Abstract
Background
Despite certain levels of clinical efficacy, current autografts and synthetic materials for soft-tissue reconstruction and/or augmentation suffer from donor-site morbidity, rupture, dislocation, and volume reduction. Human adult stem cells can self-replicate and differentiate into adipogenic cells in response to appropriate signaling cues. This study investigated the shape and dimension maintenance of engineered adipose tissue from adult human mesenchymal stem cells.
Methods
Human mesenchymal stem cells were isolated from bone marrow of a healthy donor and differentiated into adipogenic cells. Adipocytes derived from these cells were encapsulated in a poly(ethylene glycol)-based hydrogel shaped into a generic cylinder (n = 6 implants), with hydrogel encapsulating human mesenchymal stem cells (n = 6) and cell-free hydrogel (n = 6) as controls. Porous collagen sponges were also used to seed human mesenchymal stem cell–derived adipocytes (n = 6), human mesenchymal stem cells (n = 4), or without cells (n = 4). All poly(ethylene glycol) and collagen constructs were implanted subcutaneously in athymic mice for 4 weeks.
Results
In vivo grafts demonstrated the formation of substantial adipose tissue encapsulating human mesenchymal stem cell–derived adipogenic cells in either poly(ethylene glycol)-based hydrogel or collagen sponge and a lack of adipose tissue formation in cell-free or human mesenchymal stem cell–derived grafts. Engineered adipose tissue in poly(ethylene glycol)-based hydrogel maintained approximately 100 percent of the original dimensions after 4-week in vivo implantation, significantly higher than the approximately 35 to 65 percent volume retention by collagen sponge.
Conclusions
These findings demonstrate that the predefined shape and dimensions of adipose tissue engineered from human mesenchymal stem cells can be maintained after in vivo implantation. These data further indicate the potential for autologous applications in reconstructive and plastic surgery procedures.
Soft-tissue reconstruction or augmentation represents one of the most acute challenges in all surgical procedures following trauma, tumor resection operations, or congenital anomalies. Plastic surgeons are constantly burdened with the clinical needs for soft-tissue augmentations and/or reconstructions to improve the aesthetic contour of soft tissues.1–3 In 2002, a total of 6.2 million individuals in the United States underwent reconstructive surgery procedures.4 In the majority of plastic surgery procedures, subcutaneous adipose tissue is an important component of the missing soft tissue.5–7
Currently, plastic surgeons use autologous tissue grafts or synthetic implants for the reconstruction and/or augmentation of soft tissues.8–10 Autologous soft-tissue grafts are commonly used because allografts, xenografts, and synthetic materials have complications such as pathogen transmission and immune rejection issues.11–13 Various levels of clinical success have been reported with the use of autologous soft tissue.11,12,14–16 However, donor-site morbidity remains the principal drawback of autologous soft-tissue grafts.17 In addition, autologous tissue grafts have a tendency to lose volume and/or shape up to an average of 40 to 60 percent reduction in tissue volume over time.7,18,19 Synthetic materials such as silicone or saline implants have the advantage of endless supply and have been documented to replace missing soft tissues with various levels of clinical success. However, synthetic materials have drawbacks, such as rupture, leakage, dislocation, and suboptimal biocompatibility. Certain synthetic materials interfere with the detection of breast cancer.20 Engineered soft tissue from stem cells has the potential to overcome most of the deficiencies associated with autologous soft-tissue grafts and synthetic materials.6,21–23 Stem cells readily self-replicate and are therefore capable of replenishing the supply of tissue-forming cells, including adipogenic cells. In a seminal study, bone marrow–derived mesenchymal stem cells have been shown to differentiate into adipogenic cells in vitro in monolayer culture.24 Mesenchymal stem cells that can be isolated by means of minimally invasive procedures from bone marrow or other anatomical locations are highly expandable in culture and can be readily induced to differentiate into adipose tissue–forming cells after exposure to adipogenic inducing supplements.24–26
Shape and dimensions are of critical importance to plastic surgery applications of engineered soft tissue. Creation and maintenance of the shape and dimensions of the engineered soft tissue are perhaps best accomplished by scaffold biomaterials. Hydrogels are hydrophilic, three-dimensional polymer networks that absorb water or biological fluids while maintaining their distinct three-dimensional structure.27 Among the hydrogel family, poly(ethylene glycol)-based hydrogel polymers have certain advantages for tissue-engineering applications, not only because of their proven biocompatibility28–33 but also because of their demonstrated capacity to support the growth and differentiation of mesenchymal stem cells into multiple lineages such as bone, cartilage, and adipose tissue.21,34,35 A notable disadvantage of poly(ethylene glycol)-based hydrogels in bone tissue engineering is their slow degradation rate.26–28 However, the slow degradation rate of poly(ethylene glycol)-based hydrogels can be chemically and/or biologically modified and may prove to be advantageous in adipose tissue engineering (e.g., maintaining the volume of engineered adipose tissue).
The shape of the engineered soft tissue must be created to fit the missing soft tissue in the patient.7,18 Our recent work has demonstrated the molding into predefined shape and dimensions of the engineered adipose tissue that has later formed in vivo and de novo from adipogenic cells.22 Subsequently, the created shape and dimensions of the engineered soft tissue need to be maintained over the long term after surgical implantation in the host so that the outcome of plastic surgical procedures is long lasting. Several previous reports have shown that the dimensions of the engineered adipose tissue are retained to various degrees.21,34,35 For example, porous alginate hydrogel seeded with fibroblasts appears to be more facilitative in retaining the volume of engineered soft tissue than cell-free hydrogel.34 However, in all previous reports, some changes in the shape and dimensions of the engineered adipose tissue have occurred.21,22,34,35 We have recently demonstrated that the shape and dimensions of the engineered adipose tissue by encapsulating adipogenic cells in a poly(ethylene glycol)-based hydrogel with pore sizes that are sufficiently small to disallow host cell invasion have been maintained virtually 100 percent following 4 weeks of in vivo implantation.23 The goal of the current study was to compare the poly(ethylene glycol)-based hydrogel with a porous collagen sponge in their relative efficacy toward shape and dimension maintenance in adipose tissue engineering from bone marrow–derived human mesenchymal stem cells.
