Abstract
Purpose
We performed this study to evaluate the predictive value of pretreatment F-18 FDG PET/CT for progression-free survival (PFS) in patients with gastric cancer.
Methods
Of 321 patients with a diagnosis of gastric cancer, we retrospectively enrolled 97 patients (men:women = 61:36, age 59.8 ± 13.2 years), who underwent pretreatment F-18 fluoro-2-deoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) from January 2009 to December 2009. Maximum standardized uptake value (SUVmax) was measured for each case with detectable primary lesions. In the remaining non-detectable cases, SUVmax was measured from the corresponding site seen on gastroduodenoscopy for analysis. In subgroup analysis, metabolic tumor volume (MTV) was measured in 50 patients with clearly distinguishable primary lesions. SUVmax, stage, depth of tumor invasion and presence of lymph node metastasis were analyzed in terms of PFS. Receiver operating characteristic (ROC) curves were used to find optimal cutoff values of SUVmax and MTV for disease progression. The relationship between SUVmax, MTV and PFS was analyzed using the Kaplan-Meier with log-rank test and Cox’s proportional hazard regression methods.
Results
Of 97 patients, 15 (15.5 %) had disease progression. The mean follow-up duration was 29.6 ± 10.2 months. The mean PFS of low SUVmax group (≤5.74) was significantly longer than that of the high SUVmax group (>5.74) (30.9 ± 8.0 vs 24.3 ± 13.6 months, p = 0.008). In univariate analysis, stage (I vs II, III, IV), depth of tumor invasion (T1 vs T2, T3, T4), presence of lymph node metastasis and SUVmax (>5.74 vs ≤5.74) were significantly associated with recurrence. In multivariate analysis, high SUVmax (>5.74) was the only poor prognostic factor for PFS (p = 0.002, HR 11.03, 95 % CI 2.48–49.05). Subgroup multivariate analysis revealed that high MTV (>16.42) was the only poor prognostic factor for PFS (p = 0.034, HR 3.59, 95 % CI 1.10–11.71).
Conclusion
In gastric cancer, SUVmax measured by pretreatment F-18 FDG PET/CT has a significant predictive value for PFS. In addition, if MTV is measurable, high MTV is an independent factor for disease progression.
Keywords: Gastric cancer, Progression-free survival, Prognosis, F-18 FDG PET/CT, Maximum standardized uptake value, Metabolic tumor volume
Introduction
Gastric cancer is the most commonly diagnosed cancer in Korea and the fourth most common cancer in the world [1, 2]. Recently, mortality and 5-year survival rate associated with gastric cancer have markedly decreased in Korea, possibly due to early detection and surgery [2, 3]. Nevertheless a considerable number of patients are diagnosed in advanced stage and have a poor prognosis. Surgery is the only way to cure gastric cancer. The stage of gastric cancer, depth of tumor invasion and extent of lymph node metastasis are the most significant factors for predicting recurrence [4, 5]. But pretreatment assessment of prognosis in patients with gastric cancer is not well established.
F-18 fluoro-2-deoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) has become an increasingly important method for the detection, staging, and assessment of treatment response of a variety of malignancies, including squamous cell carcinoma of head and neck and non-small cell lung cancer [6–8]. Maximum standardized uptake value (SUVmax), a semiquantitative parameter in integrated F-18 FDG PET/CT, is a significant factor for prognosis and treatment guidance in many types of cancer [9–11]. Metabolic tumor volume (MTV) is a volumetric PET parameter, defined as the summed volume of tumor tissues with increased FDG uptake. Several studies suggest that MTV is an independent factor of prognosis in many types of malignancies [12–17]. F-18 FDG PET/CT is useful to detect recurrence after surgery in patients with gastric cancer [18], but the role of pretreatment PET/CT scan is still unclear, because of low sensitivities of primary tumor and lymph node metastasis [19, 20]. We hypothesized that PET parameters, SUVmax and MTV measured by pretreatment F-18 FDG PET/CT can be valuable for predicting the prognosis of patients with gastric cancer.
