1. Introduction
Bisphenol A (BPA) is a high volume production chemical that is used in a wide variety of consumer products, including polycarbonate and other forms of plastics, resins used to line food and beverage containers, thermal printed papers, and composites used in dentistry. As a result of its widespread use, humans are exposed to BPA on a virtually constant basis [1]. Although estimates of daily exposure differ markedly [2–4], BPA contaminates our air, water, and soil [5], and the pervasiveness of human exposure is not disputed [3, 6]. Relevant to our research, there is extensive evidence that BPA crosses the placenta in humans and animals, resulting in measurable concentrations of unconjugated (bioactive) BPA in placenta, fetal tissues and blood [3, 7–9].
BPA is an endocrine disrupting chemical (EDC) that has been demonstrated to affect signaling mechanisms involving estrogen, androgen, aryl hydrocarbon and thyroid hormone receptors [10, 11]. Animal studies have demonstrated that maternal exposure can significantly alter fetal development, resulting in a variety of adverse outcomes in the adult [12–15]. In addition, numerous epidemiological studies have reported associations between BPA and adverse health effects [16], including when exposure occurs during fetal life [17], which has been a main focus of research with laboratory animals [18]. In response, regulatory agencies in some countries have begun to restrict the uses of BPA. For example, Canada has declared BPA a “toxic chemical”, the US-FDA banned BPA for use in baby bottles (although this was requested by the baby bottle industry), and the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) has called for the elimination of BPA in food packaging in 2014 [19].
Despite the evidence that BPA induces a wide range of adverse effects whether exposure occurs during development or in adulthood, debate about the level of concern appropriate for BPA continues, with discussion centering on two issues that are addressed in our current study: 1) the routes by which humans are exposed and thus how estimates of the current total daily exposure levels relate to the amount of BPA in blood that is unconjugated vs. conjugated [20], and 2) the relevance of animal models for predicting human pharmacokinetics and pharmacodynamics [2, 21].
The limited information about BPA metabolism during pregnancy in primates and its importance in assessing developmental exposure, together with the controversy regarding potential routes of exposure to BPA, prompted us to undertake the present set of studies in pregnant female rhesus monkeys. We first conducted pharmacokinetic studies of pregnant females. We used in the present study the same oral dose of deuterated BPA (dBPA) on a subset of the rhesus monkey females from our initial study of non-pregnant females [2] that became pregnant and carried a female fetus during the following breeding season. This allowed us to compare dBPA metabolism in the same females in a non-pregnant and pregnant state; we also examined dBPA at multiple times in pregnancy. We then initiated a second study with a separate group of pregnant monkeys using a different exposure paradigm of continuous exposure via subcutaneously (sc) implanted Silastic capsules containing dBPA (Figure 1). Our hypothesis was that the continuous exposure paradigm would more accurately mimic some of the potential sources of human exposure (transdermal, sublingual/buccal, inhalation) than the single daily oral bolus gavage administration commonly used in toxicological research [1, 22–24]. Specifically, there is evidence that human exposure to BPA is likely from multiple sources and multiple routes [1] including dermal exposures from BPA-containing receipt paper [25, 26], inhalation exposure to BPA on dust [27–29], iatrogenic exposures from medical devices [30], and also sublingual absorption from food while in the mouth [20]. Thus, subcutaneously implanted Silastic capsules may provide a better model for the exposure of humans that is not accounted for by a single gavage administration, which results in a very low percent of the administered dose being bioavailable relative to other routes of exposure [20].
Figure 1.
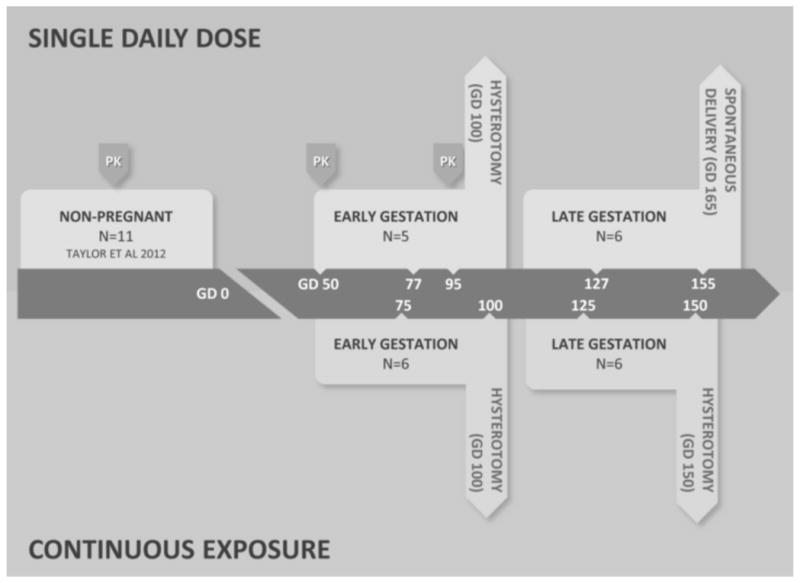
Routes and duration of dBPA exposure. Two routes of exposure were used in these studies; single daily oral doses of 400-μg/kg body weight dBPA (top panel) and continuous exposure via sc Silastic implants (bottom panel). For each treatment both early and late pregnancy exposure groups were included as detailed in the methods. Four of the females in the single daily oral exposure group were the same females used in previous pharmacokinetic studies of non-pregnant females [2], and similar pharmacokinetic studies of these females were conducted during early pregnancy as indicated by PK arrows (data in Table 1). Large arrows at the end of each treatment period indicate the day of gestation and method of collection of maternal and fetal blood and fetal tissues (oral exposure data in Tables 4 and 5). The top row of numbers within the arrow indicate the gestation days (GD) on which maternal blood was collected 4 hr after oral administration of dBPA (data in Table 2). The bottom row of numbers within the arrow indicate the GD on which just maternal blood (GD 75 and 125) and both maternal and fetal blood (GD 100 and 150) was collected (data in Table 3).
The pharmacokinetic results of our study, together with a series of publications showing significant adverse effects on the ovaries, mammary glands, brain and lungs of fetuses carried by the same dBPA-treated monkey females [31–34], indicate that there is no mechanism to protect the developing fetus from maternal exposure to BPA during pregnancy. Our data also suggest that continuous exposure to BPA via Silastic capsules produces a profile of conjugated vs. unconjugated BPA in serum similar to that observed in cross-sectional studies in people. In contrast, the corresponding profile of conjugated vs. unconjugated BPA in serum observed following a single daily oral bolus administration in monkeys (both prior to and during pregnancy) is markedly different from what is observed in humans [35, 36].
2. Methods
2.1. Animals
Adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center. Animal protocols were reviewed and approved in advance by the Animal Care and Use Committee of the University of California, Davis; all studies were conducted in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were caged individually with a 0600- to 1800- hour light cycle and at a temperature maintained at 25–27°C. Animals were fed a diet of Purina Monkey Chow (Purina-Mills, St. Louis, MO, USA) and provided with water ad libitum. Seasonal produce, seeds, and cereal were offered as supplements for environmental enrichment. Cages were made of stainless steel, and water was delivered to each cage by rigid polyvinyl chloride pipes and a water nipple.
Only females with a history of normal menstrual cycles were selected for this study. Females ranged in age from 6 to 13 years, and body weights ranged from 6.25 to 11.25 kg throughout pregnancy (mean, 8.75 kg). All females were naturally mated according to standard California National Primate Research Center procedures. Pregnancy was detected by ultrasound examination, and an estimated day of conception (gestation day - GD 0) was assigned. At approximately GD 40, the sex of all fetuses was determined, and those with female fetuses were continued on the current study and also to determine the effects of dBPA on ovarian, mammary gland, lung and brain development. Fetal growth rate was also monitored by ultrasound every 2 to 3 weeks during treatment [37]. Cephalic vein blood samples were collected from unanesthetized, cage-restrained animals that were trained to present an arm for the procedure.
2.2 Rationale for deuterated BPA (dBPA) administration
Deuterated (d6) BPA (dBPA, CDN Isotopes, Quebec, Canada) was used in these studies because it can be clearly distinguished from BPA by liquid chromatography mass spectrometry (LC/MS), thus eliminating concern about potential BPA contamination from materials used in the preparation, handling or shipment of samples, although in practice, by using appropriate field and assay blanks, we have been able to rule out contamination with background BPA in our studies, as have others; for example [8, 38].
2.3. Treatment Groups
Two routes of BPA exposure were used for these experiments (Figure 1). The first cohort of animals was given small pieces of fruit containing 400-μg/kg body weight of dBPA once per day, modeling an acute oral exposure. Our second route of exposure was via Silastic implants, which models exposure routes that bypass first-pass metabolism in the liver [1, 20]. These implants were demonstrated in a preliminary study to release dBPA at a fairly constant rate for 30 days, after which release rate begins to drop; the capsules produced serum levels of about 3.5 ng/ml unconjugated dBPA in non-pregnant females, close to the median reported in pregnant women [8, 39]. Both the oral and continuous dose cohorts were further subdivided into early and late pregnancy treatment groups as shown in Figure 1. Early pregnancy animals were dosed from GD 50 to 100 (Early Pregnancy, Oral Dose: N=5; Early Pregnancy, Continuous Dose: N=6). Late pregnancy animals were dosed from GD 100 to 155 (Silastic implant; N=6) or from GD 100 to natural birth on about GD 165 (oral dose; N=6). Female fetuses carried by the early and late pregnancy oral dose females and by additional control females were examined for effects on various tissues that are reported elsewhere [31–34].
