Abstract
The brain’s “reward circuit” has been widely implicated in the pathophysiology of mental illness. Although there has been significant progress in identifying the functional characteristics of individual nodes within the circuit and linking dysfunction of these brain areas to various forms of psychopathology, there remains a substantial gap in understanding how the nodes of the circuit interact with one another, and how the growing neurobiological knowledge may be applied to improve psychiatric patient care. In this article, we summarize what is currently known about the functions and interactions of two key nodes of this circuit—the ventral striatum and the ventromedial prefrontal/orbital frontal cortex—in relation to mental illness.
Keywords: reward, prefrontal cortex, striatum, addiction, depression
Introduction
The brain’s “reward circuit”—the putative network of regions encoding various aspects of pleasure, motivation, value, and decision-making—is a major focus of research on the pathophysiology of mental illness. Clinical neuroimaging studies have consistently identified abnormalities in reward circuit function across a range of psychiatric disorders, including substance use disorder, depression, schizophrenia, obsessive-compulsive disorder, autism, and attention deficit hyperactive disorder. The involvement of the reward circuit in these various disorders suggests that this circuit underlies some crucial domain (or domains) of function that cuts across traditional diagnostic categories. In order for psychiatric medicine to advance toward a more neuropathophysiologically-based system of diagnosis and treatment, it will be necessary to more fully elucidate how particular elements of social, cognitive, and affective dysfunction relate to disordered activity in key brain networks, such as the reward circuit. Although neuroscientific studies have made considerable progress in identifying functional characteristics of individual nodes of the reward circuit, there remain critical unanswered questions about how these brain areas interact, and how disordered interactions in this circuit may give rise to particular symptoms of psychiatric illness. In this article, we focus on what is currently known about the interactions between two central nodes of the reward circuit—the ventral striatum (VS) and the ventromedial prefrontal/orbital frontal cortex (vmPFC/OFC). We will review findings from pre-clinical animal models as well as humans and describe how dysfunction in the communication between these regions may contribute to psychopathology, with particular attention to major depressive disorder (MDD) and substance use disorder (SUD).
The reward circuit
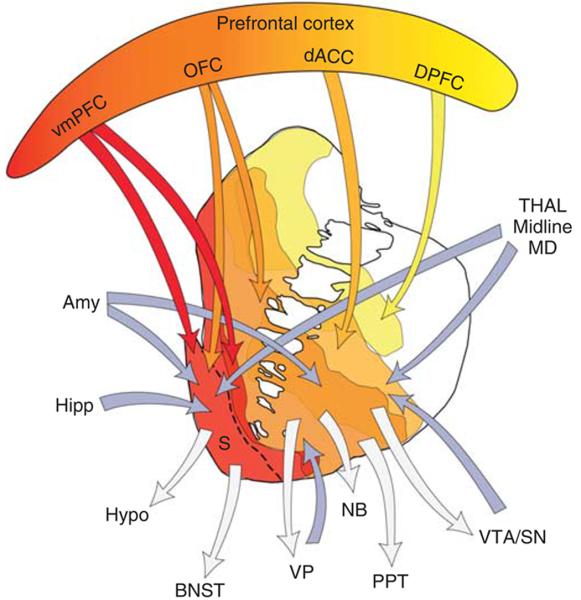
Identification of the brain’s reward circuit can be traced back to the pioneering work of Olds and Milner (1954), who demonstrated that the placement of electrodes at particular areas of the brain in rats could elicit repetitive behavioral responses to trigger electrical stimulation. This seminal finding, which first mapped positive reinforcement to specific brain sites, initiated decades of research aimed at teasing apart the neural underpinnings of how a pleasurable outcome (“reward”) can shape an organism’s behaviors or decisions. Today, various brain regions including the prefrontal cortex, striatum, ventral tegmental area (VTA), ventral pallidum, thalamus, hypothalamus, hippocampus, amygdala, and habenula (Haber & Knutson 2010) (Figure 1) interconnected by transmitter systems involving dopamine, serotonin, glutamate, GABA, and opioids (Koob and others 1994) have been incorporated into a conceptual “reward circuit”, which mediates aspects of value representation and behavioral reinforcement.
Figure 1.
A diagram showing the connectivity among brain regions implicated in a putative “reward circuit,” with emphasis on striatum and prefrontal cortex. Blue arrows indicate inputs and gray arrows indicate outputs to and from the striatum. Amy=amygdala; BNST=bed nucleus of the stria terminalis; dACC=dorsal anterior cingulate cortex; Hipp=hippocampus; Hypo=hypothalamus; MD=medio-dorsal nucleus of the thalamus; OFC=orbitofrontal cortex; PPT=pedunculopontine nucleus; S=shell; SNc=substantia nigra, pars compacta; STN=subthalamic nucleus; Thal=thalamus; VP=ventral pallidum; VS=ventral striatum; VTA=ventral tegmental area; vmPFC=ventral medial prefrontal cortex. Figure adapted from Haber & Knutson 2010.
This review will focus specifically on two key nodes of the reward circuit that are thought to play critical roles in human social affective function, and, by extension, psychiatric illness: the ventral striatum (VS) and the ventromedial prefrontal cortex (vmPFC)/orbitofrontal cortex (OFC) (Figure 2). In the following two sections, we summarize findings from animal and human research that demonstrate the importance of each of these areas for various aspects of reward processing. In the subsequent section, we highlight research that has begun to illuminate the functional interactions between these regions. We continue with a section describing how disrupted interactions between VS and vmPFC/OFC could contribute to particular dimensions of dysfunction across a variety of mental illnesses. We conclude with a section outlining future research directions that could help translate the neurobiological findings into clinical benefit.
