Abstract
Poly(ADP-ribose) polymerases (PARPs) are involved in many aspects of the cellular response to various forms of damage. PARP-1 and PARP-2, the most abundant PARPs, are central to the response to specific types of DNA damage, especially single-strand breaks. Inhibition of PARP activity may sensitize the cell to exogenous agents such as chemotherapy and radiation. In circumstances where rescue pathways are deficient, particularly the homologous recombination (HR)-directed DNA repair pathway, inhibition of PARP may result in “synthetic lethality.” BRCA mutation-associated breast cancers are a paradigm of HR-directed repair deficient tumors. Early clinical trials have demonstrated significant activity of single-agent PARP inhibitors in BRCA-deficient breast and ovarian cancer. Because of phenotypic similarities between some “triple-negative” breast cancers (TNBC) and the most prevalent type of breast cancer seen in BRCA1 mutation carriers, some have hypothesized that TNBC might also be specifically sensitive to PARP inhibition. The activity of single-agent PARP inhibitors in TNBC has not been reported. One trial did suggest significant enhancement of the activity of platinum-based combination chemotherapy, without incremental toxicity. These studies indicate that PARP inhibition is an exciting new approach to the treatment of breast cancers in women with underlying BRCA mutations and possibly in sporadic cancers with defects in HR-directed repair. Future studies will be necessary to determine whether the effectiveness of PARP inhibitors in nonhereditary cancer requires an underlying HR defect or whether these agents may improve the activity of conventional chemotherapy by other means. In addition, studies will be required to determine whether PARP inhibitors may induce synthetic lethality in tumors with defects in pathways other than the BRCA-dependent DNA repair pathway. If either or both of these prove to be the case, then PARP inhibition may benefit a wide spectrum of cancer patients.
Keywords: poly(ADP-ribose) polymerase (PARP); BRCA1; BRCA2; “basal-like,” DNA repair; base excision repair; homologous recombination-directed repair; synthetic lethality
Poly(ADP-ribose) polymerases (PARPs) are a family of enzymes that share the ability to transfer adenosine phosphate (ADP)-ribose subunits from nicotinamide dinucleotide+ onto acceptor proteins, creating long, branched, negatively charged poly-ADP ribose (PAR) polymers.1,2 The first member of the family (PARP-1) was described in 1963,3 and the superfamily now contains 18 members.2 The most abundant members are PARP-1 and PARP-2, which localize to the nucleus and seem to be the most relevant to the treatment of cancer. PARP-1 is a 113-kDa protein with a 2 DNA-binding RING-finger domains, a nuclear localizing signal, and a BRCT-repeat domain in addition to the catalytic domain that it shares with other members of the family.4 PARP-2 (62 kDa) is a smaller protein that seems to lack the RING-finger and BRCT-repeat domains found in PARP-1 but retains DNA-binding capacity.5
PARPs are involved in a number of process, including inflammation and the cellular response to response to various insults, such as ischemia or oxidative stress.6 For instance, PARP activation, with consequent depletion of nicotinamide dinucleotide+, may be an important determinant of cell death after ischemia.7 However, it is the role of PARPs in DNA damage repair that is of greatest interest to oncology therapeutics.
INVOLVEMENT OF PARPS IN DNA DAMAGE REPAIR
The maintenance of genomic integrity is a critical cellular function. Endogenous and exogenous insults result in various forms of DNA damage on a regular basis, and a number of pathways have evolved to address these lesions.8,9 PARP-1, and probably PARP-2, are specifically involved in the response to single-strand DNA breaks. PARP-1 is a major component of the base excision repair (BER) pathway.10,11 PARP-1 seems to detect single-strand DNA damage through binding mediated by the N-terminus RING finger domains (“nick sensing”). Thereafter, PARP-1 forms a homodimer, and the catalytic activity of the protein is engaged. PARP itself is a main target for poly-ADP-ribosylation, with PAR polymers being attached to the BRCT repeat domains. The creation of a bulky, negatively charged chain in the region of the DNA break likely has a number of effects. First, the PAR chain serves as a “beacon” to recruit other repair proteins such as XRCC1 to the sites of damage. Second, PAR modification of histone proteins may lead to local chromatin remodeling, improving access of DNA repair proteins to the site of damage.12 The presence of automodified PARP may also serve “antirecombinogenic” function, preventing the conversion of a single-strand break (SSB) into a more genomically unstable double-strand break.4
The BER pathway is central to the repair of endogenous DNA damage. Thousands of single-strand lesions form every day as a result of normal oxidative stresses. From this, one would assume that PARP activity is critical to survival, and it is somewhat surprising that PARP-1 knockout mice are viable, fertile, and do not seem to suffer from any obvious cancer susceptibility. They are not entirely normal, as PARP-1 deficient animals are quite sensitive to DNA-damaging agents such as N-methyl-N-nitrosourea and ionizing radiation.13 The apparently mild phenotype of PARP-1-deficient animals likely results from 2 factors. First, the fact that PARP-1/PARP-2 double knockout mice are not viable suggests that PARP-2 activity can rescue PARP-1 deficient cells, at least under normal conditions.14 Second, single-strand DNA lesions can be repaired by mechanisms other than the BER pathway.
