separate functional roles in the CBGP (Carrino et al. 2003). Second, only one specific level of mechanical stress and frequency has been used in this study. The presently observed gene and matrix expression patterns may change if another mechanical stress parameter is used. In reference to mechanical loading of calvarial bone and sutures, mechanical stresses at various levels induce different patterns of molecular responses (Mao 2005). Third, extensive biochemical analysis can be performed on harvested CBGP samples, although this undoubtedly requires additional experiments. Within these constraints, the present data represent the first identification of the expression of cartilage- and bone-related genes and matrix molecules in the CBGP. Consistent with several recent reports (Elliott et al. 2003; Chen et al. 2004; Robinson et al. 2004), decorin appears to be involved in mechanotransduction and to have important roles during both normal development and upon mechanical modulation of chondral growth and development. Nonetheless, additional work is warranted to demonstrate the role of decorin in mechanotransduction pathways.
Acknowledgements
Aurora Lopez and John Collins are gratefully acknowledged for their technical assistance. We thank Dr. Larry Fisher of NIDCR for generously providing the decorin antibody. Valuable comments from two anonymous reviewers helped improve the quality of our manuscript.
This research was supported by USPHS Research Grants EB02332 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and DE13964 and DE15391 from the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health.
References
- Alhadlaq A, Mao JJ. Tissue engineered osteochondral constructs in the shape of articular condyle. J Bone Joint Surg Am. 2005;11:556–566. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- Alini M, Roughley PJ. Changes in leucine-rich repeat proteoglycans during maturation of the bovine growth plate. Matrix Biol. 2001;19:805–813. doi: 10.1016/s0945-053x(00)00129-3. [DOI] [PubMed] [Google Scholar]
- Allen DM, Mao JJ. Heterogeneous nanostructural and nanoelastic properties of pericellular and interterritorial matrices of chondrocytes by atomic force microscopy. J Struct Biol. 2004;145:196–204. doi: 10.1016/j.jsb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Almubarak R, Da Silveira A, Mao JJ. Expression of matrix metalloproteinase genes 1 and 2 in rat facial and cranial sutures. Cell Tissue Res. 2005;321:465–471. doi: 10.1007/s00441-005-1136-2. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Baume LJ. Tooth and investing bone: a developmental entity. J Oral Surg. 1956;9:736–741. doi: 10.1016/0030-4220(56)90250-x. [DOI] [PubMed] [Google Scholar]
- Carrino DA, Onnerfjord P, Sandy JD, Cs-Szabo G, Scott PG, Sorrell JM, Heinegard D, Caplan AI. Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem. 2003;278:17566–17572. doi: 10.1074/jbc.M300124200. [DOI] [PubMed] [Google Scholar]
- Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop. 1998;355(Suppl):S41–S55. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–583. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Cohen B, Lai WM, Mow VC. A transversely isotropic biphasic model for unconfined compression of growth plate and chondroepiphysis. J Biomech Eng. 1998;120:491–496. doi: 10.1115/1.2798019. [DOI] [PubMed] [Google Scholar]
- Collins JM, Ramamoorthy K, Silvereira AD, Patson P, Mao JJ. Expression of matrix metalloproteinase genes in the rat intramembraneous bone during postnatal growth and upon mechanical stresses. J Biomech. 2005;38:485–492. doi: 10.1016/j.jbiomech.2004.04.018. [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31:599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- Enlow DH. Normal craniofacial growth. In: Cohen MMJ, Mac-Lean RE, editors. Craniosynostosis: diagnosis, evaluation, and management. 2nd edn. New York: Oxford University Press; 2000. pp. 35–50. [Google Scholar]