MATERIALS AND METHODS
Isolation and Culture of Human Bone Marrow–Derived Mesenchymal Stem Cells
Human mesenchymal stem cells were isolated from fresh bone marrow samples of an anonymous adult male donor (AllCells, Berkeley, Calif.) by following our previous methods.24 After transferring bone marrow sample to a 50-ml tube, a total of 750 μl of RosetteSep (StemCell Technologies, Vancouver, B.C., Canada) was added with a ratio of 50 μl/ml of bone marrow and incubated for 20 minutes at room temperature. Then, 15 ml of phosphate-buffered saline in 2% fetal bovine serum and 1 mM ethylenediaminetetraacetic acid solution was added to the bone marrow sample to a total volume of approximately 30 ml. The bone marrow sample was then layered on 15 ml of Ficoll-Paque (StemCell Technologies) in two 50-ml tubes that were centrifuged 25 minutes at 3000 g at room temperature. The entire layer of enriched cells was removed from the Ficoll-Paque interface. The cocktail was centrifuged at 1000 rpm for 10 minutes. The solution was aspirated into 500 μl of
Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, Inc., St. Louis, Mo.) with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, Ga.) and 1% antibiotic-antimycotic (Gibco, Carlsbad, Calif.), referred to as basal medium hereafter. The isolated mononuclear cells were counted under an inverted microscope, plated at approximately 0.5 to 1 × 106 cells per 100-mm Petri dish, and incubated in basal medium at 37°C and 5% carbon dioxide. After 24 hours, nonadherent cells were discarded, whereas adherent cells were washed twice with phosphate-buffered saline and incubated for 12 days with fresh medium change every 3 to 4 days.26 On 80 to 90 percent confluence, primary human mesenchymal stem cells were trypsinized with 0.25% trypsin and 1 mM ethyl-enediaminetetraacetic acid for 5 minutes at 37°C, counted using a hemacytometer, and replated in 100-mm Petri dishes.
Inducing Human Mesenchymal Stem Cells to Differentiate into Adipocytes
First-passage human mesenchymal stem cells were induced to differentiate into adipocyte-like cells by exposure to 10% human mesenchymal stem cell adipogenic differentiation medium (StemCell Technologies) with 1% antibiotic-antimycotic (Gibco) for 1 week (in vitro observations) and 4 weeks (for in vivo implantation). Adipogenic stimulating medium consisted of MesenCult medium for human mesenchymal stem cells (StemCell Technologies), 10% human mesenchymal stem cell adipogenic stimulatory supplements (StemCell Technologies), and 1% basal medium (Gibco). The adipogenic differentiation medium consisted of basal medium, 50 nM dexamethasone, 10 mM insulin, and 5 mM isobutyl-methylxanthine. A parallel population of human mesenchymal stem cells was continuously cultured in basal medium also in 95% air and 5% carbon dioxide at 37°C with medium changes every 3 to 4 days. Oil-Red O staining (Sigma) was used to demonstrate whether lipid vacuoles had formed in the monolayer cultures of human mesenchymal stem cells treated with adipogenic stimulatory supplemented medium or basal medium. For in vitro assessment of adipogenic differentiation, bone marrow–derived human mesenchymal stem cells were treated with adipogenic differentiation supplemented medium for 1 week. Monolayer cultured human mesenchymal stem cells with or without adipogenic differentiation were fixed in 10% formalin for 15 minutes and subjected to Oil-Red O staining. The plates were examined under an inverted microscope at 10× magnification for the presence or absence of lipid vacuoles with Oil-Red O.
Preparation of Poly(Ethylene Glycol)-Based Hydrogels and Cell Encapsulation
Poly(ethylene glycol) diacrylate (molecular weight, 3400; Shearwater Polymers, Huntsville, Ala.) was dissolved in sterile phosphate-buffered saline supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) to a final solution of 10% w/v. A photoinitiator, 2-hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (Ciba, Tarrytown, N.Y.), was added to the poly(ethylene glycol) diacrylate solution, yielding a final photoinitiator concentration of 0.05% w/v. After 4-week adipogenic differentiation or culture in basal medium, human mesenchymal stem cells were trypsinized with 0.25% trypsin and 1 mM ethylenediaminetet-raacetic acid for 5 minutes at 37°C, counted, and resuspended separately in poly(ethylene glycol) diacrylate polymer/photoinitiator solutions at a density of 3 × 106 cells/ml. An aliquot of 150 μl cell/polymer/photoinitiator suspension was loaded into sterilized plastic caps of 1.5 ml microcentrifuge tubes (9-mm diameter) (Fisher Scientific, Hampton, N.H.), followed by photopolymerization with long-wave, 365-nm ultraviolet lamp (Glo-Mark, Upper Saddle River, N.J.) at an intensity of approximately 4 mW/cm2 for 5 minutes. The photopolymerized cell-poly(ethylene glycol) constructs were removed from the plastic caps and transferred into 100-mm Petri dishes in corresponding adipogenic or basal medium.
Preparation of Collagen Constructs and Cell Seeding
Sterile collagen sponges (Helistat, Plainsboro, N.J.) were each trimmed into 4 × 4 × 4-mm cubes and immersed in basal medium for 1 hour. After 4-week adipogenic induction in adipogenic supplemented medium or basal medium as described above, human mesenchymal stem cells or human mesenchymal stem cell–derived adipocytes were trypsinized with 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid for 5 minutes at 37°C, counted, and resuspended in basal medium or adipogenic medium, respectively, and seeded into collagen sponges at a cell density of 2 × 106 cells/ml. Gentle vacuum was applied with a 20-ml syringe to facilitate seeding the cells throughout the collagen sponges, followed by short incubation at 37°C for 2 hours. The rationale to have used a higher cell density in poly(ethylene glycol)-based hydrogel (3 × 106 cells/ml) than in collagen sponge (2 × 106 cells/ml) is that whereas host cells do not infiltrate poly(ethylene glycol) on in vivo implantation, they do infiltrate collagen sponges. Thus, the initially high cell density in poly(ethylene glycol)-based hydrogel may be neutralized by infiltrating host cells into collagen sponges.
Cell Viability Assays for Cell-Encapsulated Poly-(Ethylene Glycol) Scaffold and Cell-Seeded Collagen Sponges
Cell viability of human mesenchymal stem cell-derived adipocytes encapsulated in poly(ethylene glycol) or seeded in collagen sponges was assessed after 24-hour incubation in vitro using a live/dead viability/cytotoxicity kit (L-3224; Molecular Probes, Eugene, Ore.) by following the manufacturer’s recommended protocol.23,26 Calcein diffuses through cell membrane and reacts with intracellular esterase to produce green fluorescence, whereas ethidium homodimer diffuses only through damaged cell membrane and bonds to nucleic acids to produce red fluorescence. After labeling, the number of live/dead cells was viewed and counted under an inverted optical microscope (Leica Microsystems, Wetzlar, Germany) equipped with a long-pass dual-emission fluorescent filter (Chroma, Rockingham, Vt.).