2.4. Comparison of the metabolism of single daily oral BPA doses in non-pregnant monkeys and pregnant monkeys on GD 50 and GD 95
To directly compare dBPA metabolism in the non-pregnant and pregnant females, the eleven rhesus monkey females used in our recent pharmacokinetic study [2] were mated during the next breeding season. Four females that became pregnant were determined to be carrying female fetuses, which were the focus of analysis of fetal tissues. The dose of dBPA administered to these four females in our previous study, prior to pregnancy, was the same 400 μg/kg/day dose used here [2]. One additional adult female that had not been examined prior to pregnancy was added to the BPA-exposed group to achieve a sample size of 5 for the pharmacokinetic analysis of dBPA during pregnancy. On GD 50 and then again on GD 95, the concentrations of unconjugated and conjugated dBPA in maternal serum were determined over the 24-hr period following oral administration of 400 μg/kg dBPA. Specifically, blood was collected from the 5 pregnant females for determination of serum dBPA levels at 0.5, 1, 2, 4, 8 and 24 hours following oral dBPA administration in a piece of fruit. The GD 50 blood collections occurred after the first oral dose of dBPA during pregnancy, and the GD 95 collection occurred after 45 daily oral doses of dBPA, which allowed us to assess the possibility of a time-dependent disposition of BPA during pregnancy.
2.5. Fetal blood, amniotic fluid, placenta and decidua collection on GD 100
The fetal placenta and the maternal (endometrial) portion of the placenta were collected along with maternal and fetal blood and amniotic fluid on GD 100, after 50 days of oral dosing and 5 days after the 24-hr maternal blood collection on GD 95. For the Early Pregnancy, Oral Dose group, three fetuses were collected by cesarean delivery 1 hr after the mother was fed dBPA, while the other two fetuses were collected at 3 hr after the mother was fed dBPA. The Late Pregnancy, Oral Dose group had dBPA dosing beginning on GD 100 and treatment ended with spontaneous vaginal delivery at approximately GD 165; in addition to maternal blood, blood and tissues were collected from the newborn monkeys; amniotic fluid was not available for analysis of dBPA from these naturally delivered babies. For the Late Pregnancy, Continuous Dose group, the fetuses were removed by Cesarean section on GD 150, and amniotic fluid was collected in addition to maternal and fetal blood.
2.6. Silastic implants
For the continuous dose group, each animal was implanted subcutaneously with three Silastic capsules that were 3.0-inches between the capped ends (0.132 in ID × 0.183 in OD; Fisher Scientific, cat. #508-011). dBPA was suspended at 50 mg/ml in tocopherol-stripped corn oil (Fisher Scientific; Pittsburgh, PA). Each 3-inch Silastic capsule contained 1.5 ml between the capped ends, resulting in a total for the 3 capsules of 225 mg dBPA/4.5 ml/animal. For implantation, animals were anesthetized, a small incision was made in the skin of the upper back, the three implants were inserted, after which the incision was closed. Implants were initially placed into pregnant monkeys on GD 50 (Early Pregnancy Group) and on GD 100 (Late Pregnancy Group). The capsules were removed and replaced with freshly prepared implants after 25 days of the 50-day treatment period (GD 75 or GD 125) to assure that dBPA levels remained near the maximum release rate (based on preliminary data for rate of release decreasing after 30 days). Specifically, the in vitro dBPA release rate of Silastic capsules was measured at day 12, 15, 19, 22, 26 and 30 while capsules were incubated in physiological saline in order to determine when to replace old capsules with new capsules prior to when the release rate would begin to drop.
Blood was collected from females prior to removal of the capsules after the first 25 days of exposure (on GD 75 for Early Pregnancy Group and on GD 125 for Late Pregnancy Group), and at the end of the second 25 days of exposure (on GD 100 for Early Pregnancy Group and on GD 150 for Late Pregnancy Group) for analysis of dBPA; at this time fetal blood and amniotic fluid were also collected for dBPA analysis.
3. Sample preparation and dBPA assay procedures
3.1. Chemicals
Methanol, water and tert-butyl methyl ether were HPLC grade and obtained from Fisher Scientific. D6-BPA was purchased from C/D/N Isotopes Inc. (Pointe-Claire, Quebec, Canada).
3.2. Sample Preparation
For all groups, maternal blood was allowed to stand at room temperature briefly to allow clotting. Preliminary studies indicated no deconjugation of conjugated dBPA into unconjugated dBPA over this time, and extraction of unconjugated dBPA did not lead to deconjugation of either glucuronidated or sulfated dBPA; details of a NIEHS-sponsored validation study of LC/MSMS analytical methods, including these preliminary data, will be published elsewhere. All blood and amniotic fluid samples were centrifuged at 1800 × g for 10 min at 4°C. Sera, tissue and amniotic fluid samples were stored at −80°C and shipped overnight on dry ice to the University of Missouri-Columbia.
3.3 Isotope-dilution LC/MS analysis of unconjugated and conjugated dBPA
Serum and amniotic fluid were analyzed using procedures described previously in Taylor et al. (2011). Samples (~1.5 ml) were spiked with 13C-BPA (Cambridge Isotopes Laboratories, Andover, MA) as an internal standard, and extracted twice with methyl tert-butyl ether (MTBE) for determination of unconjugated dBPA. The ether extract was dried under nitrogen and reconstituted in 60:40 methanol:water. After extraction of unconjugated dBPA, for analysis of conjugated dBPA (glucuronidated and sulfated forms), the remainder of the previously extracted samples were treated for ~18 hr at 37°C with 100 U of β-glucuronidase/aryl sulfatase (Sigma) and then the deconjugated dBPA was extracted by the same procedure described above. Preliminary studies indicated that an 18 hr treatment with 100 U of β-glucuronidase/aryl sulfatase resulted in maximal deconjugation. For placenta and decidua, tissues were homogenized in PBS, and dBPA was extracted with MTBE (10:1), after which conjugated dBPA was hydrolyzed using the same deconjugation procedure described for serum with 13CBPA as the internal standard.
Extracted dBPA was assayed by LC/MS using a Thermo Finnigan Surveyor MSQ plus connected to an integrated Thermo-Accela LC system; analytes were detected using electrospray ionization with negative polarity, a cone voltage of 70V, and probe temperature of 600°C. Separations were performed on a 1.9 micron Hypersil Gold HPLC column (50×2.1 mm) with a mobile phase gradient running from 20% to 95% acetonitrile over 6 minutes, at 550 μl/minute. dBPA and 13C-BPA were detected using selected ion monitoring for m/z 233 and m/z 239 respectively. Thermo Xcalibur software was used to autotune, acquire, and process the LC/MS data. Isotope dilution quantitation was made against a standard curve of at least 5 calibration standards (dBPA and 13C-BPA) to adequately cover the expected dBPA concentration range. The limit of detection (LOD) and the limit of quantitation (LOQ), calculated as 3 and 10 times, respectively, the standard deviations of the lowest calibration standard from three replicate analyses, were 0.06 and 0.2 ng/ml, respectively, for all assays of extracted dBPA.
4. Statistical methods and calculation of pharmacokinetic parameters
Serum concentration profiles were analysed with a Non-Compartmental Analysis (NCA) using WinNonlin (WinNonlin® professional version 5.3 Pharsight Corporation, Cary, NC, USA). Area under the curve (AUC) up to the last measured serum concentration above the LOQ, i.e. AUC(0-Clast), was calculated by using the linear trapezoidal rule. Extrapolation to infinity to obtain AUC(0-infinity) was calculated by dividing the last observed measurable serum concentration above the LOQ by the slope of the terminal phase as estimated by linear regression using the best fit option of WinNonlin. Terminal half-life (HL_Lambda_z) was obtained by dividing ln(2) by the terminal slope, based on the best fit option of WinNonlin; Mean Residence Time (MRT) was obtained with and without extrapolation to infinity using statistical moments [40]. The apparent oral clearance (CL/F) was obtained by dividing the administered dBPA dose by the corresponding AUC(0-infinity). Time (Tmax) of maximal plasma dBPA concentration (Cmax) was directly obtained from the raw data. For the mean residence time (MRT) and terminal half-life measures for unconjugated dBPA based on the data over the 24 hr after the dBPA oral exposure groups, reciprocals of the data were analysed by ANOVA. Comparisons for the parameters at different times in pregnancy were conducted using Proc GLM followed by the LSmeans test in SAS 9.3.
5. Results
The experimental design is shown in Figure 1.
