Abstract
Objectives
To determine whether ethanol infusion in the vein of Marshall (VOM) can ablate intrinsic cardiac nerves (ICN).
Background
ICN cluster around the left atrial epicardium, and are implicated in the genesis of atrial fibrillation (AF).
Methods
Patients undergoing catheter AF ablation underwent adjunctive ethanol injection in the VOM. A multipolar catheter was introduced in the VOM and used for high-frequency stimulation (HFS), either as SynchHFS (P-wave-synchronized, 30 pulses, 100 Hz, n=8) or as BurstHFS (5–10 second bursts, 33 Hz, n=72) at 25 mA, 1 ms duration. Atrioventricular (AV) nodal conduction slowing (asystole >2s or R-R interval prolongation >50%) and AF inducibility were assessed before and after VOM ethanol infusion. Up to four 1mL infusions of 98% ethanol were delivered via an angioplasty balloon in the VOM.
Results
SynchHFS induced AF in 8/8 patients. In 4/8 AF initiated spontaneously without VOM capture. No parasympathetic responses were elicited by SynchHFS. BurstHFS was performed in 32 patients undergoing de novo AF ablation (Group 1) and 40 patients undergoing repeat ablation (group 2). Parasympathetic responses were found in all 32 Group 1 patients and in 75% of Group 2 patients. After VOM ethanol infusion, parasympathetic responses were abolished in all patients (both groups). There were no acute complications related to VOM ethanol infusion.
Conclusions
The VOM contains ICN that connect with the AV node and can trigger AF. Retrograde ethanol infusion in the VOM reliably eliminates local ICN responses. The VOM is a vascular route for ICN-targeting therapies.
Keywords: Atrial fibrillation, Vein of Marshall, Ethanol, Intrinsic cardiac nerves
Introduction
Instrinsic cardiac nerves (ICN) can modulate atrial muscle physiology in a pro-fibrillatory manner. (1) ICN ablation has been proposed as an adjunctive (2) or stand-alone (3) therapy for AF. Strategies for ICN ablation have involved the use of radiofrequency at endocardial sites where parasympathetic reflexes were elicited, or epicardial ablation during surgery or epicardial access. To date, the clinical impact of ICN ablation in the overall procedural success is unclear, in part due to unreliable ablation techniques.
ICNs cluster in discrete ganglia located in the proximity of the pulmonary veins (PV). The ligament of Marshall is considered part of the ICN: (4) it has been shown to contain sympathetic (5) and parasympathetic (6) innervation and it coincides with regions known to harbor ICN, specifically the left dorsal nerve. (7) It becomes the vein of Marshall (VOM) caudally as it connects with the coronary sinus. The ligament of Marshall has been implicated in the genesis of atrial fibrillation (AF) by multiple mechanisms: as a source of ectopic beats initiating AF, (8–10) as a connection pathway with neighboring myocardium and left pulmonary veins (PV), (5,11) and as a source of arrhythmogenic autonomic innervation. (5,6) Animal (12) and human (3) surgical open-chest studies have shown that high-frequency electrical stimulation (HFS) at the ligament of Marshall area may induce parasympathetic responses characterized by significant slowing of atrioventricular (AV) nodal conduction and AF induction. It is not known whether the VOM can anatomically connect with the ICN associated with the ligament of Marshall. If so, then the VOM could be used as a closed-chest endovascular route to the ICN for therapeutic purposes. We have developed a technique for retrograde VOM ethanol infusion and have shown its feasibility and safety in humans. (13,14) We hypothesized that: 1) HFS performed within the VOM can elicit parasympathetic responses; and 2) the VOM can be used as a vascular route to target these epicardial ICN and regionally denervate the LA with chemical ablation.
Methods
Patient population
We enrolled 133 patients for participation in the VOM ethanol infusion procedure. Patients were undergoing clinically indicated PV antral isolation (PVAI), and gave consent for adjunctive ethanol injection in the VOM in a protocol that was approved by the local institutional review board, overseen by the Food and Drug Administration (IND # 105083), and an external Data Safety Monitoring Board.
Procedural strategy
After obtaining informed consent, patients were subjected to general anesthesia and vascular access was obtained. A quadripolar catheter was positioned in the His bundle, and a decapolar catheter in the coronary sinus via a femoral vein.
The VOM was cannulated as previously described. (13,14) Briefly, the right internal jugular vein was accessed with a 9F sheath. Then, a sheath designed for left ventricular pacing lead delivery was inserted in this sheath and into the coronary sinus (CPS sheath, St Jude Medical). A sub-selector catheter (LIMA angioplasty guide) was then inserted through the CPS sheath and manipulated so that its tip faced posteriorly and superiorly. Angiographic contrast was injected through the LIMA guide and the VOM was identified as an atrial branch of the coronary sinus that arose at the level of the valve of Vieussens and that was directed posteriorly.
A quadripolar catheter (1.7 F Pathfinder Mini, Cardima, or 4 F IBI, St Jude Medical, Minneapolis, MN, or 2.4F over-the-wire Internova Medical, Chiba, Japan) was inserted in the VOM through the LIMA guide. This catheter was used to record baseline VOM electrograms and to perform HFS. A transseptal puncture was then performed under intracardiac echocardiographic guidance and a circular duodecapolar catheter was inserted in the left atrium. Heparin was administered to maintain the activated clotting time between 350 and 400 seconds throughout the procedure. Three-dimensional maps of the left atrial geometry and regional bipolar voltage amplitude were performed at baseline and after VOM ethanol administration with either NavX (St Jude Medical, Minneapolis, MN) or Carto 3 (Biosense-Webster, Diamond Bar, CA) mapping systems.
High- frequency stimulation protocols
Protocol 1
To avoid atrial capture, VOM HFS was performed during atrial refractoriness, (15) delivering atrial-synchronized (sensing in the proximal coronary sinus) stimulation (30 pulses at 100 Hz and, SynchHFS). Spontaneous (non-captured) atrial activity was monitored subsequently.