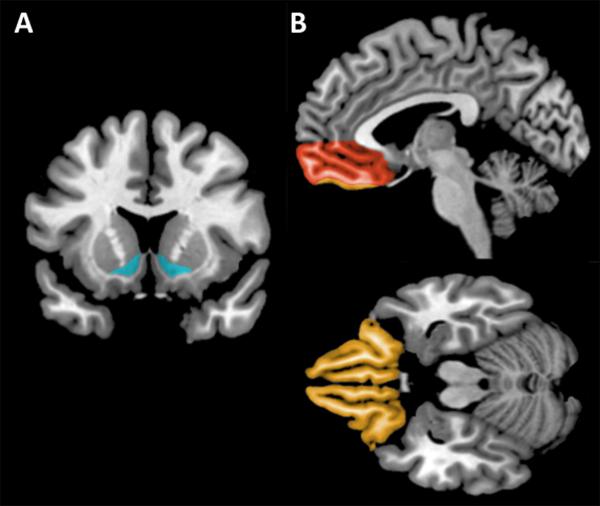
Figure 2.
A: Coronal view depicting the ventral striatum (in blue). B: Sagittal (top panel) and axial (bottom panel) views depicting the vmPFC (in red) and OFC (in orange).
Ventral Striatum (VS)
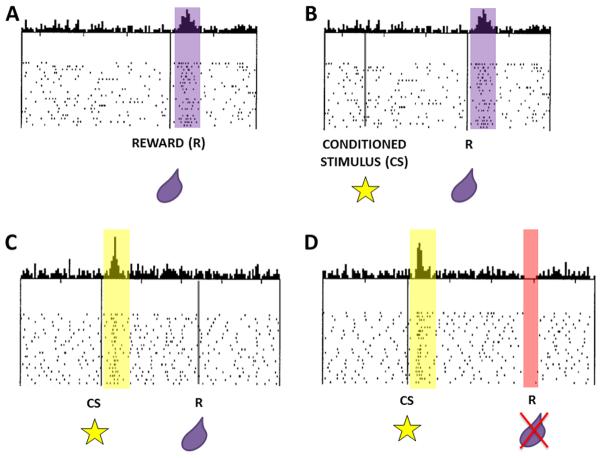
The VS (Figure 1A) is known as the “limbic-motor” interface of the brain because of its connections to limbic and cortical brain regions to serve as an integrator for affective and cognitive processes influencing motor output (Mogenson and others 1980). A major source of input to the VS comes from ascending dopaminergic pathways originating in the VTA of the midbrain. These pathways play a crucial role in signaling information about rewards to the VS (Schultz 1998). Dopamine neurons in the midbrain are characterized by phasic, short-latency spiking activity that corresponds to the temporal presentation of a reward, such as a drop of juice. If a stimulus is repeatedly paired with a rewarding outcome, a learned association occurs between the stimulus and the reward that it predicts. VTA neurons reflect this learned association by eventually firing more in response to the reward-predicting stimulus rather than the reward itself (Figure 3A-C). Moreover, when an expected reward or reward-predicting stimulus is omitted or temporally delayed, there is a depression in dopaminergic cell firing (Figure 3D). These signals about the presence and absence of reward have been termed “prediction error” signals and seem to be guiding reward-based learning (Schultz 1998). Because of this neuronal input from VTA, dopamine seems to be a potent modulator of reward-related activity in VS.
Figure 3.
Adapted from Schultz and others 1998 to demonstrate “Prediction Error” signaling in dopaminergic VTA neurons. (A) Phasic firing of neurons occurs in response to a reward (represented as a drop of juice in purple). (B) Initial pairing of conditioned stimulus (represented as a star cue) with reward yields phasic firing of dopaminergic neurons to the reward. (C) Neurons eventually fire in response to the cue predicting the reward but not to the reward itself. (D) When an expected reward is omitted, there is a depression in dopaminergic cell firing.
In a complementary line of work done in rodents, the VS has been shown to be essential for mediating responses to rewards. Functional mapping studies have linked behavioral reward responses to a smaller subregion within the VS known as the Nucleus Accumbens (NAc) (Robbins & Everitt 1996, Voorn and others 2004). Influential work by Kent Berridge in rodent models has served to distinguish between hedonic or ‘liking’ processes associated with unconditioned responses upon reward consumption and motivational or ‘wanting’ processes during the anticipation of reward (Berridge & Kringelbach 2008). Hedonic or ‘liking’ responses are primarily mediated by opioid transmission within a specialized subregion of the NAc called the NAc ‘Shell’ (NAcS), which is distinguished from another NAc subregion called the ‘Core’ (NAcC) on the basis of function. Many so-called ‘hedonic hotspots’ have been found in the NAcS because of increased behavioral ‘liking’ responses to sucrose when stimulated (Berridge & Kringelbach 2013). Meanwhile, motivational or ‘wanting’ behaviors are mediated by opioids and dopamine in both the NAcS and NAcC. The link between neuromodulatory effects of dopamine on the NAc in relation to the animal’s behavior was not established until seminal work by Salamone (1994), which shows that dopamine depletion from the NAc drives an animal away from a state of motivation for reward-seeking without affecting the consumption of freely available but less appetitive food. Extracellular NAc dopamine levels are also enhanced during anticipatory or ‘wanting’ phases of reward learning (Robbins & Everitt 1996).
Studies involving loss-of-function following NAc lesions have established crucial roles for NAc (and its subregions) in performances on value-based tasks. In a discrimination learning task, control rats are able to withhold responses to odor cues that predict negative outcomes while increasing responses to odor cues predicting positive outcomes, as indicated by increased difference in response latency to negative versus positive odor cues (Schoenbaum & Setlow 2003). Rats with NAc lesions failed to discriminate responses based on cue-outcome value information, since these animals did not show a decrease in the latency of responses to negative cues. Thus, the NAc must be intact for discrimination learning, especially about negative outcomes. Rats with NAcC lesions also tend to favor smaller, immediate rewards over larger, delayed rewards (Cardinal & Howes 2005, Cardinal and others 2001), ascribing a role for the NAcC in withholding impulsive responses. More broadly, the NAc (and especially the NAcC) seems to be crucial for guiding approach/avoidance behaviors based on salient features of a reward (such as probability and valence), which is information conveyed to VS by VTA dopaminergic neurons.