5.1. Unconjugated dBPA and conjugated dBPA pharmacokinetics are altered during early pregnancy relative to pre-pregnancy in monkeys given a single daily oral dose of dBPA
To determine if pregnancy alters the metabolism of BPA in the rhesus monkey, 5 pregnant females were fed BPA in early pregnancy beginning on GD 50 (Figure 1). Four of the 5 females had been examined in our previous pharmacokinetic study of non-pregnant females [2]; one additional pregnant female not examined prior to pregnancy was added to this group. Equivalent pharmacokinetic data to those obtained for non-pregnant females was collected on GD 50 and GD 95 for these 5 pregnant females.
Figure 2 shows a semi-logarithmic plot of unconjugated and conjugated serum dBPA levels over the 24 hr following oral administration of 400-μg/kg/day dBPA to the 4 females prior to pregnancy and the 5 females during pregnancy. Serum levels of unconjugated dBPA were maximal or near-maximal at the first time point and declined thereafter, and did not exceed the LOQ at 12 and 24 hr; levels of conjugated dBPA similarly increased early in the collection period, and declined beginning at 4 hr, and then in contrast to unconjugated dBPA, remained above the LOQ throughout the 24 hr period for all monkeys.
Figure 2.
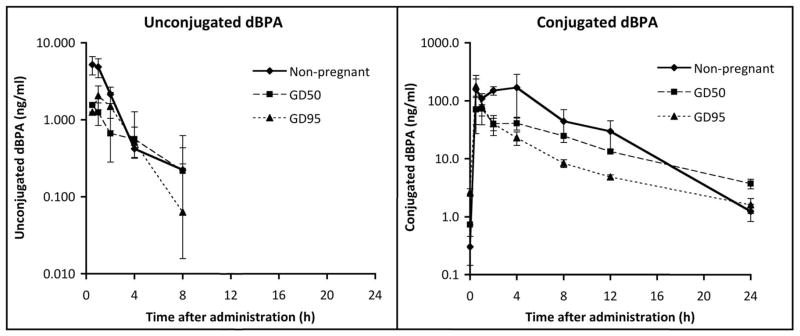
Semilog plot of concentrations (mean±SEM) of unconjugated and conjugated dBPA in serum from adult rhesus females during the 24 hr following oral administration of 400-μg/kg body weight. For pregnant females, values were obtained at GD 50 after the first oral dose and at GD 95 after 45 daily doses. Because 4 of the 5 pregnant females were previously studied [2] in the non-pregnant state, the non-pregnant data are shown for comparison. For unconjugated dBPA, concentrations at 12 and 24 hr after administration were below the LOQ.
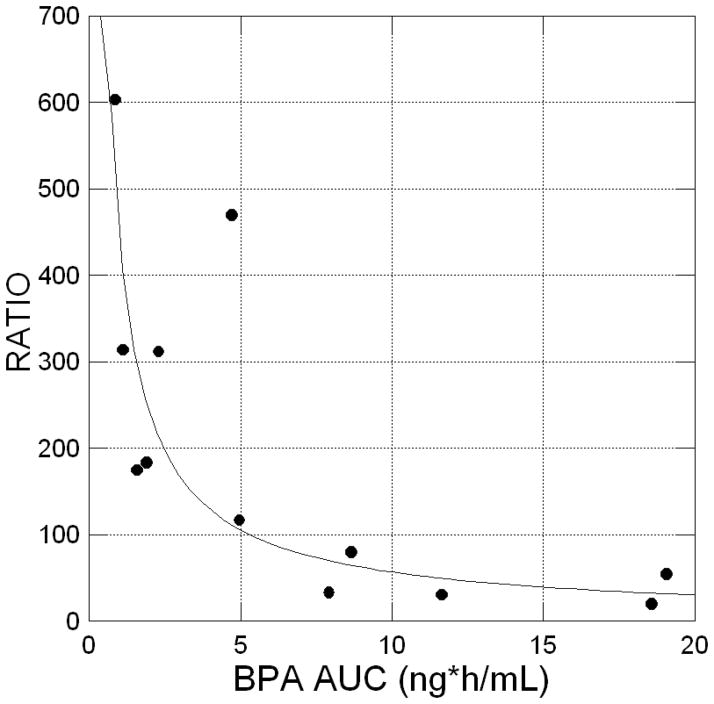
Importantly, it was observed (Figure 3) that the ratio of conjugated dBPA over unconjugated dBPA for individual monkeys was inversely proportional to the achieved level of serum unconjugated dBPA exposure based on AUC(0-infinity). This relationship was fitted with a negative power function with an exponent equal to 0.896 with an R2 of 0.713 (p<0.01). Recently published data [20] indicate that buccal absorption of BPA leads to a higher unconjugated BPA serum concentration and a lower conjugated BPA/unconjugated BPA ratio, because of the lack of a hepatic first-pass effect; our data thus suggest that a fraction of the administered dBPA dose was directly absorbed in the buccal cavity. For the monkeys with the highest serum unconjugated dBPA, a likely explanation is food hoarding, since some monkeys appear to retain small pieces of food in their cheek and thus not immediately swallow the entire dose of dBPA.
Figure 3.
Arithmetic plot showing the curvilinear relationship between unconjugated dBPA AUC (0-infinity; ng*h/mL), the independent variable, vs. the conjugated/unconjugated ratio of dBPA, the dependent variable, after oral administration in a piece of fruit in both non-pregnant monkeys reported previously [2] and pregnant monkeys (total n=12). Data were fitted to a power model of the form: Y=444.2*×−0.896 with an R2 of 0.7134 (P<0.01). The figure identifies that high serum concentrations of dBPA are associated with a low ratio of conjugated/unconjugated dBPA. High serum unconjugated dBPA is predicted to be due to these females keeping some of the dBPA-containing fruit in their cheek (referred to as “hoarding behavior”), resulting in an extended period of buccal/sublingual dBPA absorption that bypasses the liver prior to reaching target tissues [20].
Mean pharmacokinetic parameters of unconjugated dBPA are given in Table 1, and several important findings emerge from these data. First, the overall exposure AUC(0-infinity) was similar for the 3 stages, with no significant differences based on pregnancy status (non-pregnant, GD 50 or GD 95). However, the mean residence times (MRT 0 - infinity), which measure the average total time a molecule of dBPA spends in the body, were significantly longer during pregnancy (P < 0.05). Similarly, the terminal half-lives (HL) for unconjugated dBPA were significantly longer in pregnant females (P < 0.05), while maximum unconjugated dBPA plasma concentrations (Cmax) were significantly lower during pregnancy (P < 0.05).
Table 1.
Early Gestation, Oral Exposure from GD 50 – 100: Pharmacokinetic disposition of unconjugated and conjugated dBPA after oral administration of 400 μg/kg/day in a piece of fruit in females fed dBPA one time per day from gestation day (GD) 50 – 100. Blood was collected at three different times: prior to pregnancy (Non-pregnant), GD 50 and GD 95. There were 4 non-pregnant females, and one female not examined prior to pregnancy was added for examination during pregnancy. There were 3 females for which there were data at all time points, and two other females for which data were missing at one time point. Means that were significantly different (P < 0.05) are indicated by different letters (a, b). Cmax, maximal dBPA concentration; Tmax, Time of maximal plasma dBPA concentration; HL_Lamda_z, Terminal half-life; MRT, Mean Residence Time; CL/F, oral clearance; AUC(0-infinity) Area under the curve extrapolated to infinity; AUC/24 h, Area under the curve up to the last measured serum concentration above the LOQ for the 24h test period.
