The possible involvement of α-defensins in CD8 T-cell-mediated anti-HIV activities has been the subject of recent investigations [1–3]. HIV host defence mechanisms are partly mediated by CD8 T-cell non-cytotoxic antiviral responses [4]. Walker et al. [5] first demonstrated that this anti-HIV activity involves a soluble factor(s) designated as CD8 cell antiviral factor (CAF) whose identity remains unknown [4]. Zhang et al. [1] proposed that α-defensins are produced by CD8 T cells and contribute to CAF-mediated anti-HIV activities. In contrast, the recent studies by Mackewicz et al. [2] and Chang et al. [3] demonstrated that the α-defensins are not produced by CD8 T cells but unexpectedly were found to be expressed by monocytes [2].
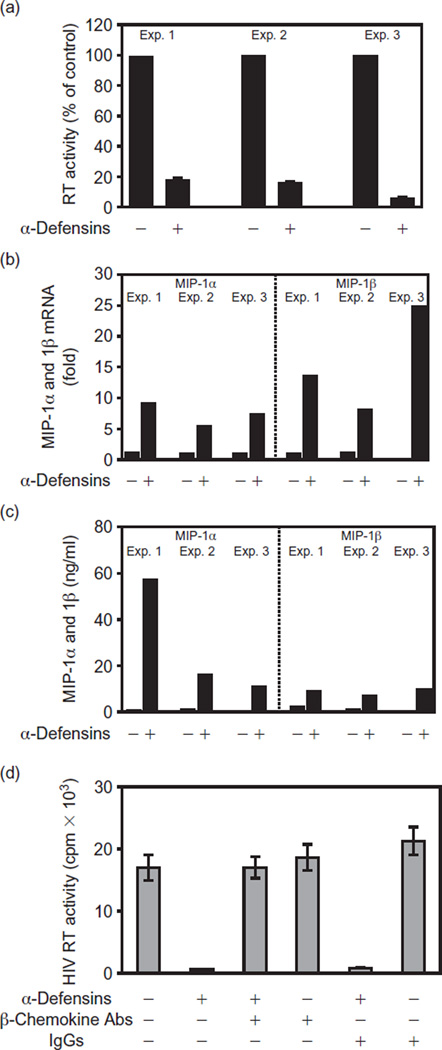
As CAF-mediated anti-HIV activity is also observed for macrophages [6,7] and monocytes express α-defensins [2], we investigated the capacity of α-defensins to suppress HIV infection of macrophages. The addition of α-defensins to peripheral blood monocyte-derived macrophage cultures markedly suppressed HIV Bal replication (Fig. 1a) [8,9]. In order to determine the mechanism(s) responsible for α-defensin-mediated HIV inhibition in macrophages, we investigated whether α-defensins regulate the expression of CC-chemokines. CC-chemokines [macrophage inflammatory protein (MIP)-1α, MIP-1β and Rantes] inhibit infection by competing with HIV M-tropic strains for the CCR5 receptor on macrophages [10,11]. Our experiments demonstrated that α-defensins dramatically enhance expression (as much as a 25-fold increase) of MIP-1α and MIP-1β messenger RNA in macrophages (Fig. 1b) [12]. This increased CC-chemokine gene expression by α-defensins was further confirmed by the demonstration of increased production (as much as a 57-fold increase) of MIP-1α and MIP-1β proteins in α-defensin-treated macrophage cultures (Fig. 1c). In addition, the antibodies to CC-chemokines completely abrogated α-defensin-mediated HIV inhibition in macrophages (Fig. 1d). Our data, therefore, indicate that the α-defensin-mediated inhibition of HIV infection of macrophages is mediated through the upregulation of CC-chemokines. This pathway is distinct from the anti-HIV activity of CAF in macrophages, because CC-chemokines are not responsible for the ability of CAF to suppress HIV infection of these cells [6,7].
Fig. 1. Effect of α-defensins on HIV infection and β-chemokine expression in macrophages.