Most of what is currently known about the role of the VS in reward in humans comes from functional neuroimaging studies. Due to the limited spatial resolution of functional magnetic resonance imaging (fMRI), subregional NAc specificity is difficult if not impossible to attain. Thus, the VS in the context of fMRI is more inclusive of a larger swathe of the basal ganglia, including the NAc, the ventral medial caudate, and the rostroventral putamen (Haber & Knutson 2010). The VS in fMRI studies has been reliably activated by stimuli predicting rewards (Knutson and others 2001, Knutson & Cooper 2005). The VS has also shown increased activation during the consumption of a reward (O’Doherty and others 2002, Yacubian and others 2006), especially when a reward is unpredicted or of a higher magnitude than expected (Berns and others 2001, Yacubian and others 2006). In accord with the animal electrophysiology results, human neuroimaging studies have shown that activity in the VS correlates with prediction errors (Daw and others 2011, Hare and others 2008, McClure and others 2003, O’Doherty and others 2003, Pessiglione and others 2006, Tobler and others 2006) (Figure 4).
Figure 4.
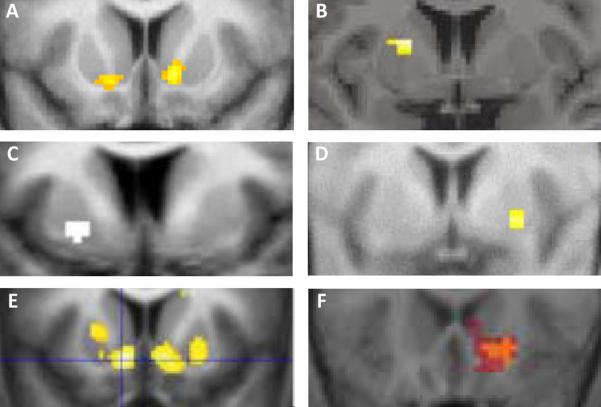
Examples from human neuroimaging studies showing that activity in the striatum, typically in VS, correlates with prediction errors, Adapted from (A) Daw and others 2011 (y=10, right, y=16, left); (B) McClure and others 2003 (y=4); (C) O’Doherty and others 2003 (y=6); (D) Tobler and others 2006 (y=3); (E) Pessiglione and others 2006 (y=12); (F) Hare and others 2008 (y=21).
Overall, the human functional imaging data demonstrating associations between reward-related behaviors and VS activity align closely with animal evidence for the role of VS in reward. The data from both experimental approaches converge to indicate that VS is important for the hedonic and anticipatory responses to reward—processes which may be critical for guiding adaptive responses to rewards or the stimuli that predict rewards.
Ventromedial Prefrontal Cortex/Orbitofrontal Cortex (vmPFC/OFC)
The vmPFC and OFC are two overlapping subregions of PFC that together comprise the lower medial wall and ventral surface of the frontal lobe, respectively (Figure 1B). Although there may be important differences in the functions of these PFC subregions (Rudebeck & Murray 2011), vmPFC and OFC are densely interconnected, subserve related processes and representations, and are functionally and structurally distinct from other regions of the PFC (Grabenhorst & Rolls 2011, Ongur & Price 2000, Wallis 2012). Moreover, in human research methodologies, such as neurological lesion studies and fMRI, the inherent limitations in spatial resolution often do not permit clear distinctions between the two areas. For the purposes of the present article, we thus refer to this region of PFC collectively as vmPFC/OFC.
Whereas electrophysiological recording and stimulation techniques provided the initial insight into the reward processing characteristics of VS, studies of focal brain lesions offered the first evidence that vmPFC/OFC is critical for certain aspects of value-based decision-making (see Figure 5 for examples of vmPFC/OFC lesions in humans). Dating back to the landmark case of Phineas Gage (Harlow 1868), and corroborated by a series of subsequent neurological case reports throughout the twentieth century (Blumer & Benson 1975, Eslinger & Damasio 1985), it has become well-established that damage to the vmPFC/OFC precipitates significant impairments in processing risk, uncertainty, reward, and punishment. The essence of the real-world decision-making deficits observed in vmPFC/OFC lesion patients was first captured in the laboratory with the Iowa Gambling Task (IGT). In the IGT, subjects play cards from four decks that vary with respect to the relative frequency and amount of monetary gain or loss. Through trial-and-error, subjects must learn to adapt their card choices to enact advantageous selections in their subsequent turns. In the first-ever demonstration of performance on the IGT following vmPFC/OFC damage, Bechara and others (1994) report a so-called “myopia for the future,” wherein lesion patients base their choices on risky (and ultimately disadvantageous) prospects of large, immediate payouts, as opposed to more modest but consistent payouts that are advantageous in the long-term. Since this initial finding, impairments in the IGT and related gambling tasks as a function of vmPFC/OFC damage have been replicated in rodents (Jentsch and others 2010, Paine and others 2013, Rivalan and others 2011, Zeeb & Winstanley 2011) and humans (Fellows & Farah 2003, Hsu and others 2005, Naccache and others 2005, Waters-Wood and others 2012). In a related line of work, the vmPFC/OFC has also been shown to be crucial in inhibiting prepotent responses to immediate, small rewards in favor of delayed, larger rewards—a decision-making phenomenon referred to as temporal discounting. Although human OFC lesion studies of temporal discounting have yielded mixed results (Fellows & Farah 2005b, Sellitto and others 2010), work in rodents (Cardinal and others 2001, Kheramin and others 2004, Mobini and others 2002) suggests that the vmPFC/OFC may be crucial for temporal discounting.
