Abstract
Mesenchymal stem cells (hMSCs) have been shown to differentiate into osteoblasts that, in turn, are capable of forming tissues analogous to bone. The present study was designed to investigate the inhibition of osteogenesis by hMSCs. Bone marrow-derived hMSCs were treated with transforming growth factor β-3 (TGFβ3) at various doses during or after their differentiation into osteogenic cells. TGFβ3 was encapsulated in poly(DL-lactic-co-glycolic acid) (PLGA) microspheres and released via controlled delivery in the osteogenic culture of hMSCs and hMSC-derived osteoblasts for up to 28 days. Controlled release of TGFβ3 inhibited the osteogenic differentiation of hMSCs, as evidenced by significantly reduced alkaline phosphatase activity and staining, as well as decreased mineral deposition. After hMSCs had been differentiated into osteoblasts, controlled release of TGFβ3 further inhibited not only alkaline phosphatase and mineral deposition but also osteocalcin expression. These findings demonstrate the potential for sustained modulation of the behavior of stem cells and/or stem cell-derived lineage-specific cells via controlled release of growth factor(s). The attenuation of osteogenic differentiation of MSCs may facilitate understanding not only the regulation and patterning of osteogenesis in development but also several pathological models such as osteopetrosis, craniosynostosis, and heart valve calcification.
Mesenchymal stem cells (MSCs) were first isolated in the 1970s and named fibroblast colony-forming units.1–3 The bone marrow is known to be a rich source for heterogeneous cell populations including hematopoietic stem cells and MSCs.4,5 Recently, a great deal of effort has been focused on the application of MSCs for therapeutic models such as skeletal, cardiac, and neuronal disorders. MSCs in the adult can be readily extracted by bone marrow aspiration and expanded over more than 20 cumulative population doublings without losing some of their capacity to differentiate into osteoblasts, adipocytes, and chondrocytes.6–9 Recently, embryonic stem cells have also been shown to differentiate into osteoblast-like and chondrocyte-like cells.8,10–12
Osteogenic differentiation of MSCs is one of the most studied differentiation pathways.9,13,14 In critical size defects resulting from trauma, congenital anomalies, and tumor resection, bone healing is incomplete without a sufficient number of bone-forming cells. Osteoblasts differentiated from MSCs are potentially critical during bone healing and have been seeded or encapsulated in polymeric scaffolds in 3D for bone tissue engineering.15–18 In many instances, mineralized tissue or even bone-like tissues have been formed.19–21 MSC differentiation into osteoblasts is known to be regulated by an array of molecular cues. For example, osteoblastic differentiation of hMSCs is stimulated by epidermal growth factor.9 Replacement of insulin and triiodothyronine with bone morphogenetic protein-4 (BMP-4)-conditioned medium results in the suppression of adipogenesis and increased osteogenic development of embryonic stem cells.22 Canonical wingless (Wnt) signaling has been increasingly demonstrated to be a critical regulatory pathway of osteogenic differentiation.23 Inhibition of Wnt signaling may prevent osteogenesis and predispose hMSCs to enter the cell cycle.14 In addition, osteogenesis is stimulated by BMP-2 and further sustained by Wnt signaling. Down-regulation of Wnt signaling in maturing osteoblasts is necessary for the formation of a mineralized bone matrix.24,25 Osteoblastic differentiation controlled by Wnt signaling or transforming growth factor (TGF)β signaling is regulated by the activation of Runx2 gene expression in mesenchymal cells.26 Deletion of the two Runx2 isoforms, Runx2-II and Runx2-I, results in complete cessation of bone development.27
Previous effort has overwhelmingly focused on the forward differentiation of somatic or embryonic stem cells toward the osteoblastic lineage. Little is known about the reverse process of osteogenesis, i.e., inhibition of osteogenic differentiation of stem cells. In addition to the potential revelation of regulatory cues in the differentiation mechanisms, several clinical models clearly demand our improved understanding of the inhibition of osteogenic differentiation of stem cells. For instance, craniosynostosis is a cluster of heterogeneous congenital anomalies commonly characterized by the premature ossification of cranial sutures.28–32 Accelerated osteogenic differentiation in cranial sutures is proposed to be one of the potential causes for craniosynostosis.31,33 Ossification of heart valves is a common cause of their degeneration and failure, attributable to the mineralization of interstitial cells and matrices.34 Understanding the inhibition of osteogenic differentiation may help reveal the mechanisms of heart valve ossification. Undesirable ectopic bone formation also occurs in engineered rabbit tendons from MSCs, and needs to be rectified.35 In short, there are substantial needs to improve our understanding of the inhibition of osteoblastic differentiation from stem cells, for the understanding of bone development, as well as for devising potential therapeutic approaches for several pathological models as described above. The present study was designed to determine whether osteogenesis from not only MSCs but also MSC-derived osteoblasts can be inhibited by long-term controlled release of TGFβ3 from biodegradable microspheres.
MATERIALS AND METHODS
In vitro osteogenic differentiation of hMSCs
hMSCs were isolated from donated human bone marrow samples (AllCells, Berkeley, CA) and plated in monolayers in six-well plates at a density of 3×104 cells/well per our previous methods36 (Figure 1A and B). The isolated hMSCs were cultured-expanded at 37 °C, 95% humidity, and 5% CO2, using Dulbecco’s Modified Eagle’s Medium (DMEM-c; Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), and 1% antibiotic and antimycotic (10,000 U/mL penicillin [base], 10,000 μg/mL streptomycin [base], 25 μg/mL amphotericin B) (Atlanta Biologicals).5,36 Following expansion, hMSC culture was supplemented with osteogenic stimulants (OS) including 100 nM dexamethasone, 0.05 mM ascorbic acid-2-phosphate, and 10 mM β-glycerophosphate, per our previous work.36,37
Figure 1.