Replication forks are unable to progress normally through unrepaired SSB until they are repaired. If replication forks along opposing strands meet at a SSB, a discontinuous double-strand end may form.15 Such double-strand ends are potentially highly recombinogenic and could contribute to significant genomic instability. To avoid these outcomes, the cell engages repair mechanisms that are normally involved in the repair of double-strand breaks, such as homologous recombination (HR)-directed repair and non-homologous end joining (NHEJ). HR-directed repair is a highly accurate DNA repair mechanism that is engaged during S phase, using the available sister chromatid as a template for resolving the break. NHEJ is available throughout the cell cycle but is less accurate and has more potential to contribute to genomic instability. Bone marrow cells from PARP-1-deficient mice manifest increased numbers of sister chromatid exchanges under both normal and stress conditions,13 indicating that HR-directed repair mechanisms are engaged in response to endogenous and exogenous DNA damage when SSB repair is impeded by loss of PARP activity. It is likely this redundancy and overlap of repair mechanisms that preserves cells lacking PARP1.
RATIONALE FOR PARP INHIBITION IN BRCA-ASSOCIATED CANCER
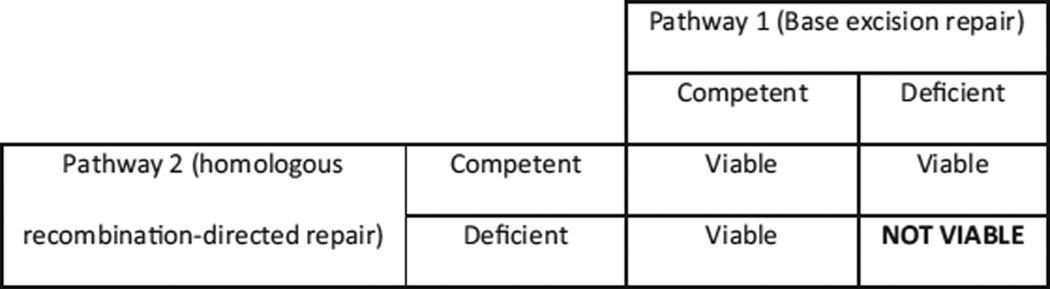
“Synthetic lethality” is the phenomenon whereby cell death results from the loss of function of 2 different gene products, when loss of either gene product in isolation does not lead to cell death (Fig. 1).16 Because double-strand break repair pathways, such as HR-directed repair and NHEJ, seem to be important to the repair of SSB in PARP-deficient cells, one would predict that cells lacking these pathways would be sensitive to the loss of PARP function.17
FIGURE 1.
Concept of synthetic lethality.
The products of BRCA1 and BRCA2 are critical to the process of HR-directed DNA repair.18 If PARP activity were to be lost in BRCA-deficient cells, such as BRCA-associated breast and ovarian cancers, one would expect that exogenous or endogenous DNA damage that is normally addressed by the BER pathway would result in genomic instability and cell death as the HR-directed pathway is not available to rescue those cells. Noncancer cells from BRCA mutation carriers would not be expected to experience such an effect, because these cells still have enough functional BRCA gene product to remain competent in double-strand break repair. Pharmacologic inhibition of PARP should, therefore, have a high therapeutic index in the treatment of BRCA-related breast and ovarian cancer.