In Vivo Implantation of Cell-Scaffold Constructs
For in vivo implantation, 4-month-old athymic nude mice (Harlan, Indianapolis, Ind.) were placed under general anesthesia with intraperitoneal injection of 100 mg/kg ketamine plus 5 mg/kg xylazine. The dorsal skin was disinfected with 10% povidone-iodine and 70% alcohol. After skin incision along the dorsal midsagittal plane, subcutaneous pockets were prepared by blunt dissection. The polymerized poly(ethylene glycol)-based hydrogel constructs encapsulating human mesenchymal stem cell–derived adipogenic cells (n = 6), or with expanded human mesenchymal stem cells (n = 6), or cell-free poly-(ethylene glycol) control constructs (n = 6) were implanted in the subcutaneous pouches of athymic mice. Each mouse received at least all three types of constructs: poly(ethylene glycol)-based hydrogel constructs containing human mesenchymal stem cell-derived adipogenic cells, poly(ethylene glycol)-based hydrogel constructs containing human mesenchymal stem cells, and cell-free poly(ethylene glycol)-based hydrogel construct. Collagen sponge constructs loaded with human mesenchymal stem cell–derived adipogenic cells (n = 6), expanded human mesenchymal stem cells (n = 4), and cell-free collagen constructs (n = 4) were implanted in subcutaneous pouches in the dorsum of athymic mice (Harlan). In each athymic mouse, a total of four constructs were placed in four adjacent pouches, all with a combination of cell-seeded constructs and cell-free constructs. Our previous work has shown that placement of cell-seeded and cell-free constructs in one athymic mouse, while minimizing the number of experimental animals, does not lead to cross-interactions.23,32,33 The incision was closed by a sterile absorbable 3-0 surgical suture. The entire procedure was performed under sterile conditions. The athymic mice were closely monitored until they gained sternal position. Four weeks after implantation, the animals were killed by carbon dioxide overdose. After incision and careful retrieval of the implanted grafts, the fibrous capsule surrounding each construct was carefully removed. The harvested grafts were then fixed in 10% formalin for further histologic analysis. All animal experiments received the appropriate approval from the institutional animal care committee.
Histologic Analysis of In Vivo Harvested Poly-(Ethylene Glycol)-Based Hydrogel and Collagen Grafts
After 4-week in vivo implantation in the dorsum of athymic mice, poly(ethylene glycol) and collagen grafts were embedded in optimum cutting temperature tissue freezing mixture (IMEB, Chicago, Ill.). Thin sections (10 μm) were cut using standard histologic technique, according to our previous methods.23,32 Sections were stained with hematoxylin and eosin and Oil-Red O (Sigma), mounted with glycerol gelatin (Sigma), and viewed under a light microscope (Leica).
Statistical Analysis of Dimensions of In Vivo Harvested Poly(Ethylene Glycol) and Collagen Adipose Tissue Grafts
The study design was to compare the dimensions of collagen and poly(ethylene glycol)-based hydrogel constructs without cells and seeded with human mesenchymal stem cells and human mesenchymal stem cell-derived adipocytes after in vivo implantation with analysis of variance and Bonferroni tests at an alpha level of 0.05. Analysis of variance was used for both within- and between-group comparisons.
RESULTS
All surgical procedures were uneventful. The athymic mice tolerated in vivo implantation procedures well and did not show any major complications. All results described below were consistent among all samples in the same category.
Differentiation of Human Mesenchymal Stem Cells into Adipogenic Cells
Typical monolayer culture of bone marrow–derived human mesenchymal stem cells placed in basal medium (without adipogenic stimulation) is shown in Figure 1, above, left. The monolayer human mesenchymal stem cells were stained negatively to Oil-Red O, a typical adipogenic marker (Fig. 1, below, left). In contrast, the same population of human mesenchymal stem cells treated with adipogenic stimulating medium for 1 week showed different cell morphology and the presence of rounded intracellular droplets (Fig. 1, above, right). Oil-Red O staining indicated that extracellular matrix droplets seen in Figure 1, above, right, were lipid vacuoles (Fig. 1, below, right), suggesting that these bone marrow–derived human mesenchymal stem cells had differentiated into adipogenic cells. This is consistent with previous findings of adipogenic differentiation from mesenchymal stem cells from several species, including humans.23,24 This in vitro adipogenic differentiation from human mesenchymal stem cells is a prerequisite for adipose tissue engineering in vivo.
Fig. 1.
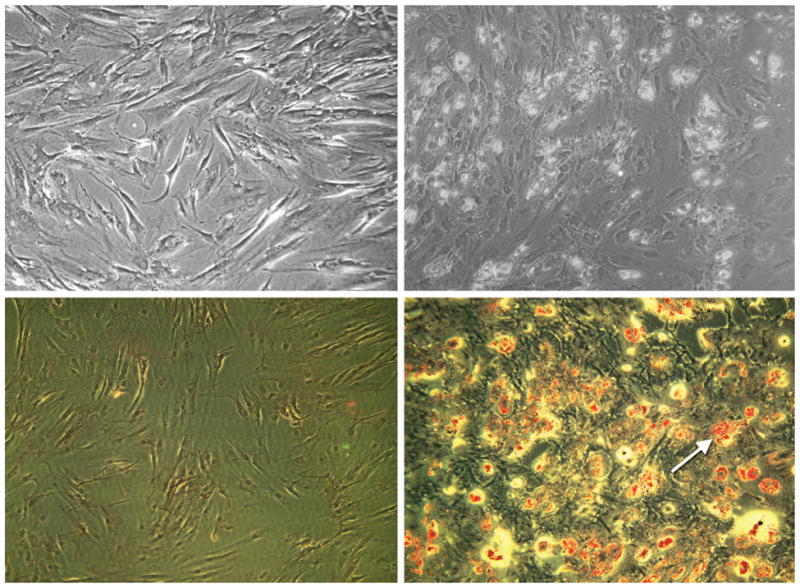
Human mesenchymal stem cells and their adipogenic differentiation in monolayer culture. (Above, left) Phase-contrast image of human mesenchymal stem cells undergoing proliferation. Note the fibroblast-like appearance of human mesenchymal stem cells (cf. Alhadlaq and Mao, 2004). (Below, left) Negative Oil-Red O staining of human mesenchymal stem cells without adipogenic induction, as in above, left. (Above, right) After treatment with adipogenic supplements of dexamethasone, insulin, and isobutyl-methylxanthine, the same population of human mesenchymal stem cells as in above, left treated with adipogenic stimulating medium for 1 week showed different cell morphology and the presence of rounded extracellular matrix vacuoles. (Below, right) Positive Oil-Red O staining of lipid vacuoles (arrow) of human mesenchymal stem cells treated with adipogenic induction indicates that human mesenchymal stem cells had differentiated into adipogenic cells.