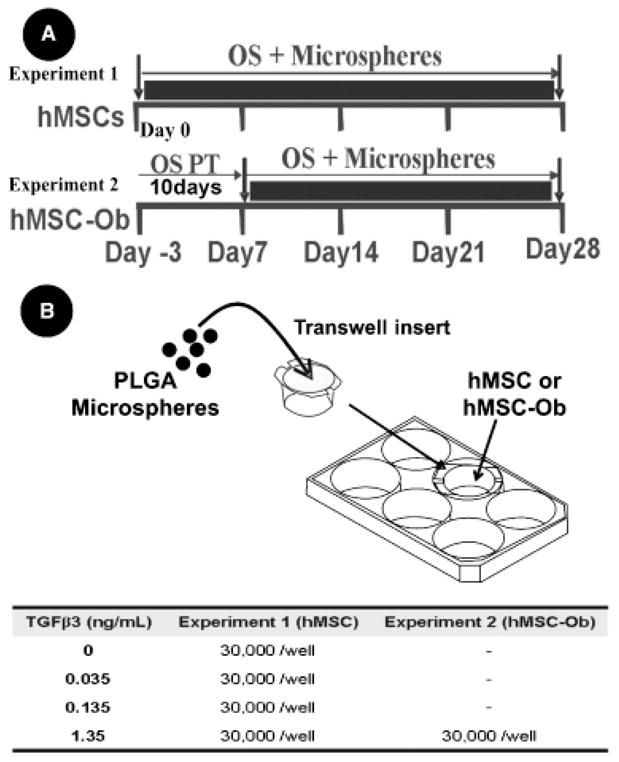
Experimental design. (A) Human mesenchymal stem cells (hMSCs—Experiment 1) and hMSC-derived osteoblasts (hMSC-Ob—Experiment 2) cultured with osteogenic-supplemented medium (OS) to promote osteogenesis. hMSCs in Experiment 1 were cultured under osteogenic conditions plus transforming growth factor β-3 (TGFβ3)-loaded PLGA microspheres or empty microspheres (control) for 4 weeks in vitro. In Experiment 2, a different group of hMSCs was allowed to differentiate into osteoblast-like cells by pre-treatment (OS PT) with OS for ten days with no microspheres (hMSC-Ob). hMSC-Ob were then cultured in OS for another 3 weeks in the presence of TGFβ3 or empty PLGA microspheres. (B) Poly(DL-lactic-co-glycolic acid) (PLGA) microspheres are placed on a porous membrane of a transwell insert, which is subsequently placed in the well of a six-well cell culture plate, where hMSC or hMSC-Ob are plated in monolayers, allowing released TGFβ3 to diffuse through the porous membrane and come in contact with the underlying cells. Tabulated doses of released TGFβ3 from PLGA microspheres refer to the amount of TGFβ3 released after 1 week. Cumulative release from the present PLGA microspheres is non-linear and decreases in subsequent weeks.
Microsphere preparation and TGFβ3 encapsulation
Microspheres of poly(DL-lactic-co-glycolic acid) (PLGA; Sigma) of 50: 50 copolymer ratio encapsulating recombinant human TGFβ3 (R&D Systems, Minneapolis, MN) were prepared using the double-emulsion technique ([water-in-oil]-in-water) (Figure 2A–C) as per our previously reported technique.36 Briefly, PLGA (250 mg) was dissolved into 1 mL dichloromethane. A total of 0.5 or 2.5 μg TGFβ3 was diluted in 50 μL of 1% Bovine serum albumin phosphate-buffered saline (PBS) solution and added to the PLGA solution, forming a mixture (primary emulsion) that was emulsified for 1 minute (water-in-oil). The primary emulsion was added to 2 mL of 1% polyvinyl alcohol solution (PVA, MW 30,000–70,000) and mixed for 1 minute ([water-in-oil]-in-water). One hundred milliliters of 0.1% PVA solution was added to the mixture and stirred for 1 minute using an electric stirrer at 450 r.p.m. Lastly, 100 mL of 2% isopropanol was added to the final emulsion and continuously stirred for 2 hours under a chemical hood to remove the solvent (450 r.p.m.). The 0.5 μg TGFβ3 was considered as the low loading dose, while 2.5 μg TGFβ3 the high loading dose. A control group of “empty” microspheres was fabricated using the same PLGA microsphere fabrication procedures but encapsulated distilled water, instead of TGFβ3. PLGA microspheres encapsulating TGFβ3 or distilled water were isolated using filtration (2 μm filter) and washed three times with distilled water. Microspheres were frozen in liquid nitrogen for 30 minutes and freeze-dried for 48 hours. Lyophilized microspheres were sterilized using ethylene oxide gas. Our previous experience with this fabrication method demonstrated that the average diameter of microspheres falls between 20 and 200 μm.36 We previously demonstrated that that ethylene oxide gas was nondetrimental and preserved the morphology of PLGA microspheres and release kinetics of growth factors encapsulated.36 The sterilized PLGA microspheres were maintained at −20 °C until use.
Figure 2.
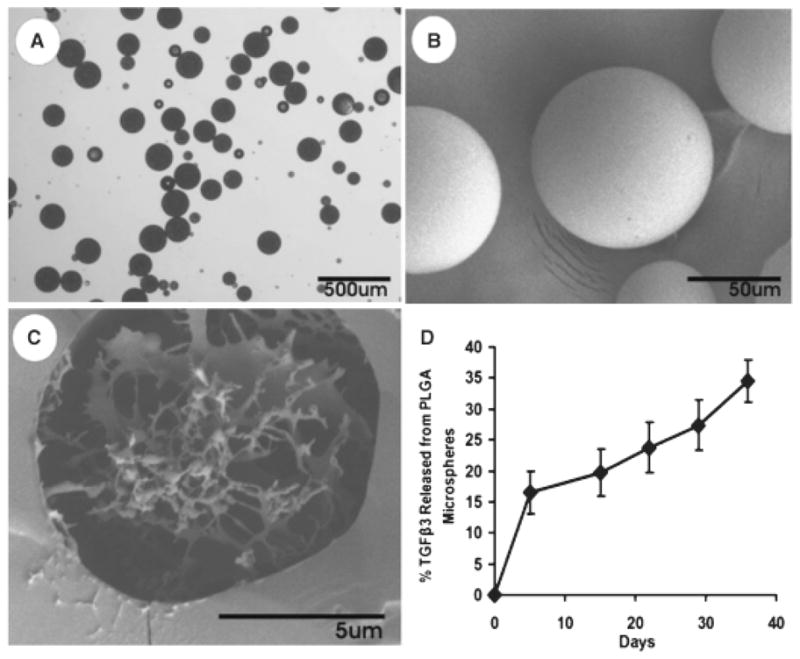
Fabrication and controlled release of poly(DL-lactic-co-glycolic acid) (PLGA) microspheres. (A) Light microscopy of PLGA microspheres encapsulating transforming growth factor β-3 (TGFβ3). (B) Scanning electron microscopy (SEM) showing smooth spherical surface of PLGA microspheres with average diameter of 102 ± 45 μm. (C) SEM of the cross-section of a PLGA microsphere illustrating the crystal-like structure of encapsulated molecules. (D) Cumulative release profile of TGFβ3 from PLGA microspheres.