Two groups showed in 2005 that PARP inhibition results in synthetic lethality in BRCA-deficient cells.19,20 Farmer et al.20 showed that pharmacologic PARP inhibitors were highly cytotoxic to BRCA2-deficient VC-8 cells and that siRNA “knockdown” of BRCA2 in MCF-7 and MDA-MD-231 cells resulted in sensitivity to PARP inhibition. In a simultaneously published article, Bryant et al19 showed that BRCA-deficient embryonic stem cells are sensitive to PARP inhibition. Preclinical studies then demonstrated that treatment with a PARP inhibitor had a significant impact on orthotopic transplants of BRCA-null breast cancers transplanted from conditional mutant mice.21–23
Taken together, these studies have provided the preclinical rationale for exploring PARP inhibition as a strategy to treat malignancies in which the cells are deficient in HR-directed repair. The concept of using PARP inhibitors as single agents to induce cell death through synthetic lethality represents a novel approach to cancer treatment but may not be the only mechanism by which PARP inhibitors could improve cancer therapy. When used in combination therapy, PARP inhibitors may also enhance the effectiveness of conventional treatments by impairing the repair of damage caused by those agents (eg, impeding repair of SSB induced by radiotherapy). Finally, combination therapies that include PARP inhibitors may increase cytotoxicity by increasing the overall burden of DNA breaks, both single and double stranded, and thus promote cell death even in cells with relatively competent DNA repair mechanisms.
CLINICAL STUDIES OF PARP INHIBITION IN BRCA-ASSOCIATED CANCER
The only published studies directly evaluating the hypothesis that PARP inhibitors may be effective single-agent treatment for BRCA-associated cancers have been conducted with olaparib (AZD2281 and KU0059436). Olaparib is a substituted 4-benzyl-2Hphthalazin-1-one that possesses high-inhibitory enzyme and cellular potency for both PARP-1 and PARP-2. Fong et al24 recently published the results of an expanded single-agent phase I study in which an effort was made to oversample patients with BRCA-associated malignancy. After reaching maximal tolerated dose (MTD) in the traditional phase I portion, the investigators expanded the study to include a number of additional patients with documented BRCA-associated cancer. A total of 60 patients enrolled, 22 of whom had mutations in BRCA1 or BRCA2. Another woman with ovarian cancer had a strongly suggestive family history but declined testing. The patients had breast (15%), ovarian (35%), colorectal (13%), melanoma (7%), sarcoma (7%), or prostate cancer (5%). The MTD was noted to be 400 mg twice daily (BID), with reversible dose-limiting toxicity noted at a dose of 400 mg per os (po) BID (grade 3 mood alteration and fatigue in 1 of 8 patients) and, more prominently, at 600 mg po BID (grade 4 thrombocytopenia and grade 3 somnolence in 2 of 5 patients). The investigational agent was well tolerated overall, with mild gastrointestinal side effects being noted in a minority of patients below the MTD. Objective tumor response was noted in 9 of 60 patients, although some clinical benefit was noted in 17 of 60 (including 12/19 individuals with BRCA-associated breast, ovarian, or prostate cancer). Only mutation carriers seemed to experience durable antitumor activity.
In response to the significant findings of the phase I study, a follow-up multicenter proof of principle phase II trial of olaparib in BRCA-deficient advanced breast cancers was performed.25 A total of 54 women with BRCA mutation-associated breast cancer who had received at least 1 prior regimen for the treatment of metastatic breast cancer were enrolled. In a single-arm, 2 sequential patient cohort design, the first 27 patients received continuous oral olaparib in 28 day cycles at 400 mg BID. In the second cohort, 27 patients received 100 mg of olaparib po BID. The primary end point was overall response rate. Subjects had received a median of 3 prior regimens and 44 of 54 had been treated with an anthracycline and taxane. Fatigue was the most common adverse event, although usually grades 1 to 2. Grades 1 to 2 nausea were also common. In the intention-to-treat cohort, objective response rates were 41% (n = 11) and 22% (n = 6) in those patients receiving 400 mg BID and 100 mg BID, respectively. The median progression-free survival (PFS) in the 400 mg BID cohort was 5.7 months and 3.8 months in the 100 mg BID cohort. Similar results were observed in a concurrent study of nearly identical design evaluating the effectiveness of single-agent olaparib in the treatment of BRCA-associated ovarian cancer.26
Taken together, these studies serve as important proof of the principle that certain cancers can be treated effectively by approaches designed to induce synthetic lethality. Further clinical studies are underway, using other agents, to confirm the activity of PARP inhibitors in the treatment of BRCA mutation-associated cancers and other cancers that may be deficient in HR-directed repair.