Figure 5.
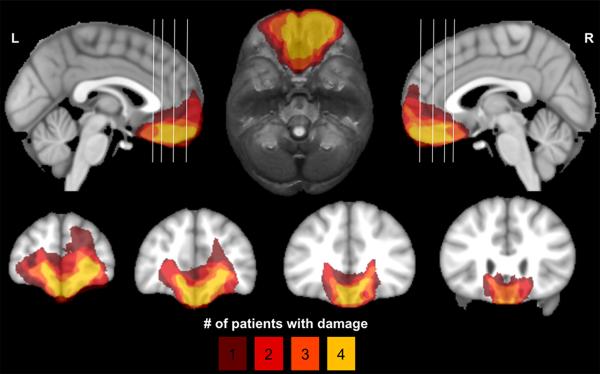
Lesion overlap map showing four neurological patients with bilateral damage to the vmPFC/OFC. Color indicates the number of overlapping lesions. The top row shows mid-sagittal views (left and right) and an axial view (middle). The coronal slices in the bottom row correspond to the white vertical lines on the sagittal views.
A study by Jones and Mishkin (1972) has provided key evidence in non-human primates suggesting that impairments following damage to the vmPFC/OFC may specifically reflect an inability to update new information due to changes in contingencies (reversal learning), as opposed to an inability to learn from initial action-outcome contingencies (discrimination learning). This deficit in reversal learning has since been replicated in additional vmPFC/OFC lesion studies of primates (Chudasama and others 2007, Dias and others 1996, Man and others 2009), rodents (Bissonette and others 2008, Churchwell and others 2009, Izquierdo and others 2013, McAlonan & Brown 2003, Schoenbaum and others 2003, Stalnaker and others 2007), and humans (Fellows & Farah 2003, Fellows & Farah 2005a, Tsuchida and others 2010). The robustness of these effects indicates a role for the vmPFC/OFC in updating information regarding the value of an outcome, with evidence in primates and humans suggesting that vmPFC/OFC may be more critical for performance in stimulus-outcome as opposed to action-outcome reversal learning tasks (Rudebeck and others 2008).
Yet another way to assess the role of the vmPFC/OFC in updating values of outcomes is through learning tasks that use extinction trials following post-training alterations in the value of an outcome. One such task known as outcome or reinforcer devaluation involves initial discrimination learning using cues predicting at least two separate rewarding stimuli, one of which is subsequently devalued through either satiation or induced aversion via pairing with a noxious stimulus. This paradigm has revealed devaluation impairments following vmPFC/OFC lesion in rodents (Gallagher and others 1999, Pickens and others 2003, West and others 2013) and non-human primates (Izquierdo and others 2004, Machado & Bachevalier 2007, West and others 2011). Although we are not aware of any human lesion data on the effect of vmPFC/OFC damage on reinforcer devaluation task performance, human fMRI studies show that OFC activity tracks the devaluation of an outcome and the cue predicting a devalued outcome through satiety (Gottfried and others 2003, Valentin and others 2007). Work involving other tasks requiring updating of stimulus-outcome associations provides further evidence for the importance of the vmPFC/OFC for this function. Takahashi and others (2009) used a Pavlovian overexpectation task in rats to show that OFC lesions impair the ability to adjust response behaviors based on violations of expected reward magnitudes. Similarly, contributions of the OFC in related reinforcement tasks such as ‘blocking’ have been demonstrated in work with rodents (Burke and others 2007) and humans (Tobler and others 2006) [For a detailed review of these studies, see (Schoenbaum and others 2011)].
One important caveat to keep in mind when comparing results across species is that vmPFC/OFC may not be directly homologous between rodents and primates (Wallis 2012). Regardless, taken together, lesion studies in both rodents and primates on reinforcement learning and decision-making which involve changing contingencies and outcomes demonstrate a clear role for the vmPFC/OFC in integrating information about the magnitude or value of a specific outcome to guide future actions. This conclusion is further supported by human functional imaging work, which has shown that, across a wide variety of contexts, stimuli, and outcomes, vmPFC/OFC activity commonly represents reward value (Diekhof and others 2012, Grabenhorst & Rolls 2011, Levy & Glimcher 2012, Liu and others 2011).
Interactions between VS and vmPFC/OFC
Collectively, the extant data on the functions of vmPFC/OFC and VS in reward processing provides evidence for complementary roles that may be crucial for the control and execution of value-based decisions. A critical unresolved question, however, is how these two areas interact to mediate the observed functions. In this section, we outline research findings suggesting that vmPFC/OFC serves to modulate VS activity during reward-related behavior.
First, we note that anatomical and functional connectivity data from animals and humans are consistent with putative interactions between vmPFC/OFC and VS. Rodent studies have demonstrated direct glutamatergic projections from vmPFC/OFC to VS (Gabbott and others 2005, Sesack and others 1989, Voorn and others 2004), while human fMRI studies indicate highly correlated activity between the vmPFC/OFC and VS at rest (Di Martino and others 2008) and during tasks involving favorable outcomes or rewards (Cauda and others 2011, Diekhof and others 2012). While consistent with vmPFC/OFC modulation of VS activity, these circumstantial and correlational findings do not provide evidence of causality. Evidence corroborating the exact causal functional dynamics between the VS/NAc and vmPFC/OFC has only recently begun to emerge as a result of novel technological advances, which, at present, are only available for non-human animal studies.