| Unconjugated dBPA | Conjugated dBPA | Conjugated/Unconjugated | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Non-pregnant | GD 50 | GD 95 | Non-pregnant | GD 50 | GD 95 | Non-pregnant | GD 50 | GD 95 | |
| Cmax | Mean | 5.79a | 1.62b | 2.25b | 311.49 | 82.21 | 182.78 | 53.79 | 50.91 | 81.39 |
| (ng/mL) | SE | 1.21 | 0.36 | 0.85 | 83.92 | 40.77 | 56.37 | |||
| Median | 5.56 | 1.53 | 1.25 | 389.02 | 51.70 | 133.20 | ||||
| N | 4 | 4 | 5 | 3 | 4 | 5 | ||||
| Tmax(h) | Mean | 0.75 | 0.63 | 0.80 | 1.83 | 2.38 | 0.80 | |||
| SE | 0.14 | 0.13 | 0.12 | 1.09 | 0.94 | 0.30 | ||||
| Median | 0.75 | 0.50 | 1.00 | 1.00 | 2.50 | 0.50 | ||||
| N | 4 | 4 | 5 | 3 | 4 | 5 | ||||
| HL_Lambda_z | Mean | 1.39a | 3.94b | 3.48b | 2.70a | 6.07b | 7.62b | 1.95 | 1.54 | 2.19 |
| (h) | SE | .29 | 1.09 | 1.03 | 0.06 | 0.71 | 0.88 | |||
| Median | 1.15 | 4.21 | 3.19 | 2.69 | 6.18 | 7.72 | ||||
| N | 4 | 3 | 4 | 3 | 4 | 5 | ||||
| MRT(0-∞) | Mean | 2.20a | 6.08b | 5.47b | 4.99a | 8.71b | 5.88a | |||
| (h) | SE | 0.35 | 1.81 | 1.64 | 1.08 | 0.90 | 0.69 | |||
| Median | 1.92 | 6.00 | 5.06 | 5.86 | 8.85 | 5.81 | ||||
| N | 4 | 3 | 4 | 3 | 4 | 5 | ||||
| Cl/F | Mean | 42766.33 | 69717.60 | 58927.98 | 382.24a | 817.36 | 1115.14b | |||
| (mL/kg/h) | SE | 12352.12 | 30475.37 | 18785.16 | 116.14 | 132.28 | 130.97 | |||
| Median | 38017.76 | 55319.90 | 58966.10 | 384.80 | 849.13 | 1043.84 | ||||
| N | 4 | 3 | 4 | 3 | 4 | 5 | ||||
| AUC(0-∞) | Mean | 12.09 | 8.65 | 10.69 | 1317.05 | 534.52b | 380.81b | 108.95 | 61.77 | 35.61 |
| (ng*h/mL) | SE | 3.53 | 3.68 | 4.53 | 464.97a | 94.21 | 48.20 | |||
| Median | 10.66 | 7.23 | 7.55 | 1039.50 | 491.40 | 383.21 | ||||
| N | 4 | 3 | 4 | 3 | 4 | 5 | ||||
| Mean serum concentration AUC/24 h (ng/mL) | Mean | 0.50 | 0.36 | 0.45 | 54.88 | 22.27 | 15.87 | |||
Levels of conjugated dBPA also differed in non-pregnant, GD 50 and GD 95 females (Figure 2). Serum levels of conjugated dBPA based on the data for all females were lower during pregnancy relative to pre-pregnancy levels (this was also true for the subset of 3 females for which there were data at all time points; data not shown). The conjugated dBPA AUC(0 to infinity) was about 2.5–3.5 times higher in non-pregnant females than during pregnancy (P < 0.05; Table 1). The overall ratio of conjugated to unconjugated dBPA was lower during pregnancy relative to pre-pregnancy, but the difference did not reached statistical significance. The conjugated dBPA terminal half-lives (HL) were over 2-times longer during pregnancy than in non-pregnant females (P < 0.05). Relative to non-pregnant females, the apparent conjugated dBPA clearance (CL/F) increased significantly on GD 50 (P < 0.05) as well as on GD 95 (P < 0.01), while mean residence time (MRT 0 - infinity) was significantly increased on GD 50 (P < 0.05) but not on GD 95 relative to values prior to pregnancy (Table 1).
5.2. Maternal serum unconjugated and conjugated dBPA: Comparison during pre-pregnancy, early pregnancy and late pregnancy in monkeys given a single daily oral dose of dBPA
Because our initial data provided evidence of a number of changes in BPA disposition during early pregnancy, we compared levels of dBPA in serum at 5 different time points during early through late pregnancy. For these comparisons, we chose to collect maternal serum 4 hr after feeding because our previous studies of non-pregnant females [2] demonstrated that serum concentrations of dBPA at this time are close to the average AUC (0–24) value (AUC/24 hr; Table 1). Serum levels of unconjugated and conjugated dBPA were evaluated in early pregnancy: oral dose animals at GD 50 (at 4 hr after administering dBPA on the first day of oral administration), GD 77 and GD 95. Another group of females carrying female fetuses was examined in late pregnancy: oral dose animals fed dBPA one time per day beginning on GD 100 were examined at 4 hr after oral administration of dBPA on GD 127 and GD 155. Table 2 provides a comparison of the data for these collection times during pregnancy with levels obtained previously in non-pregnant females 4 hr after the same oral dBPA dose using the same administration procedure [2].
Table 2.
Early and Late Gestation, Oral Exposure: Maternal serum unconjugated and conjugated (as well as the ratio of conjugated/unconjugated dBPA at 4 h after consumption of a piece of fruit containing 400 μg/kg/day dBPA from gestation day (GD) 50 – 100 (early pregnancy group) or from GD 100 – natural parturition (about GD 165). Serum was collected for analysis on GD 50, 77 and 95 for the early pregnancy treated females, and on GD 127 and 155 for the late pregnancy treated females. The data are not presented as molar concentrations because conjugated BPA consists of an unknown proportion of glucuronidated and sulfated BPA.
| N | UNCONJUGATED (ng/ml) | CONJUGATED (ng/ml) | CONJUGATED/UNCONJUGATED | ||
|---|---|---|---|---|---|
|
|
|||||
| EARLY PREGNANCY | NonPreg | 4 | 0.42±0.09 | 168.52±116.57 | 402.70 |
| GD 50 | 4 | 0.56±0.41 | 40.68±9.57 | 72.71 | |
| GD 77 | 5 | 0.17±0.03 | 13.46±2.78 | 80.33 | |
| GD 95 | 5 | 0.51±0.20 | 22.93±6.06 | 44.85 | |
|
|
|||||
| LATE PREGNANCY | GD 127 | 6 | 0.24±0.12 | 18.70±4.85 | 77.07 |
| GD 155 | 5 | 0.31±0.13 | 11.85±3.19 | 38.01 | |
Unconjugated dBPA in maternal serum: NS
Conjugated dBPA in maternal serum: Non-Pregnant > All Gestation Days; P < 0.01
Conjugated dBPA in maternal serum: GD 50 > all later gestation days (P < 0.05)
Levels of unconjugated dBPA did not differ significantly between non-pregnant females and any time point during early or late pregnancy. In contrast, the concentration of conjugated dBPA in non-pregnant females was significantly higher than at any time in pregnancy (P < 0.01). In addition, serum conjugated dBPA was significantly (P < 0.05) higher on GD 50 relative to later days in pregnancy. Thus, there are reduced levels of serum conjugated dBPA throughout pregnancy compared to non-pregnant females at 4 hr after oral exposure to dBPA, and serum conjugated dBPA levels are also higher on GD 50 than at any subsequent time in pregnancy (Table 2). We also collected blood from females in the late pregnancy group prior to the initiation of daily oral doses of dBPA on GD 100, and, as expected, no unconjugated or conjugated dBPA was found.
5.3. Pharmacokinetic studies of pregnant rhesus monkeys continuously exposed to dBPA via Silastic capsules
5.3.1. BPA release from Silastic capsules in vitro
To determine the dose of dBPA released per day, we first calculated the average release rate of dBPA from the capsules per 24 hours over a 7-week period by placing 3 test capsules containing the dose of BPA spiked with 3H-BPA in physiological saline solution. The in vitro release rate of 3H-BPA began to drop rapidly beginning on day 30 of incubation. Thus, the capsules were implanted in pregnant females and changed after 25 days so that two different sets of capsules were used for the 50 days of treatment.
5.3.2. Continuous exposure via subcutaneous implants results in markedly different conjugated to unconjugated ratios of dBPA
We examined serum dBPA levels in a non-pregnant female monkey exposed to dBPA continuously via sc implanted Silastic capsules. Serum dBPA was measured every other day (6 collections) for 12 days and averaged 3.57 ng/ml and 12.74 ng/ml for unconjugated and conjugated dBPA, respectively; unconjugated dBPA values were within the range of serum unconjugated BPA that has been reported in some human studies [3, 8].
For pregnant females implanted with Silastic capsules, the average steady-state serum unconjugated dBPA concentrations achieved at GD 100 during early pregnancy and at GD 150 during late pregnancy were 0.45±0.23 and 0.91±0.13 ng/mL, respectively (Table 3). These constant levels contrasted markedly with values obtained using the oral dosing strategy where unconjugated serum dBPA levels briefly reached higher levels (mean Cmax on GD 95 was 2.25 ng/ml) but rapidly decreased and were below the LOQ by 12 hr after the oral bolus administration (Table 1; Figure 1). However, the average AUC(0 – infinity) for unconjugated dBPA on GD 95 (0.45 ng/ml) following oral treatment was identical to the mean steady state unconjugated dBPA concentration on GD 100 resulting from treatment via Silastic capsules (Table 3).
Table 3.
Early and Late Gestation, Silastic Capsule Exposure: Maternal serum unconjugated and conjugated dBPA (ng/mL) at gestation day (GD) 75, 100, 125 and 150, as well as the ratio of conjugated/unconjugated dBPA (n = 6/Group). Silastic capsules were implanted in the “Early pregnancy” females on GD 50, and blood was collected prior to these capsules being replaced on GD 75. After implantation of new Silastic capsules for another 25 days, blood was collected from these females on GD 100, at which time fetal blood, tissues and amniotic fluid were also collected. Another group of females in the “Late pregnancy” treatment group were implanted with Silastic capsules on GD 100, and blood was collected prior to these capsules being replaced on GD 125. After implantation of new Silastic capsules for another 25 days, blood was collected from these females on GD 155, at which time fetal blood, tissues and amniotic fluid were also collected. The data for non-pregnant female was based on 6 serum measurements over 12 days from one female implanted with Silastic capsules containing the same dose of dBPA. P values are based on Fisher’s LSD comparing all groups if the overall ANOVA was statistically significant. NC = not calculated.
| UNCONJUGATED | CONJUGATED | CONJUGATED/UNCONJUGATED | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Status | Maternal Serum | Fetal Serum | Amniotic Fluid | Maternal Serum | Fetal Serum | Amniotic Fluid | Maternal Serum | Fetal Serum | Amniotic Fluid | |
|
|
||||||||||
| EARLY PREGNANCY | NonPreg | 3.57±0.30 | 12.74±1.63 | 3.58±0.52 | ||||||
| GD 75 | 0.76±0.20 | 1.41±0.34 | 1.86±0.34 | |||||||
| GD 100 | 0.45±0.23 | 0.37±0.16 | <LOQ | 1.74±0.38 | 17.51±4.15 | 39.26±8.68 | 3.87±0.42 | 38.91±3.85 | NC | |
|
|
||||||||||
| LATE PREGNANCY | GD 125 | 0.70±0.15 | 1.32±0.36 | 1.89±0.52 | ||||||
| GD 150 | 0.91±0.13 | <LOQ | 3.00±0.66 | 0.90±0.15 | 6.68±0.94 | 171.46±56.32 | 0.99±0.15 | NC | 57.15±19.82 | |
- Unconjugated dBPA in maternal serum: GD 100 vs. 150; P < 0.05
- Unconjugated dBPA in fetal serum: GD 100 vs. 150; NS
- Unconjugated dBPA in amniotic fluid: GD 100 vs. 150; P < 0.001
- Conjugated dBPA in maternal serum: GD 100 vs. 150; P < 0.05
- Conjugated dBPA in fetal serum: GD 100 vs. 150; P < 0.05
- Conjugated dBPA in amniotic fluid: GD 100 vs. 150; P < 05.
