Abstract
Introduction
The benefit of an operation to remove the primary tumor among patients with synchronous stage IV colorectal cancer is controversial. This study analyzed the survival benefits associated with primary tumor resection among chemotherapy-treated stage IV colorectal cancer patients.
Methods
The study analyzed 11,716 chemotherapy-treated stage IV colorectal cancer patients in the California Cancer Registry between 1996 and 2007, with follow-up through 2009. Patients were stratified into operation and non-operation groups. Estimates of median overall and colorectal cancer-specific survival were generated.
Results
Patients undergoing operation compared to those who are not had higher median overall and colorectal cancer-specific survival, 21 versus 10 months (p <0.0001) and 22 versus 12 months (p <0.0001), respectively. Patients who were offered surgery but refused had decreased median overall and colorectal cancer-specific survival when compared to patients who underwent resection, 8 versus 21 months (p <0.001) and 7 versus 22 months (p <0.001), respectively. In multivariate regression models, patients who underwent resection of primary tumor had improved overall (hazard ratio (HR), 0.42; 95 % confidence interval (CI) 0.40–0.44, p <0.0001) and colorectal cancer-specific survival (HR, 0.43; 95 % CI, 0.41–0.45; p <0.0001).
Conclusion
Primary tumor resection is associated with improved survival among stage IV chemotherapy-treated colorectal cancer patients.
Keywords: Primary tumor resection, Metastatic colorectal cancer
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death in the USA.1 In 2012, it was estimated that 143,000 new cases of CRC have been diagnosed and 28,000 had metastatic disease at the time of presentation.1 A small number of patients (approximately 6 %) with metastatic disease present with acute complications related to their primary tumors such as obstruction, significant hemorrhage, and perforation, where an urgent intervention (such as colectomy or diverting colostomy) is needed prior to starting systemic chemotherapy.2 However, the majority of patients with unresectable metastatic disease do not have symptoms related to the primary tumor that mandate surgical treatment,3 and the decision whether to resect the primary tumor is controversial.4.
Research thus far on this topic is mostly limited to small case– control or retrospective studies, and the results are conflicting— especially regarding survival. Cirocchi et al. summarized the results of seven studies on survival benefits of non-resection versus resection for asymptomatic primary tumors in patients with unresectable stage IV CRC in a Cochrane database study.2 Among the studies described were articles by Galizia et al. and Ruo et al., who reported a statistically significant overall survival benefit of 9 versus 16 months and 12.3 versus 15.2 months, respectively, favoring the resection group.5, 6 Likewise, the Konyalian et al. retrospective study of 109 patients reported a median survival of 375 days for those who underwent resection versus 138 days for those who did not.7 Other studies, including manuscripts by Scoggins et al., Tebutt et al., Michel et al., and Benoist et al., did not report any significant difference in survival.8–11 However, these negative studies were limited by a small sample size and a retrospective approach and were likely not powered to detect survival differences. Although there are two randomized controlled clinical trials underway that have been designed to investigate this topic further, the results will not be available for several more years. In the interim, clinicians have to make decisions about primary tumor resection without clear evidence.
Given the limitations noted in prior studies, we designed a comprehensive survival analysis using data from a large population-based cancer registry over a 12-year time period. The aims of this study were to analyze overall survival (OS) and CRC-specific survival (CRC-SS) associated with operation on the primary tumor versus non-operative treatment among chemotherapy-treated stage IV CRC patients.
Materials and Methods
This study was performed using data from the population-based California Cancer Registry (CCR), which is a statewide cancer surveillance program and is also part of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program. Patients' personal information is de-identified and confidentiality is maintained.