Protocol 2
Prolonged (3–10 second) bursts at 33 Hz, (BurstHFS) were delivered via the VOM. Both protocols were delivered at 25 mA of amplitude, 1 ms pulse width. A positive parasympathetic response was defined as either AV block or asystolic pause >2 seconds, or a bradycardic response manifested as R-R interval prolongation by > 50% when averaging 15 beats before and after HFS. Local AF inducibility with HFS was assessed pre and post ethanol infusion in the VOM. If AF persisted beyond the VOM ethanol administration, local AF inducibility with HFS could not be tested.
VOM ethanol infusion procedure
The quadripolar catheter was then retracted from the VOM and an angioplasty wire (BMW, Abbott, Abbott Park, Illinois) was advanced into the VOM as distally as possible. A pre-loaded angioplasty balloon (8 mm length, 2 mm nominal diameter, Voyager OTW, Abbott or 6 mm length, 1.5 mm diameter, Medtronic, Minneapolis, MN) was advanced over the wire, as distally as possible. Depending on the length of the VOM, up to 4 injections of 98% ethanol (1 cc over 2 minutes) were delivered. Starting in the most distal VOM, the balloon was slightly retracted sequentially after each injection, so that the last injection was given from the most proximal VOM. After ethanol infusion, the angioplasty wire and balloon were retracted and the quadripolar catheter was re-inserted to in the VOM to record signals and repeat HFS with the same protocol used prior to ethanol administration. Ethanol levels were measured in mixed venous blood at the end of the procedure. Beyond VOM instrumentation, the procedure continued with radiofrequency ablation using a Thermocool® catheter (Biosense-Webster, Diamond Bar, CA) navigated with the Artisan® robotic sheath (Hansen Medical, Mountain View, CA). Radiofrequency ablation with a power of 25–35 W and saline irrigation of 17–30 cc/min was performed as needed in each case to isolate the PVs, ablate complex fractionated potentials, or LA flutters if present.
Statistical analyses
Data are presented as mean ± standard deviation. Student’s t test was used to compare means. Proportions were compared with Χ2 or Fisher’s exact test where appropriate. A P value of less than 0.05 was considered significant.
Patient follow-up
Patients were followed clinically at 1, 3, 6 and 12 months post procedure, and as needed for clinical recurrences. Continuous 4-week event monitors were connected routinely at 3 and 12 months, and as needed for symptomatic arrhythmias.
Results
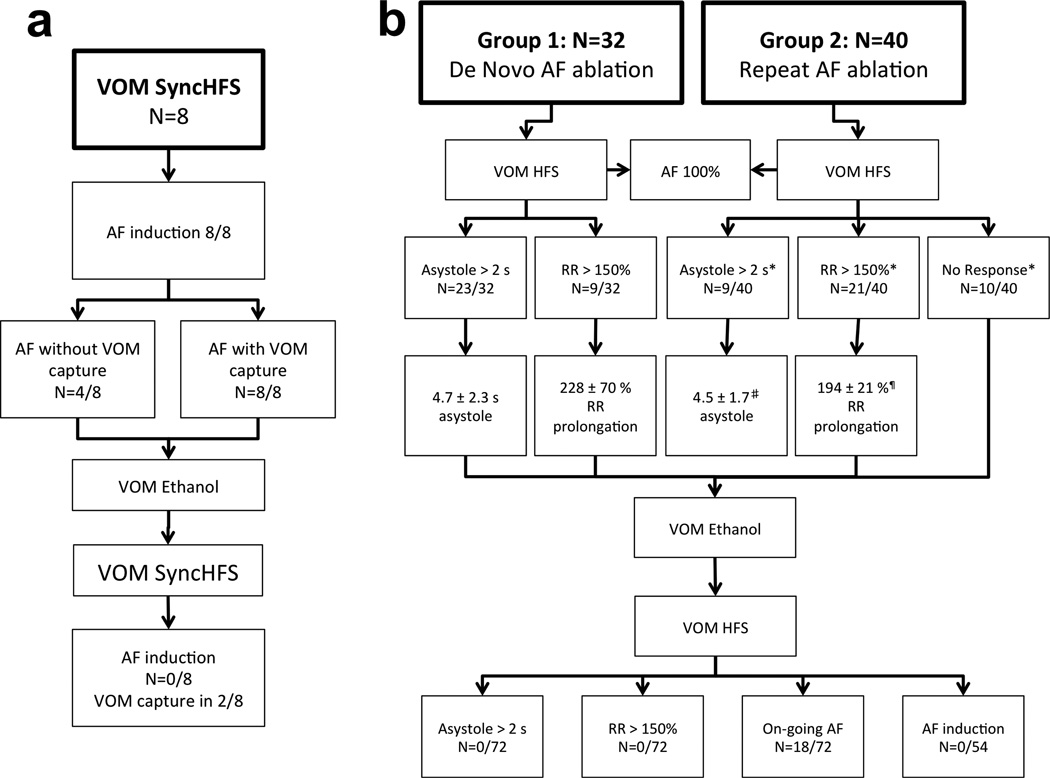
All patients were brought to the electrophysiology laboratory for clinically indicated catheter ablation of AF, after being treated with antiarrhythmic drugs and cardioversions as necessary prior to the procedure. Of 133 patients, the VOM was successfully cannulated in 119 (89%). We were able to complete either HFS protocol before and after VOM ethanol in a total of 80 patients (8 in protocol 1, and 72 in protocol 2), which are reported in this study. In the remainder, HFS protocols were not completed due to inability to introduce a quadripolar catheter in the VOM (Pathfinder Mini® catheter not manufactured after 2010), or to complete the pre- and post ethanol VOM HFS protocols. Figure 1 summarizes the results.
Figure 1. Results summary.
a. SyncHFS protocol. b. BurstHFS protocol. See text for details.