- Conjugated/Unconjugated dBPA in maternal serum: GD 100 vs. 150; P < 0.05
- Unconjugated dBPA in maternal serum: non-Preg > All Gestation Days; P < 0.001
- Conjugated dBPA in maternal serum: non-Preg > All Gestation Days; P < 0.001
- Conjugated/Unconjugated dBPA in maternal serum: non-Preg > All Gestation Days; P < 0.05
- GD 100: Unconjugated dBPA, maternal vs. fetal vs. amniotic fluid; all comparisons, P > 0.1
- GD 100: Conjugated dBPA, maternal vs. fetal vs. amniotic fluid; all comparisons, P < 0.05
- GD 150: Unconjugated dBPA, maternal vs. fetal vs. amniotic fluid; all comparisons, P < 0.01
- GD 150: Conjugated dBPA, maternal vs. fetal vs. amniotic fluid; all comparisons, P < 0.001
The most dramatic difference based on route of exposure was in the conjugated/unconjugated dBPA ratio between the oral dose and continuous subcutaneous dose pregnant females. The ratio of conjugated/unconjugated dBPA in maternal serum throughout pregnancy as a result of continuous exposure from Silastic capsules ranged from 1.03:1 (on GD 150) to 1.97:1 (on GD 100; Table 3), which was dramatically lower than this ratio in serum collected 4 hr after oral exposure, which ranged from a mean of 38 (on GD 155) to 80 (on GD 77; Table 2); the mean conjugated/unconjugated ratios based on AUC(0 – infinity) on GD 50 and 95 were 62 and 36 ng/ml, respectively (Table 1).
5.4. Maternal and fetal serum, placenta and amniotic fluid comparisons following dBPA exposure during early and late pregnancy
How rapidly bioactive BPA from maternal exposure reaches the fetal compartment and whether levels of unconjugated BPA in maternal serum are an accurate indication of fetal exposure are important considerations for which there are limited data. To address these questions, we compared levels of dBPA in maternal and fetal serum, placenta and amniotic fluid using data from both oral and continuous exposure animals.
5.4.1. dBPA reaches the fetal compartment after oral exposure
We compared levels of unconjugated and conjugated dBPA in maternal and fetal serum and amniotic fluid on GD 100 at 1 and 3 hr after daily oral dBPA administration to the dam between GD 50 – 100 during early pregnancy (Table 4). The levels of conjugated dBPA in maternal serum appeared to decrease between 1 and 3 hr after maternal ingestion, such that the higher levels of conjugated dBPA in maternal relative to fetal serum at 1 hr were not found at 3 hr (Table 4). Specifically, at 1 hr after administration, we found similar levels of unconjugated dBPA in maternal and fetal serum but lower levels of conjugated dBPA in fetal serum relative to maternal serum, as well as a lower ratio of conjugated to unconjugated dBPA in fetal serum relative to maternal serum. However, at 3 hr after maternal ingestion, unconjugated dBPA was below the LOQ in fetal serum, and conjugated BPA in fetal serum was similar to or higher than in maternal serum.
Table 4.
Early Gestation, Oral Exposure from GD 50 – 100: Levels of unconjugated and conjugated dBPA in serum of mothers and fetuses as well as amniotic fluid at 2 times (about 1 h and 3 h) after the last oral exposure: on GD 100. No data are presented for unconjugated dBPA in amniotic fluid or for the conjugated/unconjugated ratio in amniotic fluid because unconjugated dBPA in all amniotic fluids was below the LOQ. NC = not calculated.
| UNCONJUGATED
|
CONJUGATED
|
CONJUGATED/UNCONJUGATED
|
||||||
|---|---|---|---|---|---|---|---|---|
| ID | Mother | Fetus | Mother | Fetus | Amniotic fluid | Mother | Fetus | Hrs till Collection |
| #3 | 1.71 | 1.11 | 158.13 | 57.83 | 6.82 | 96.26 | 52.10 | 1.15 |
| #14 | 1.84 | 0.72 | 225.02 | 33.57 | 8.44 | 122.09 | 46.72 | 1.38 |
| #16 | 2.70 | 3.08 | 144.50 | 83.76 | 3.97 | 53.57 | 27.20 | 1.15 |
|
| ||||||||
| mean | 2.08 | 1.64 | 175.88 | 58.39 | 6.41 | 90.64 | 42.01 | 1.23 |
| SEM | 0.31 | 0.73 | 24.88 | 14.49 | 1.31 | 19.98 | 7.56 | |
| #2 | 2.51 | <LOQ | 72.95 | 69.11 | 11.88 | 29.80 | NC | 3.20 |
| #6 | 1.19 | <LOQ | 97.51 | 212.733 | 24.84 | 82.35 | NC | 3.00 |
|
| ||||||||
| mean | 1.85 | <LOQ | 85.23 | 140.92 | 18.36 | 56.08 | NC | 3.10 |
In amniotic fluid, unconjugated dBPA was not above the LOQ of 0.2 ng/ml at either 1 or 3 hr after maternal ingestion of dBPA. However, conjugated dBPA concentrations increased from a mean of 6.4 ng/ml to 18.4 ng/ml between 1 and 3 hr (Table 4). Notably, the concentrations of conjugated dBPA in amniotic fluid were consistently lower than serum conjugated dBPA concentrations in either mother or fetus.
Examination of maternal decidual tissue and fetal placental tissue (Table 5) revealed similar concentrations of unconjugated and conjugated dBPA in both tissues, with conjugated dBPA being about 5-fold higher than unconjugated dBPA at both 1 and 3 hr after the mother was fed dBPA on GD 100. Taken together, these findings suggest that maternally ingested dBPA enters maternal blood and crosses the placenta into the fetal blood, and the rate of clearance of conjugated dBPA appears slower in fetal relative to maternal serum (Table 4).
Table 5.
Early Gestation, Oral Exposure from GD 50 – 100: Levels of BPA in maternal decidua and fetal placenta at 2 times (about 1 h and 3 h) after the last oral exposure on GD 100. NC = not calculated.
| Decidua
|
Placenta
|
||||||
|---|---|---|---|---|---|---|---|
| ID | Unconjugated (ng/g) | Conjugated (ng/g) | Conjugated: Unconjugated | Unconjugated (ng/g) | Conjugated (ng/g) | Conjugated: Unconjugated | Hrs till Collection |
| #3 | 2.42 | 4.62 | 1.91 | 1.09 | 6.61 | 6.06 | 1.15 |
| #14 | 3.59 | 25.30 | 7.05 | 1.97 | 17.96 | 9.12 | 1.38 |
| #16 | 3.49 | 9.5 | 2.72 | 4.09 | 12.31 | 3.01 | 1.15 |
|
| |||||||
| Mean | 3.17 | 13.14 | 4.15 | 2.38 | 12.29 | 5.16 | 1.23 |
| SEM | 0.37 | 6.24 | 16.86 | 0.89 | 3.28 | 3.69 | |
| #2 | <LOQ | 7.26 | <LOQ | <LOQ | 4.95 | NC | 3.20 |
| #6 | 2.68 | 10.28 | 3.84 | 0.97 | 11.98 | 12.35 | 3.00 |
|
| |||||||
| Mean | NC | 8.77 | NC | NC | 8.47 | NC | 3.10 |
5.4.2. Continuous dosing via Silastic capsules during early and late pregnancy: dBPA in maternal and fetal serum and amniotic fluid on GD 100 and GD 150
In animals continuously dosed via Silastic capsules, the profiles of unconjugated dBPA in maternal and fetal serum were different on GD 100 and on GD 150 (Table 3). Specifically, levels of unconjugated dBPA were similar on GD 100 in maternal serum (range: <:LOQ-1.19 ng/ml; 3 samples<LOQ) and fetal serum (range: <LOQ-1.03 ng/ml; 2 samples<LOQ). However, on GD 150 only one of 6 fetuses had serum unconjugated dBPA that was above the LOQ, while all mothers had unconjugated dBPA above the LOQ (range: 0.58–1.51 ng/ml). In contrast, levels of unconjugated dBPA in amniotic fluid showed the opposite pattern, with levels below the LOQ for all but one fetus on GD 100, while on GD 150 all amniotic fluid dBPA values were above the LOQ (range: 0.81–5.83 ng/ml). In addition, on GD 150, amniotic fluid unconjugated dBPA levels were significantly (P < 0.01) higher than levels in both maternal and fetal serum (Table 3).