Delivery of TGFβ3-encapsulated microspheres to hMSCs and hMSC-derived osteoblasts
hMSC (Experiment 1) or hMSC-derived osteoblasts (Experiment 2) were incubated with TGFβ3-encapsulated PLGA microspheres at concentrations of 0, 0.035, 0.135, and 1.35 ng/mL (Figure 1A and B). Doses of released TGFβ3 from PLGA microspheres refer to the amount of TGFβ3 released after a 1 week exposure of microspheres to an aqueous environment. Both Experiment 1 and Experiment 2 included OS as described above throughout all time points. The main difference between the two cell types in Experiments 1 and 2 is that in the latter, hMSCs were pretreated with osteogenic supplements for 10 days (hMSC-Ob) before introduction of controlled delivery of TGFβ3 from PLGA microspheres or empty microspheres (control). Osteogenic-supplemented medium was changed every 3–4 days. Sterilized PLGA microspheres were added to hMSC or hMSC-Ob cultures using 0.4 μm pore size transwell inserts (Figure 1B) (Becton Dickinson Labware, Franklin Lakes, NJ).38 The transwell inserts allowed the released growth factor to act on the underlying cells without direct contact with microspheres.36,38 Released TGFβ3 was allowed to diffuse through the transwell insert pores via medium solution and interact with the underlying cell monolayer (Figure 1B). Referencing the release kinetics of TGFβ3 from PLGA microspheres (Figure 2D), values for the amount of TGFβ3 released over the first 7 days were used to form four experimental groups: control (empty microspheres), 3 mg of low-concentration microspheres (0.035 ng TGFβ3/mL released during first week), 3 mg of high-concentration microspheres (0.135 ng TGFβ3/mL released during first week), and 30 mg of high-concentration microspheres (1.35 ng TGFβ3/mL released during first week) (Figure 1B). The cumulative release of molecules from PLGA microspheres is nonlinear and typically attenuated after the first week; however, sustained exposure to control-released TGFβ3 continues throughout the experiment at doses established in release profile studies (Figure 2D).
Analysis of inhibitory effects during osteogenesis of hMSCs and hMSC derived osteoblasts
At 7, 14, 21, and 28 days, cell culture samples were collected by the addition of detergent Triton-X 100 (Sigma) to the cell culture for 15 minutes, after the medium had been removed and the cells washed twice with PBS. Using cell scrapers, samples were physically detached and pipetted into a glass test tube. Samples were then lysed by sonication for 20 seconds on ice, and frozen at −70 °C until samples at all time points were collected, and then thawed and assayed. In order to evaluate the potential of TGFβ3 in reducing the osteogenic behavior of hMSC-Ob, the above methods were performed not only using (undifferentiated) hMSCs but also hMSC that were pretreated with osteogenic supplements for 10 days (Figure 1A) and presented high levels of osteogenic differentiation markers before the addition of TGFβ3-loaded PLGA microspheres. Only the highest dose of TGFβ3 (1.35 ng/mL released during first week) was used for hMSC-derived osteoblasts in experiment 2. Pretreated hMSC-Ob were cultured with OS for 21 days post pretreatment to complete the experimental time-line (Figure 1).
All assays were evaluated using thawed-lysed samples. DNA content was determined using the fluorescent DNA quantification kit (BioRad Labs, Hercules, CA) and expressed in μg DNA per mL of sample. Alkaline phosphatase (ALP) activity was quantified using ALP reagent (RaiChem, San Diego, CA) and expressed as mU/mL, normalized to DNA concentration. ALP staining and Von Kossa staining were used to assess ALP activity and mineral deposition qualitatively.5,37 Cell culture wells were fixed with 5% formaline and stained in situ. Osteocalcin was measured using an osteocalcin enzyme linked immunosorbent assay (ELISA) kit (Metra, San Diego, CA).
Statistical analysis
Paired t-tests were used to compare the quantitative alkaline phosphatase activity and osteocalcin levels of hMSCs and hMSC-Ob between the control group (no TGFβ3) and each experimental group (TGFβ3 released from PLGA microspheres). All statistical analyses were performed with an α level of 0.05 using Minitab 14 software (State College, PA).
RESULTS
PLGA microsphere-encapsulated TGFβ3
TGFβ3 was encapsulated by PLGA microspheres fabricated using the double-emulsion-solvent-extraction technique (Figure 2A and B). The topology of the PLGA microspheres encapsulating TGFβ3 showed smooth spherical surfaces by scanning electron microscope (SEM) imaging (Figure 2B). SEM imaging of a cross-section of microspheres allowed visualization of the crystal-like structure of encapsulated molecules (encapsulation of bovine serum albumin, in Figure 2C). The average diameter of TGFβ3-encapsulating PLGA microspheres was 102 ± 45 μm (Figure 2A and B). Total encapsulation of TGFβ3 in PLGA microspheres resulted in 0.2 and 0.75 ng TGFβ3/mL/mg of microspheres for the low and high initial loading concentrations, respectively (low: 0.5 μg TGFβ3; high: 2.5 μg TGFβ3), as quantified by ELISA. TGFβ3 was released up to 35 days/ELISA (Figure 2D).
