Abstract
The present study was designed to explore an ex vivo culturing protocol for fibroblastic differentiation of human mesenchymal stem cells (hMSCs) using connective tissue growth factor (CTGF). Fibroblastic differentiation from stem cells is of widespread significance in the engineering of virtually all tissues including tendons, ligaments, periodontal ligament, cranial sutures and as interstitial filler of all organs. The treatment with 100 ng/ml of recombinant human CTGF and 50 μg/ml ascorbic acids on monolayer cultured hMSCs showed significant increases in type I collagen and tenascin-C (Tn-C) contents by 2 and 4 wks. In addition, CTGF-treated hMSCs failed to show osteogenic or chondrogenic differentiation. The present data show that CTGF is an effective induction factor for fibroblastic differentiation of hMSCs. These findings have implications for engineering fibrous tissue by providing the initial evidence of a reproducible protocol for fibroblastic differentiation of hMSCs.
I. Introduction
Bone marrow derived mesenchymal stem cells (MSCs) can differentiate into multiple cell lineages such as fibroblasts, osteoblasts, chondrocytes, and adipocytes [1]. However, differentiation of human MSC (hMSCs) into fibroblasts has been assumed to be the default pathway, yet with little experimental evidences. Altman et al. [2] and Noth et al. [3] showed that hMSCs are differentiated into ligament fibroblasts by cyclic mechanical stimulation. Also, it was reported that various growth factors and their combinations could promote the differentiation of hMSCs into fibroblasts [4], [5]. Nonetheless, the evidences of chemical induction factors are still isolated in comparison with those for other differentiating lineages. Thus, further systematic information on chemical factors inducing fibroblastic differentiation of hMSCs is still required. The present study was designed to explore an ex vivo culturing protocol for fibroblastic differentiation of hMSCs. Establishment of a reliable approach to induce ex vivo differentiation of hMSCs into fibroblasts has widespread implications in the tissue engineering of tendons, ligaments, cranial sutures, fascia, periosteum and any interstitial fibrous tissue [6], [7]. We selected connective tissue growth factor (CTGF) as the induction factor for fibroblastic differentiation of hMSCs on the basis of previous evidence that CTGF promotes fibroblasts proliferation and matrix formation in vitro [8]. CTGF, a CCN family growth factor, is also known to be implicated in physiological processes related to embryo development and differentiation [9]. Our findings in this study provide the initial and yet original evidence of a reproducible protocol for fibroblastic differentiation of hMSCs that are capable of differentiating into osteoblasts and chondrocytes. The hMSC-derived fibroblasts expressed a number of different molecular markers than their parent cells (hMSCs).
II. Materials and Methods
A. Isolation and Culture-expansion of hMSCs
Human bone marrow samples taken from healthy donors ranging from 18 to 45 years of age were purchased from AllCells (Berkeley, CA). The bone marrows were prepared to isolate hMSCs per our previous methods [10], [11]. Briefly, the whole bone marrow sample was incubated with mesenchymal cell enrichment cocktail (RosetteSep™; StemCell Technologies, Inc., Vancouver, Canada). After incubation, the marrow sample was diluted with PBS containing 2% FBS and 1 mM EDTA. Diluted sample was then layered on top of a density gradient solution (Ficoll-Paque®; StemCell Technologies, Inc., Vancouver, Canada). Following centrifugation, enriched cells were removed and resuspended in cell culture media consisting of 89% DMEM, 10% FBS, and 1% penicillin–streptomycin (basal cell culture media). Cells were passaged up to two times each time upon confluency. Upon the second passage, the isolated hMSCs were grown in monolayer (5,000 cells/well) in 12-well culture plates in basal cell culture media, with fresh medium change every 3–4 days.
B. Treatment with Connective Tissue Growth Factor
Upon near confluence, hMSCs were treated with DMEM supplemented with 0 and 100 ng/ml of recombinant human CTGF (BioVendor, Candler, NC) and 50 μg/ml ascorbic acid, with conditioned medium change every third day. Two to 4 wks following CTGF and ascorbic acid treatment, the following molecular markers were assayed by ELISA: collagen type I (Chondrex, Redmond, WA) and tenascin-C (Tn-C; IBL-America, Minneapolis, MN), selected markers for ligament fibroblasts [2]. The lysis of the selected extracellular matrix was performed using 0.5 M acetic acid solution. Collagen deposition was visualized using Trichrome staining.