Using computational modeling, Frank and Claus (2006) provided a mechanistic account of fronto-striatal interactions derived from theories of reward and reinforcement learning. In this model, a striatal system receives dopaminergic inputs from the midbrain, which monitor the frequency of positive and negative decision outcomes via go and no-go (‘trial-and-error’) learning to refine motor actions. The OFC receives information about these positive and negative decision outcomes from the VS and constitutively updates information about the magnitude of an outcome to facilitate subsequent action selection on the basis of these updated value terms. Taking the OFC ‘off-line’ in this OFC-striatal neural network model results in deficits in decision-making, reversal learning, and devaluation, much like the effects seen in these tasks following lesions to the vmPFC/OFC across species. This model thus proposes that the expression of fast, flexible, and adaptive decision-making relies on intact interaction between the OFC and striatum. More specifically, it indicates a role for the OFC in the top-down biasing of striatal activity for action selection, wherein information about the magnitude or value of an outcome becomes integrated with information about simple frequencies of positive and negative outcomes to quickly and efficiently influence differentiation between ‘go’ and ‘no-go’ responses. This intriguing computational model preceded direct, in vivo tests of VS-vmPFC/OFC interactions by several years, owed to the recent emergence of novel applications of multimodal techniques used to test the behavioral consequences of causal interactions between these two brain regions.
Novel combinations of techniques have only recently been used to relate the causal interactions between vmPFC/OFC and VS to animal behavior. Ghazizadeh and others (2012) did this for the first time by combining electrophysiological recording of neurons in the NAcS with concurrent inactivation of the vmPFC in rodents during performance on a reward learning task. In the task, pressing an ‘active’ lever was required during the presentation of a tone to procure a sucrose reward and terminate the tone. Rats concurrently learned to inhibit responses to an ‘inactive’ lever which produced no reward if pressed during the presentation of a different tone. In contrast to control animals that were able to respond with greater frequency to the active lever while reducing lever-pressing during the neutral tone presentation, animals with inactivated vmPFCs lever-pressed during both tone presentations. This disinhibition following vmPFC inactivation was temporally specific to the presentation of the neutral tone, since responding was higher during the neutral tone presentation compared to other task epochs, such as the time delay between reward consumption and a new trial.
These compelling behavioral data call to question the exact influence that vmPFC can have on NAcS activity to affect appropriate time-sensitive responding. As expected, vmPFC inactivation results in direct modulation of NAcS neuronal activity and corresponds with response disinhibition during tone presentations (Ghazizadeh and others 2012). vmPFC is specifically responsible for both a suppression of phasic firing that promotes behavioral cue responding and for providing excitatory input to increase the basal firing of NAcS neurons that inhibit responses generalized to reward-seeking (i.e., responses with no bearing on actual outcome). Putting this in the context of the computational model described above, vmPFC seems to control at least two distinct populations of neurons in the NAcS to mediate appropriate responding: (1) one population which facilitates actions (“go”) and (2) one population that inhibits responses (“no-go”). The dual nature of this modulation suggests a process of summation or integration of opposing signals for the ultimate expression of motoric output.
In a parallel effort to characterize the importance of the VS-vmPFC/OFC neural pathway on value-based decision-making, St. Onge and colleagues (2012) performed concurrent inactivation of both brain regions and assessed the effects of this functional disconnection on rodent performance in a probabilistic discounting task. In this task, larger, uncertain rewards were pitted against smaller, sure rewards. Rats learned that pressing the lever that corresponded to large/risky outcomes was disadvantageous over time as the probability of obtaining a reward decreased over the course of the task. Although disconnection of the vmPFC and NAc did not impair the acquisition of probabilistic reward learning, the animals were less accurate and had slower response times and reduced locomotor activity. The authors suggest that these findings reflect impaired attention or vigilance.
This interpretation finds support in an earlier study showing that mPFC and NAc inactivation or disconnection resulted in attentional impairments in a five-choice serial reaction time task (Christakou and others 2004). However, re-evaluating these findings in the context of the previously described working model of VS-vmPFC/OFC interactions provides an alternative interpretation of the data. If both VS and vmPFC/OFC are taken ‘off-line,’ there is no information from vmPFC/OFC about value to integrate with dopamine signals from the midbrain to influence and efficiently guide discrimination of ‘go’ or ‘no go’ responding. In effect, the most efficient (and perhaps most direct) pathway involved in guiding value-based decision-making has been “wiped out,” and it could be the case that other brain regions (i.e., amygdala, hippocampus, thalamus, dorsolateral PFC) are processing these reward-outcome associations, but to a much less efficient degree. This would explain the paradoxical nature of these findings, wherein learning is still intact but occurs at a much slower rate and is subject to error [or, in the context of Christakou and others, 2004, perseveration].
Despite these promising initial findings, substantially more work needs to be done to characterize the interactions within the VS-vmPFC/OFC circuit. Virtually no human studies have been done to understand the causal nature of these interactions and how these interactions relate to behavior. Future directions exploring the causal role of vmPFC/OFC activity on VS activity in humans could, like the previously described studies, combine multiple techniques. For example, in a recent study by Cohen and others (2012), NAc activity was recorded simultaneously with surface electroencephalogram (EEG) on patients with obsessive-compulsive disorder undergoing deep brain stimulation in the NAc during performance on a task of reward anticipation and motivation. Granger causality analyses showed that “top-down” or frontal cortical to NAc synchrony was stronger when rewards were being anticipated. As another example, a combined fMRI-lesion approach has been employed with amygdala-damaged patients (n=2) to assess the causal role of the amygdala on vmPFC activity during engagement in a reversal learning task (Hampton and others 2007). These studies presage future opportunities to examine the effects of manipulations of vmPFC/OFC function (e.g., through deep brain stimulation or neurological damage) for detectable differences in VS activity (compared to healthy controls) during their engagement in a reward processing task.