RATIONALE FOR USE OF PARP INHIBITORS IN “TRIPLE-NEGATIVE” BREAST CANCER
The studies described above demonstrated the effectiveness of PARP inhibition as a therapeutic strategy in cancers arising in BRCA mutation carriers. Because only a small fraction of breast cancers arise in women with such mutations, the impact of the approach on the more global breast cancer problem is likely to be limited unless subsets of “sporadic” breast cancers can be identified that may be amenable to the same line of attack. Somatic mutations in BRCA1 or BRCA2 seem to be rare in nonhereditary breast or ovarian cancer, although loss of heterozygosity of the genomic regions encompassing these genes is not uncommon. Several studies have demonstrated reduced expression of BRCA1 protein in some nonhereditary breast cancers, especially poorly differentiated disease.27,28 Reduced expression seems to result from either somatic BRCA1 promoter hypermethylation or downregulation consequent to overexpression of regulatory proteins such as Id4.29–32 Sporadic breast cancers with reduced BRCA1 expression usually do not express estrogen or progesterone receptors and do not overexpress HER2 [triple-negative breast cancer (TNBC)]. In addition, many TNBC (but not all) demonstrate a “basal-like” gene expression pattern, as do most breast cancers arising in BRCA1-mutation carriers. These commonalities have led some to suggest that a subset of TNBC may have a BRCA1 pathway defect that is severe enough to compromise HR repair.33 If this is the case, then a subset of TNBC may be sensitive to synthetic lethality induced by PARP inhibition. However, there is incomplete phenotypic overlap, between TNBC, basal-like breast cancer defined by gene expression pattern, and the cancers that arise in BRCA1 mutation carriers.34 Not all TNBCs or basal-like cancers necessarily have reduced BRCA1 expression. Even in those that do, HR-directed repair has not been directly assessed, and it is not clear whether this repair mechanism is sufficiently compromised to confer susceptibility to a synthetic lethal approach. Therefore, it is premature to assume that PARP inhibition alone will be broadly effective in TNBC by the same synthetic lethality mechanism as in BRCA-associated disease.
As a therapeutic strategy, PARP inhibition may have merit even if it does not lead to synthetic lethality by exploiting defects in HR repair. Numerous chemotherapy agents (and ionizing radiation) induce DNA damage that is usually repaired by the BER pathway, and inhibition of that pathway would be expected to result in increased cytotoxicity. As described above, PARP-1 knockout mice were sensitive to treatment with N-methyl-N-nitrosourea or ionizing radiation,13 suggesting that the ability of the double-strand break repair system to compensate for loss of PARP function is not infinite. Therefore, combinations that increase the stress on that system may be effective even without engaging a true synthetic lethality mechanism.
The most actively investigated partners for combination with PARP inhibitors are platinum-based agents. The major form of DNA damage induced by these drugs is thought to be repaired by the nucleotide excision repair pathway.35 However, platinum agents also cause double-strand crosslinks that require double-strand break repair pathways for resolution. Cells that are defective in double-strand break repair, such as those lacking BRCA1 or BRCA2, are extremely sensitive to platinum-based agents.36 Preclinical studies of combination therapy with PARP inhibitors and platinum agents animal models of BRCA-deficient breast cancers have shown enhanced efficacy (and toxicity).21,37 The lack of HR-directed repair capability leads to cell death after exposure to the aggregate insult of double-strand breaks resulting from the inability to repair platinum-induced crosslinks and similar breaks resulting from replication fork collapse at the site of SSB that are not repaired due to PARP inhibition.
If there is a defect in HR-directed repair in subsets of TNBC, one would expect exquisite platinum sensitivity in that setting as well. Clinical studies examining this hypothesis are not conclusive, perhaps in part due to the heterogeneity of TNBC and the design of the available studies. However, the effectiveness of combination platinum/PARP inhibitor therapy may not require a HR repair defect. Combining a platinum agent with a PARP inhibitor might increase effectiveness by increasing double-strand breaks to the point where even a competent repair system is overwhelmed. By extension, PARP inhibitors may enhance the effectiveness of non-platinum agents and combinations may be effective in settings other than those in which a HR repair defect is predicted to exist.