Several significant differences in the levels of conjugated dBPA were also evident in continuously dosed animals. Of particular interest was the significantly higher mean conjugated dBPA concentration in amniotic fluid on GD 150 relative to GD 100 (Table 3; P < 0.01). In addition, the relationship between maternal serum levels of conjugated dBPA and levels in fetal serum and amniotic fluid was striking; on GD 100, conjugated dBPA levels in amniotic fluid were more than 20 times higher and levels in fetal serum were 10 times higher than levels in maternal serum (all comparisons P < 0.05). By GD 150, the increased levels of conjugated dBPA in amniotic fluid relative to maternal or fetal serum were even more pronounced, with mean levels in amniotic fluid 150-fold higher than levels in maternal serum and 26-fold higher than in fetal serum (all comparisons P < 0.001; Table 3).
6. Discussion
To determine if pregnancy affects BPA pharmacokinetics, we performed studies at multiple times in pregnancy and compared our findings to results from non-pregnant rhesus monkeys, a primate that is an experimental model for human xenobiotic pharmacokinetics [41]. We eliminated concerns about environmental contamination by administering dBPA instead of BPA, but also conducted preliminary tests to ensure that all equipment used did not leach detectable BPA. Thus, contamination of samples with environmental BPA can be controlled. Due to concerns that gavage administration does not provide a pharmacokinetic profile relevant to human exposure to BPA, as clearly demonstrated in a recent experiment [20], we used two different dosing protocols: a single daily oral administration in a piece of fruit and continuous exposure via sc implanted Silastic capsules. In addition, because the validity of models used by the US FDA to estimate the routes involved in daily human exposures [42] has been challenged by data from multiple studies [1, 2, 20, 22, 24], the doses used in our studies were selected on the basis of data from biomonitoring studies. Importantly, for both treatment protocols, the dose used resulted in serum unconjugated dBPA in rhesus monkeys within the median concentrations (range, 0.3–4.4 ng/mL, or 1–19.4 nM) reported in humans [3, 12].
Our study is unique in two important respects: pharmacokinetic studies were conducted prior to pregnancy on females used in the early pregnancy oral dosing experiment, allowing us to assess the effects of pregnancy by making direct comparisons of dBPA metabolism before and during early to mid (GD 50–100) pregnancy in the same females. Second, tissues (ovary, mammary gland, brain and lung) from the female fetuses carried by the dams in our early (GD 50 – 100) and late pregnancy (GD 100 – parturition) oral dBPA-exposure studies were evaluated in 4 separate published studies [31–34]. Significant adverse effects were evident in dBPA-exposed fetuses in all studies, and the findings for ovary, mammary gland and brain recapitulate previously reported effects from numerous studies in rodents. This underscores the importance of our pharmacokinetic data, since data from this small cohort of monkeys demonstrate that low, human-relevant concentrations of unconjugated dBPA in maternal and fetal serum disrupt normal fetal development in a primate model.
6.1. Oral dosing: Pharmacokinetics of dBPA are altered during pregnancy
By conducting identical pharmacokinetic studies of orally ingested dBPA in maternal serum prior to and at multiple times during pregnancy, our data clearly demonstrate that, in the rhesus monkey, while some aspects of the disposition of dBPA were significantly altered by pregnancy, there was no specific barrier to protect the fetus against maternal exposure to dBPA. Alterations observed to dBPA disposition during pregnancy were not unexpected, as pregnancy is associated to multiple physiological changes that could affect the disposition of unconjugated and conjugated BPA and thus explain what we observed in the present study. In women, these include expansion of body fluids, an increase of cardiac output (+30–50%), alteration of regional blood flow, an increase of glomerular filtration rate (GFR), and changes of the activity of hepatic drug metabolizing enzymes that can affect the clearance of chemicals. These effects are most pronounced in the last part of pregnancy during the period of rapid fetal growth [43, 44].
Despite the potential for differences in the disposition of unconjugated dBPA due to pregnancy, the overall exposure to unconjugated dBPA after oral bolus administration of a 400-μg/kg/day dose (Table 2) was similar in pregnant compared to the same non-pregnant monkeys [based on the AUC(0-infinity)], confirming previous findings from studies in rats [45]. This suggests that pregnancy does not significantly alter first-pass metabolism in the liver following absorption of BPA from the gastrointestinal system. These data contrast with what has been reported in some studies of pregnant women where there is evidence of an increase in serum unconjugated BPA relative to levels reported in non-pregnant women [3, 8, 39]. However, unlike the present study, where the same monkeys were evaluated before and during pregnancy, the human data are from independent cross-sectional studies.
Using the AUC of the IV route reported previously in rhesus monkeys (i.e. 180±76nM*h/L or 41ng*h/mL) for an IV BPA dose of 100 μg/kg [46], we estimated the absolute bioavailability of dBPA by the oral route to be about 7.3% in our non-pregnant females and about 5% for pregnant females at GD 50. This is a rather high bioavailability for an oral route, as Doerge et al. reported a 0.94% bioavailability; the difference between our results and those of Doerge et al. is very likely due to the method of oral dBPA administration. Oral gavage by Doerge et al. would result in the entire administered dBPA dose being subjected to a hepatic first-pass effect. In contrast, our more physiologically relevant method of administration in a piece of fruit created the possibility that individual animals would engage in hoarding behavior (keeping a portion of the fruit in their cheek). Thus, a significant fraction of the dBPA dose would likely be directly absorbed in the buccal cavity and escape the hepatic first-pass effect [20]. This hypothesis is supported by the inverse relationship between the level of serum unconjugated dBPA exposure over the 24 hr after oral administration and the corresponding conjugated/unconjugated dBPA ratio (Figure 3).
In contrast to the data for unconjugated dBPA, a comparison for AUC(0-infinity) of conjugated dBPA in maternal serum prior to and during pregnancy showed significant differences. Specifically, there were significantly lower AUC(0-infinity) values on GD 50 and GD 95 to 40% and 28%, respectively, of the pre-pregnancy values (Table 1). This is at variance with what was reported in rats [45], a species where the main pathway of BPA elimination is the feces, not urine as in primates. We propose two possible interpretations of these results: 1) plasma clearance of conjugated dBPA increases during pregnancy, leading to a lower conjugated dBPA [based on AUC(0-infinity)] for a given amount of unconjugated dBPA, or 2) the metabolic pathway of dBPA is different during pregnancy, with a larger fraction of dBPA not being transformed to a hydrolysable phase II glucuronidated or sulfated metabolite. Our prediction is that the most likely explanation for the relatively lower conjugated dBPA serum concentration after oral bolus exposure is an increase of renal conjugated dBPA clearance during pregnancy; this is consistent with the phenomenon in women of renal adaptation that is characterized by an increase in glomerular filtration rate, typically 50% above the pre-pregnancy value [47].
The mean residence times (MRT(0 - infinity) refers to the average total time dBPA molecules of a given dBPA dose spend in the body; this parameter takes into account all phases of dBPA disposition (absorption, distribution and elimination). The MRT(0-infinity) values were significantly longer when measured on both GD 50 and 95 relative to non-pregnant females (Table 1), and the most likely explanation is a slower rate of absorption of dBPA during pregnancy. This also may explain the finding that terminal half-lives (HL_Lambda_z (h)) for unconjugated dBPA were significantly longer on both GD 50 and 95 of pregnancy than prior to pregnancy (Table 1). We interpret the terminal half-life during pregnancy as a half-life of dBPA absorption, not a half-life of dBPA elimination. This is identified in pharmacokinetics as the presence of a so-called “flip-flop”, where the rate of absorption is the rate-limiting step in the processes of absorption, distribution and elimination of a chemical or drug, and the plasma concentration-time profile tends to closely parallel the rate of absorption [48]. The fact that absorption rate was likely the limiting step of dBPA disposition during pregnancy also explains a significantly lower maximum unconjugated dBPA plasma concentration (Cmax) on GD 50 and GD 95 of pregnancy than prior to pregnancy, even though the AUC(0-infinity) did not differ between pregnancy and pre-pregnancy. An alternative explanation could be an expansion of the volume of distribution during pregnancy, as the terminal half-life is a hybrid parameter reflecting both clearance and volume of distribution.