Cell Viability in Poly(Ethylene Glycol)-Based Hydrogel and Collagen Sponge Scaffolds
On confirmation that human mesenchymal stem cells were differentiated into adipogenic cells in monolayer culture, the next concern was whether encapsulated cells were able to survive in polymer scaffolds such as the presently used collagen or poly(ethylene glycol). Live and dead cell assay (data not shown) confirmed that the majority of human mesenchymal stem cell–derived adipogenic cells, such as those seen in Figure 1, below, right, encapsulated in poly(ethylene glycol)-based hydrogel were labeled with green calcein, indicating their survival after hydrogel encapsulation. The majority of human mesenchymal stem cell–derived adipogenic cells also survived cell seeding in the collagen sponge. Therefore, adipogenic cells derived from human bone marrow mesenchymal stem cells survived seeding in both a synthetic and biocompatible poly(ethylene glycol)-based hydrogel and a natural collagen sponge scaffold in three dimensions.
Shape and Dimensions of Tissue-Engineered Adipose Tissue
After 4-week in vivo implantation, both poly(ethylene glycol)-based hydrogel grafts and collagen sponge grafts were harvested from the subcutaneous pouches in the dorsum of athymic mice. Figure 2 demonstrates the retrieval process of poly(ethylene glycol)-based hydrogel scaffolds. The cell-free poly-(ethylene glycol)-based hydrogel scaffold (arrows in Fig. 2, above) was isolated from the subcutaneous pouch of athymic mouse. Note the somewhat pale color of the cell-free poly(ethylene glycol)-based hydrogel scaffold and that the diameter of the cell-free poly(ethylene glycol)-based hydrogel was approximately 9 mm, as originally fabricated (Fig. 2, above). In contrast, poly(ethylene glycol)-based hydrogel scaffold encapsulating human mesenchymal stem cells (without adipogenic differentiation) appeared to be darker in color and yet attached to the host subcutaneous tissue (Fig. 2, center). The diameter of the poly(ethylene glycol)-based hydrogel encapsulating human mesenchymal stem cells (without adipogenic differentiation) was also approximately 9 mm, as originally fabricated (Fig. 2, center). Most interestingly, poly(ethylene glycol)-based hydrogel encapsulating human mesenchymal stem cell–derived adipogenic cells, such as those in Figure 1, below, right, appeared darker in color and well attached to the host subcutaneous tissue (Fig. 2, below). Note that the diameter of the poly(ethylene glycol)-based hydrogel encapsulating human mesenchymal stem cell–derived adipogenic cells was cylindrical in shape and approximately 9 mm, as originally fabricated (different magnification in Fig. 2, below). Thus, macroscopic examination qualitatively revealed that poly(ethylene glycol)-based hydrogel contributed to the shape and dimension maintenance of human mesenchymal stem cell–derived adipogenic cells.
Fig. 2.
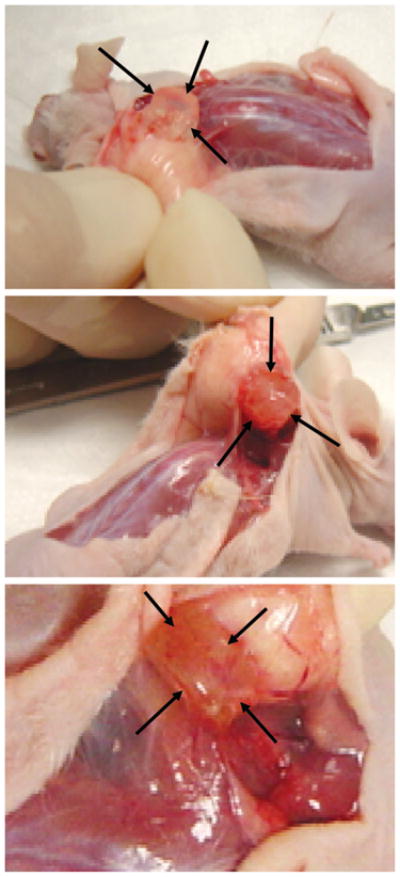
Harvest of adipogenic poly(ethylene glycol)-based hydrogel grafts from human mesenchymal stem cells and control groups after in vivo implantation in athymic mice. (Above) Representative cell-free poly(ethylene glycol)-based hydrogel construct (between arrows) showing retention of the original size (9-mm diameter). (Center) Representative poly(ethylene glycol)-based hydrogel construct encapsulating human mesenchymal stem cells without adipogenic differentiation (between arrows) showing that poly(ethyleneglycol)-based hydrogel adhered to surrounding host tissue and retained the original size (9-mm diameter). (Below) Representative poly(ethylene glycol)-based hydrogel construct (between arrows) encapsulating adipogenic cells derived from human mesenchymal stem cells showing its adhesion to surrounding host tissue and retention of the original size (9-mm diameter; greater magnification).
Figure 3 demonstrates the shape, dimensions, and different photo-opaqueness of poly(ethylene glycol)-based hydrogel grafts after in vivo implantation. Figure 3, above, left shows a plastic cap of a 1.5-ml microcentrifuge tube (9-mm diameter) that was used as a generic mold of the shape and dimensions for tissue-engineered adipose tissue. The representative cell-free poly(ethylene glycol)-based hydrogel scaffold, despite its general maintenance of the shape and diameter, was largely transparent (Fig. 3, above, right). The representative poly(ethylene glycol)-based hydrogel scaffold encapsulating human mesenchymal stem cells without adipogenic differentiation showed photo-opaqueness and general maintenance of the original shape and dimensions (Fig. 3, below, left). Most interestingly, the representative poly(ethylene glycol)-based hydrogel construct encapsulating human mesenchymal stem cell–derived adipogenic cells not only maintained the original shape and dimensions but also demonstrated substantial photo-opaqueness (Fig. 3, below, right), suggesting that a substantial amount of extracellular matrix had been synthesized in the human mesenchymal stem cell–derived adipogenic poly(ethylene glycol)-based hydrogel construct.