Protocol 1
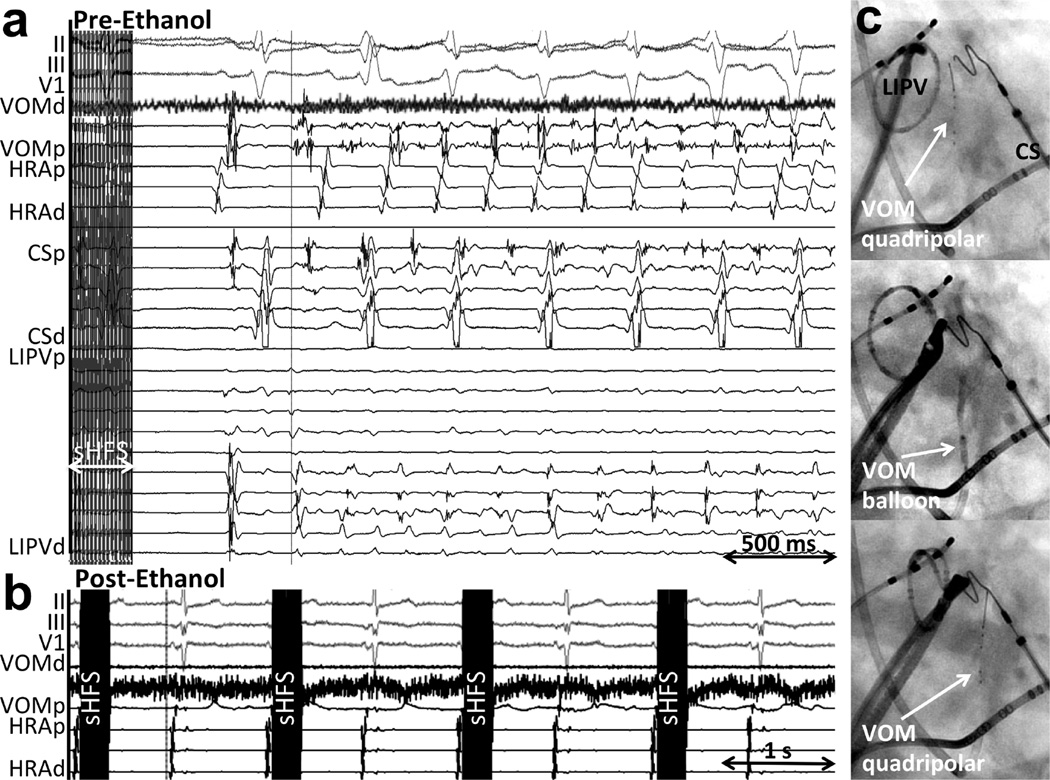
A total of 8 patients with a previous catheter ablation procedure underwent SynchHFS, which induced AF in all patients when atrial capture was obtained. In 4/8 patients, AF spontaneously initiated after non-capturing short SynchHFS trains, and in all 4 cases, AF-initiating atrial premature contractions were recorded first in the VOM. Figure 2 shows an example. VOM ethanol infusion abolished AF induction upon repeating SynchHFS (Figure 2b). Because of the inability to assess parasympathetic responses (which relied on effects on AV conduction) with this protocol, it was abandoned for subsequent patients.
Figure 2. Example of P-wave-synchronized high frequency stimulation (SynchHFS) inducing AF without atrial capture, and abolition of AF induction after VOM ethanol.
a. At baseline (Pre-ethanol), 30 stimuli at 100 Hz following the P wave (sHFS) did not capture the atrium. After a sinus beat, AF ensues, with the first arising from the VOM location. b. After VOM ethanol infusion, SynchHFS fails to induce AF. c. Procedural sequence: Right anterior oblique fluoroscopic views of the intracardiac catheters. Top panel, a quadripolar catheter is inserted in the VOM for baseline HFS and VOM signal recording. Mid panel, an angioplasty balloon is inserted in the VOM for selective angiograms and ethanol infusion. Botton panel, repeat quadripolar catheter insertion in the VOM for post-ethanol HFS. CS: coronary sinus; HRA: high right atrium, LIPV: left inferior pulmonary vein; VOM: vein of Marshall; p, proximal; d, distal.
Protocol 2
Group 1. De novo AF ablation
Group 1 included 32 patients undergoing AF catheter ablation for the first time. AF was paroxysmal in 19/32 patients and persistent in 13. Mean age was 63±8 years and 9 were female. BurstHFS led to AF induction in all patients. Parasympathetic responses were assessed during induced AF, and were elicited in all patients, either as an asystolic response (4.7 ± 2.3 s) in 23/32 patients, or as an RR prolongation (of 228 ± 70%) in 9/32 patients. Figures 3 and 4 show examples of asystole and RR prolongation elicited upon VOM BurstHFS, respectively. AF was induced in all patients during HFS and subsequently terminated in 27. After VOM ethanol infusion, and AF –or any atrial arrhythmia- was not re-inducible by VOM HFS in those 27 patients (Figures 3b and 4b), and parasympathetic responses were not obtained in any of the 32 patients.