Inhibition of osteogenic differentiation of hMSC
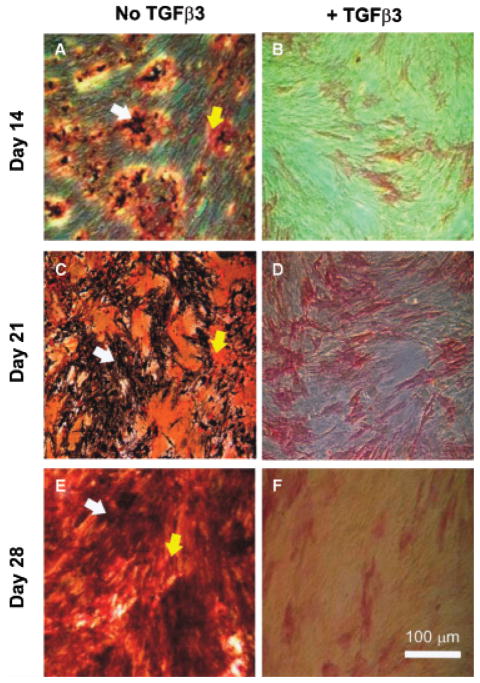
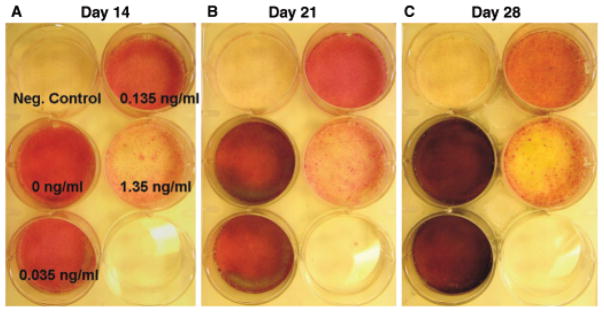
Bone marrow-derived hMSCs in monolayer culture with osteogenic supplements showed a large increase in ALP activity up to 21 days (open bars in Figure 3) (p < 0.01). The ALP activity of hMSCs exposed to TGFβ3 released from PLGA microspheres at various doses was significantly inhibited by 14 and 21 days (shaded bars in Figure 3) (p < 0.01). This is further exemplified by combined ALP and von Kossa staining of TGFβ3 incubated hMSCs in culture at 14, 21, and 28 days (Figure 4B, D, and F), in comparison with gradually increasing ALP staining of control hMSCs without TGFβ3 treatment (Figure 4A, C, and E). ALP was not measured at the late time point of 28 weeks, given its typical effective expression during early osteogenesis (3–21 days). Increasing mineral deposition was detected by von Kossa staining in the control group up to 28 days (no TGFβ3) (Figure 5A–C), whereas the high TGFβ3 dose group (1.35 ng/mL) effectively inhibited mineralization at days 14, 21, and 28 (Figure 5A–C). Experimental cell culture wells stained for ALP and mineral deposition (von Kossa) included negative control (hMSCs cultured in DMEM-c), as well as osteogenic cultures with 0, 0.035, 0.135, or 1.35 ng/mL, of TGFβ3 up to 28 days (Figure 5A–C). Although the inhibition of mineral deposition also occurred in response to lower TGFβ3 doses, the high dose was more effective. Since much higher doses of TGFβ3 (> 10 ng/mL) are known to induce chondrogenic differentiation of MSCs,5 we checked chondrogenesis by safranin-O staining for sulfated glycosaminoglycans that are typically found in cartilage. No chondrogenic differentiation was observed in any of the present experimental groups (data not shown).
Figure 3.
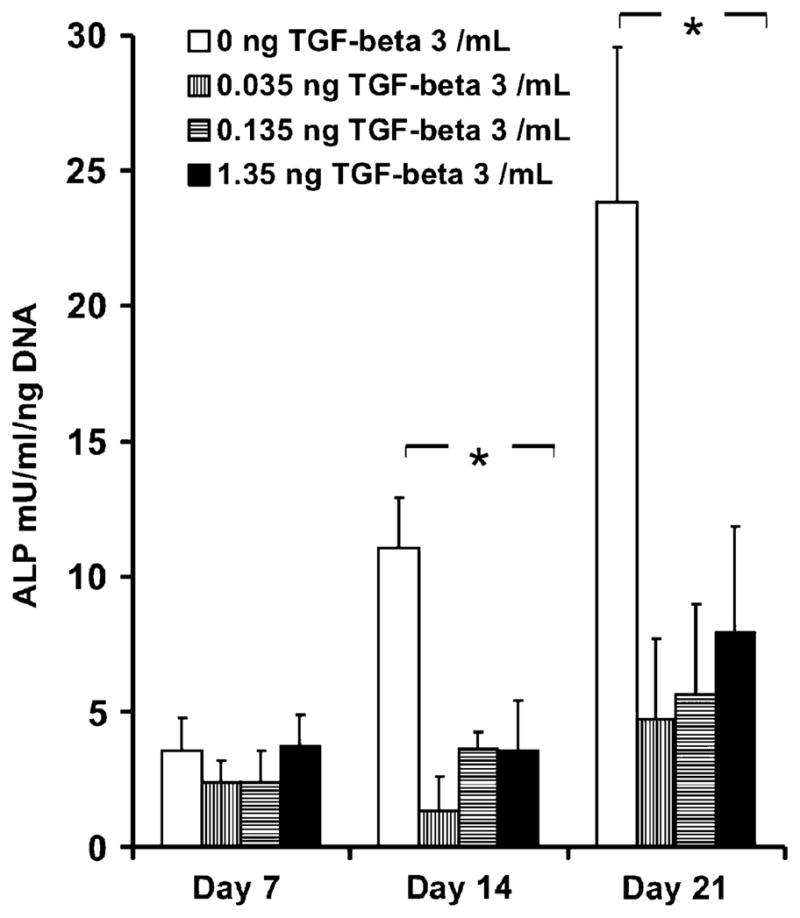
Alkaline phosphatase (ALP) activity of human mesenchymal stem cells (hMSCs) osteogenic cultures with various doses of transforming growth factor β-3 (TGFβ3) released from poly(DL-lactic-co-glycolic acid) (PLGA) microspheres (Experiment 1). Increased activity of ALP, an early marker for osteogenic differentiation up to 3 weeks in osteogenic culture was observed. Various doses of TGFβ3 (3 mg of low-concentration spheres: 0.035 ng/mL released after 1 week; 3 mg of high-concentration spheres: 0.135 ng/mL released after 1 week; and 30 mg of high-concentration spheres: 1.35 ng/mL released after 1 wk) inhibited increases in ALP activity up to 3 wks, indicating inhibition of osteogenic differentiation of hMSCs (n=3, p < 0.05).