Fig. 3.
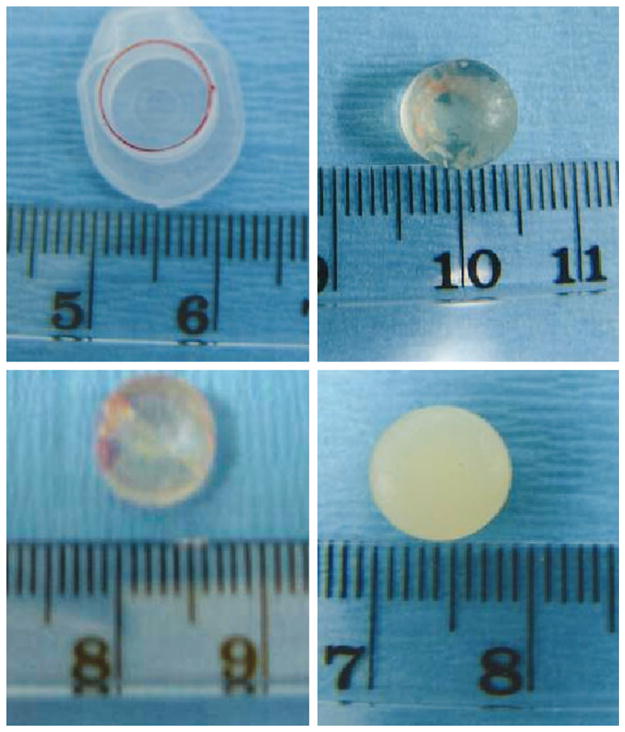
Shape, dimensions, and photo-opaqueness of in vivo harvested poly(ethylene glycol)-based hydrogel construct encapsulating engineered adipose tissue from human mesenchymal stem cells and control groups. (Above, left) Plastic cap of a 1.5-ml microcentrifuge tube (9-mm diameter) used as a generic mold of the shape and dimensions for engineered adipose tissue. (Above, right) Harvested cell-free poly(ethylene glycol)-based hydrogel is largely transparent. (Below, left) Poly(ethylene glycol)-based hydrogel encapsulating human mesenchymal stem cells (without adipogenic differentiation) showing some photo-opacity. (Below, right) Poly(ethylene glycol)-based hydrogel encapsulating adipogenic cells derived from human mesenchymal stem cells showing substantial photo-opacity. All poly(ethylene glycol) grafts maintained the original shape and dimensions (cf. above, left).
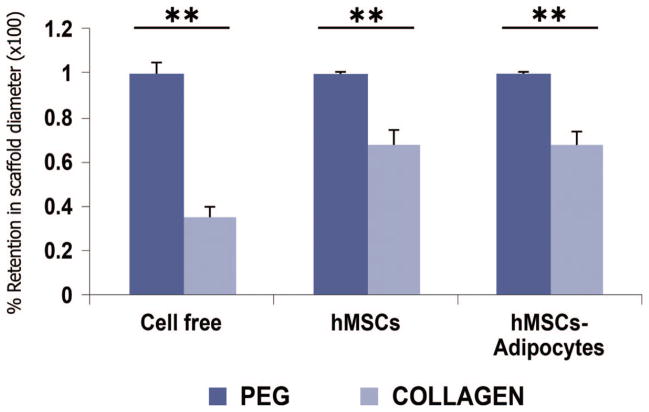
Quantification of the diameters of in vivo harvested poly(ethylene glycol)-based hydrogel scaffolds and collagen sponge scaffolds confirmed that poly(ethylene glycol)-based hydrogel scaffolds maintained the original shape and dimensions and that collagen sponge scaffolds shrunk and lost the original dimensions (Fig. 4). The collagen scaffolds, whether cell-free, seeded with human mesenchymal stem cells, or seeded with human mesenchymal stem cell–derived adipogenic cells, were on average 35.0 ± 5.0 percent, 68.3 ± 5.8 percent, and 68.3 ± 5.4 percent of the original dimension. In contrast, cell-free poly(ethylene glycol)-based hydrogels, human mesenchymal stem cell–encapsulated poly(ethylene glycol)-based hydrogels, and poly(ethylene glycol)-based hydrogel scaffolds encapsulating human mesenchymal stem cell–derived adipogenic cells maintained 99.5 ± 0.05 percent, 98.9 ± 0.05 percent, and 99.4 ± 0.05 percent of the original dimensions, respectively. The collagen scaffolds without cells, seeded with human mesenchymal stem cells, or seeded with human mesenchymal stem cell–derived adipogenic cells were significantly smaller than corresponding poly(ethylene glycol)-based hydrogels scaffolds without cells, encapsulating human mesenchymal stem cells, or human mesenchymal stem cell–derived adipogenic cells (analysis of variance, p < 0.01) (Fig. 4).
Fig. 4.
Quantitative analysis of retention of scaffold diameters (×100 percent ± SD) of harvested poly(ethylene glycol) and collagen grafts after 4 weeks of in vivo implantation (hMSCs, human mesenchymal stem cells). Dark blue bar graphs represent poly(ethylene glycol)-based hydrogel grafts; the lighter bar graphs represent collagen grafts. Poly(ethylene glycol)-based hydrogel without cells (n = 6), encapsulating human mesenchymal stem cells (n = 6), or encapsulating human mesenchymal stem cell– derived adipocytes(n = 6)maintained the original diameter of the poly(ethyleneglycol) scaffold nearly 100 percent. In comparison, collagen sponges without seeded cells (n = 4) lost the original diameter by 65.0 ± 5.0 percent, whereas collagen sponges seeded with human mesenchymal stem cells (n = 4) and with human mesenchymal stem cell– derived adipocytes (n = 6) lost the original diameters by 31.7 ± 5.8 percent and 31.7 ± 5.4 percent, respectively. **p < 0.01 (analysis of variance with Bonferroni). Analysis of variance was used for both within- and between-group comparisons, although p < 0.01 is only shown for within-group comparisons.