To this point, we have summarized evidence that adaptive value-based learning and decision-making depends critically on efficient integration of reward-related information within the VS-vmPFC/OFC pathway. A corollary of this supposition is that variation in the integrity of this circuit would be associated with variation in overt levels of social and affective functions related to reward processing. In the next section, we describe evidence that numerous psychiatric illnesses may involve deficiencies in VS-vmPFC/OFC circuit function.
Evidence of abnormal VS and vmPFC/OFC activity and structure in mental illness
Dysfunction within the VS-vmPFC/OFC circuitry may be a key neuropathophysiological mechanism underlying certain symptoms of mental illness. Here we provide examples of clinical research findings that associate dysfunction in reward processing with abnormal structural and/or functional characteristics of VS and vmPFC/OFC. In particular, we focus on SUD and MDD, which together illustrate how disorder in the VS-vmPFC/OFC circuit can be linked to distinct symptoms related to reward processing.
We begin with SUD, as abuse and dependence on drugs and alcohol is virtually defined by maladaptive reward-related decision-making. Perhaps not surprisingly, VS activity in response to rewards and reward-predicting cues has been found to be altered in individuals with SUD for a number of substances, including cocaine (Hyatt and others 2012, Jia and others 2011, Konova and others 2012), nicotine (David and others 2005, Franklin and others 2007, Peters and others 2011, Rose and others 2012), alcohol (Beck and others 2009, Braus and others 2001, Schacht and others 2011, Volkow and others 2007, Wrase and others 2007) and other drugs (Bjork and others 2008, Nestor and others 2010). Across disorders, there seems to be a general dissociative trend of either reduced or no detectable change in VS activity during reward anticipation (Beck and others 2009, Bjork and others 2008, Jia and others 2011, Peters and others 2011, Rose and others 2012, Wrase and others 2007) and increased VS activity during reward receipt/‘consumption’ (Bjork and others 2008, Braus and others 2001, David and others 2005, Franklin and others 2007, Jia and others 2011, Konova and others 2012, Wrase and others 2007) (Figure 6). The remarkable similarities in VS activation patterns during different stages of reward processing speak to a possible common underlying dysfunction for users of different substances. However, inconsistencies in experimental paradigms to test reward processing in individuals with SUD necessitates future work that carefully examines differences in the types of rewards used to elicit activity as well as the specific drug of abuse in question. Overall, non-normative VS activity across substance use groups appears to reflect the marked changes in appetitive and hedonic experiences of SUD patients.
Figure 6.
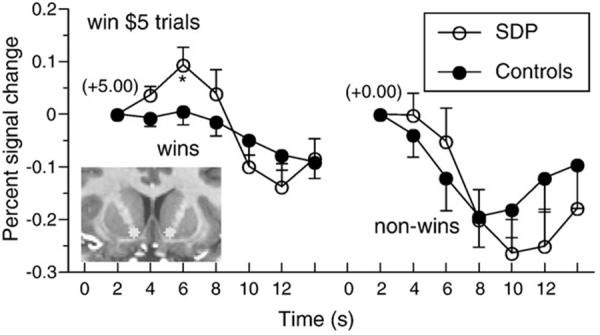
An example of reward circuit dysfunction in SUD. Greater activation over time (percent signal change) occurred in bilateral VS in response to receipt of reward (+5.00) in substance dependent patients (SDP) versus healthy participants. Bilateral VS activation does not differ between groups for a neutral outcome (+0.00). Figure adapted from Bjork and others 2008.
Many human neuroimaging studies of SUD patients have implicated the PFC, specifically the OFC, as a region showing reward-related impairments (Goldstein & Volkow 2011, London and others 2000). For instance, cocaine administration was shown to induce activations in the OFC along with other PFC regions (Kufahl and others 2005). SUD subjects also show reduced gray matter volumes of the vmPFC/OFC (Durazzo and others 2011, Ersche and others 2011, Konova and others 2012, Tanabe and others 2009). VS volume is relatively less well-characterized but shows reductions in alcohol abusers (Sullivan and others 2005) and increases in cocaine users (Ersche and others 2011).
There are indications that interactions between VS and vmPFC/OFC may be important for the development of SUD. OFC activity is negatively associated with methylphenidate-induced DA increases in the VS, while low dopamine D2 receptor availability was associated with increased mPFC responses to rewards (Asensio and others 2010). Further, decreased functional connectivity between the NAc and OFC is associated with duration of opioid dependence (Upadhyay and others 2010). Deep brain stimulation of the NAc in a patient with severe alcoholism resulted in less risky, more careful choices during NAc stimulation, with recruitment of a region of vmPFC (Heldmann and others 2012).
In combination, these studies suggest that impaired communication between the VS and vmPFC/OFC during reward processing may be a critical neural substrate for the dysfunctional decision-making apparent in SUD.
Next we consider MDD. One of the core symptoms of MDD is anhedonia—the loss of interest or pleasure in most, if not all, activities (Der-Avakian & Markou 2012). Anhedonia is a governing symptom for ‘reward circuit’ studies of the disorder because of its relation to impaired emotional and motivational responses to positively-valenced stimuli. When compared to healthy controls, patients with MDD show markedly different VS activity in response to money and other rewarding or pleasant stimuli, with the majority of these studies implicating VS hypoactivity (Diener and others 2012, Greening and others 2013, Heller and others 2009, Kumar and others 2008, McCabe and others 2009, Robinson and others 2012, Stoy and others 2012) (Figure 7).
Figure 7.