In this study, the current CCR database was obtained as an Excel file and imported into the SAS 9.2 statistical software (SAS Inc., Cary, NC, USA) for analyses. Statistical codes were written to create a new subset database for our analysis. Inclusion criteria include the following: (1) stage IV CRC, (2) treatment with chemotherapy, (3) diagnosis between 1996 and 2007 with follow-up through December 31, 2009, (4) age of 20 years or above, and (5) histology codes consistent with CRC primary tumor (see Supplemental Table 1 for a list of the histology codes included). Patients who had contraindications to undergo surgery were excluded using the existing “reason for no surgery” variable in CCR. Other variables analyzed include demographic data such as gender, age, race/ethnicity socioeconomic status, as well as the type of surgery performed, tumor histology, histologic grades, location of primary tumor, reason for no resection, and survival time. As recorded in CCR, “surgery” is defined as surgery on the primary tumor site, which must occur as part of the first course of therapy within 6 months of diagnosis. The overall survival duration (in months) was calculated using dates of diagnosis and either death from any cause or last contact. Details about chemotherapy such as type, timing, and dosing are unavailable in CCR. The treatment recorded in CCR refers to that received during the first 6 months after cancer diagnosis.
For our data analysis, 11,716 patients who met our inclusion criteria were selected. Patients were stratified into operation and non-operation groups for comparison of clinical characteristics, which were analyzed with Pearson's chi-square test or Fisher's exact test for categorical and dichotomous variables, respectively, and Student's t test for comparison of continuous variables. Estimates of median OS and CRC-SS were generated using the Kaplan–Meier method to compare patients who did and did not undergo surgical resection. A subset univariate analysis was done to compare the median OS and CRC-SS of patients who underwent operation versus patients who refused operation, as well as survival data based on age (greater vs. less than 70 years) and primary tumor site (colon vs. rectal cancer). In addition, multivariate OS and CRC-SS analyses were performed using Cox proportional hazard ratios adjusted for age, gender, race/ethnicity histology, primary tumor site, surgery, and time period of diagnosis (1996–1999, 2000–2003, 2004–2007). Both the Institutional Review Board of the University of California, Irvine, and the Committee for the Protection of Human Subjects of the California Health and Human Services Agency have determined that this work is exempt from IRB review and approval (letters on February 28, 2012 and February 15, 2012, respectively).
Results
Surgical resection of the primary tumor was performed in 73.4 % of patients (8,599 of 11,716) (Table 1). Partial colectomy was the most frequent type of surgery performed (45.9 %). The median age was 61 years in both the operation and the non-operation groups. Adenocarcinoma was the most common histologic subtype. The proximal/transverse colon was the most likely primary tumor site in the operation group (43.8 %), followed by the sigmoid colon and rectum. The rectum was the most likely primary tumor site in the non-operation group (36.2 %). The most common reason why the first course of treatment did not include resection of the primary tumor was that an operation was not recommended by the provider (Table 2). Thirty-eight patients were recommended to have operation but refused (Table 2).
Table 1. Demographic information for stage IV colorectal cancer cases, 1996–2007, California Cancer Registry.
| Total | Operation | Non-operation | p value | |
|---|---|---|---|---|