Histologic Features of Engineered Adipogenic Tissues
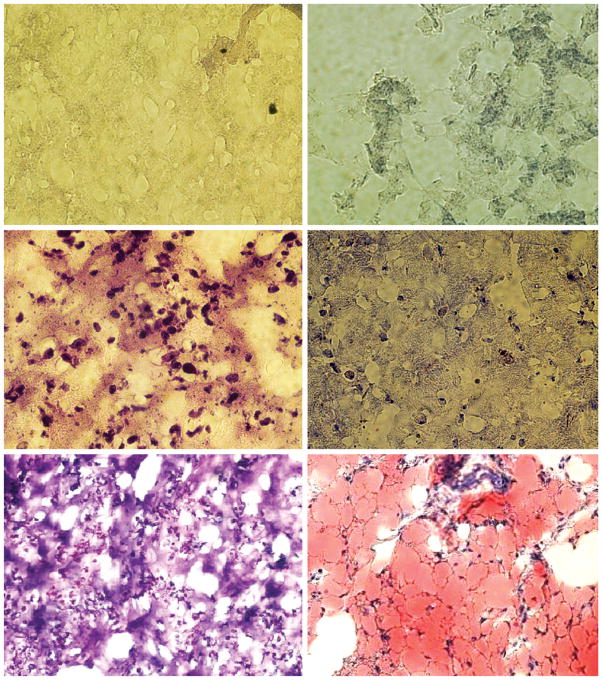
In vivo adipogenesis in poly(ethylene glycol)-based hydrogels and collagen sponges showed distinctive features. Cell-free poly(ethylene glycol)-based hydrogel stained with both hematoxylin and eosin and Oil-Red O showed typical gel-like structures (Fig. 5, above), consistent with our previous findings. Poly(ethylene glycol)-based hydrogels encapsulating human mesenchymal stem cells (without adipogenic differentiation) showed cellular structures surrounded by hydrogel (Fig. 5, center, left). However, Oil-Red O staining showed negative label of lipid vacuoles (Fig. 5, center, right). Poly(ethylene glycol)-based hydrogels encapsulating human mesenchymal stem cell–derived adipogenic cells after 4-week in vivo implantation showed cellular structures surrounded by hydrogel and multiple irregular islands of space (Fig. 5, below, left). Oil-Red O staining revealed widespread lipid vacuoles surrounded by flattened cells that resembled adipocytes (Fig. 5, below, right). The irregular islands of space in hematoxylin and eosin–stained section in Figure 5, below, left are likely filled with lipid vacuoles as shown in Figure 5, below, right. The lack of host cell invasion as evidenced in Figure 5, above indicates that all the adipose tissue in Figure 5, below, right has derived from the delivered adipogenic cells that differentiated from human mesenchymal stem cells, instead of host mouse cells.
Fig. 5.
Representative hematoxylin and eosin and Oil-Red O staining of tissue-engineered poly(ethylene glycol)-based hydrogel grafts retrieved after 4-week in vivo implantation in the dorsum of athymic mice (hMSCs, human mesenchymal stem cells). (Above) Representative hematoxylin and eosin– and Oil-Red O–stained micrographs of cell-free control poly(ethylene glycol)-based hydrogels construct showing neither resident cells nor lipid vacuoles. (Center, left) Representative hematoxylin and eosin–stained micrograph of poly(ethylene glycol)-based hydrogel construct encapsulating human mesenchymal stem cells demonstrates abundant resident cells. (Center, right) Representative Oil-Red O–stained micrograph of poly(ethylene glycol)-based hydrogel construct encapsulating human mesenchymal stem cells demonstrates a lack of lipid vacuoles. (Below, left) Representative hematoxylin and eosin–stained micrograph of poly(ethylene glycol)-based hydrogel construct encapsulating human mesenchymal stem cell– derived adipogenic cells demonstrates abundant resident cells among irregular islands of space. (Below, right) Representative Oil-Red O–stained micrograph of poly-(ethylene glycol)-based hydrogel construct encapsulating human mesenchymal stem cell– derived adipogenic cells demonstrates abundant lipid vacuoles among flattened cells that resemble adipocytes. It is probable that lipid vacuoles occupied the irregular islands of space seen in below, left (original magnification, ×10).
Cell-free collagen sponges stained with hematoxylin and eosin showed the presence of apparent collagen fiber bundles along with sparsely distributed cells that invaded the scaffold from the host (Fig. 6, above). However, Oil-Red O staining indicated a lack of adipogenesis in cell-free collagen sponges (Fig. 6, center). Collagen sponges seeded with human mesenchymal stem cells without adipogenic differentiation showed cellular structures among apparent collagen fiber bundles (Fig. 6, below). Similarly, Oil-Red O staining showed negative label of lipid vacuoles. Collagen sponges seeded with human mesenchymal stem cell–derived adipogenic cells after 4-week in vivo implantation showed sparsely distributed cells among collagen fiber-like structures and irregular islands of space. Oil-Red O staining disclosed clusters of lipid vacuoles surrounded by flattened cells with resemblance to adipocytes.
Fig. 6.
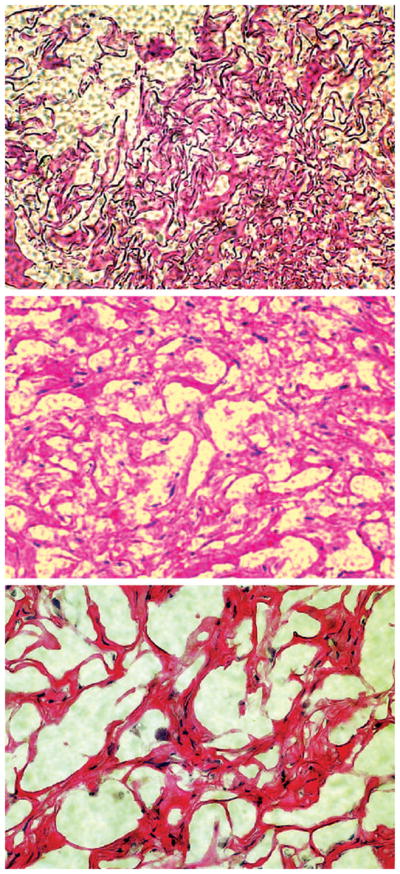
Representative hematoxylin and eosin and Oil-Red O staining of tissue-engineered collagen sponge grafts retrieved after 4-week in vivo implantation in the dorsum of athymic mice (hMSCs, human mesenchymal stem cells). (Above and center) Representative hematoxylin and eosin– and Oil-Red O–stained micrographs of cell-free control collagen sponge construct showing the presence of resident cells among apparent collagen bundles. (Below) Representative hematoxylin and eosin–stained micrograph of collagen sponge construct seeded with human mesenchymal stem cells demonstrates resident cells among apparent collagen bundles.