C. Osteogenic and Chondrogenic Differentiation
hMSCs were separately induced to differentiate into osteoblasts and chondrocytes to serve as controls for the above-described fibroblastic differentiation. Chondrogenic medium contained a supplement of 10 ng/ml TGF-β3 (R&D Systems Inc., Minneapolis, MN), whereas osteogenic medium contained 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate (Sigma-Aldrich, St. Lois, MO) per our prior methods with osteogenic or chondrogenic supplemented medium [10], [11]. At Days 14 and 28, von Kossa staining and calcium content assay (Calcium reagents, Raichem, Columbia, MD) were performed to evaluate osteogenic differentiation, whereas safranin O straining and glycosaminoglycan (GAG) assay were performed to evaluate chondrogenic differentiation (Blyscan™, Biocolor, UK).
III. Results and Discussion
A. Collagen and Tenascin-C Synthesis Increases When hMSCs Are Treated With CTGF
Exposure of 100 ng/ml recombinant human CTGF and 50 μg/ml ascorbic acid to hMSCs induced remarkable increases in collagen deposition in 4 wks as revealed by Trichrome straining (Fig. 1B), in comparison with hMSCs without CTGF and ascorbic acid supplements. These qualitative data were substantiated by quantitative analysis of collagen content. By 2 wks, collagen content of hMSCs treated with 100 ng/ml of CTGF increased significantly by approx. 4.5 fold in comparison with hMSCs without CTGF (p<0.01). By 4 wks, this increase was approx. 9.5 fold (p<0.001) (Fig. 2). Tn-C content, another indication of fibroblastic differentiation, in hMSCs treated with CTGF was approx. 2 folds higher than hMSCs without CTGF by 2 wks (Fig. 3). By 4 wks of CTGF treatment, Tn-C content of hMSCs treated with CTGF remained approx. 1.8 folds higher than hMSCs without CTGF.
Fig. 1.
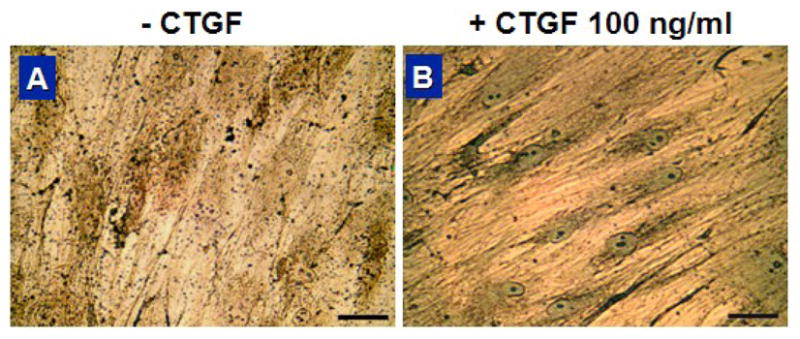
Trichrome staining showing marked collagen synthesis upon CTGF for 4 wks. Treatment with 100 ng/ml of CTGF induced an increase in collagen deposition (scale=50 μm).
Fig. 2.
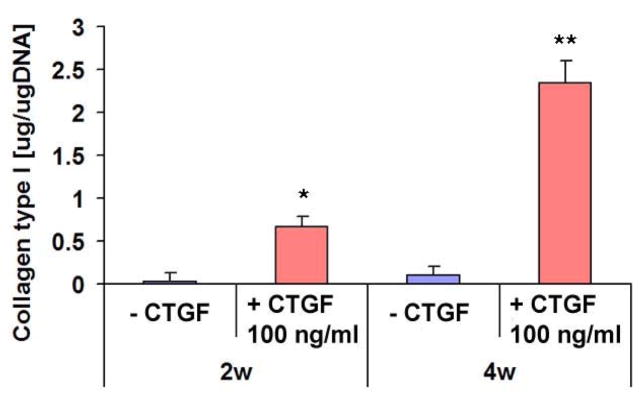
Collagen type I contents with or without CTGF treatment. Type I collagen contents in monolayer cultured hMSCs were significantly increased by the treatment with 100 ng/ml CTGF and 50 μg/ml ascorbic acid by 2 & 4 wks (n=3, *: p<0.01, **: p<0.001).
Fig. 3.