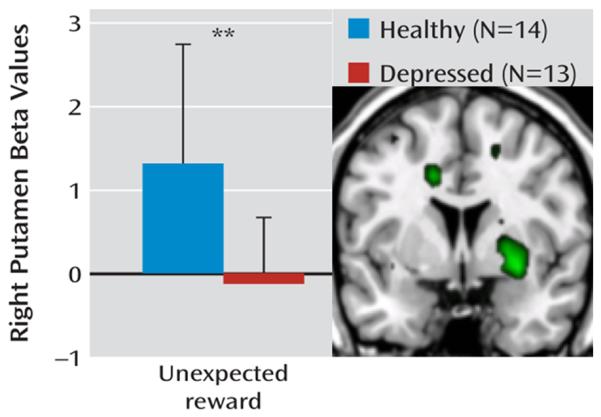
An example of reward circuit dysfunction in MDD. Relative to healthy controls, unmedicated individuals with major depressive disorder exhibit reduced activity (beta values) in right anteroventral putamen, an area which corresponds to VS, in response to unexpected rewards.
Likewise, MDD patients display differences in vmPFC/OFC engagement when processing both money and other rewards (Knutson and others 2008, McCabe and others 2009, Smoski and others 2009, Smoski and others 2011) and positively valenced stimuli (Heller and others 2009, Johnstone and others 2007, Keedwell and others 2009) compared to controls. Consistency in these findings on vmPFC/OFC engagement during reward processing in MDD is less established than it is for VS, with some studies reporting hyperreactivity and others reporting hyporeactivity of these regions. However, studies of OFC gray matter volume consistently report gray matter reductions in MDD patients compared to controls (Bremner and others 2002, Lacerda and others 2004, Lee and others 2003). Interestingly, Koenigs and others (2008) report markedly low levels of depression in patients with bilateral vmPFC lesions. Taken together, converging evidence of structural and functional impairments of VS and vmPFC/OFC in MDD provides the basis for targeting VS-vmPFC/OFC pathways for clinical treatments.
Emerging evidence on neural outcomes following treatment interventions support the involvement of these nodes of the reward circuit in MDD. The subgenual anterior cingulate cortex (sgACC), a region of the vmPFC, is a main target of deep brain stimulation for MDD treatment (Giacobbe and others 2009). A recent investigation of deep brain stimulation in treatment-resistant MDD has confirmed sgACC and the VS as primary targets based on successful treatment outcome (Taghva and others 2012). Pharmacological intervention studies have shown normalization of VS activity to monetary rewards (Stoy and others 2012), vmPFC/sgACC activity to emotionally salient pictures (Keedwell and others 2010, Rosenblau and others 2012), and sustained frontostriatal connectivity during emotion regulation (Heller and others 2013) following treatment.
As in SUD, the totality of evidence on MDD strongly implicates dysfunction in the VS-vmPFC/OFC circuit as a critical neural substrate for the pathogenesis of the disorder. Dysfunction in this circuit may also underlie a number of other disorders that present a wide range of social and affective symptoms. Although it is beyond the scope of the present review to describe the individual findings in detail, multiple studies have demonstrated alterations in VS and vmPFC/OFC structure and/or functional engagement in reward-related fMRI tasks in schizophrenia, obsessive compulsive disorder, autism, and attention deficit hyperactive disorder, among other disorders (Dichter and others 2012).
Conclusion and Future Directions
In this article, we have outlined evidence that sectors of the basal ganglia and prefrontal cortex (VS and vmPFC/OFC, respectively) interact to mediate aspects of processing reward and positive affect that are critical dimensions of mental illness. One pressing issue currently facing this field of research is how advances in our understanding of these brain-behavior relationships can ultimately translate into improved psychiatric patient care. In the near future, additional research could answer a number of outstanding questions related to the potential clinical benefit of these findings. For example, could clinically useful neurobiological and/or psychological assessments of reward processing be developed? These assessments could include behavioral test performance (e.g., reversal learning, temporal discounting, etc.) as well as functional or structural imaging of the VS-vmPFC/OFC circuit. Could these assessments provide information about the risk for developing a particular disorder? If so, how early during the lifespan could these assessment techniques be useful? Could the diagnostic neurobiological and/or psychological assessment information be used to predict a patient’s response to the various treatment options? Moreover, could the information be used to develop novel treatments (e.g., cognitive-behavioral therapies to specifically modify risk/reward sensitivity or cultivate and sustain positive affect, or possibly pharmacological or brain stimulation techniques to modulate activity in the reward circuit)? In some cases, research projects designed to address these important questions are well underway. As the translation from basic neuroscience to psychiatric patient care continues to progress, the brain’s reward circuit figures to play a prominent role.