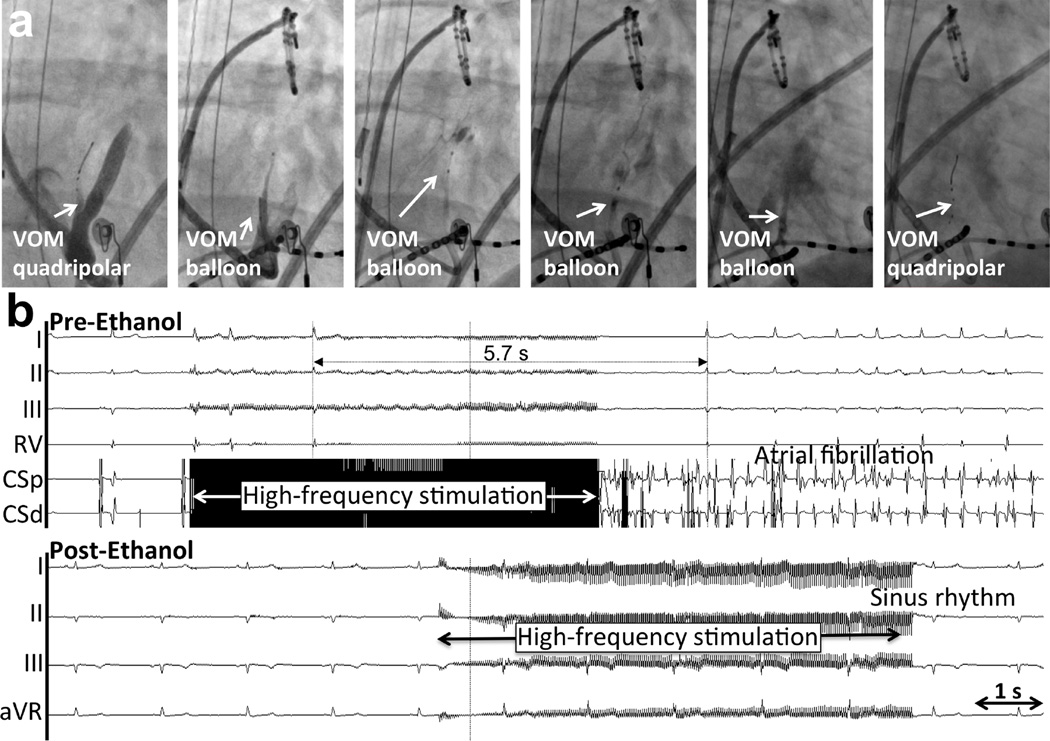
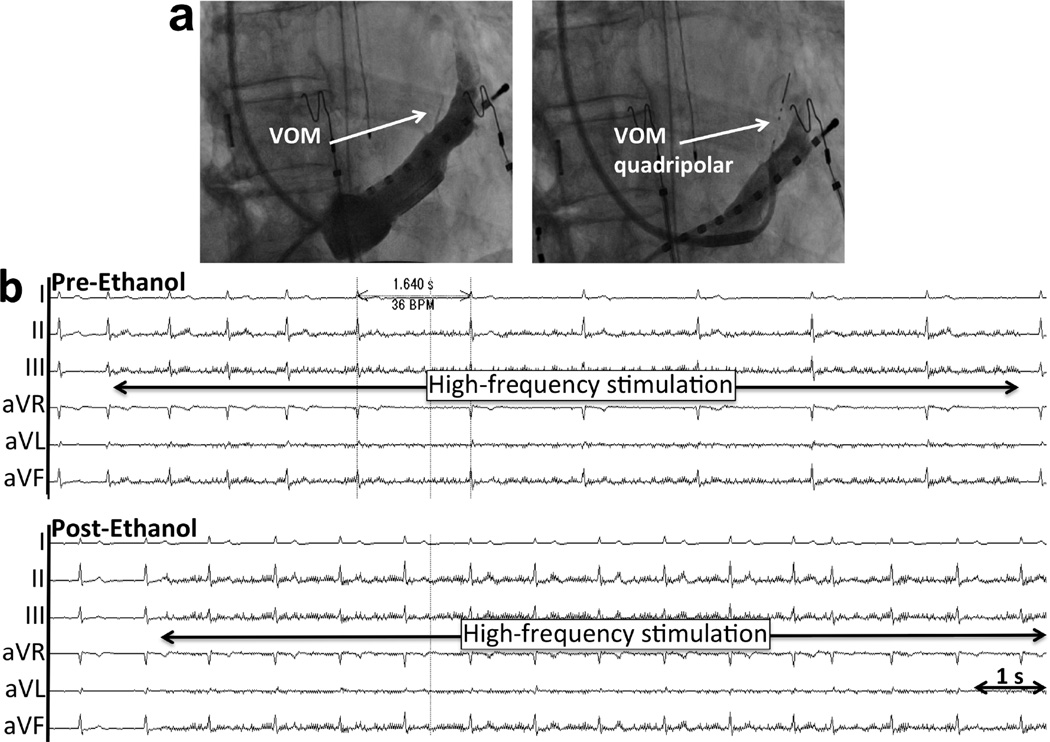
Figure 3. Asystolic response to BurstHFS in a patient undergoing de novo AF ablation (Group 1).
a. Procedural sequence from left to right. Right anterior oblique views. A quadripolar 1.7F catheter is inserted in the VOM, identified by nonselective contrast injection through the LIMA angiographic guide in the coronary sinus. Baseline HFS is performed. Next, an angioplasty balloon is inserted for VOM venogram and ethanol infusion at up to 4 locations, from distal to proximal. Finally, repeat insertion of the quadripolar catheter for HFS is done. b. Pre-ethanol response to BurstHFS. Surface electrocardiogram and coronary sinus (CS) signals are shown. BurstHFS leads to asystole of 5.7 s and induction of AF. c. After ethanol infusion, BurstHFS fails to induce parasympathetic responses or AF.
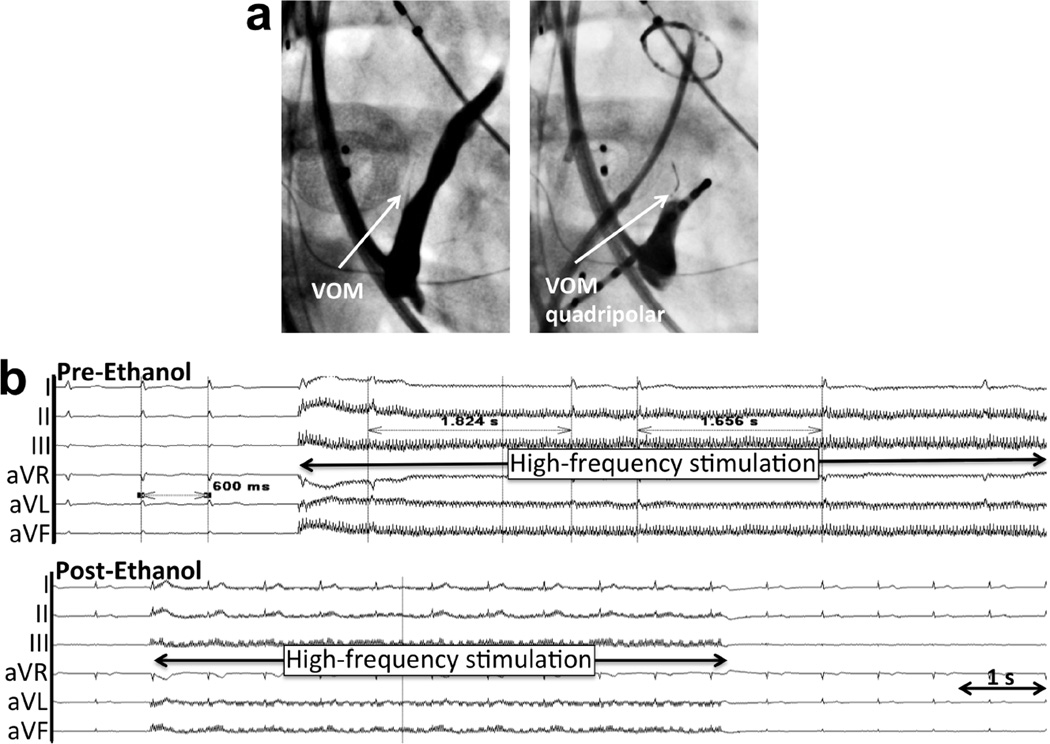
Figure 4. Bradycardic (RR prolongation) response to BurstHFS in a patient undergoing de novo AF ablation (Group 1).