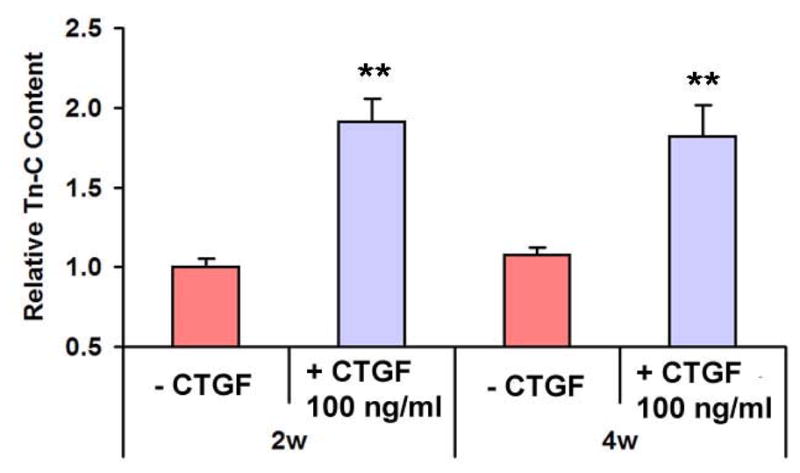
Tn-C contents with CTGF treatment. Tn-C contents, a maker for ligament fibroblasts, were significantly increased by the treatment with 100 ng/ml CTGF and 50 μg/ml ascorbic acid by 2 & 4wks (n=3, *: p<0.01, **: p<0.001).
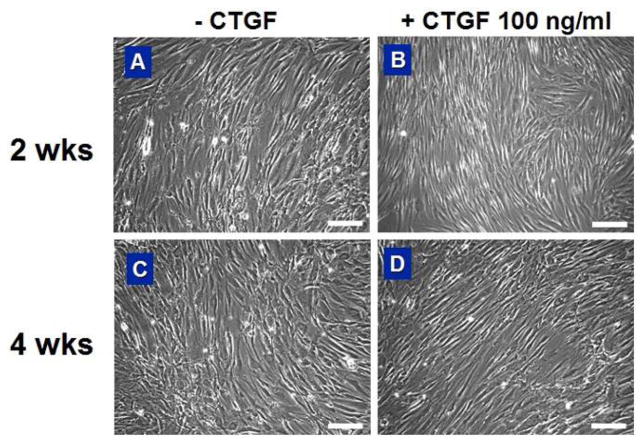
Thus, the parallel increases in type I collagen and Tn-C contents suggest that hMSCs treated by CTGF have differentiated into cells that synthesize abundant type I collagen even though there were no significant changes in cellular morphology (Fig. 4). However, one must at least rule out the possibility of osteogenic differentiation of these CTGF-treated hMSCs, given that osteoblasts also synthesize abundant type I collagen.
Fig. 4.
Morphology of hMSCs with or without CTGF treatment. Both hMSCs and CTGF-treated hMSCs showed fibroblast-like spindle shape. However, there were no significant differences in cellular morphology caused by CTGF-treatment by 2 & 4 wks (scale = 200 μm).
B. CTGF-Treated hMSCs Fail to Show Osteogenic or Chondrogenic Differentiation
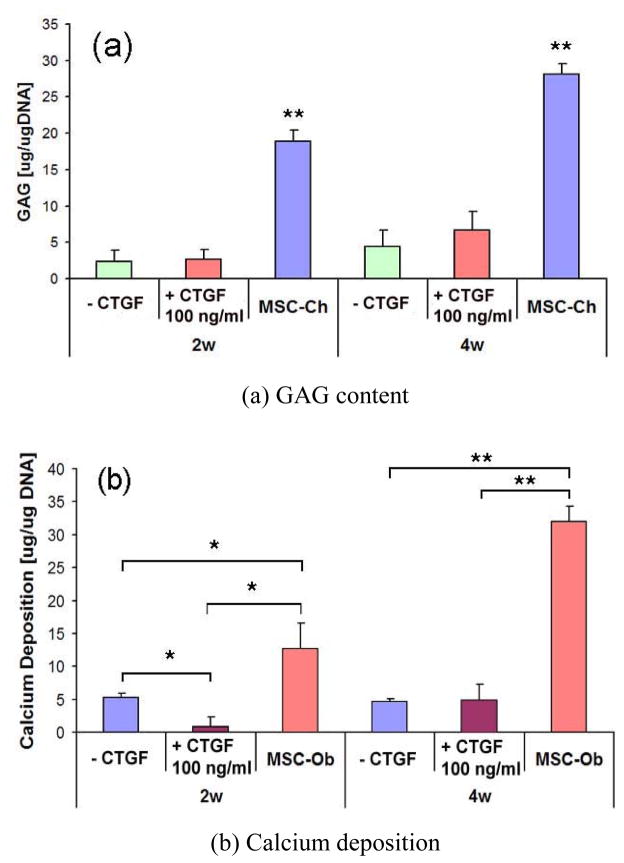
hMSCs cultured in chondrogenic or osteogenic supplemented medium showed corresponding chondrogenic or osteogenic differentiation (Fig. 5C and 6C, respectively). However, neither hMSCs nor hMSCs treated with 100 ng/ml of CTGF showed any positive label of chondrogenic or osteogenic differentiation (Fig. 5A, B and Fig. 6A, B). GAG content of hMSC-derived chondrocytes was significantly higher than hMSCs with or without CTGF (Fig. 7A). Calcium content of hMSC-derived osteoblasts was significantly higher than hMSCs with or without CTGF (Fig. 7B).