CLINICAL STUDIES OF PARP INHIBITION IN THE TREATMENT OF TNBC
To date, there are few clinical studies specifically describing the activity of PARP inhibitors in TNBC. At the 2009 meeting of the American Society of Clinical Oncology, O’Shaughnessy et al38 presented a randomized phase II study of gemcitabine and carboplatin with or with BSI-201 in patients with metastatic TNBC. BSI-201 is an intravenous PARP inhibitor whose structure has not been published and for which there is extremely limited peer-reviewed preclinical data. A total of 120 patients were enrolled in this study, whose primary end points were clinical benefit rate and toxicity. Secondary end points included objective response rate, progression-free survival, and overall survival. At an interim analysis of 109 patients, the experimental arm demonstrated statistically significant improvements in PFS (median 6.9 vs. 3.3 months, HR 0.342, P < 0.002) and overall survival (9.2 vs. 5.7 months, HR 0.348, P < 0.0001). There were also significant improvements in objective response rate (48% vs. 16%) and clinical benefit (62% vs. 21%). Remarkably, these results were obtained without any evidence of incremental toxicity in the investigation treatment arm. These exciting results have lead to a confirmatory phase III randomized study that is currently accruing (ClinicalTrials.gov identifier NCT00938652).
FUTURE DIRECTIONS
Although olaparib and BSI-201 are the PARP inhibitors that are most advanced in clinical development, a number of agents with similar activity are in early clinical trials (Table 1). Formulations and routes of delivery (oral vs. intravenous) differ, but it remains to be seen whether different agents manifest significant differences in efficacy or toxicity. A number of other questions remain. It is not clear whether tumors with HR-directed repair defects will be more effectively treated with PARP inhibitors as single agents or in combination with conventional cytotoxics, such as cisplatin or carboplatin. It is also not yet clear whether the incremental benefit of PARP inhibition is restricted to cancers that have putative defects in HR-directed repair or upregulation of PARP. It is possible that inhibition of BER may produce a more general sensitization to conventional chemotherapy (or radiation) that does not depend on the engagement of a synthetic lethality mechanism. If an underlying defect in HR-directed repair is required for a benefit from PARP inhibition, it would be helpful to have an assay for HR-directed repair competency. Such an assay does not yet exist, although a number of investigators are exploring novel approaches to this problem.39
TABLE 1.
Active U.S. Trials of PARP Inhibitors (Breast Cancer Eligible)
| Agents | Phase | Combination Agent |
ClinicalTrials.Gov Number |
|---|---|---|---|
| BSI-201 | 3 | Gemcitabine/carboplatin | NCT00938652 |
| 2 (neoadjuvant) | Gemcitabine/carboplatin | NCT00813956 | |
| 1 | Irinotecan | NCT00298675 | |
| 1 | Various | NCT00422082 | |
| Olaparib (AZD2281) | 1 | Carboplatin | NCT00647062 |
| 1 | Gemcitabine/cisplatin | NCT00678132 | |
| ABT888 | 1 | Single agent | NCT00810966 |
| NCT00892736 | |||
| 1 | Cyclophosphamide ± doxorubicin | NCT00740805 | |
| 1 | Paclitaxel/carboplatin | NCT00535119 | |
| 1 | Topotecan | NCT00553189 | |
| MK-4827 | 1 | Single | NCT00749502 |
| AG014699 (no active U.S. studies) | |||
| INO1001 (no active U.S. studies) |
The mechanisms of resistance to PARP inhibitor treatment must be defined. In the setting of treatment of BRCA mutation-associated cancers, 1 group has identified resistance associated with reversion of the underlying germline mutation, resulting in restoration of HR-directed repair capability.40 Interestingly, a similar mechanism has been described as being associated with resistance to platinum-based chemotherapy in BRCA2-deficient cancer.41 This is not likely to be a relevant mechanism in the setting of sporadic TNBC, but animal studies have indicated that resistance may be associated with upregulation of multidrug resistance proteins.37 This may present challenges for treatment of disease that is not associated with BRCA mutations.
Finally, PARP inhibition may induce synthetic lethality in a wider universe of cancers beyond those that are known or believed to harbor defects in the BRCA-associated DNA repair pathways. Preclinical data suggest sensitivity in cells with defects in other components of HR-directed repair.42,43 There may also sensitivity in cells without obvious defects in this pathway. For instance, recent data suggest that PTEN-null cells may also be sensitive to this approach.44 These observations suggest that there may be a broader application of PARP inhibition in cancer therapy than has been appreciated so far. Indeed, there may be other applications of the synthetic lethality approach to cancer treatment that remain to be discovered.45 This conceptual advance, and the proof-of-principle that has been provided by the results of PARP inhibition in BRCA-associated cancer, presents an exciting opportunity to develop an entirely new approach to cancer therapeutics that builds on recent discoveries in cancer genetics and cell biology.
Acknowledgments
This work was supported by Astra-Zeneca (M.R.) to conduct a clinical trial of olaparib in BRCA-associated breast cancer.