a. Catheter location in right anterior oblique view. A septal occluder device is also present. b. Pre- and post-ethanol recordings. AF had been previously induced, and significant RR prolongation is induced by BurstHFS. Such response is abolished after ethanol injection.
Group 2. Repeat AF ablation
Group 2 included 40 patients undergoing a repeat AF catheter ablation procedure. AF had been paroxysmal in 19/40, and persistent in 21. Mean age was 64±9 years and 12 were female. Except for the proportion of persistent vs paroxysmal AF (p<0.001), none of the patient characteristics were statistically different from those of Group 1. Clinical failures of a previous catheter ablation procedure (1.3 ± 0.5 prior procedures) had been due to flutter in 22/40 patients.
BurstHFS in the VOM during sinus rhythm or atrial flutter led to AF induction in all patients at the initiation of HFS (4 required previous cardioversion for AF). Parasympathetic responses were triggered by BurstHFS in 30/40 patients: in 9 patients an asystolic response was obtained (4.5 ± 1.7 s, P=NS compared to asystolic responses of Group 1 patients); and in 21 patients an RR prolongation was obtained (of 194 ± 21 %, p<0.05 compared to RR prolongation obtained in Group 1 patients). Figure 5 and 6 show examples of asystolic and RR-prolongation responses, respectively. The distribution of asystolic vs RR-prolongation responses of Group 2 contained a greater proportion of RR prolongation responses (and of no parasympathetic responses) than that of Group 1 patients (p<0.05).
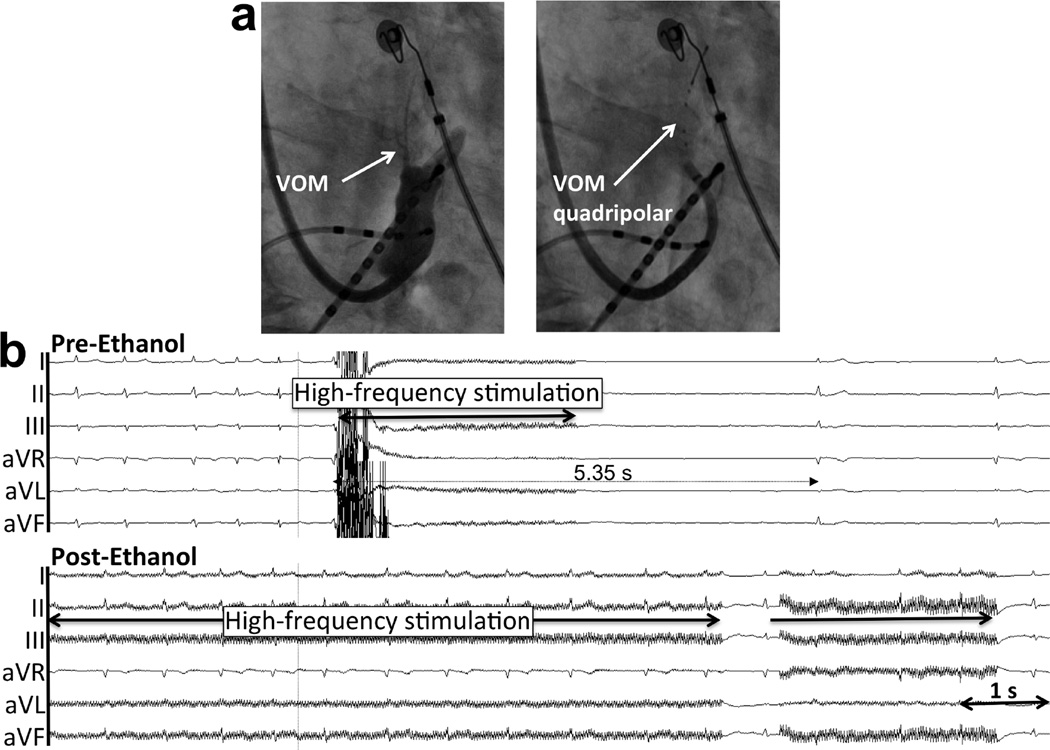
Figure 5. Asystolic response to BurstHFS in a patient undergoing repeat AF ablation (Group 2).
a. Catheter location in right anterior oblique view. b. Pre- and post-ethanol recordings. AF had been previously induced, and a 5.35 s asystole is induced by BurstHFS. Such response is abolished after ethanol injection.
Figure 6. Bradycardic (RR prolongation) response to BurstHFS in a patient undergoing repeat AF ablation (Group 2).
a. Catheter location in right anterior oblique view. b. Pre- and post-ethanol recordings. AF had been previously induced, and significant RR prolongation is induced by BurstHFS. Such response is abolished after ethanol injection.
Local AF inducibility with VOM HFS was re-assessed post-ethanol injection. Thirteen patients remained in sustained AF after ethanol injection in the VOM and inducibility could not be tested with repeat HFS (cardioversion was not performed). In the other 27 patients in whom either cardioversion was performed or the initial AF was non-sustained, inducibility was re-assessed. In this group, AF –or any atrial arrhythmia- remained non-inducible via VOM HFS in 27/27 patients (100%) (Figures 1, 5b and 6b). Parasympathetic responses were eliminated in all patients after VOM ethanol injection.