DISCUSSION
A portion of the present findings confirms our previous data that the shape and dimensions of engineered adipose tissue from adipocytes derived from adult human mesenchymal stem cells can be maintained after in vivo implantation.23 We envision that the present approach or a close approximation can lead to therapeutic applications. It is conceivable that autologous adult human stem cells will be isolated by using minimally invasive approaches from the patient; by means of the present approach, engineered adipose tissue with predefined shape and dimensions can be implanted in the patient. Volume retention by the present hydrogel system encapsulating adipogenic cells derived from human mesenchymal stem cells demonstrates the promise of the present hydrogel/cell encapsulation system for potential clinical application in soft-tissue reconstruction and augmentation procedures. A lack of host cell invasion into the implanted cell-free poly(ethylene glycol)-based hydrogels, as evidenced in our previous work23 and confirmed in the current work, indicates that all the engineered adipose tissue results from human mesenchymal stem cell–derived adipocytes instead of host mouse cells. In comparison, host cell invasion likely has occurred in porous collagen sponges.
The presently demonstrated adipogenic potential of human mesenchymal stem cells is consistent with previous studies designed to explore their multipotentiality.24,36,37 Adult mesenchymal stem cells can be obtained autologously from the same patient for whom adipose tissue is to be engineered, thus eliminating immunologic rejection issues.24,26 Because of their high expandability in culture and established protocols for multiple lineages of differentiation, mesenchymal stem cells have been increasingly used as the cell source for cell-based tissue-engineering approaches.7,9,33,38 Our previous work also showed the expression of an adipocyte-related gene (PPAR-g2) in poly(ethylene glycol) grafts encapsulating human mesenchymal stem cell–derived adipogenic cells harvested after 4 weeks of subcutaneous implantation in athymic mice.23 Thus, bone marrow–derived adult stem cells are a reliable cell source for adipose tissue engineering, and can be autologous from the patient who needs adipose tissue.
Shape and dimension formation of engineered adipose tissue is of critical importance to reconstructive and plastic surgical procedures, similar to the maintenance of the created shape and dimension for prolonged duration after in vivo implantation. An advantage of poly(ethylene glycol)-based hydrogel is that it is malleable into a given shape and dimension.23,32,33 A previously regarded disadvantage of the slow degradation rate of poly(ethylene glycol)-based hydrogel appears to be advantageous for adipose tissue engineering in that poly(ethylene glycol)-based hydrogel enables the maintenance of shape and dimensions of engineered adipose tissue. It is notable that poly-(ethylene glycol) grafts without cells, encapsulating human mesenchymal stem cells, and human mesenchymal stem cell–derived adipocytes all maintained virtually 100 percent of their original shape and dimensions after 4 weeks of in vivo implantation. Thus, the maintenance of shape and dimensions of engineered adipose tissue by 4-week in vivo implantation is likely attributable to the properties of poly(ethylene glycol)-based hydrogel. Longer term studies using the present systems are necessary to determine the maintenance of shape and dimensions of poly(ethylene glycol)-based hydrogel grafts to determine the parallel processes of poly(ethylene glycol)-based hydrogel degradation and adipose tissue synthesis by human mesenchymal stem cell–derived adipocytes. It is important in longer term studies that poly-(ethylene glycol) grafts encapsulating human mesenchymal stem cells and cell-free poly(ethylene glycol) are included to determine the contribution of poly(ethylene glycol)-based hydrogel in shape and dimension maintenance. Previous work has demonstrated that poly(ethylene glycol) supports cell viability and the differentiation of mesenchymal stem cells into multiple mesenchymal lineages such as chondrogenic, osteogenic,33,39 and adipogenic.23 Because of its large water content, poly(ethylene glycol)-based hydrogel possesses superior diffusion properties and provides the encapsulated cells with an extracellular matrix–like environment.28,29,40
In contrast to shape and dimension maintenance by poly(ethylene glycol)-based hydrogel encapsulating adipogenic cells derived from human mesenchymal stem cells, collagen sponges seeded with the same cells shrink substantially and lose the predefined shape and dimensions. The invaded host cells into collagen sponges likely consist of heterogeneous populations such as fibroblasts, adipocytes, macrophages, and others. Although host cell invasion may be useful in the long run, only selective cell types are desirable at appropriate time points in the engineered tissue.41 Poly(ethylene glycol)-based hydrogel is advantageous in this regard in that its initial property not to allow host cell invasion can be modulated by copolymerizing with another biocompatible material.42 A key to long-term vitality of engineered soft tissue is angiogenesis. Our ongoing experiments show that angiogenesis and host tissue ingrowth can be engineered in hydrogel scaffolds in vivo. Alternatively, other hydrogel materials may also be useful in adipose tissue engineering. For example, peptide-linked alginate implants supported the adhesion and proliferation of seeded sheep preadipocytes and adipose tissue formation.21 Seeding dermal fibroblasts in alginate implants leads to significant ingrowth of fibrovascular stroma, with cells thoroughly distributed within the gel.34 The volume of the in vivo implanted alginate constructs has varied between 19 and 88 percent after 8 weeks of subcutaneous implantation in rats, with the highest percentage of volume maintenance on seeding fibroblasts in alginate and in situ solidification after subcutaneous injection.34
It is notable that most previous adipose tissue engineering work has used porous scaffolds such as polyglycolic acid, hyaluronic acid–based scaffolds, Matrigel, alginate, collagen sponges, or fibrin.21,22,35 A common feature associated with the use of porous scaffolds in adipose tissue engineering is ready resorption and/or degradation of the porous scaffold, potentially associated with enzymes produced by invading host cells. Poly(ethylene glycol)-based hydrogel that has been used in our previous and current work has the advantage of not allowing host cell invasion initially and yet retaining virtually 100 percent of the predefined shape and dimensions after in vivo implantation.23
CONCLUSIONS
Based on the premise of our current approaches, engineered adipose tissue not only has survived in vivo in the host, likely by means of nutrient diffusion, but has maintained its predefined shape and dimensions. From the progress in adipose tissue engineering within a short period of time,19 it appears that multiple technological approaches will become available for clinical trials in reconstructive and augmentative surgical procedures in the foreseeable future.