| Number of patients (n (%)) | 11,716 (100) | 8,599 (73.4) | 3,117 (26.6) | <0.0001 |
| Sex (n (%)) | <0.0001 | |||
| Male | 6,419 (54.8) | 4,564 (53.1) | 1,855 (59.5) | |
| Female | 5,297 (45.2) | 4,035 (46.9) | 1,262 (40.5) | |
| Agea | <0.0001 | |||
| Median (years) | 61.0 | 61.0 | 61.0 | |
| Range (years) | 20–84 | 20–84 | 20–84 | |
| <70 years | 8,593 (73.3) | 6,354 (73.9) | 2,239 (71.8) | |
| ≥70 years | 3,123 (26.7) | 2,245 (26.1) | 878 (28.2) | |
| Year of diagnosis (n (%)) | <0.0001 | |||
| 1996–1999 | 3,542 (30.2) | 2,768 (32.2) | 774 (24.8) | |
| 2000–2003 | 3,775 (32.2) | 2,770 (32.2) | 1,005 (32.2) | |
| 2004–2007 | 4,399 (37.6) | 3,061 (35.6) | 1,338 (42.9) | |
| Histologic subtype (n (%)) | <0.0001 | |||
| Adenocarcinoma | 10,276 (87.7) | 7,456 (86.7) | 2,820 (90.5) | |
| Mucinous adenocarcinoma | 1,372 (11.7) | 1,140 (13.0) | 258 (8.3) | |
| Other | 68 (0.6) | 29 (0.3) | 39 (1.3) | |
| Histologic gradeb (n (%)) | <0.0001 | |||
| Well differentiated | 466 (4.6) | 322 (3.9) | 144 (7.4) | |
| Moderately differentiated | 6,490 (63.5) | 5,198 (62.9) | 1,292 (66.0) | |
| Poorly differentiated | 3,158 (30.9) | 2,656 (32.2) | 502 (25.6) | |
| Undifferentiated | 105 (1.0) | 85 (1.0) | 20 (1.0) | |
| Site of primary tumor (n (%)) | <0.0001 | |||
| Proximal and transverse | 4,607 (39.3) | 3,770 (43.8) | 837 (26.9) | |
| Descending | 514 (4.4) | 414 (4.8) | 100 (3.2) | |
| Sigmoid | 2,674 (22.8) | 2,195 (25.5) | 479 (15.4) | |
| Rectosigmoid | 1,121 (9.6) | 864 (10.1) | 257 (8.3) | |
| Rectum | 2,293 (19.6) | 1,164 (13.5) | 1,129 (36.2) | |
| Unknown | 507 (4.3) | 192 (2.2) | 315 (10.1) | |
| Type of surgeryc (n (%)) | <0.0001 | |||
| No surgery | 3,117 (26.6) | 0 | 3,117 (100) | |
| Local excision | 80 (0.7) | 80 (0.9) | 0 | |
| Partial colectomy | 3,941 (33.7) | 3,941 (45.9) | 0 | |
| Hemicolectomy | 3,420 (29.2) | 3,420 (39.8) | 0 | |
| Total/procto-colectomy | 985 (8.4) | 985 (11.5) | 0 | |
| Unknown colectomy | 170 (1.5) | 170 (2.0) | 0 | |
| Socioeconomic status (n (%)) | <0.0001 | |||
| First quintile | 1,524 (13.0) | 1,065 (12.4) | 459 (14.7) | |
| Second quintile | 2,161 (18.4) | 1,561 (18.2) | 600 (19.3) | |
| Third quintile | 2,457 (21.0) | 1,776 (20.7) | 681 (21.9) | |
| Fourth quintile | 2,726 (23.3) | 2,028 (23.6) | 698 (22.4) | |
| Fifth quintile | 2,848 (24.3) | 2,269 (25.2) | 679 (21.8) |
The table only includes patients who received chemotherapy and had no contraindication to surgical resection of the primary tumor
Age at diagnosis
Missing data, 1,842
Missing data, 5
Table 2. Reason why the first course of treatment did not include resection of the primary tumor.
| Reason for no resection of primary tumor | Number (%) |
|---|---|
| Not recommended | 2,828 (90.1) |
| Recommended but not given for unknown reason | 181 (5.8) |
| Recommended, unknown if given | 60 (1.9) |
| Recommended but refused | 38 (1.2) |
| Unknown if operation was either recommended or given | 6 (0.19) |
| Missing data | 4 (0.13) |
| Total number of patients without resection | 3,117 (100) |
In univariate analyses, patients who underwent resection were found to have significantly improved overall survival compared to those who did not undergo resection (21 vs. 10 months; 95 % confidence interval (CI), 20–21 months vs. 10–11 months; p <0.0001) (Fig. 1). A similar trend was observed for CRC-SS (22 vs.12 months;95% CI, 22–23 months vs. 11–12 months; p <0.0001) (Fig. 2). In the subset analysis, patients who were offered operation but refused had decreased median OS and CRC-SS when compared to patients who underwent resection: 17.5 versus 21 months (95 % CI, 12– 23 months vs. 20–21 months; p <0.0001) and 17.5 versus 22 months (95 % CI, 12–23 months vs. 22–23 months; p <0.0001), respectively. Of note, patients who refused operation had greater median OS and CRC-SS when compared to patients who did not undergo resection.
Fig. 1.