Acute procedural outcomes
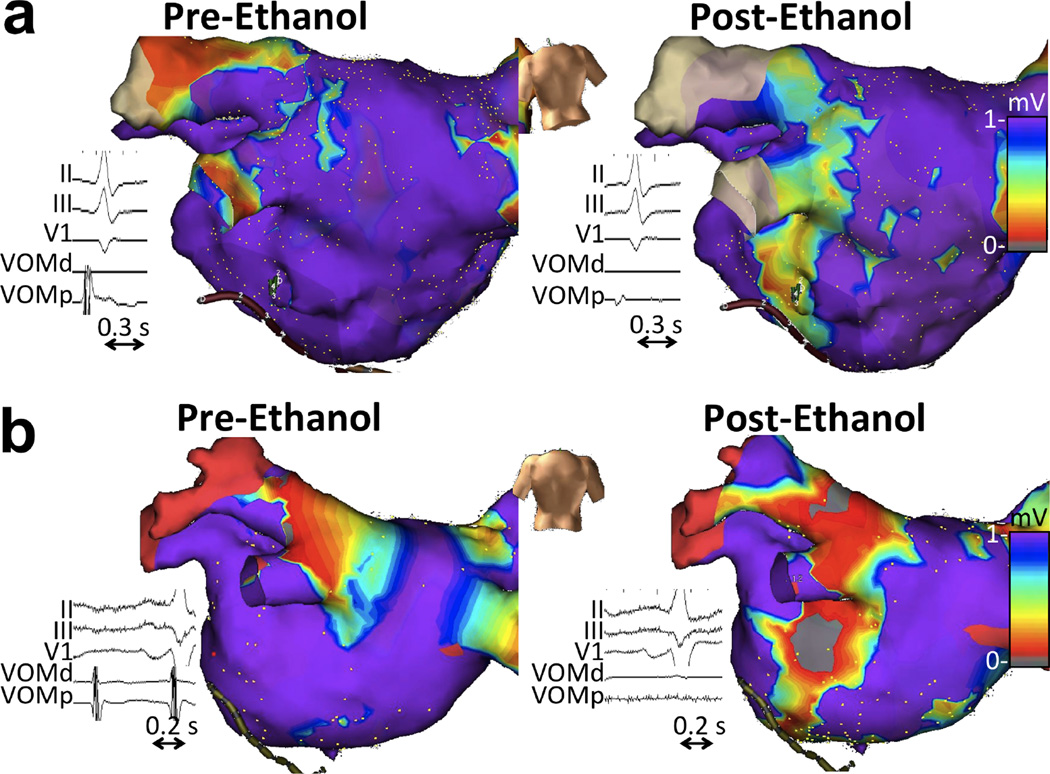
There were no complications directly attributable to VOM instrumentation or ethanol infusion. Ethanol levels measured in mixed venous blood at the end of the procedure were undetectable in all patients. Fluoroscopy and procedure times required to complete the VOM component of the procedure were 9.6±5.2 min and 62.5±12.5 min, respectively. Ethanol lead to elimination of VOM signals in all patients and to the creation of a low-voltage area (bipolar voltage less than 0.1 mV) in the endocardial surface of 8.8±4.1 cm2, located between the coronary sinus and the left inferior pulmonary vein. Figure 7 shows examples. The left inferior pulmonary vein was disconnected in 18/32 patients in group 1 solely with VOM ethanol infusion. In group 2, 32 out of 40 patients had LIPV reconnection and in 23/32 patients VOM ethanol led to redisconnection. (16) Overall, average radiofrequency time required to disconnect the LIPV was 2.9±1.2 min, compared with 16.1±8.2 min required to isolate each of the remaining veins (p<0.05). (17)
Figure 7. VOM electrograms and endocardial bipolar voltage maps before and after VOM ethanol infusion.
a. De novo ablation (group 1). b. Repeat ablation (group 2). VOM signals are abolished and a large endocardial low-voltage area is created by ethanol.
Patient follow-up
Patients were followed-up for an average of 24.6±12.5 months. On follow-up, one patient developed pleuro-pericarditis similar to Dressler’s syndrome: two weeks post ablation, he presented with bilateral pleural effusions and chest pain consistent with pericarditis but without pericardial effusion or any other abnormal findings on chest contrast computerized tomography. He responded to anti-inflammatory agents and colchicine. Two patients had chest pain and pericardial effusions requiring drainage 2 and 4 weeks post ablation. All patients had received conventional radiofrequency ablation concomitantly (with the use of a robotic sheath) that may have contributed to pericardial effusions. Seventy-eight percent of all patients completed electrocardiographic monitoring as requested (100% of the 14 patients with symptoms). AF recurrence occurred in 6/32 patients of group 1, and all had pulmonary vein reconnections on a repeat procedure, including one in whom the left inferior pulmonary vein had been disconnected with ethanol. In group 2, 8/40 patients had recurrent atrial tachyarrhythmias, 2 had AF and 6 had atrial flutters (roof-dependent, LA appendage atrial tachycardia, and right atrial flutter, 2 each) which underwent successful repeat ablation.
Discussion
The main findings of our study are that: 1) The VOM and its neighboring atrial myocardium contain ICN that can reach the AV node and induce parasympathetic responses; 2) Stimulation of VOM-associated ICN can induce AF without local atrial capture; 3) VOM HFS-induced parasympathetic responses are less common in patients with prior catheter AF ablation, suggesting that neuronal damage can occur during a PVAI procedure; and 4) VOM ethanol infusion eliminates parasympathetic responses and AF induction, consistent with regional left atrial denervation. These findings support the feasibility of VOM cannulation as a percutaneous technique for endovascular delivery of ICN-targeted therapies.
The anatomical distribution of the ICN is complex. ICNs are reported to cluster in distinct ganglionated plexi in the vicinity of the PV ostia. It is important to recognize the distinction between the extracardiac ligament of Marshall, a remnant of the left superior vena cava, and the intracardiac VOM. The ligament of Marshall is known to be a conduit for sympathetic (5) and parasympathetic (6) extrinsic cardiac nerves, and it coincides with the left dorsal cardiac nerve.