REFERENCES
- 1.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 3.Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of a new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11:39–43. doi: 10.1016/0006-291x(63)90024-x. [DOI] [PubMed] [Google Scholar]
- 4.Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair. 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Yelamos J, Schreiber V, Dantzer F. Toward specific functions of poly(ADPribose) polymerase-2. Trends Mol Med. 2008;14:169–178. doi: 10.1016/j.molmed.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Gerö D, Szabó C. Poly(ADP-ribose) polymerase: a new therapeutic target? Curr Opin Anaesthesiol. 2008;21:111–121. doi: 10.1097/ACO.0b013e3282f63c15. [DOI] [PubMed] [Google Scholar]
- 7.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 9.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 10.Almeida KH, Sobol RW. A unified view of base excision repair: lesiondependent protein complexes regulated by post-translational modification. DNA Repair (Amst) 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldecott KW. Mammalian single-strand break repair: mechanisms and links with chromatin. DNA Repair (Amst) 2007;6:443–453. doi: 10.1016/j.dnarep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Murcia JM, Niedergang C, Trucco C, et al. Requirement of poly(ADPribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menissier de Murcia J, Ricoul M, Tartier L, et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 16.Dobzhansky T. Genetics of natural populations. Xiii. Recombination and variability in populations of Drosophila Pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 18.Venkitaraman AR. Targeting the molecular defect in BRCA-deficient tumors for cancer therapy. Cancer Cell. 2009:89–90. doi: 10.1016/j.ccr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 20.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 21.Evers B, Drost R, Schut E, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14:3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 22.Hay T, Matthews JR, Pietzka L, et al. Poly(ADP-ribose) polymerase-1 inhibitor treatment regresses autochthonous BRCA2/p53-mutant mammary tumors in vivo and delays tumor relapse in combination with carboplatin. Cancer Res. 2009:3850–3855. doi: 10.1158/0008-5472.CAN-08-2388. [DOI] [PubMed] [Google Scholar]
- 23.Rottenberg S, Jonkers J. Modeling therapy resistance in genetically engineered mouse cancer models. Drug Resist Updat. 2008;11:51–60. doi: 10.1016/j.drup.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Fong PC, Boss DS, Yap TA, Tutt A, Wu P. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;2009:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 25.Tutt A, Robson M, Garber JE, et al. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer. J Clin Oncol (Meet Abstr) 2009;27:CRA501. [Google Scholar]
- 26.Audeh MW, Penson RT, Friedlander M, et al. Phase II trial of the oral PARP inhibitor olaparib (AZD2281) in BRCA-deficient advanced ovarian cancer. J Clin Oncol (Meet Abstr) 2009;27:5500. [Google Scholar]
- 27.Rakha EA, El-Sheikh S, Kandil MA, El-Sayed ME, Green AR, Ellis IO. Expression of BRCA1 protein in breast cancer and its prognostic significance. Hum Pathol. 2008;39:857–865. doi: 10.1016/j.humpath.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Wilson CA, Ramos L, Villaseñor MR, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 29.Catteau A, Harris WH, Xu CF, Solomon E. Methylation of the BRCA1 promoter region in sporadic breast and ovarian cancer: correlation with disease characteristics. Oncogene. 1999;18:1957–1965. doi: 10.1038/sj.onc.1202509. [DOI] [PubMed] [Google Scholar]
- 30.Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 31.Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21:1761–1765. doi: 10.1093/carcin/21.9.1761. [DOI] [PubMed] [Google Scholar]
- 32.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2006;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 33.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 34.Schneider BP, Winer EP, Foulkes WD, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8018. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 35.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 37.Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105:2–7. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Shaughnessy J, Osborne C, Pippen J, et al. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): results of a randomized phase II trial. ASCO Meet Abstr. 2009;27:3. [Google Scholar]
- 39.Willers H, Taghian AG, Luo C-M, Treszezamsky A, Sgroi DC, Powell SN. Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol Cancer Res. 2009;7:1304–1309. doi: 10.1158/1541-7786.MCR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 41.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lord CJ, McDonald S, Swift S, Turner NC, Ashworth A. A high-throughput RNA interference screen for DNA repair determinants of PARP inhibitor sensitivity. DNA repair. 2008;7:2010–2019. doi: 10.1016/j.dnarep.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Turner NC, Lord CJ, Iorns E, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27:1368–1377. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:1–8. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaelin WG., Jr Synthetic lethality: a framework for the development of wiser cancer therapeutics. Genome Med. 2009;1:99. doi: 10.1186/gm99. [DOI] [PMC free article] [PubMed] [Google Scholar]