Our data demonstrate that orally ingested dBPA readily crosses the placenta in early pregnancy (GD 100), with a significant amount of both unconjugated and conjugated dBPA detectable in the decidua and placenta 1 hr after feeding dams (Table 5). Further, while unconjugated dBPA was detected in fetal serum (although with one exception at a lower concentration than in the mother), it was not detectable in amniotic fluid (Table 4). In addition, conjugated dBPA was generally lower in fetal serum and markedly lower in amniotic fluid relative to maternal serum. Prior studies have shown that in a pregnant sheep model, glucuronidated BPA does not cross the placental barrier either from maternal to fetal compartment or from the fetal to maternal compartment [9].In rats only a small percentage of glucuronidated BPA in the maternal circulation crosses the placenta, and the placenta also can deconjugate a small percentage of glucuronidated BPA in maternal serum [49].
It is likely that the elimination of fetal unconjugated dBPA was by a back transfer of dBPA to the mother rather than due to an in situ dBPA transformation to conjugated dBPA. UDP-glucuronosyltransferase is not expressed in the human fetal liver until after birth, and infant rhesus monkeys have markedly (~4-fold) higher AUC(0-infinity) unconjugated serum BPA values relative to adult monkeys after gavage administration of the same dose [46], revealing a virtually identical age-related increase in phase II metabolism of BPA in rhesus monkeys and mice, based on our prior studies with infant and adult mice [2, 50]. There appears to be a steady increase in phase II metabolism of BPA between infancy and adulthood in rhesus monkeys [46], although this remains unexamined in humans [51].
In these studies we did not distinguish between the two conjugates of dBPA (glucuronidated dBPA and sulfated dBPA) due to a lack of standards. The standards and isotopes for BPA-glucuronide and BPA-monosulfate, as well as methods for measuring these two conjugates together with unconjugated BPA (without hydrolysis) are now available, and simultaneous assays for unconjugated and conjugated BPA have been validated [9, 36]. Future studies will be able to confirm whether the proportion of glucuronidated and sulfated BPA changes during pregnancy and between the maternal and fetal compartments in animal models, consistent with recently published data from pregnant women [36]. This is important given that significant amounts of both unconjugated and conjugated dBPA were evident 1 hr after oral administration. We are particularly interested in the possibility that the proportion of conjugated BPA that is sulfated might be much higher in pregnancy than in non-pregnant females because the placenta contains both sulfotransferase and sulfatase [36]. The critical issue here is that there is a considerable exchange between the placenta, which expresses sulfatases that hydrolyze sulfated estrogens, and fetal tissues, which can conjugate estrogens, providing a continuous cycling pool of unconjugated and conjugated estrogens. The sulfated BPA may act as a “reserve”, yielding active BPA following hydrolysis [52]. While preliminary evidence suggests that this may be the case in human pregnancies [36], the evidence is that BPA-sulfation is less important during pregnancy in sheep [9]. Finally, while the degree to which different fetal tissues can bioactivate glucuronidated or sulfated BPA remains unknown, recent evidence shows that glucuronidated, sulfated and chlorinated BPA can rapidly alter phosphorylation of ERK and JNK pathways via membrane estrogen receptor-α [53]. This raises the possibility that even without biotransformation, conjugated BPA could impact tissues during fetal and postnatal life.
6.2. Continuous subcutaneous dosing via Silastic capsules: dBPA metabolism changes during the course of pregnancy
The results of our experiments using continuous subcutaneous dBPA administration via Silastic capsules yielded a complex pattern of dBPA levels in mother, fetus and amniotic fluid in early and late pregnancy (Table 3). The data are, however, understandable if two factors are considered: first, fetal urination (~ 300 ml/kg fetal weight/day in humans) and swallowing (200–250 mL/kg fetal weight/day in humans) contribute to amniotic fluid composition only after the second half of pregnancy. Second, from mid-gestation on, fetal dBPA conjugating capacity may mainly be supported by sulfotransferases, given that BPA like endogenous estrogens and other xenoestrogens, is a substrate for sulfotransferases [36]. In contrast, in early pregnancy, BPA exposure in the fetus and amniotic fluid is mainly governed by bidirectional diffusion between the mother, placenta, fetus and amniotic fluid without active fetal metabolic contribution. Thus, in early pregnancy, BPA likely gains access to the fetus through passive diffusion and is cleared primarily by back-diffusion to the mother via the placenta. In contrast, in late pregnancy, we predict that BPA would be actively eliminated by the fetal kidney and by conjugation. This would explain the relatively low concentration of unconjugated dBPA in fetal serum and the presence of higher concentrations of unconjugated and conjugated dBPA in amniotic fluid in late relative to early pregnancy.
In a pregnant sheep model, fetal exposure to BPA from maternal IV infusion for 24 hr or from direct BPA infusion into the fetal jugular vein for 24 hr led to high levels of glucuronidated BPA in the fetal plasma that decreased very slowly. In amniotic fluid, unconjugated BPA was not detected, but significant levels of conjugates were detected and remained stable during several hours after the end of the infusion before decreasing slowly as in fetal plasma [9]. These results are in agreement with ours here and show that in late pregnancy, conjugated BPA eliminated by the fetal kidney remained trapped in the fetal compartment, likely due to the urination-amniotic fluid swallowing cycle. Thus, the elimination of dBPA from the fetal compartment is slow due to poor capacities of water-soluble compounds to cross the placental barrier, which leads to fetal overexposure to BPA metabolites. In addition, an increase in the capacity of the fetal kidneys to clear conjugated BPA in late pregnancy may explain both a lower conjugated BPA serum level in fetuses associated with higher levels of dBPA conjugates in amniotic fluid on GD 150 relative to GD 100 (Table 4). In summary, in early pregnancy, the fetal compartment can be viewed as a deep peripheral compartment exchanging slowly and passively with the maternal compartment, while in late pregnancy, urination, swallowing and fetal metabolism are responsible for a dynamic equilibrium between the mother and the fetus as well as within the different fetal compartments.
After continuous dBPA administration using sc implanted Silastic capsules, the steady-state serum concentration in a non-pregnant monkey was higher (3.57 ng/mL) than in pregnant females. As serum concentration is controlled by both serum clearance and the amount of BPA actually released in vivo by the capsule, which cannot be estimated from the in vitro release rate into buffer, we cannot speculate on the origin of this difference. Nevertheless, the two different routes of exposure used in our studies provide evidence that BPA metabolism is dependent upon both the route of exposure and the stage of pregnancy analyzed. Importantly, the route of exposure (oral vs. Silastic capsule) affects the ratio of unconjugated and conjugated BPA in maternal serum. Our data show that continuous exposure to BPA via Silastic capsules produces a profile of conjugated/conjugated BPA in maternal serum that ranged from 0.99:1 to 3.87:1 during pregnancy compared to 3.58:1 prior to pregnancy (Table 3). Because the ratios obtained from continuously exposed animals are more similar to the profiles observed in cross-sectional studies in people, where the ratio of conjugated to unconjugated BPA is less than 10:1 [35, 36], our results suggest that continuous exposure (via subcutaneously implanted capsule) may better model human exposures than oral bolus exposure one time per day.
Gayrard and colleagues have shown that BPA is rapidly absorbed in the mouth, which is a known method for rapid (and virtually complete) uptake of drugs such as nitroglycerine. The Gayrard et al. study was conducted in dogs, which are an accepted model for human oral exposures [20]. In contrast to the Gayrard et al. findings, others have proposed that virtually all BPA exposure is via the oral route and that these exposures can be modeled by gavage administration, which bypasses sublingual absorption [42]. The only attempt at a human pharmacokinetic study involved placing BPA directly into the stomach by administering it in a capsule; this experiment was also of limited value because of the insensitivity (10-fold less sensitive than our assay) of the BPA assay that did not allow levels of serum unconjugated BPA to be determined [54]. We have thus had to rely on primate and rodent pharmacokinetic studies to estimate human pharmacokinetics of BPA, and the available evidence is that both rodent and primate data are relevant to human pharmacokinetics [2].
7. Conclusions
In the absence of requirements for the identification of product components on the part of producers, many uses of the estimated >10-billion pounds of BPA produced per year remain unknown. This information gap has greatly limited the ability to adequately estimate the routes of exposure to BPA, but it seems likely that non-oral exposures, such as from thermal receipt paper coated with milligram amounts of free BPA, are significant contributors [25, 55, 56]. Importantly, absorption of BPA into the blood stream by routes other than via the gut results in higher unconjugated BPA than predicted on the basis of models that assume all uptake of BPA is from the gut [20]. This is because BPA uptake from the gut passes through the hepatic portal vessels directly to the liver where first-pass metabolism (conjugation) occurs. Conjugation of BPA due to this “first pass” effect is often misrepresented as complete, which is not an accurate description following absorption from the gut and is clearly not the case for the greater amount of unconjugated BPA that can reach target tissues via other routes.
In summary, these experiments show unequivocally that biologically active BPA passes from the mother to the fetus and that a daily oral dose of 400 μg/kg/day was required to produce an internal BPA concentration that was within the median concentration detected in pregnant women and fetuses in a number of studies [3]. Thus, based on monitoring of the unconjugated dBPA concentration, the exposures used in our studies are clearly relevant to human exposures because they led to serum unconjugated dBPA concentrations within the range reported in human biomonitoring studies, and use of the dBPA isotope precludes the possibility of confounding environmental contamination. Furthermore, the fact that continuous exposure via Silastic capsules produces a ratio of conjugated/unconjugated BPA that is more similar to that seen in humans than the ratio obtained using oral exposure suggests that the impact of non-oral routes of human exposure has been greatly underestimated. Lastly, the pharmacokinetics of BPA during pregnancy are complex and change during the course of pregnancy. Thus, assumptions about BPA clearance and metabolism cannot be directly extrapolated using values obtained from a non-pregnant state or at a single time point in pregnancy, especially with respect to concentrations in the fetal compartment. Indeed, it is possible that high levels of conjugated BPA in the fetal compartment during late pregnancy may act as a reservoir for potential cycling between unconjugated and conjugated BPA [36] even if this hypothesis is not supported by results obtained in the ewe [9].