Stimulation of the ligament of Marshall can induce atrial fibrillation, and even ventricular arrhythmias (15) presumably by direct activation of such extrinsic nerves. The VOM, although in continuity with the ligament of Marshall, is an intracardiac structure in direct connection with the coronary sinus. ICN reached by VOM HFS may include not only those ICN clustered in the VOM –posterolateral left atrial ganglionated plexus (18) - but also those associated with the inferior left ganglionated plexus. Depending of the VOM length, even extrinsic cardiac nerves of the ligament of Marshall may be reached. Regardless of which ICN are stimulated by VOM HFS, in order to lead to AV node conduction slowing, those VOM-associated ICN need to communicate with the right inferior PV plexus, which is the one directly communicating with the AV node. (19,20) Thus, eliciting a parasympathetic response in the AV node by VOM HFS proves not only local ICN stimulation, but also the existence and activation of interneuronal communications between different ICN. These findings are consistent with those of Lin et al, (12) who found, in a canine study using HFS, that both the ligament of Marshall and the inferior left ganglionated plexus modulate AV nodal conduction.
Induction of AF by local VOM HFS without local atrial capture is a more complex phenomenon. A conceivable pathophysiological conjecture is that SynchHFS can selectively stimulate local ICN leading to acetylcholine release and action potential shortening. Coincidental stimulation of local adrenergic nerves could lead to local afterdepoloarizations and AF induction as postulated by Patterson et al. (21) The relative inconsistency of AF induction by this SynchHFS (4/8 patients) may thus illustrate the multiple pathophysiological steps required in its mechanism. AF induced by BurstHFS was a consistent phenomenon, but atrial capture is expected to have played a major role in this setting. Either by local ICN ablation, or atrial tissue ablation, VOM ethanol eliminated AF inducibility by VOM HFS.
Reduction of parasympathetic responses by previous AF catheter ablation has been previously reported. (22) Our data showing a reduced incidence of parasympathetic responses by VOM HFS is consistent with these previous observations.
VOM ethanol successfully eliminated parasympathetic responses as well as AF inducibility. Selective radiofrequency ablation of ICN has been shown to abolish the inducibility of AF in humans, (3) and to eliminate stimulation-induced vagal responses. (22) Additionally, autonomic denervation with radiofrequency applications at sites that elicited a parasympathetic response considerably increased the effectiveness of pulmonary vein antral isolation (PVAI) in patients with paroxysmal AF. (23) Even anatomically remote modulation of the autonomic nervous system such as renal denervation may impact ablation success. (24) Our study is the first to show abolition of parasympathetic responses using chemical ablation rather than radiofrequency energy in humans. Ethanol is highly cytotoxic when infused intravascularly and creates a myocardial scar when delivered through either the coronary venous or arterial route. It has been therapeutically used in septal ablation for hypertrophic cardiomyopathy, (25) ablation of ventricular tachycardia via the coronary arteries (26,27) or coronary veins, (29) and ablation of the atrioventricular node. (30) The vagal denervating effects of ethanol infusion in the VOM were initially studied by our group in dogs. We showed that it can selectively abolish vagal innervation of the left atrium by blunting vagally mediated decreases in LA effective refractory periods. (13) We have subsequently shown the clinical usefulness of VOM ethanol infusion in humans on both AF recurrences after catheter ablation, (16) and in the treatment of perimitral flutter. (31) This is, to our knowledge, the first demonstration of percutaneous chemical denervation of the left atrium in humans.
Clinical implications
Although still controversial, ablation of ICN is a potential target in AF ablation. (2) Ethanol infusion in the VOM is attractive in ablation of AF because local toxicity of ethanol is limited to tissues in direct contact with it, and spares neighboring structures such as the esophagus. VOM ethanol infusion directly addresses mechanistic sources of AF located in the VOM, including ectopic triggers. (32) Our current data adds atrial denervation as an additional therapeutic effect of VOM ethanol. Radiofrequency ablation can effectively eliminate parasympathetic responses, when identified, (23) it is technically simpler than VOM cannulation, which does not eliminate its need. However, it is unclear whether ICN in the VOM region can be readily ablated conventionally, given the fact that VOM signals are often present after endocardial radiofrequency in this region. (16) The ultimate role of VOM ethanol infusion in the treatment of AF remains to be determined and would only be established in a randomized clinical trial assessing rhythm control outcomes.
An important implication of our study is that the VOM can be used as a vascular route to deliver therapies targeting the ICN. Ethanol is nonspecifically toxic to all tissues exposed. However, other pharmacological strategies targeting specifically the ICN could be utilized in the future using the VOM as a route.
Limitations
Due to its technical complexity, the protocol (VOM cannulation, HFS pre- and post ethanol) could only be completed in 80/133 patients. Autonomic nervous system activity analysis with tools such as heart rate variability was not performed. The functional relevance of ICN ablation remains to be determined, but that was beyond the scope of our study. The physiological relevance of interrupting neuronal connections is unclear, and it may potentially be deleterious for AF (33) or ventricular arrhythmias, (34) although these were not observed on follow-up. The patient population is widely heterogeneous and included a wide range of left atrial sizes and AF patterns. Although the acute procedural safety of VOM ethanol infusion is clear, the long-term effects of ethanol and the permanence of ethanol-induced ablation lesions are unknown. It is clear that VOM ethanol infusion is an unconventional procedure that requires additional experience. Most clinically relevant procedural endpoints achieved can currently be achieved by radiofrequency. Only a randomized clinical trial can prove whether VOM ethanol infusion add clinical benefit to catheter ablation of AF.
Conclusion
The VOM contains ICN that connect with the AV node and can trigger AF. Retrograde ethanol infusion in the VOM causes regional LA parasympathetic denervation, by abolishing local ICN responses and AF inducibility in humans. The VOM is a vascular route for ICN-targeting therapies.
Acknowledgments
Financial Support: NIH/NHLBI R21HL097305 and R01 HL115003 (to MV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Heart Rhythm Society 2011 32nd Annual Scientific Sessions in San Francisco, CA, and awarded The Eric N. Prystowsky Fellows Clinical Research Award.