Figure 4.
Transforming growth factor β-3 (TGFβ3) induced inhibition of osteogenic differentiation (Experiment 1). (A, C, and E) Human mesenchymal stem cells in osteogenic culture and empty PLGA microspheres increased alkaline phosphatase (ALP) staining and mineral deposition. (White arrows, mineral deposition; yellow arrows, alkaline phosphatase). (B, D, and F) Suppression of osteogenesis by released TGFβ3 from PLGA microspheres (30 mg of high-concentration microspheres: 1.35 ng/mL released after 1 week) (ALP stain, red; von Kossa, black). Scale bar=100 μm.
Figure 5.
Dose dependence of human mesenchymal stem cells (hMSCs) to transforming growth factor β-3 (TGFβ3) in osteogenic culture (Experiment 1). Red staining of alkaline phosphatase (ALP) and black coloring of mineral deposition by von kossa stain were progressively increased in osteogenic cultures of groups with no TGFβ3 (0 ng/mL) over the 28-day period (A → B → C). Groups with highest dose of released TGFβ3 (1.35 ng/mL in the first week) had less staining intensity of both von kossa and ALP up to 28 days of osteogenic culture, indicating the inhibition of osteogenic differentiation of hMSCs. Negative controls were hMSCs cultured in non-osteogenic growth medium.
Inhibition of osteogenesis of hMSC-derived osteoblasts
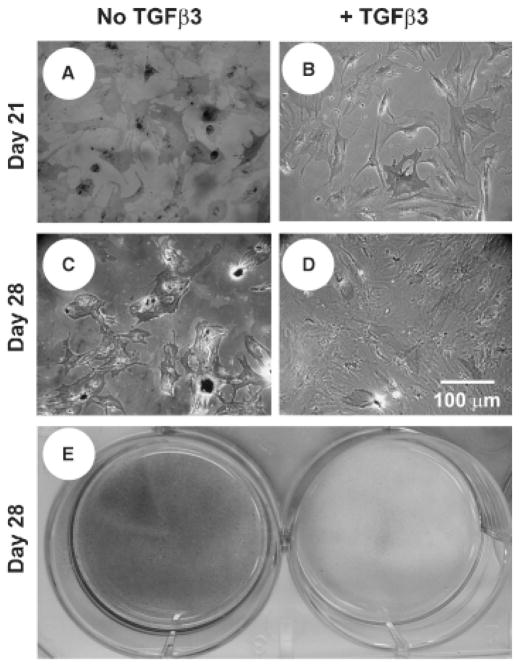
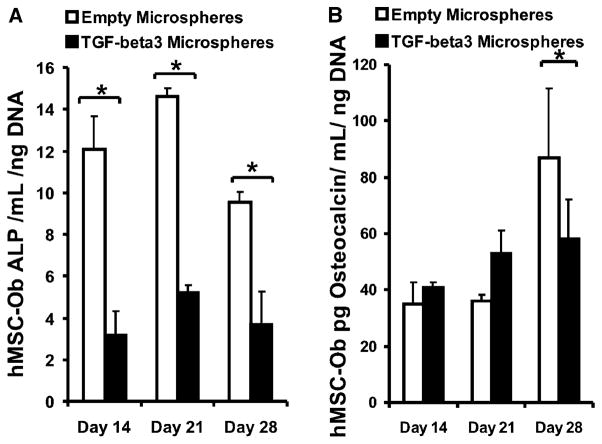
hMSCs are known to begin differentiating into osteoblasts within 1–14 days of in vitro treatment in osteogenic medium (100 nM dexamethasone, 0.05 mM ascorbic acid-2-phophate, and 100 mM β-glycerophosphate), as per our previous work.15,36 In the present study, we differentiated hMSCs into osteoblast-like cells in vitro for 10 days, per our previous approach. These hMSC-Ob showed substantial activity inhibition upon delivery of TGFβ3 in PLGA microspheres for up to 28 days in culture, as evidenced by a significant reduction in mineralization (Figure 6B and D and right plate in Figure 6E), in comparison with the corresponding controls (no TGFβ3) (Figure 6A and C and left plate in Figure 6E). Quantitatively, the controlled release of TGFβ3 from 30 mg of high-concentration microspheres (1.35 ng/mL released during the first week) significantly reduced the ALP activity at days 14, 21, and 28 (Figure 7A), as well as osteocalcin activity by day 28 (Figure 7B) (p < 0.05).
Figure 6.
TGFβ3 inhibits further differentiation of human mesenchymal stem-cell derived osteoblasts (hMSC-Ob). Representative von Kossa stain image of osteogenic culture of hMSC-Ob with placebo microspheres (A and C), or in the presence of TGFβ3 from microspheres (30 mg of high-concentration microspheres: 1.35 ng/mL released after 1 week) (B and D) (Experiment 2). E. Representative hMSC-Ob osteogenic culture wells with empty microspheres (left) and TGFβ3-loaded microspheres (right) (mineral deposition=black).
Figure 7.