The aim of this study was to evaluate the predictive value of the pretreatment F-18 FDG PET/CT for PFS in patients with gastric cancer.
Materials and Methods
Patients
From January 2009 to December 2009, we reviewed retrospectively the electrical medical records of 321 patients with pathologically proven gastric cancer at Chonbuk National University Hospital, Jeonju, South Korea. We enrolled 97 patients (men:women = 61:36, age 59.8 ± 13.2 years) who underwent pretreatment F-18 FDG PET/CT. All patients underwent gastroduodenoscopy, contrast-enhanced abdominal CT and blood test (preoperative tumor markers: CEA [carcinoembryonic antigen], CA 19–9 [carbohydrate antigen 19–9]) for cancer staging. After diagnosis, all patients underwent surgery, (subtotal gastrectomy, total gastrectomy, or exploration) and most (90/97, 92.8 %) received adjuvant chemotherapy.
Acquisition of F-18 FDG PET/CT Scan
F-18 FDG PET/CT scans were obtained on a Biograph Truepoint 40 (Siemens, Berlin, Germany) or Biograph 16 (Siemens, Berlin, Germany). All patients fasted at least 6 h before the intravenous injection of F-18 FDG (3.7–5.5 MBq/kg). Prior to injection of F-18 FDG, blood glucose levels were checked in order to determine whether the levels were within the reference range (<130 mg/dl for nondiabetic patients and <200 mg/dl for diabetic patients). A CT scan from the skull base to the upper thigh was performed prior to PET for attenuation correction and anatomic localization using a standard protocol of 120 kVp, 80 mA (adjusted for body thickness), and a section thickness of 3 mm. Immediately after CT, whole-body PET images were acquired at 50-60 min after intravenous administration of F-18 FDG. Standard PET protocol was used to scan from the skull base to the upper thighs with an acquisition time of 2.5 min per bed position in three-dimensional (3-D) mode. Patients sat quietly in a separate room during the uptake phase and during the scan, patients were supine with their arms raised above their head in the fused PET/CT scanner with a single gantry and table. A limited breath-hold technique was used in order to avoid motion-induced artifacts near the diaphragm.
Measurement of PET Parameters
All images were reviewed at a workstation (Syngo MI applications, Flexible Display 7.0.7.7; Siemens Medical Solutions, Erlangen, Germany). Two types of PET parameters, SUVmax and MTV were measured. First, SUVmax was measured in all patients. In visual analysis, SUVmax was measured from each case with detectable primary lesion (Fig. 1). Detectable primary lesion was defined as FDG-avid focus localized in the gastric wall greater than surrounding gastric wall regardless of intensity of uptake. A region of interest (ROI) was drawn in 3-D planes where the lesion seemed to have the highest uptake according to intensity. Calculation of SUVmax was as follows: SUVmax = Cmax/(IA/TBW) (where Cmax is the activity concentration in the voxel of highest tumor activity (Bq/ml), TBW is the total body weight [kg], and IA is the injected activity [kBq]). In the remaining non-detectable cases, SUVmax was measured based on the corresponding site seen on gastroduodenoscopy or contrast-enhanced CT (Fig. 2). On the other hand, MTV was measured in 50 patients with clearly distinguishable images. MTV was defined as the summed volume in cubic centimeters in the primary tumor. We used a fixed threshold method of SUV 2.5 to measure MTV like previous studies [12, 13]. MTV was measured from attenuation-corrected F-18 FDG PET/CT images using an SUV-based automated contouring program (Syngo MI applications, Volumetric Analysis 7.0.7.7; Siemens Medical Solutions). The ROI was drawn slightly large enough to incorporate tumor in the axial, coronal and sagittal FDG PET/CT images. The contour around the tumor inside the ROI was automatically produced, and voxels presenting SUV > 2.5 within the contouring margin were incorporated to define the tumor volumes (Fig. 1).