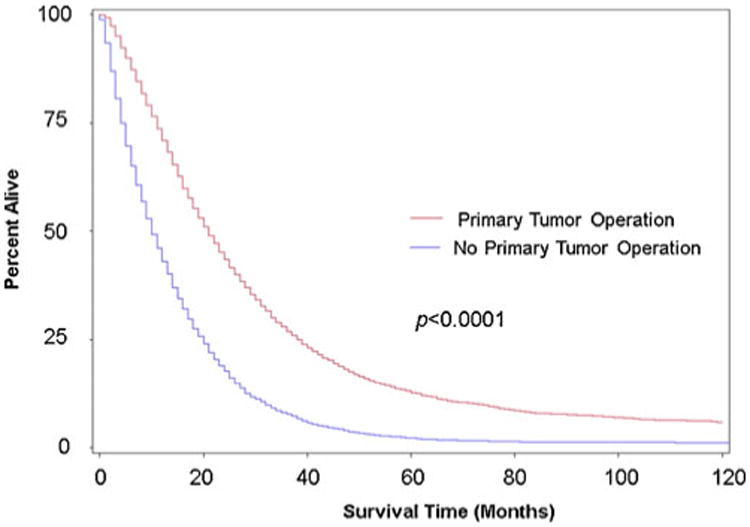
Kaplan–Meier overall survival rate estimates for chemotherapy-treated stage IV CRC patients based on whether or not they underwent operation of the primary tumor. Includes data from the California Cancer Registry during 1996–2006 with follow-up through December 31, 2009. Red line, received primary tumor operation (n =8,599); blue line, no primary tumor operation (n =3,117)
Fig. 2.
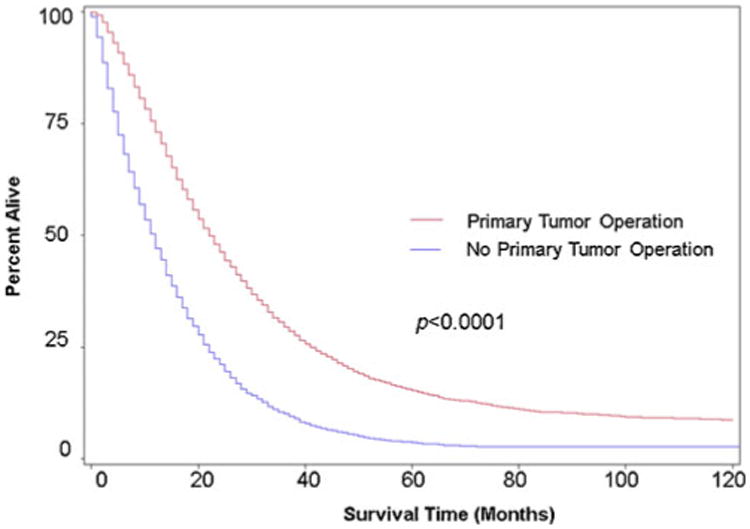
Kaplan–Meier colorectal cancer-specific rate estimates for chemotherapy-treated stage IV CRC patients based on whether or not they underwent operation of the primary tumor. Includes data from the California Cancer Registry during 1996–2006 with follow-up through December 31, 2009. Red line, received primary tumor operation (n =8,599); blue line, no primary tumor operation (n =3,117)
Elderly patients (defined as ≥70 years of age) accounted for 26.7 % of the study population. Elderly patients who underwent resection had improved OS and CRC-SS compared to elderly patients who did not have resection: 17 versus 7 months (95 % CI, 16–18 months vs. 7–8 months; p <0.0001) and 19 versus 9 months (95 % CI, 18–20 months vs. 8–10 months; p <0.0001), respectively. For non-elderly patients (defined as <70 years of age), the resection group had improved median OS and CRC-SS compared to the non-resection group: 22 versus 11 months (95 % CI, 22–23 months vs. 11–12 months; p <0.0001) and 24 versus 13 months (95 % CI, 23–24 months vs. 12–13 months; p <0.0001), respectively.
In the full regression model, surgical resection of the primary tumor was associated with a statistically significant improvement in OS (hazard ratio (HR), 0.42; 95 % CI, 0.40–0.44; p <0.0001) (Table 3). In this model, age ≥70 years was associated with decreased overall survival compared to patients <70 years of age. Hispanic ethnicity was observed to have improved overall survival compared to Whites (8 % decreased risk of death from any cause). Proximal and transverse colon cancer subsite locations were associated with poor survival compared to descending, sigmoid, rectosigmoid, and rectal cancers. In addition, diagnosis of CRC in later time periods (2000–2003, 2004–2007) was associated with improved overall survival compared to that in earlier periods (1996–1999).