- Farnum CE, Nixon A, Lee AO, Kwan DT, Belanger L, Wilsman NJ. Quantitative three-dimensional analysis of chondrocytic kinetic responses to short-term stapling of the rat proximal tibial growth plate. Cells Tissues Organs. 2000;167:247–258. doi: 10.1159/000016787. [DOI] [PubMed] [Google Scholar]
- Farnum CE, Lee AO, O’Hara K, Wilsman NJ. Effect of short-term fasting on bone elongation rates: an analysis of catch-up growth in young male rats. Pediatr Res. 2003;53:33–41. doi: 10.1203/00006450-200301000-00009. [DOI] [PubMed] [Google Scholar]
- Friede H, Alberius P, Lilja J, Lauritzen C. Trigonocephaly: clinical and cephalometric assessment of craniofacial morphology in operated and nontreated patients. Cleft Palate J. 1990;27:362–367. doi: 10.1597/1545-1569(1990)027<0362:tcacao>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Handa K, Solchaga LS, Caplan AI, Hering TM, Goldberg VM, Yoo JU, Johnstone B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81:284–294. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hoyte DA. The cranial base in normal and abnormal skull growth. Neurosurg Clin North Am. 1991;2:515–537. [PubMed] [Google Scholar]
- Kobayashi ET, Hashimoto F, Kobayashi Y, Sakai E, Miyazaki Y, Kamiya T, Kobayashi K, Kato Y, Sakai H. Force-induced rapid changes in cell fate at midpalatal suture cartilage of growing rats. J Dent Res. 1999;78:1495–1504. doi: 10.1177/00220345990780090301. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Potter-Perigo S, Hall KL, Wight TN, Schwartz SM. Distinct rat aortic smooth muscle cells differ in versican/PG-M expression. Arterioscler Thromb Vasc Biol. 1996;16:821–829. doi: 10.1161/01.atv.16.6.821. [DOI] [PubMed] [Google Scholar]
- Lisignoli G, Zini N, Remiddi G, Piacentini A, Puggioli A, Trimarchi C, Fini M, Maraldi NM, Facchini A. Basic fibroblast growth factor enhances in vitro mineralization of rat bone marrow stromal cells grown on non-woven hyaluronic acid based polymer scaffold. Biomaterials. 2001;22:2095–2105. doi: 10.1016/s0142-9612(00)00398-7. [DOI] [PubMed] [Google Scholar]
- Lo IKH, Marchuk L, Hart DA, Frank CB. Messenger ribonucleic acid levels in disrupted human anterior cruciate ligaments. Clin Orthop Relat Res. 2003;407:249–258. doi: 10.1097/00003086-200302000-00034. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Integrins and cell signaling in chondrocytes. Biorheology. 2002;39:119–124. [PubMed] [Google Scholar]
- Ludwig MS, Ftouhi-Paquin N, Huang W, Page N, Chakir J, Hamid Q. Mechanical strain enhances proteoglycan message in fibroblasts from asthmatic subjects. Clin Exp Allergy. 2004;34:926–930. doi: 10.1111/j.1365-2222.2004.01980.x. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Mechanobiology of craniofacial sutures. J Dent Res. 2002;81:810–816. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Calvarial development: cells and mechanics. Curr Opin Orthop. 2005;16:331–337. [Google Scholar]
- Mao JJ, Nah H-D. Growth and development: hereditary and mechanical modulations. Am J Orthod Dentofac Orthop. 2004;125:676–689. doi: 10.1016/j.ajodo.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Rahemtulla F, Scott PG. Proteoglycan expression in articular tissues of the rat temporomandibular joint in response to bite raise. J Dent Res. 1998;77:1520–1528. doi: 10.1177/00220345980770070701. [DOI] [PubMed] [Google Scholar]
- Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85(A Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26:1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- Mochida Y, Duarte WR, Tanzawa H, Paschalis EP, Yamauchi M. Decorin modulates matrix mineralization in vitro. Biochem Biophys Res Commun. 2003;305:6–9. doi: 10.1016/s0006-291x(03)00693-4. [DOI] [PubMed] [Google Scholar]
- Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, Denhardt DT, Noda M. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res. 2003;18:1706–1715. doi: 10.1359/jbmr.2003.18.9.1706. [DOI] [PubMed] [Google Scholar]
- Oberheim MC, Mao JJ. Bone strain patterns of the zygomatic complex in response to simulated orthopedic forces. J Dent Res. 2002;81:608–612. doi: 10.1177/154405910208100906. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. 2002;17:284–292. doi: 10.1359/jbmr.2002.17.2.284. [DOI] [PubMed] [Google Scholar]