Fig. 5.
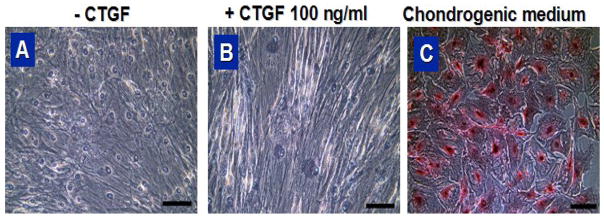
Safranin O staining of hMSCs treated with CTGF (a, b) and chondrogenic medium (c) for 4 wks (scale = 100 μm).
Fig. 6.
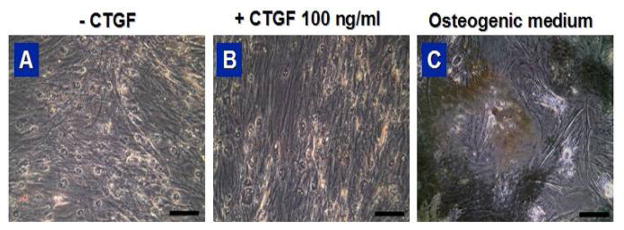
von Kossa staining of hMSCs treated with CTGF (a, b) and osteogenic medium (c) for 4 wks (scale = 100 μm).
Fig. 7.
GAG and calcium deposition in hMSCs monolayer treated with CTGF or corresponding differentiation medium. GAG content and calcium deposition were not affected by the treatment with 100 ng/ml of CTGF by 2 & 4 wks (n=3, *: p<0.01, **: p<0.001).
IV. Conclusion
In this study, CTGF appeared to be a potent stimulator for fibroblastic differentiation of human mesenchymal stem cells. CTGF increases Type I collagen and tenascin-C syntheses but do not induce hMSCs into chondrogenic or osteogenic lineages as evidence by little increase in the syntheses of chondrogenic and osteogenic matrix molecules. The presently demonstrated CTGF stimulation of fibroblastic differentiation of hMSCs is reproducible. Therefore, CTGF may be an essential factor in a reliable and reproducible protocol to induce ex vivo differentiation of hMSCs into fibroblasts. Ongoing studies will verify gene expressions as well as additional molecular markers.
Acknowledgments
This work was supported in part by the U.S. National Institute of Health under Grants DE13964, DE15391, and EB02332.
Contributor Information
Chang H. Lee, Email: chl2109@columbia.edu, Department of Orthodontics and Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60612 USA. He is now with the College of Dental Medicine and Department of Biomedical Engineering, Columbia University, New York, NY 10032 USA (phone: 212-305-9393
Eduardo K. Moioli, Email: emoiol1@uic.edu, Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60612 USA
Jeremy J. Mao, Email: jmao@columbia.edu, Department of Orthodontics and Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60612 USA. He is now with the College of Dental Medicine and Department of Biomedical Engineering, Columbia University, New York, NY 10032 USA
References
- 1.Caplan AI. Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1210. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 2.Altman GH, Horan RL, Martin I, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 3.Noth U, Schupp K, Heymer A, et al. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy. 2005;7:447–455. doi: 10.1080/14653240500319093. [DOI] [PubMed] [Google Scholar]
- 4.Moreau JE, Chen J, Bramono DS, et al. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164–174. doi: 10.1016/j.orthres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Hankemeier S, Keus M, Zeichen J, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41–49. doi: 10.1089/ten.2005.11.41. [DOI] [PubMed] [Google Scholar]
- 6.Butler DL, Shearn JT, Juncosa N, et al. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop Relat Res. 2004;427(Suppl):S190–S199. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- 7.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 8.Wang JF, Olson ME, Ball DK, et al. Recombinant connective tissue growth factor modulates porcine skin fibroblast gene expression. Wound Repair Regen. 2003;11:220–229. doi: 10.1046/j.1524-475x.2003.11311.x. [DOI] [PubMed] [Google Scholar]
- 9.Moussad EA, Brigstock DR. Connective tissue growth factor: what’s in a name? Mole Gen Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 10.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 11.Marion NW, Liang W, Reilly G, et al. Borate Glass Supports the In Vitro Osteogenic Differentiation of Human Mesenchymal Stem Cells. Mech Adv Mat Struct. 2005;12:1–8. [Google Scholar]