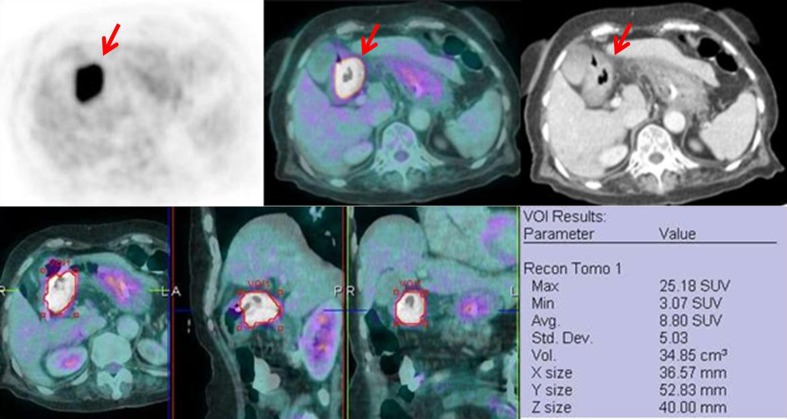
Fig. 1.
The upper panels show F-18 FDG PET, fused F-18 FDG PET/CT and CT images of an 81-year-old man with gastric cancer. Intense FDG-avid wall thickening (SUVmax = 25.18) is shown at antrum (arrows). The lower panels show an example of measuring metabolic tumor volume (MTV) in axial, sagittal and coronal planes from fused images in the same patients (MTV = 34.85 cm3)
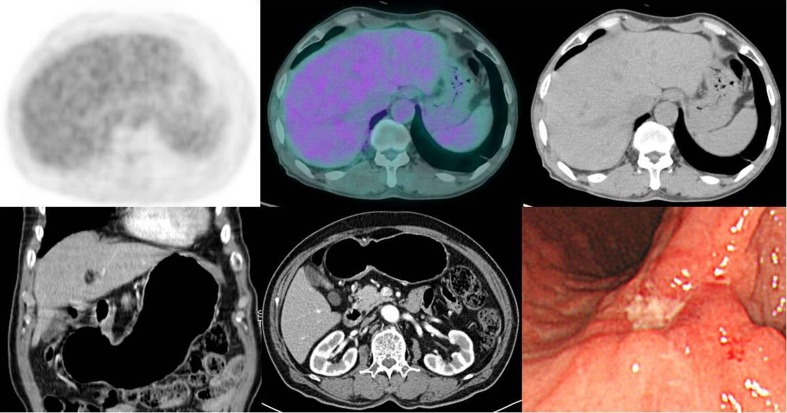
Fig. 2.
Upper endoscopy shows a flat depressed and deep ulcerative lesion with irregular margin at gastric angle in a 75-year-old man with gastric cancer (right bottom). The upper panels show F-18 FDG PET, fused F-18 FDG PET/CT and CT images, but no abnormal focal FDG-avid lesion is seen. Neither abnormal mass nor wall thickening is seen in contrast-enhanced CT images (left and middle bottom)
In this method, we could obtain a highly constant MTV value in repetitive measurement, and only these 50 patients were included in MTV measurable group.
Clinical Follow-Up
All patients were evaluated regularly by physical examination, blood sampling (tumor marker: CEA and CA 19–9), gastroduodenoscopy and imaging study (contrast-enhanced abdominal CT and/or F-18 FDG PET/CT), for follow-up every 6 months in the first year and then 12 months every subsequent year. When abnormality was detected, further evaluations such as pathologic confirmation or additional imaging study were performed. Recurrence was defined as the reappearance of disease during the follow-up period and was confirmed by cytologic or histopathologic examination or by a suggestive lesion in imaging studies. Progression-free survival (PFS) was defined as the time interval from the date of surgery to the date of the disease progression detected on imaging study or pathologic confirmation, or if no event occurred, to the date of the last follow-up.