- Pyke C, Kristensen P, Østergaard PB, Oturai PS, Rømer J. Proteoglycan expression in the normal rat kidney. Nephron. 1997;77:461–470. doi: 10.1159/000190325. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P, Lewis NW, Mao JJ. Zone-specific micromechanical properties of the intercellular matrices of growth plate cartilage. Ann Biomed Eng. 2004;32:284–291. doi: 10.1023/b:abme.0000012748.41851.b4. [DOI] [PubMed] [Google Scholar]
- Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons—a computational study from molecular to micro-structural level. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Rivas R, Shapiro F. Structural stages in the development of the long bones and epiphyses: a study in the New Zealand white rabbit. J Bone Joint Surg Am. 2002;84:85–100. doi: 10.2106/00004623-200201000-00013. [DOI] [PubMed] [Google Scholar]
- Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Arlis HR, Haworth RD, Heier L, Hoffman L, LaTrenta G. The role of the cranial base in facial growth: experimental craniofacial synostosis in the rabbit. Plast Reconstr Surg. 1997;99:1396–1407. doi: 10.1097/00006534-199704001-00030. [DOI] [PubMed] [Google Scholar]
- Stevens SS, Beaupre GS, Carter DR. Computer model of endochondral growth and ossification in long bones: biological and mechanobiological influences. J Orthop Res. 1999;17:646–653. doi: 10.1002/jor.1100170505. [DOI] [PubMed] [Google Scholar]
- Stokes DG, Liu G, Coimbra IB, Piera-Velazquez S, Crowl RM, Jimenez SA. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002a;46:404–419. doi: 10.1002/art.10106. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Mente PL, Iatridis JC, Farnum CE, Aronsson DD. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am. 2002b;84A:1842–1848. doi: 10.2106/00004623-200210000-00016. [DOI] [PubMed] [Google Scholar]
- Sundaramurthy S, Mao JJ. Modulation of endochondral development of the distal femoral condyle by mechanical loading. J Orthop Res. 2006 doi: 10.1002/jor.20024. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Vesentini S, Redaelli A, Montevecchi FM. Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J Biomech. 2005;38:433–443. doi: 10.1016/j.jbiomech.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Wang X, Mao JJ. Accelerated chondrogenesis of the rabbit cranial base growth plate by oscillatory mechanical stimuli. J Bone Miner Res. 2002a;17:1843–1850. doi: 10.1359/jbmr.2002.17.10.1843. [DOI] [PubMed] [Google Scholar]
- Wang X, Mao JJ. Chondrocyte proliferation of the cranial base cartilage upon in vivo mechanical stresses. J Dent Res. 2002b;81:701–705. doi: 10.1177/154405910208101009. [DOI] [PubMed] [Google Scholar]
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29–35. doi: 10.1016/s0306-9877(03)00100-2. [DOI] [PubMed] [Google Scholar]
- Woodard JC, Donovan GA, Fisher LW. Pathogenesis of vitamin (A and D)-induced premature growth-plate closure in calves. Bone. 1997;21:171–182. doi: 10.1016/s8756-3282(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Wu QQ, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res. 2000;256:383–391. doi: 10.1006/excr.2000.4847. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kamiya N, Harada D, Takagi M. Effects of transforming growth factor-beta1 on the gene expression of decorin, biglycan, and alkaline phosphatase in osteoblast precursor cells and more differentiated osteoblast cells. Histochem J. 1999;31:687–694. doi: 10.1023/a:1003855922395. [DOI] [PubMed] [Google Scholar]
- Yeh L-C, Lee JC. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol Cell Endocrinol. 1999;153:113–124. doi: 10.1016/s0303-7207(99)00076-3. [DOI] [PubMed] [Google Scholar]
- Zohar R, Lee W, Arora P, Cheifetz A, Mcculloch C, Sodek J. Single cell analysis of intracellular osteopontin in osteogenic cultures of fetal rat calvarial cells. J Cell Physiol. 1997;170:88–100. doi: 10.1002/(SICI)1097-4652(199701)170:1<88::AID-JCP10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]