Table 3. Cox proportional hazards model for overall survival and CRC-specific survival of stage IV colorectal cancer patients.
| Variables | Overall survival | CRC-specific survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95 % CI | p value | HR | 95 % CI | p value | |
| Age | 1.000 (referent) | 1.000 (referent) | ||||
| <70 years | ||||||
| ≥70 years | 1.15 | 1.083–1.226 | <0.0001 | 1.130 | 1.058–1.206 | 0.0003 |
| Gender | 1.020 | 0.982–1.060 | 0.31 | 1.018 | 0.978–1.060 | 0.377 |
| Ethnicity/race | ||||||
| White | 1.000 (referent) | 1.000 (referent) | ||||
| Black | 1.073 | 0.999–1.151 | 0.052 | 1.056 | 0.980–1.138 | 0.153 |
| Hispanic | 0.919 | 0.869–0.972 | 0.003 | 0.904 | 0.852–0.959 | 0.0008 |
| Asian | 0.966 | 0.908–1.030 | 0.28 | 0.934 | 0.875–0.998 | 0.0443 |
| Native Americans | 1.021 | 0.768–1.358 | 0.88 | 1.018 | 0.757–1.371 | 0.904 |
| Histology | ||||||
| Adenocarcinoma | 1.000 (referent) | 1.000 (referent) | ||||
| Mucinous | 1.007 | 0.949–1.068 | 0.823 | 0.989 | 0.929–1.053 | 0.74 |
| Others | 1.070 | 0.834–1.373 | 0.60 | 0.977 | 0.743–1.285 | 0.87 |
| Primary site | ||||||
| Proximal/transverse | 1.000 (referent) | 1.000 (referent) | ||||
| Descending | 0.777 | 0.706–0.855 | <0.0001 | 0.801 | 0.725–0.885 | <0.0001 |
| Sigmoid | 0.732 | 0.697–0.769 | <0.0001 | 0.753 | 0.715–0.794 | <0.0001 |
| Rectosigmoid | 0.715 | 0.668–0.765 | <0.0001 | 0.726 | 0.676–0.780 | <0.0001 |
| Rectal | 0.641 | 0.607–0.676 | <0.0001 | 0.655 | 0.619–0.694 | <0.0001 |
| Time period | ||||||
| 1996–1999 | 1.000 (referent) | 1.000 (referent) | ||||
| 2000–2003 | 0.870 | 0.83–0.912 | <0.0001 | 0.877 | 0.835–0.922 | <0.0001 |
| 2004–2007 | 0.684 | 0.653–0.718 | <0.0001 | 0.671 | 0.639–0.705 | <0.0001 |
| Tumor resection | ||||||
| No | 1.000 (referent) | 1.000 (referent) | ||||
| Yes | 0.417 | 0.398–0.436 | <0.0001 | 0.429 | 0.409–0.450 | <0.0001 |
| Socioeconomic status | ||||||
| First quintile | 1.000 (referent) | 1.000 (referent) | ||||
| Second quintile | 0.948 | 0.885–1.015 | 0.127 | 0.975 | 0.906–1.049 | 0.490 |
| Third quintile | 0.904 | 0.844–0.968 | 0.037 | 0.947 | 0.881–1.0118 | 0.139 |
| Fourth quintile | 0.860 | 0.804–0.920 | <0.0001 | 0.882 | 0.821–0.948 | 0.0007 |
| Fifth quintile | 0.823 | 0.769–0.882 | <0.0001 | 0.871 | 0.810–0.937 | 0.0002 |
Similar results were observed in the multivariate model for CRC-SS (Table 3). Resection of the primary tumor was associated with improvement in CRC-SS (HR, 0.43; 95 % CI, 0.41–0.45; p <0.0001). In the CRC-SS model, both Asian and Hispanic ethnicities were observed to have improved CRC-SS compared to Whites.