Transforming growth factor β-3 (TGFβ3) inhibits the alkaline phosphatase activity and osteocalcin content of hMSC-derived osteoblasts. Alkaline phosphatase (ALP) activity (A) and osteocalcin content (B) of osteogenic culture of human mesenchymal stem cells (hMSC)-derived osteoblasts with empty PLGA microspheres (empty bars) or TGFβ3-loaded poly(DL-lactic-co-glycolic acid) (PLGA) microspheres (1.35 ng/mL released during first 1 week) (black bars) up to 3 weeks after a 10-day pretreatment with osteogenic supplements (Experiment 2) (n=3, p < 0.05).
DISCUSSION
Osteogenic differentiation from progenitor cells indicates a commitment to lineage specification and is the first step in osteogenesis. The present data represent an original demonstration of the inhibition of osteogenic differentiation of hMSCs and matrix synthesis of hMSC-derived osteoblasts by the controlled release of TGFβ3 for up to 21–28 days. The advantage of controlled release of TGFβ3, as opposed to addition in culture medium, is that controlled release enables continuous exposure of cells to the growth factor, and has the potential to avoid rapid denaturation and, if in an in vivo model, rapid diffusion. In order to inhibit osteogenesis, both osteogenic differentiation of progenitor cells and mineral deposition should be attenuated. The present data demonstrate that osteogenic differentiation from stem cells and mineral depposition were both inhibited. TGFβ3 is one of the three mammalian isoforms of the TGFβ superfamily, and a key regulator of skeletogenesis during development and tissue regeneration.39–42 TGFβ1 and TGFβ2 appear to stimulate osteogenesis and are required for osteoblastic differentiation.43,44 In contrast, the actions of TGFβ3 on osteogenic differentiation and mineral deposition are yet to be fully understood. The present findings suggest that TGFβ3 has dose dependent inhibitory effects on osteogenic differentiation and osteoblastic matrix synthesis.
TGFβ3 may have reciprocal actions to the roles of TGFβ1 and TGFβ2 in osteogenesis. During cranial suture morphogenesis, antibody blockage of TGFβ3 receptors results in premature ossification.45–47 Moreover, the delivery of exogenous TGFβ3 in collagen sponges to cranial sutures results in a decrease in the proliferation of osteoblasts.48 On the other hand, TGFβ2 delivery increases the proliferation of sutural osteoblasts and results in premature sutural ossification.45–48 Thus, TGFβ2 and TGFβ3 seem to mediate different processes of osteogenesis in a reciprocal fashion in cranial sutures. Additional studies utilizing the present approaches are warranted to incorporate other TGFβ isoforms for exploring the interplay between TGFβ3 and other cytokines. Investigations of cellular activation and regulation by these growth factors, including receptor activity, intracellular signaling pathways, and protein synthesis may elucidate novel regulatory strategies for osteogenesis. Another implication of the present approach involves the regulation of stem cell fate. For instance, embryonic stem cells implanted in mice and rats may form teratomas, indicating that differentiation of progenitors must be controlled postimplantation.49,50 Undesired ectopic bone formation occurs in approximately 28% of tissue-engineered rabbit tendon repairs using MSCs.35 Heterotopic ossification in ligaments, tendons, and muscles such as in fibrodysplasia ossificans progressiva patients leads to stiffness in the affected areas and may also limit movement in affected joints (e.g., knees, wrists, shoulders, spine, and/or neck).51–54 Thus, the use of stem cells in skeletal tissue engineering demands their controlled differentiation postimplantation. The present data demonstrate a system that may be used in regulating stem cells during osteogenesis by maintaining their undifferentiated state, and preventing ectopic bone formation in soft-tissue engineering.
Many parameters of microsphere fabrication and delivery to cell culture may be tailored for more accurate and encompassing clinical applications. The present fabrication of PLGA microspheres only included a single PLA: PGA ratio. Variations in the polymer composition and structure may alter the proposed TGFβ3 release profile, as shown in our previous work.36 Different release profiles can be advantageous in the modulation of differentiation behavior of stem cells and/or stem-cell derived lineage-specific cells. Functionalization of PLGA microspheres with agents such as heparin or incorporation into hydrogels may diminish the initial burst effect and linearize the TGFβ3 release profile.55–57 The fabrication of more similar-sized microspheres may be a potential procedure to decrease the variation of released TGFβ3 concentrations. Additionally, the inhibitory effects presented here on the osteogenesis of hMSCs and hMSC-Ob may not be reciprocally correlated, given that the culture time of each experiment was offset by the pre-treatment time of 10 days necessary to initiate osteogenic differentiation of hMSCs in Experiment 2. hMSCs in Experiment 1 were tested for 28 days in osteogenic culture and continuous delivery of TGFβ3, whereas hMSCs in Experiment 2 were initially differentiated into hMSC-Ob for 10 days, and then introduced to TGFβ3 delivery for another 21 days (total of 31 experimental days). Thus, small timely discrepancies must be considered. Nonetheless, the present approach takes advantage of the versatile and widespread applications of controlled delivery of bioactive molecules from degradable polymer microspheres, in particular the regulation of stem cell fate during osteogenesis and the inhibition of bone matrix formation of differentiated osteoblasts.
The inhibition of osteogenic differentiation from progenitor cells may have implications in bone development. For locally resident MSCs, regional TGFβ3 concentration likely regulates their differentiation pathways. This speculation can be tested by improving our understanding of potential pathways of TGFβ3 actions in the regulation of osteogenesis, and likely parallel pathways such as adipogenesis and angiogenesis.58–60 Whether TGFβ3 regulates stem cell fate and osteogenesis by regulating intracellular SMAD pathways or by the interactions with other cytokines at the cell surface level warrants additional studies. In wound healing, TGFβ3 attenuates the synthesis of collagen type I and furthermore inhibits scar tissue formation.61,62 Attenuation of type I collagen synthesis may also be one of the pathways of inhibition of osteogenesis. Such demonstrations elicit many implications in inhibiting keloid formation, skin tissue engineering, and cardiovascular procedures, where fibrous tissue formation and stem cell fate must be regulated.