Monocytes were purified from peripheral blood of three healthy HIV-negative adult donors according to our previously described techniques and were maintained as monocyte-derived macrophages [8]. Monocytes (> 98% purity) were plated in 48-well culture plates at a density of 5 × 105 cells/well in Dulbecco’s modified essential medium containing 10% fetal calf serum. (a) Macrophages maintained for 7 days were preincubated with or without α-defensins (25 µM, hNP-1 and hNP-2; Chemi-Con International, Inc., Temecula, CA, USA) for 24 h and were then infected with HIV Bal strain. HIV replication in infected macrophage cultures was analysed by measuring reverse transcriptase (RT) activity in culture supernatants [9] at day 8 post-infection and was expressed as a percentage of control (infected and untreated macrophage cultures), which was defined as 100%. (b) Macrophages were incubated with or without α-defensins (25 µM) for 3 h and total RNA iso-isolated from the cells was subjected to real-time reverse trancriptase–polymerase chain reaction [12] for quantification of macrophage inflammatory protein (MIP)-1α and MIP-1β messenger RNA. (c) Macrophages were incubated with or without α-defensins for 24 h, the culture supernatants were collected for CC-chemokine production using enzyme-linked immunosorbent assay kits (Endogen, Inc., Cambridge, MA, USA). (d) Macrophages were incubated with or without α-defensins or goat neutralizing polyclonal antibodies (25 µg/ml each) to human CC-chemokines (MIP-1α, MIP-1β and regulated upon activation: normal T cell expressed/secreted [Rants]; R&D Systems, Minneapolis, MN, USA) and goat IgG (control antibody; 75 µg/ml) for 24 h and were then infected with HIV Bal strain. HIV reverse transcriptase activity in the culture supernatants was measured at day 8 post-infection.
The biological interaction of defensins with chemokines and chemokine receptors has been documented. Defensins functionally overlap with chemokines in microbicidal activity [13]. The treatment of dendritic cells with β-defensin-2 upregulated the expression of CC-chemokines (MIP-1α and MIP-1β) and down-regulated CCR5 expression [14]. By utilizing chemokine receptors on immune cells, defensins may contribute to the regulation of host adaptive immunity against microbial invasion [15]. Taken together, our data provide evidence that α-defensins could play a role in host defence against HIV infection of macrophages. The biological interaction of α-defensins with CC-chemokines may constitute a unique mechanism of innate immunity against HIV disease.
Acknowledgments
Sponsorship: This work was supported by grants from the National Institutes of Health (DA12815 and DA16022 to W.Z.H., MH49981 and AA13547 to S.D.D.).
References
- 1.Zhang L, Yu W, He T, Yu J, Caffrey RE, Dalmasso EA, et al. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 2.Mackewicz CEYJ, Tran P, Diaz L, Mack E, Selsted ME, Levy JA. α-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17:F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 3.Chang TL, Francois F, Mosoian A, Klotman ME. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from alpha-defensin-1 HIV inhibition. J Virol. 2003;77:6777–6784. doi: 10.1128/JVI.77.12.6777-6784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy JA, Mackewicz CE, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 5.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 6.Moriuchi H, Moriuchi M, Combadiere C, Murphy PM, Fauci AS. CD8+ T-cell-derived soluble factor(s), but not beta-chemokines RANTES, MIP-1 alpha, and MIP-1 beta, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci U S A. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker E, Bossart KN, Levy JA. Primary CD8+ cells from HIV-infected individuals can suppress productive infection of macrophages independent of beta-chemokines. Proc Natl Acad Sci U S A. 1998;95:1725–1729. doi: 10.1073/pnas.95.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 9.Ho WZ, Lioy J, Song L, Cutilli JR, Polin RA, Douglas SD. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol. 1992;66:573–579. doi: 10.1128/jvi.66.1.573-579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 12.Guo CJ, Douglas SD, Lai JP, Pleasure DE, Li Y, Williams M, et al. Interleukin-1beta stimulates macrophage inflammatory protein-1alpha and -1beta expression in human neuronal cells (NT2-N) J Neurochem. 2003;84:997–1005. doi: 10.1046/j.1471-4159.2003.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T. Defensins and host defense. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- 14.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]