References
- Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–42. doi: 10.1016/j.biopsych.2009.04.035. others. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–8. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–80. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–21. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer D, Benson D. Personality changes with frontal and temporal lobe lesions. Grune & Stratton; New York: 1975. [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–94. doi: 10.1007/s007020170038. others. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. others. [DOI] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Miller DN, Schoenbaum G. Conditioned reinforcement can be mediated by either outcome-specific or general affective representations. Front Integr Neurosci. 2007;1:2. doi: 10.3389/neuro.07.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–77. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–80. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Kralik JD, Murray EA. Rhesus monkeys with orbital prefrontal cortex lesions can learn to inhibit prepotent responses in the reversed reward contingency task. Cereb Cortex. 2007;17:1154–9. doi: 10.1093/cercor/bhl025. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–96. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Bour L, Mantione M, Figee M, Vink M. Top-down-directed synchrony from medial frontal cortex to nucleus accumbens during reward anticipation. Hum Brain Mapp. 2012;33:246–52. doi: 10.1002/hbm.21195. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–94. doi: 10.1016/j.biopsych.2005.04.028. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–15. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. others. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–86. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–66. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–85. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–200. doi: 10.1111/j.1530-0277.2011.01452.x. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–24. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–7. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005a;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005b;43:1214–21. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–26. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–9. doi: 10.1038/sj.npp.1301371. others. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–4. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh A, Ambroggi F, Odean N, Fields HL. Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J Neurosci. 2012;32:726–37. doi: 10.1523/JNEUROSCI.3891-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe P, Mayberg HS, Lozano AM. Treatment resistant depression as a failure of brain homeostatic mechanisms: implications for deep brain stimulation. Exp Neurol. 2009;219:44–52. doi: 10.1016/j.expneurol.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DG. The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–55. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the Passage of an Iron Bar Through the Head. Bulletin of the Massachusetts Medical Society; 1868. pp. 327–47. [Google Scholar]
- Heldmann M, Berding G, Voges J, Bogerts B, Galazky I. Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS One. 2012;7:e36572. doi: 10.1371/journal.pone.0036572. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry. 2013;170:197–206. doi: 10.1176/appi.ajp.2012.12010014. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–50. doi: 10.1073/pnas.0910651106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–3. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Hyatt CJ, Assaf M, Muska CE, Rosen RI, Thomas AD. Reward-related dorsal striatal activity differences between former and current cocaine dependent individuals during an interactive competitive game. PLoS One. 2012;7:e34917. doi: 10.1371/journal.pone.0034917. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Darling C, Manos N, Pozos H, Kim C. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. J Neurosci. 2013;33:4105–9. doi: 10.1523/JNEUROSCI.4942-12.2013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–8. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Woods JA, Groman SM, Seu E. Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharmacology. 2010;35:1797–806. doi: 10.1038/npp.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–60. doi: 10.1016/j.biopsych.2011.05.008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp Neurol. 1972;36:362–77. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Keedwell P, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol. 2009;23:775–88. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord. 2010;120:120–5. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho MY, Velázquez-Martinez DN, Bradshaw CM. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 2004;175:206–14. doi: 10.1007/s00213-004-1813-y. others. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–92. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–48. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Parvaz MA, Alia-Klein N. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. Eur J Neurosci. 2012;36:2979–88. doi: 10.1111/j.1460-9568.2012.08211.x. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Rassnick S, Heinrichs S, Weiss F. Alcohol, the reward system and dependence. EXS. 1994;71:103–14. doi: 10.1007/978-3-0348-7330-7_11. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–14. doi: 10.1016/j.neuroimage.2005.06.039. others. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–8. doi: 10.1016/j.biopsych.2003.08.021. others. [DOI] [PubMed] [Google Scholar]
- Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54:529–33. doi: 10.1016/s0006-3223(03)00063-5. others. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22:1027–38. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and biobehavioral reviews. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334–42. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Man MS, Clarke HF, Roberts AC. The role of the orbitofrontal cortex and medial striatum in the regulation of prepotent responses to food rewards. Cereb Cortex. 2009;19:899–906. doi: 10.1093/cercor/bhn137. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–77. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160:290–8. doi: 10.1007/s00213-001-0983-0. others. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Naccache L, Dehaene S, Cohen L, Habert MO, Guichart-Gomez E. Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia. 2005;43:1318–28. doi: 10.1016/j.neuropsychologia.2004.11.024. others. [DOI] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49:1133–43. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- OLDS J, MILNER P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paine TA, Asinof SK, Diehl GW, Frackman A, Leffler J. Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: reversal by D1 receptor antagonist administration. Behav Brain Res. 2013;243:247–54. doi: 10.1016/j.bbr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–5. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–9. doi: 10.1176/appi.ajp.2010.10071024. others. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–84. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F. Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front Behav Neurosci. 2011;5:22. doi: 10.3389/fnbeh.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–36. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012;169:152–9. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM. Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiatry. 2012;71:206–13. doi: 10.1016/j.biopsych.2011.09.013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblau G, Sterzer P, Stoy M, Park S, Friedel E. Functional neuroanatomy of emotion processing in major depressive disorder is altered after successful antidepressant therapy. J Psychopharmacol. 2012;26:1424–33. doi: 10.1177/0269881112450779. others. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–85. doi: 10.1523/JNEUROSCI.3541-08.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values. Ann N Y Acad Sci. 2011;1239:1–13. doi: 10.1111/j.1749-6632.2011.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–33. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage. 2011;56:61–8. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci. 2003;23:9833–41. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–40. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci. 2010;30:16429–36. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Rittenberg A, Dichter GS. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Res. 2011;194:263–70. doi: 10.1016/j.pscychresns.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 2012;32:2886–99. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–8. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–88. doi: 10.1177/0269881111416686. others. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57:768–76. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Taghva AS, Malone DA, Rezai AR. Deep Brain Stimulation for Treatment-Resistant Depression. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.10.068. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Stalnaker TA, Haney RZ, Calu DJ. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron. 2009;62:269–80. doi: 10.1016/j.neuron.2009.03.005. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–4. doi: 10.1016/j.biopsych.2008.07.030. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’doherty JP, Dolan RJ, Schultz W. Human neural learning depends on reward prediction errors in the blocking paradigm. J Neurophysiol. 2006;95:301–10. doi: 10.1152/jn.00762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–75. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–114. doi: 10.1093/brain/awq138. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27:4019–26. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends in neurosciences. 2004;27:468–74. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15:13–9. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters-Wood SM, Xiao L, Denburg NL, Hernandez M, Bechara A. Failure to learn from repeated mistakes: persistent decision-making impairment as measured by the iowa gambling task in patients with ventromedial prefrontal cortex lesions. J Int Neuropsychol Soc. 2012;18:927–30. doi: 10.1017/S135561771200063X. [DOI] [PubMed] [Google Scholar]
- West EA, DesJardin JT, Gale K, Malkova L. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. J Neurosci. 2011;31:15128–35. doi: 10.1523/JNEUROSCI.3295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Forcelli PA, McCue DL, Malkova L. Differential effects of serotonin-specific and excitotoxic lesions of OFC on conditioned reinforcer devaluation and extinction in rats. Behav Brain Res. 2013;246C:10–14. doi: 10.1016/j.bbr.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–94. doi: 10.1016/j.neuroimage.2006.11.043. others. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011;31:2197–204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]