Subset analyses were performed based on tumor subsite location within the colorectum for colon (proximal, transverse, sigmoid, descending) and rectal cancers (rectum, rectosigmoid). Similar to the primary findings for all CRC patients combined, survival estimates were significantly improved for the resection group versus the non-resection group among colon cancer (OS HR=0.39, 95 % CI 0.37–0.42; p <0.0001; CRC–SS HR=0.41, 95 % CI 0.39–0.44) and rectal cancer patients (OS HR= 0.46, 95 % CI 0.43–0.50; p<0.0001; CRC-SS HR=0.46, 95 % CI 0.42–0.50).
Discussion
In this study, we observed that chemotherapy-treated stage IV CRC patients who have undergone resection of the primary tumor had significantly improved overall and CRC-specific survival compared to those who did not undergo primary tumor resection. The magnitude of benefit from operation in this setting was substantial as we observed a nearly 60 % decreased risk of death in the OS and CRC-SS analyses after adjusting for relevant clinical factors. Additionally, in this study we compared outcomes of patients who underwent operation on the primary tumor to those who were offered operation but refused it as the latter group likely had limited comorbidities and adequate performance status such that they would be offered operation in the first place. Interestingly, among chemotherapy-treated stage IV patients, compared to patients receiving operation on the primary tumor, survival outcomes were poor for those who were offered but refused operation.
Previously, Cook et al. examined the U.S. SEER database from 1988 to 2000, noting that 17,658 (66 %) of the 26,754 patients presenting with stage IV CRC underwent primary tumor resection.12 In all age groups, patients undergoing resection had higher median and 1-year survival rates (colon: 11 vs. 2 months, 45 vs. 12 %, p <.001; rectum: 16 vs. 6 months, 59 vs. 25 %, p <.001) when compared with patients who did not undergo resection. However, chemotherapy use is not available in SEER, so the authors could not account for the role of chemotherapy in explaining these survival differences. A possible explanation for their observed results is that patients not receiving surgery were also not treated with chemotherapy, and so in fact their results compare “treated” versus “non-treated” stage IV CRC patients. Here, we limited our analyses to chemotherapy-treated stage IV CRC patients, and a substantial survival advantage of primary tumor-directed operation was observed.
Our findings are consistent with several previous reports. Ruo et al. conducted a single-institution retrospective study from 1996 to 1999 during which 127 patients underwent upfront resection and 103 had no resection.5 They found that resected patients had significantly prolonged median survival (16 vs. 9 months, p <0.001) and 2-year survival (25 vs. 6 %, p <0.001) compared to patients who were never resected. In addition, Galizia et al. reported on a single-institution retrospective analysis of 64 patients and also found a significantly improved survival in the resection group compared to the non-resection group (15.2 vs. 12.3 months, p =0.003).6 Konyalian et al. reported a median survival benefit of 375 versus 138 days in patients who underwent resection, with a HR of 0.34,7 compared to our findings of an HR of 0.42 (overall survival).
As noted previously, conflicting data exist. In a retrospective review of the Vanderbilt University Hospital tumor registry from 1985 to 1997, 66 metastatic CRC patients had resection and 23 did not. The median survival of the non-resection group was 16.6 months versus the resection group's 14.5 months (p =0.59).8 Similarly, in another retrospective study, Tebbutt et al. reviewed 362 metastatic CRC patients treated at a UK hospital from 1990 to 2000, during which 280 patients underwent upfront resection and 82 had non-operative management, and survival rate was noted to be 14 versus 8.2 months, respectively, but not statistically significant.9 It should be noted that each of these studies is limited by low numbers of patients and likely has insufficient power to detect survival differences.
We chose a time period of diagnosis beginning in 1996 because it reflects a major transition in the treatment of metastatic CRC. Irinotecan was introduced in 1996; oxaliplatin, bevacizumab, and cetuximab in 2004; and panitumumab in 2006.13 Therefore, the time period of 1996 to 2007 captures the patient population treated with modern chemotherapy regimens, although it is acknowledged that the CCR database does not report the specific chemotherapy patients received.
A subset analysis was done for elderly patients, defined as age ≥70 years. We observed that even for these patients, there were statistically significant overall and CRC-specific survival improvements associated with surgical operation.