TGFβ3 is a potent chondrogenic factor during MSC differentiation and is highly expressed in the proliferating zone of cartilaginous growth plates.63 Given its involvement during native chondrogenesis, TGFβ3 has been implemented in 2D and 3D in vitro chondrogenic differentiation-engineered models for MSCs.5,15,64 The presently demonstrated controlled delivery of TGFβ3 using PLGA microspheres provides tools for continued chondrogenic differentiation of MSCs using a single application of TGFβ3-loaded microspheres. Although the concentration of TGFβ3 in the present study for the inhibition of MSC osteogenic differentiation and osteoblast matrix synthesis (up to ~1 ng/mL) is several orders of magnitude lower than required chondrogenic doses (> 10 ng/mL), the same protocol for microsphere fabrication and encapsulation may be replicated to deliver increased amounts of TGFβ3. Increasing the amount of microspheres or the initial TGFβ3 loading concentration, as well as biomaterial parameter variations, may result in desirable sustained delivery of chondrogenic doses of TGFβ3, illustrating the versatility of this approach in tissue engineering and wound-healing models.
Acknowledgments
We would like to acknowledge Aurora Lopez and Dr. Paul A. Clark for general technical assistance. We thank two anonymous reviewers, whose insightful comments helped improve the quality of our manuscript. This research was supported by NIH grants DE15391 and EB02332 to J.J.M.
References
- 1.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 3.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–64. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 5.Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A, Mao JJ. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–23. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 6.Halleux C, Sottile V, Gasser JA, Seuwen K. Multi-lineage potential of human mesenchymal stem cells following clonal expansion. J Musculoskelet Neuronal Interact. 2001;2:71–6. [PubMed] [Google Scholar]
- 7.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–48. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 8.Deng MJ, Jin Y, Shi JN, Lu HB, Liu Y, He DW, Nie X, Smith AJ. Multilineage differentiation of ectomesenchymal cells isolated from the first branchial arch. Tissue Eng. 2004;10 (9–10):1597–606. doi: 10.1089/ten.2004.10.1597. [DOI] [PubMed] [Google Scholar]
- 9.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–7. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 10.Woll NL, Bronson SK. Analysis of embryonic stem cell-derived osteogenic cultures. Methods Mol Biol. 2006;330:149–59. doi: 10.1385/1-59745-036-7:149. [DOI] [PubMed] [Google Scholar]
- 11.Garreta E, Genove E, Borros S, Semino CE. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215–27. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 12.Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, Bishop AE, Polak JM. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro- environment. Tissue Eng. 2006;12:1687–97. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- 14.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by micro-environmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:e37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 15.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–44. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 16.Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48:913–27. doi: 10.1002/(sici)1097-4636(1999)48:6<913::aid-jbm22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Jansen JA, Vehof JW, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H, Hedberg EL, Mikos AG. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101 (1–3):127–36. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Holtorf HL, Jansen JA, Mikos AG. Ectopic bone formation in rat marrow stromal cell/titanium fiber mesh scaffold constructs: effect of initial cell phenotype. Biomaterials. 2005;26:6208–16. doi: 10.1016/j.biomaterials.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Krebsbach PH, Robey PG. Dental and skeletal stem cells: potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002;66:766–73. [PubMed] [Google Scholar]
- 20.Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A, Mao JJ. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–23. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Malaval L, Aubin JE. Global amplification polymerase chain reaction reveals novel transitional stages during osteoprogenitor differentiation. J Cell Sci. 2003;116 (Part 9):1787–96. doi: 10.1242/jcs.00376. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–69. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Tuan RS. Biology of developmental and regenerative skeletogenesis. Clin Orthop Relat Res. 2004;(Suppl):S105–17. doi: 10.1097/01.blo.0000143560.41767.ee. [DOI] [PubMed] [Google Scholar]
- 24.van der HG, van der Werf SM, Farih-Sips H, van Bezooijen RL, Lowik CW, Karperien M. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res. 2005;20:1867–77. doi: 10.1359/JBMR.050614. [DOI] [PubMed] [Google Scholar]
- 25.Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–9. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 26.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Z, Awad HA, Liu S, Mahlios J, Zhang S, Guilak F, Mayo MS, Quarles LD. Selective Runx2-II deficiency leads to low-turnover osteopenia in adult mice. Dev Biol. 2005;283:345–56. doi: 10.1016/j.ydbio.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KY, Nolen AA, Gampper TJ, Jane JA, Opperman LA, Ogle RC. Elevated levels of transforming growth factors beta 2 and beta 3 in lambdoid sutures from children with persistent plagiocephaly. Cleft Palate Craniofac J. 1997;34:331–7. doi: 10.1597/1545-1569_1997_034_0330_elotgf_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 29.Mooney MP, Moursi AM, Opperman LA, Siegel MI. Cytokine therapy for craniosynostosis. Expert Opin Biol Ther. 2004;4:279–99. doi: 10.1517/14712598.4.3.279. [DOI] [PubMed] [Google Scholar]
- 30.Opperman LA, Nolen AA, Ogle RC. TGF-beta 1, TGF-beta 2, and TGF-beta 3 exhibit distinct patterns of expression during cranial suture formation and obliteration in vivo and in vitro. J Bone Miner Res. 1997;12:301–10. doi: 10.1359/jbmr.1997.12.3.301. [DOI] [PubMed] [Google Scholar]