Highlights.
dBPA pharmacokinetics differed in non-pregnant and pregnant female monkeys
Serum conjugated/unconjugated dBPA was higher feeding dBPA than with sc capsules
Mothers with highest unconjugated serum dBPA had lowest conjugated/unconjugated ratio
Capsules led to higher unconjugated and lower conjugated dBPA in mothers than fetuses
Oral dBPA led to adverse effects in fetal lungs, brain, mammary glands and ovaries
Acknowledgments
Funding was provided by NIEHS grant (ES016770) and the Office of Research Infrastructure Programs Division of Comparative Medicine Grant (OD011107/RR00169; California National Primate Research Center) to CAV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environmental health perspectives. 2009;117:784–9. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, et al. Similarity of bisphenol a pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environmental health perspectives. 2011;119:422–30. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental health perspectives. 2010;118:1055–70. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekant W, Volkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicology and applied pharmacology. 2008;228:114–34. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.EnvironmentCanada. Screening Assessment for The Challenge Phenol, 4,4′-(1-methylethylidene)bis- (Bisphenol A) Chemical Abstracts Service Registry Number 80-05-7. Environment Canada and Health Canada; Ottawa: Oct, 2008. 2008. [Google Scholar]
- 6.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao XL, Zhang J, Goodyer CG, Hayward S, Cooke GM, Curran IH. Bisphenol A in human placental and fetal liver tissues collected from Greater Montreal area (Quebec) during 1998–2008. Chemosphere. 2012:505–11. doi: 10.1016/j.chemosphere.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbel T, Gayrard V, Viguie C, Puel S, Lacroix MZ, Toutain PL, et al. Bisphenol A disposition in the sheep maternal-placental-fetal unit: mechanisms determining fetal internal exposure. Biology of Reproduction. 2013;89(1):11, 1–9. doi: 10.1095/biolreprod.112.106369. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinol. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 12.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive toxicology. 2007;24:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:256–68. doi: 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reproductive toxicology. 2011;31:1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochester JR. Bisphenol A and human health: A review of the literature. Reproductive toxicology. 2013;42:132–55. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Braun JM, Hauser R. Bisphenol A and children’s health. Curr Opin Pediatr. 2011;23:233–9. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive toxicology. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ANSES. French Agency for Food, Environmental and Occupational Health & Safety. [Accessed: December 30, 2011];Health Effects of Bisphenol A. 2011 (Request nos. 2009-SA-0331 and 2010-SA-0197; September 2011). http://www.anses.fr/index.htm.
- 20.Gayrard V, Lacroix MZ, Collet SH, Viguie C, Bousquet-Melou A, Toutain PL, et al. High bioavailability of bisphenol a from sublingual exposure. Environmental health perspectives. 2013;121:951–6. doi: 10.1289/ehp.1206339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reproductive toxicology. 2007;24:139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Gies A, Heinzow B, Dieter HH, Heindel J. Bisphenol a workshop of the German Federal Environment Agency - March 30–31, 2009 work group report: public health issues of bisphenol A. International journal of hygiene and environmental health. 2009;212:693–6. doi: 10.1016/j.ijheh.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Sieli PT, Jasarevic E, Warzak DA, Mao J, Ellersieck MR, Liao C, et al. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environmental health perspectives. 2011;119:1260–5. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environmental health perspectives. 2010b;118:1051–4. doi: 10.1289/ehp.0901717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Analytical and bioanalytical chemistry. 2010;398:571–6. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- 26.Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82:424–30. doi: 10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 27.Rudel RA, Brody JG, Spengler JD, Vallarino J, Geno PW, Sun G, et al. Identification of selected hormonally active agents and animal mammary carcinogens in commercial and residential air and dust samples. Journal of the Air & Waste Management Association (1995) 2001;51:499–513. doi: 10.1080/10473289.2001.10464292. [DOI] [PubMed] [Google Scholar]
- 28.Geens T, Roosens L, Neels H, Covaci A. Assessment of human exposure to Bisphenol-A, Triclosan and Tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere. 2009;76:755–60. doi: 10.1016/j.chemosphere.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Loganathan SN, Kannan K. Occurrence of bisphenol a in indoor dust from two locations in the eastern United States and implications for human exposures. Arch Environ Contam Toxicol. 2012;61:68–73. doi: 10.1007/s00244-010-9634-y. [DOI] [PubMed] [Google Scholar]
- 30.Duty SM, Mendonca K, Hauser R, Calafat AM, Ye X, Meeker JD, et al. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics. 2013;131:483–9. doi: 10.1542/peds.2012-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17525–30. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, DE, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–20. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8190–5. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Winkle LS, Murphy SR, Boetticher MV, Vandevoort CA. Fetal exposure of rhesus macaques to bisphenol a alters cellular development of the conducting airway by changing epithelial secretory product expression. Environmental health perspectives. 2013;121:912–8. doi: 10.1289/ehp.1206064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao C, Kannan K. Determination of free and conjugated forms of bisphenol a in human urine and serum by liquid chromatography-tandem mass spectrometry. Environmental science & technology. 2012;46:5003–9. doi: 10.1021/es300115a. [DOI] [PubMed] [Google Scholar]
- 36.Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, et al. BPA, BPA glucuronide, and BPA sulfate in mid-gestation umbilical cord serum in a northern California cohort. Environmental science & technology. 2013;47:12477–85. doi: 10.1021/es402764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarantal AF. Ultrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fascicularis) macaques: reproductive and research applications. In: Wolfe-Coote S, editor. The Laboratory Primate. Boston: Elsevier Academic Press; 2005. pp. 317–51. [Google Scholar]
- 38.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environmental health perspectives. 2013;121:283–6. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28:258–63. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. [Google Scholar]
- 41.Slikker W, Jr, Bailey JR, Newport D, Lipe GW, Hill DE. Placental transfer and metabolism of 17 alpha-ethynylestradiol-17 beta and estradiol-17 beta in the rhesus monkey. The Journal of pharmacology and experimental therapeutics. 1982;223:483–9. [PubMed] [Google Scholar]
- 42.Patterson TA, Twaddle NC, Roegge CS, Callicott RJ, Fisher JW, Doerge DR. Concurrent determination of bisphenol A pharmacokinetics in maternal and fetal rhesus monkeys. Toxicology and applied pharmacology. 2013;267:41–8. doi: 10.1016/j.taap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Horan C. Physiological changes in pregnancy. In: Berghella V, editor. Obstetric Evidence Based Guidelines. 1. London: Informa; 2007. [Google Scholar]
- 44.Silversides CK, Colman JM. Physiological Changes in Pregnancy. In: Oakley C, Warnes CA, editors. Heart Disease in Pregnancy. 2. Malden, Massachusetts: Blackwell Publishing; 2007. pp. 6–17. [Google Scholar]
- 45.Domoradzki JY, Pottenger LH, Thornton CM, Hansen SC, Card TL, Markham DA, et al. Metabolism and pharmacokinetics of bisphenol A (BPA) and the embryo-fetal distribution of BPA and BPA-monoglucuronide in CD Sprague-Dawley rats at three gestational stages. Toxicol Sci. 2003;76:21–34. doi: 10.1093/toxsci/kfg206. [DOI] [PubMed] [Google Scholar]
- 46.Doerge DR, Twaddle NC, Woodling KA, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicology and applied pharmacology. 2010;248:1–11. doi: 10.1016/j.taap.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88:10–7. doi: 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 48.Boxenbaum H. Pharmacokinetics tricks and traps: flip-flop models. J Pharm Pharm Sci. 1998;1:90–1. [PubMed] [Google Scholar]
- 49.Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environmental health perspectives. 2010;118:1196–203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor JA, Welshons WV, Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reproductive toxicology. 2008;25:169–76. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clinical pharmacokinetics. 1999;36:439–52. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 52.Pasqualini JR. Enzymes involved in the formation and transformation of steroid hormones in the fetal and placental compartments. J Steroid Biochem Mol Biol. 2005;97:401–15. doi: 10.1016/j.jsbmb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Vinas R, Watson CS. Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environmental health : a global access science source. 2013;12:26. doi: 10.1186/1476-069X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chemical research in toxicology. 2002;15:1281–7. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 55.Mendum T, Stoler E, VanBenschoten H, Warner JC. Concentration of bisphenol A in thermal paper. Green Chemistry Letters and Reviews. 2011;4:81–6. [Google Scholar]
- 56.Liao C, Kannan K. Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure. Environmental science & technology. 2011;45:9372–739. doi: 10.1021/es202507f. [DOI] [PubMed] [Google Scholar]