Disclosures: none
References
- 1.Park HW, Shen MJ, Lin SF, Fishbein MC, Chen LS, Chen PS. Neural mechanisms of atrial fibrillation. Curr Opin Cardiol. 2012;27:24–28. doi: 10.1097/HCO.0b013e32834dc4e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–678. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S26–S34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–696. e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. Journal of the American College of Cardiology. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 6.Ulphani JS, Arora R, Cain JH, et al. The ligament of Marshall as a parasympathetic conduit. American journal of physiology. 2007;293:H1629–H1635. doi: 10.1152/ajpheart.00139.2007. [DOI] [PubMed] [Google Scholar]
- 7.Vaitkevicius R, Saburkina I, Rysevaite K, et al. Nerve supply of the human pulmonary veins: an anatomical study. Heart Rhythm. 2009;6:221–228. doi: 10.1016/j.hrthm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. Journal of cardiovascular electrophysiology. 1999;10:636–648. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Katritsis D, Ioannidis JP, Anagnostopoulos CE, et al. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. Journal of cardiovascular electrophysiology. 2001;12:750–758. doi: 10.1046/j.1540-8167.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurotobi T, Ito H, Inoue K, et al. Marshall vein as arrhythmogenic source in patients with atrial fibrillation: correlation between its anatomy and electrophysiological findings. Journal of cardiovascular electrophysiology. 2006;17:1062–1067. doi: 10.1111/j.1540-8167.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 11.Tan AY, Chou CC, Zhou S, et al. Electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall: implications for mechanisms of atrial fibrillation. American journal of physiology. 2006;290:H312–H322. doi: 10.1152/ajpheart.00369.2005. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Scherlag BJ, Niu G, et al. Autonomic elements within the ligament of Marshall and inferior left ganglionated plexus mediate functions of the atrial neural network. Journal of cardiovascular electrophysiology. 2009;20:318–324. doi: 10.1111/j.1540-8167.2008.01315.x. [DOI] [PubMed] [Google Scholar]
- 13.Valderrabano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circulation Arrhythmia and electrophysiology. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valderrabano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of Marshall: Adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–1558. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Scherlag BJ, Lu Z, et al. Inducibility of atrial and ventricular arrhythmias along the ligament of marshall: role of autonomic factors. Journal of cardiovascular electrophysiology. 2008;19:955–962. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 16.Dave AS, Baez-Escudero JL, Sasaridis C, Hong TE, Rami T, Valderrabano M. Role of the vein of Marshall in atrial fibrillation recurrences after catheter ablation: therapeutic effect of ethanol infusion. Journal of cardiovascular electrophysiology. 2012;23:583–591. doi: 10.1111/j.1540-8167.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 17.Valderrabano M, Liu XL, Sidhu J. Retrograde ethanol infusion in the vein of Marshall helps isolate left pulmonary veins during ablation of atrial fibrillation. Europace. 2009;11 Abstract 89. [Google Scholar]
- 18.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Hou Y, Scherlag BJ, Lin J, et al. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Mischke K, Zarse M, Schmid M, et al. Chronic augmentation of the parasympathetic tone to the atrioventricular node: a nonthoracotomy neurostimulation technique for ventricular rate control during atrial fibrillation. Journal of cardiovascular electrophysiology. 2010;21:193–199. doi: 10.1111/j.1540-8167.2009.01613.x. [DOI] [PubMed] [Google Scholar]
- 21.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Saliba WI, Lakkireddy D, et al. Vagal responses induced by endocardial left atrial autonomic ganglion stimulation before and after pulmonary vein antrum isolation for atrial fibrillation. Heart Rhythm. 2007;4:1177–1182. doi: 10.1016/j.hrthm.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances longterm benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 24.Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. Journal of the American College of Cardiology. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Ommen SR, Lakkis NM, et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. Journal of the American College of Cardiology. 2001;38:1701–1706. doi: 10.1016/s0735-1097(01)01614-x. [DOI] [PubMed] [Google Scholar]
- 26.Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation. 1989;79:475–482. doi: 10.1161/01.cir.79.3.475. [DOI] [PubMed] [Google Scholar]
- 27.Okishige K, Andrews TC, Friedman PL. Suppression of incessant polymorphic ventricular tachycardia by selective intracoronary ethanol infusion. Pacing Clin Electrophysiol. 1991;14:188–195. doi: 10.1111/j.1540-8159.1991.tb05089.x. [DOI] [PubMed] [Google Scholar]
- 28.Kay GN, Epstein AE, Bubien RS, Anderson PG, Dailey SM, Plumb VJ. Intracoronary ethanol ablation for the treatment of recurrent sustained ventricular tachycardia. Journal of the American College of Cardiology. 1992;19:159–168. doi: 10.1016/0735-1097(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 29.Baher A, Shah DJ, Valderrabano M. Coronary venous ethanol infusion for the treatment of refractory ventricular tachycardia. Heart Rhythm. 2012;9:1637–1639. doi: 10.1016/j.hrthm.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kay GN, Bubien RS, Dailey SM, Epstein AE, Plumb VJ. A prospective evaluation of intracoronary ethanol ablation of the atrioventricular conduction system. Journal of the American College of Cardiology. 1991;17:1634–1640. doi: 10.1016/0735-1097(91)90659-w. [DOI] [PubMed] [Google Scholar]
- 31.Baez-Escudero JL, Morales PF, Dave AS, et al. Ethanol infusion in the vein of Marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012;9:1207–1215. doi: 10.1016/j.hrthm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keida T, Fujita M, Okishige K, Takami M. Elimination of non-pulmonary vein ectopy by ethanol infusion in the vein of Marshall. Heart Rhythm. 2013;10:1354–1356. doi: 10.1016/j.hrthm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Cummings JE, Gill I, Akhrass R, Dery M, Biblo LA, Quan KJ. Preservation of the anterior fat pad paradoxically decreases the incidence of postoperative atrial fibrillation in humans. Journal of the American College of Cardiology. 2004;43:994–1000. doi: 10.1016/j.jacc.2003.07.055. [DOI] [PubMed] [Google Scholar]
- 34.He B, Lu Z, He W, et al. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int J Cardiol. 2013;168:86–93. doi: 10.1016/j.ijcard.2012.09.067. [DOI] [PubMed] [Google Scholar]