- 31.Most D, Levine JP, Chang J, Sung J, McCarthy JG, Schendel SA, Longaker MT. Studies in cranial suture biology: up-regulation of transforming growth factor-beta1 and basic fibroblast growth factor mRNA correlates with posterior frontal cranial suture fusion in the rat. Plast Reconstr Surg. 1998;101:1431–40. doi: 10.1097/00006534-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Collins JM, Ramamoorthy K, Da SA, Patston P, Mao JJ. Expression of matrix metalloproteinase genes in the rat intramembranous bone during postnatal growth and upon mechanical stresses. J Biomech. 2005;38:485–92. doi: 10.1016/j.jbiomech.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 33.De Pollack C, Renier D, Hott M, Marie PJ. Increased bone formation and osteoblastic cell phenotype in premature cranial suture ossification (craniosynostosis) J Bone Miner Res. 1996;11:401–7. doi: 10.1002/jbmr.5650110314. [DOI] [PubMed] [Google Scholar]
- 34.Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–7. [PubMed] [Google Scholar]
- 35.Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–46. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human- shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–6. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 38.Lu L, Stamatas GN, Mikos AG. Controlled release of transforming growth factor beta1 from biodegradable polymer microparticles. J Biomed Mater Res. 2000;50:440–51. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Akhurst RJ, Fitzpatrick DR, Fowlis DJ, Gatherer D, Millan FA, Slager H. The role of TGF-beta s in mammalian development and neoplasia. Mol Reprod Dev. 1992;32:127–35. doi: 10.1002/mrd.1080320208. [DOI] [PubMed] [Google Scholar]
- 40.Roberts AB, Kondaiah P, Rosa F, Watanabe S, Good P, Danielpour D, Roche NS, Rebbert ML, Dawid IB, Sporn MB. Mesoderm induction in Xenopus laevis distinguishes between the various TGF-beta isoforms. Growth Factors. 1990;3:277–86. doi: 10.3109/08977199009003670. [DOI] [PubMed] [Google Scholar]
- 41.Adab K, Sayne JR, Carlson DS, Opperman LA. TGF-beta1, TGF-beta2, TGF-beta3 and Msx2 expression is elevated during frontonasal suture morphogenesis and during active postnatal facial growth. Orthod Craniofac Res. 2002;5:227–37. doi: 10.1034/j.1600-0544.2002.02227.x. [DOI] [PubMed] [Google Scholar]
- 42.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–7. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 43.Pelton RW, Hogan BL, Miller DA, Moses HL. Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev Biol. 1990;141:456–60. doi: 10.1016/0012-1606(90)90401-4. [DOI] [PubMed] [Google Scholar]
- 44.Lehnert SA, Akhurst RJ. Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development. 1988;104:263–73. doi: 10.1242/dev.104.2.263. [DOI] [PubMed] [Google Scholar]
- 45.Longaker MT. Role of TGF-beta signaling in the regulation of programmed cranial suture fusion. J Craniofac Surg. 2001;12:389–90. doi: 10.1097/00001665-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Poisson E, Sciote JJ, Koepsel R, Cooper GM, Opperman LA, Mooney MP. Transforming growth factor-beta isoform expression in the perisutural tissues of craniosynostotic rabbits. Cleft Palate Craniofac J. 2004;41:392–402. doi: 10.1597/02-140.1. [DOI] [PubMed] [Google Scholar]
- 47.Opperman LA, Chhabra A, Cho RW, Ogle RC. Cranial suture obliteration is induced by removal of transforming growth factor (TGF)-beta 3 activity and prevented by removal of TGF-beta 2 activity from fetal rat calvaria in vitro. J Craniofac Genet Dev Biol. 1999;19:164–73. [PubMed] [Google Scholar]
- 48.Opperman LA, Adab K, Gakunga PT. Transforming growth factor-beta 2 and TGF-beta 3 regulate fetal rat cranial suture morphogenesis by regulating rates of cell proliferation and apoptosis. Dev Dyn. 2000;219:237–47. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1044>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 49.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–14. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50. doi: 10.1634/stemcells.2005-0014. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan F, Sawyer J, Connors S, Keough K, Shore E, Gannon F, Glaser D, Rocke D, Zasloff M, Folkman J. Urinary basic fibroblast growth factor. A biochemical marker for pre-osseous fibroproliferative lesions in patients with fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998:59–65. [PubMed] [Google Scholar]
- 52.Kaplan FS, Gannon FH, Hahn GV, Wollner N, Prauner R. Pseudomalignant heterotopic ossification. Clin Orthop Relat Res. 1998:134–40. [PubMed] [Google Scholar]
- 53.Kaplan FS, Shore EM. Encrypted morphogens of skeletogenesis: biological errors and pharmacologic potentials. Biochem Pharmacol. 1998;55:373–82. doi: 10.1016/s0006-2952(97)00559-5. [DOI] [PubMed] [Google Scholar]
- 54.Urtizberea JA, Testart H, Cartault F, Boccon-Gibod L, Le MM, Kaplan FS. Progressive osseous heteroplasia. Report of a family. J Bone Joint Surg Br. 1998;80:768–71. [PubMed] [Google Scholar]
- 55.Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7:E231–40. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung HJ, Kim HK, Yoon JJ, Park TG. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharm Res. 2006;23:1835–41. doi: 10.1007/s11095-006-9039-9. [DOI] [PubMed] [Google Scholar]
- 57.Chung YI, Tae G, Hong YS. A facile method to prepare heparin- functionalized nanoparticles for controlled release of growth factors. Biomaterials. 2006;27:2621–6. doi: 10.1016/j.biomaterials.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 58.Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Fetal and adult fibroblasts have similar TGF-beta-mediated, Smad-dependent signaling pathways. Plast Reconstr Surg. 2006;117:2277–83. doi: 10.1097/01.prs.0000224299.16523.76. [DOI] [PubMed] [Google Scholar]
- 59.Clark DA, Coker R. Transforming growth factor-beta (TGF-beta) Int J Biochem Cell Biol. 1998;30:293–8. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 60.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–6. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 61.Hosokawa R, Nonaka K, Morifuji M, Shum L, Ohishi M. TGF-beta 3 decreases type I collagen and scarring after labioplasty. J Dent Res. 2003;82:558–64. doi: 10.1177/154405910308200714. [DOI] [PubMed] [Google Scholar]
- 62.Levine JH, Moses HL, Gold LI, Nanney LB. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993;143:368–80. [PMC free article] [PubMed] [Google Scholar]
- 63.Matsunaga S, Yamamoto T, Fukumura K. Temporal and spatial expressions of transforming growth factor-betas and their receptors in epiphyseal growth plate. Int J Oncol. 1999;14:1063–7. doi: 10.3892/ijo.14.6.1063. [DOI] [PubMed] [Google Scholar]
- 64.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]