Patients who refused resection had significantly improved median OS and CRC-SS compared to patients who did not undergo resection. One possible explanation is that the patients who were recommended to undergo tumor resection generally might have better performance status, less comorbid conditions, and less disease burden compared to patients who did not undergo resection, and therefore have survival advantage. However, it is important to note that patients who were offered surgery were likely healthier than patients not offered surgery in terms of performance status and comorbidities. Furthermore, even those patients who “refused surgery” were likely different from those who accepted surgery, and analysis of population-based cancer registry data cannot account for such residual confounding.
The biological basis of improved survival attributed to resection of primary tumors among patients with stage IV CRC is unknown. Beyond “debulking” of overall tumor load, the primary tumor may have unique attributes that support tumor progression. For example, primary tumors frequently exhibit higher genetic variability than metastases and, therefore, may have greater capacity to generate new metastatic clones selected for resistance to ongoing therapies. 14–16.
Recent reports have shown that among stage IV CRC patients who have an asymptomatic primary tumor, leaving the primary tumor intact did not cause unacceptable complications, and survival was not significantly compromised.17,18 This is reflected in the current National Comprehensive Cancer Network guidelines, which recommend leaving the primary tumor intact and starting systemic chemotherapy first unless there is any obstruction, hemorrhage, or perforation that warrants urgent intervention.19 This approach has now been widely adopted as a common practice. Therefore, if the association of primary tumor resection and survival benefits is confirmed in future studies, it could significantly change the way clinicians manage primary tumors among metastatic CRC patients.
In this report, Hispanic ethnicity was independently associated with improved survival compared to Whites. Members of our group have previously reported that among CRC patients of all stage in the CCR, Hispanics had an 8 % decreased CRC-specific mortality compared to Whites after adjusting for age, gender, stage, histology, site within the colorectum, socioeconomic status, and treatment rendered.20 Explanations for these epidemiologic observations are unknown and could be the subject of future investigations.
Our study has the advantage of being a large population-based analysis of chemotherapy-treated patients with metastatic CRC in California, which offers sufficient power to address the study question. However, several limitations exist. CCR lacks information on patient performance status, indications for surgery, level of tumor burden, comorbid conditions, as well as the specific type (s) of chemotherapy agents received. One cannot determine from CCR data if patients had symptoms related to their cancer prior to surgery. Consistent with other large population-based analyses, pathologic confirmation of tumors is done at the local level, and there was no central pathologic review for the current analysis.
In summary, we report that resection of the primary tumor among chemotherapy-treated stage IV CRC patients is associated with improved OS and CRC-SS. Due to the study's limitations, randomized controlled trials are still needed. Two randomized studies, SYNCHRONOUS (German Intergroup, 800 CRC patients)21 and CAIRO4 (Dutch Colorectal Cancer Group, 360 colon cancer patients)22, are currently underway. We anxiously await the results of these trials and hope that they will provide valuable insights on the benefits of primary tumor resection among metastatic CRC patients.
Supplementary Material
Acknowledgments
Disclosures of Financial Support and Disclaimers The project was supported by award number P30CA062203 from the National Cancer Institute and by the UC Irvine Chao Family Comprehensive Cancer Center. B.S.L. was supported in part by Pfizer Oncology Clinical Fellowship in Translational Cancer Prevention. All authors contributed to the writing and final approval of the article. The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author (s), and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Presentation: The abstract was presented at the ASCO 2011 Annual Meeting in Chicago, IL, USA, June 4, 2011.
Electronic supplementary material: The online version of this article (doi:10.1007/s11605-013-2421-0) contains supplementary material, which is available to authorized users.
Contributor Information
Walter Y Tsang, Chao Family Comprehensive Cancer Center, University of California, Irvine, CA 92697, USA; Division of Hematology/Oncology, University of California, Irvine, CA 92697, USA; Department of Medicine, University of California, Irvine, CA 92697, USA.
Argyrios Ziogas, Department of Epidemiology, University of California, Irvine, CA 92697, USA.
Bruce S. Lin, Chao Family Comprehensive Cancer Center, University of California, Irvine, CA 92697, USA; Division of Hematology/Oncology, University of California, Irvine, CA 92697, USA; Department of Medicine, University of California, Irvine, CA 92697, USA
Tara E. Seery, Chao Family Comprehensive Cancer Center, University of California, Irvine, CA 92697, USA; Division of Hematology/Oncology, University of California, Irvine, CA 92697, USA; Department of Medicine, University of California, Irvine, CA 92697, USA
William Karnes, Division of Gastroenterology, University of California, Irvine, CA 92697, USA; Department of Medicine, University of California, Irvine, CA 92697, USA.