Acknowledgments
This research was supported by National Institutes of Health grants DE13964, DE15391, and EB02332. The authors are grateful to Aurora Lopez for general technical assistance and to three anonymous reviewers, whose insightful comments helped improve the quality of their article.
Footnotes
DISCLOSURE
None of the authors has any financial interest in the products, devices, or drugs mentioned in this article.
References
- 1.Patrick CW, Jr, Chauvin PB, Reece GP. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999;5:139. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 2.Beahm EK, Walton RL, Patrick CW., Jr Progress in adipose tissue construct development. Clin Plast Surg. 2003;30:547. doi: 10.1016/s0094-1298(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 3.Alster TS, West TB. Human-derived and new synthetic injectable materials for soft-tissue augmentation: Current status and role in cosmetic surgery. Plast Reconstr Surg. 2000;105:2515. doi: 10.1097/00006534-200006000-00034. [DOI] [PubMed] [Google Scholar]
- 4.American Society of Plastic Surgeons (Web site) [Accessed March 26, 2005.]. Available at: http//www.plasticsurgery.org.
- 5.Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587. [PubMed] [Google Scholar]
- 6.Brey EM, Patrick CW., Jr Tissue engineering applied to reconstructive surgery. IEEE Eng Med Biol Mag. 2000;19:122. doi: 10.1109/51.870241. [DOI] [PubMed] [Google Scholar]
- 7.Patrick CW., Jr Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;263:361. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 8.Atala A. Future perspectives in reconstructive surgery using tissue engineering. Urol Clin North Am. 1999;26:157. doi: 10.1016/s0094-0143(99)80013-5. [DOI] [PubMed] [Google Scholar]
- 9.Walgenbach KJ, Shestak KC. Pedicled TRAM breast reconstruction. Breast Dis. 2002;16:73. doi: 10.3233/bd-2002-16111. [DOI] [PubMed] [Google Scholar]
- 10.Garfein ES, Orgill DP, Pribaz JJ. Clinical applications of tissue engineered constructs. Clin Plast Surg. 2003;30:485. doi: 10.1016/s0094-1298(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 11.Butler DL, Awad HA. Perspectives on cell and collagen composites for tendon repair. Clin Orthop. 1999;367:S324. doi: 10.1097/00003086-199910001-00031. [DOI] [PubMed] [Google Scholar]
- 12.Eppley BL. Alloplastic implantation. Plast Reconstr Surg. 1999;104:1761. doi: 10.1097/00006534-199911000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Hart D. Overcoming complications of breast implants. Plast Surg Nurs. 2003;23:55. doi: 10.1097/00006527-200323020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 15.Erol OO, Spira M. Reconstructing the breast mound employing a secondary island omental skin flap. Plast Reconstr Surg. 1990;86:510. doi: 10.1097/00006534-199009000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Kononas TC, Bucky LP, Hurley C, Amy JWJ. The fate of suctioned and surgically removed fat after reimplantation for soft tissue augmentation: A volumetric and histologic study in the rabbit. Plast Reconstr Surg. 1993;91:763. doi: 10.1097/00006534-199304001-00001. [DOI] [PubMed] [Google Scholar]
- 17.Patrick CW, Jr, Zheng B, Johnston C, Reece GP. Long-term implantation of preadipocyte-seeded PLGA scaffolds. Tissue Eng. 2002;8:283. doi: 10.1089/107632702753725049. [DOI] [PubMed] [Google Scholar]
- 18.Lee KY, Halberstadt CR, Holder WD, Mooney DJ. Breast reconstruction. In: Lanza RP, Langer R, Vacanti J, editors. Principles of Tissue Engineering. San Diego: Academic Press; 2000. pp. 409–423. [Google Scholar]
- 19.Lorenz HP, Hedrick MH, Chang J, Mehrara BJ, Longaker MT. The impact of biomolecular medicine and tissue engineering on plastic surgery in the 21st century. Plast Reconstr Surg. 2000;105:2467. doi: 10.1097/00006534-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Patrick CW, Jr, Miller MJ. Tissue engineering. Clin Plast Surg. 2003;1:91. doi: 10.1016/s0094-1298(02)00071-8. [DOI] [PubMed] [Google Scholar]
- 21.Halberstadt C, Austin C, Rowley J, Culberson C, Loebsacl A, Wyatt S. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Eng. 2002;8:309. doi: 10.1089/107632702753725067. [DOI] [PubMed] [Google Scholar]
- 22.Fischbach A. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: Implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger MF. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Caplan AI. Embryonic development and the principles of tissue engineering. Novartis Found Symp. 2003;249:17. [PubMed] [Google Scholar]
- 26.Alhadlaq A, Mao JJ. Mesenchymal stem cells: Isolation and therapeutics. Stem Cells Dev. 2004;13:436. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 27.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 28.Poshusta AK, Anseth KS. Photopolymerized biomaterials for application in the temporomandibular joint. Cells Tissues Organs. 2001;169:272. doi: 10.1159/000047891. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Fiegel J, Krauland E, Hanes J. New polymeric carriers for controlled drug delivery following inhalation or injection. Biomaterials. 2002;23:4425. doi: 10.1016/s0142-9612(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 31.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 32.Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 33.Alhadlaq A, Elisseeff JH, Hong L, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 34.Marler JJ, Guha A, Rowley J, Koka R, Mooney D, Upton J. Soft-tissue augmentation with injectable alginate and syngeneic fibroblasts. Plast Reconstr Surg. 2000;105:2049. doi: 10.1097/00006534-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Von Heimburg D, Zachariah S, Heschel I, et al. Human preadipocytes seeded on freeze dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 2001;22:429. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 36.Caplan AI, Bruder SP. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 37.Tuan RS, Kuo CK. Tissue engineering with mesenchymal stem cells. IEEE Eng Med Biol Mag. 2003;22:51. doi: 10.1109/memb.2003.1256272. [DOI] [PubMed] [Google Scholar]
- 38.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 39.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 40.Huss FR, Kratz G. Mammary epithelial cell and adipocyte co-culture in a 3-D matrix: The first step towards tissue-engineered human breast tissue. Cells Tissues Organs. 2001;169:361. doi: 10.1159/000047903. [DOI] [PubMed] [Google Scholar]
- 41.Lee RH. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 42.Rahaman MN, Mao JJ. Stem cell based composite tissue constructs for regenerative medicine. Biotech Bioeng. 2005;91:261. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]