Michael J. Stamos, Department of Surgery, University of California, Irvine, CA 92697, USA
Jason A. Zell, Email: jzell@uci.edu, Chao Family Comprehensive Cancer Center, University of California, Irvine, CA 92697, USA; Division of Hematology/Oncology, University of California, Irvine, CA 92697, USA; Department of Medicine, University of California, Irvine, CA 92697, USA; Department of Epidemiology, University of California, Irvine, CA 92697, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Cirocchi R, Trastulli S, Abraha I, Vettoretto N, Boselli C, Montedori A, Parisi A, Noya G, Platell C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev. 2012;8:CD008997. doi: 10.1002/14651858.CD008997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costi R, Mazzeo A, Di Mauro D, Veronesi L, Sansebastiano G, Violi V, Roncoroni L, Sarli L. Palliative resection of colorectal cancer: does it prolong survival? Ann Surg Oncol. 2007;14:2567–76. doi: 10.1245/s10434-007-9444-2. [DOI] [PubMed] [Google Scholar]
- 4.Chang GJ. Challenge of primary tumor management in patients with stage IV colorectal cancer. J Clin Oncol. 2012;30:3165–6. doi: 10.1200/JCO.2012.43.5743. [DOI] [PubMed] [Google Scholar]
- 5.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722–8. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 6.Galizia G, Lieto E, Orditura M, Castellano P, Imperatore V, Pinto M, Zamboli A. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg. 2008;143:352–8. doi: 10.1001/archsurg.143.4.352. discussion 8. [DOI] [PubMed] [Google Scholar]
- 7.Konyalian VR, Rosing DK, Haukoos JS, Dixon MR, Sinow R, Bhaheetharan S, Stamos MJ, Kumar RR. The role of primary tumour resection in patients with stage IV colorectal cancer. Colorectal Dis. 2007;9:430–7. doi: 10.1111/j.1463-1318.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 8.Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651–7. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 9.Tebbutt NC, Norman AR, Cunningham D, Hill ME, Tait D, Oates J, Livingston S, Andreyev J. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut. 2003;52:568–73. doi: 10.1136/gut.52.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel P, Roque I, Di Fiore F, Langlois S, Scotte M, Teniere P, Paillot B. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol. 2004;28:434–7. doi: 10.1016/s0399-8320(04)94952-4. [DOI] [PubMed] [Google Scholar]
- 11.Benoist S, Pautrat K, Mitry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. 2005;92:1155–60. doi: 10.1002/bjs.5060. [DOI] [PubMed] [Google Scholar]
- 12.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637–45. doi: 10.1245/ASO.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Kohne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009;14:478–88. doi: 10.1634/theoncologist.2008-0202. [DOI] [PubMed] [Google Scholar]
- 14.Goranova TE, Ohue M, Shimoharu Y, Kato K. Dynamics of cancer cell subpopulations in primary and metastatic colorectal tumors. Clin Exp Metastasis. 2011;28:427–35. doi: 10.1007/s10585-011-9381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–8. [PubMed] [Google Scholar]
- 16.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–17. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD, Paty PB. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–84. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCahill LE, Yothers G, Sharif S, Petrelli NJ, Lai LL, Bechar N, Giguere JK, Dakhil SR, Fehrenbacher L, Lopa SH, Wagman LD, O'Connell MJ, Wolmark N. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. 2012;30:3223–8. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colon Cancer. National Comprehensive Cancer Network Guidelines 2013 [Google Scholar]
- 20.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:1950–62. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 21.Rahbari NN, Lordick F, Fink C, Bork U, Stange A, Jager D, Luntz SP, Englert S, Rossion I, Koch M, Buchler MW, Kieser M, Weitz J. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS–a randomised controlled multicentre trial (ISRCTN30964555) BMC Cancer. 2012;12:142. doi: 10.1186/1471-2407-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutch Colorectal Cancer Group Website. 2013 Available at: http://www.dccg.nl/node/111.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