Statistical Analysis
Statistical analysis was performed using SPSS software (Version 20.0; SPSS, Chicago, IL, USA). The independent t-test and Pearson’s chi-square test were used to evaluated the significances of differences between continuous and categorical variables, respectively. Known prognostic factors, SUVmax, stage, depth of tumor invasion and presence of lymph node metastasis were analyzed in terms of PFS. Receiver operating characteristic (ROC) curves were used to find optimal cutoff values of SUVmax and MTV for disease progression. PFS was evaluated with the Kaplan-Meier method after surgery. Comparison of the PFS between groups was examined using the log-rank test in univariate analysis. The Cox proportional hazard model using forward conditional stepwise selection was used to evaluate prognostic variables for multivariate analysis, and an estimated hazard ratio (HR) with 95 % confidence interval (95 % CI) was presented. Null hypotheses of no difference were rejected if p values were less than 0.05 or, equivalently, if the 95 % CIs of hazard ratio estimates excluded.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. A total of 97 patients were evaluated. The mean age was 59.8 ± 13.2 years. Gastric cancer was staged according to TNM classification by 7th edition of the AJCC cancer staging manual [21]. There were 46 patients with T1 (47.4 %), 13 patients with T2 (13.4 %), 24 patients with T3 (24.7 %), and 14 patients with T4 stage (14.4 %). Lymph node metastasis was positive in 36 patients (37.1 %). There were 51 patients with stage I (52.6 %, indeed one patient with Tis stage, stage 0 was included in stage I category), 16 patients with stage II (16.5 %), 22 patients with stage III (22.7 %), and 8 patients with stage IV (8.2 %). Mean SUVmax and MTV of the primary lesion were 6.8 ± 5.3 and 33.2 ± 48.5 cm3 respectively. Patients characteristics according to disease progression are summarized in Table 2. Age, T stage, presence of lymph node metastasis, TNM stage and SUVmax were significantly different between groups (Table 2). Comparison of MTV measurable and non-measurable groups are displayed in Table 3. Age, pathologic type, T stage, presence of lymph node metastasis, TNM stage and SUVmax were significantly different between groups (Table 3).
Table 1.
Patient characteristics
| Characteristics | Value (%) |
|---|---|
| Age (years, mean ± SD) | 59.8 ± 13.2 |
| Sex | |
| Male | 61 (62.9 %) |
| Female | 36 (37.1 %) |
| Pathologic type | |
| Well differentiated | 20 (20.6 %) |
| Moderately differentiated | 30 (30.9 %) |
| Poorly differentiated | 32 (33.0 %) |
| Signet ring cell/mucinous | 15 (15.5 %) |
| T stage | |
| T1a | 46 (47.4 %) |
| T2 | 13 (13.4 %) |
| T3 | 24 (24.7 %) |
| T4 | 14 (14.4 %) |
| Lymph node metastasis | |
| Positive | 36 (37.1 %) |
| Negative | 61 (62.9 %) |
| TNM stage | |
| Ia | 51 (52.6 %) |
| II | 16 (16.5 %) |
| III | 22 (22.7 %) |
| IV | 8 (8.2 %) |
| Operation | |
| Subtotal gastrectomy | 73 (75.3 %) |
| Total gastrectomy | 20 (20.6 %) |
| Exploration | 4 (4.1 %) |
| SUVmax (mean ± SD) | 6.8 ± 5.3 |
| MTVb (cm3, mean ± SD) | 33.2 ± 48.5 |
aOne patient with Tis stage was included
bMTV was measured in clearly distinguishable 50 patients
Table 2.
Comparison of disease progression and non-progression groups
| Characteristics | Disease progression | Non-progression | p value |
|---|---|---|---|
| Age (years, mean ± SD) | 69.7 ± 7.6 | 57.0 ± 14.9 | 0.002 |
| Sex | 0.136 | ||
| Male | 3 | 49 | |
| Female | 12 | 33 | |
| Pathologic type | 0.470 | ||
| Well differentiated | 3 | 17 | |
| Moderately differentiated | 7 | 23 | |
| Poorly differentiated | 4 | 28 | |
| Signet ring cell/mucinous | 1 | 14 | |
| T stage | 0.001 | ||
| T1 | 3 | 43a | |
| T2 | 2 | 11 | |
| T3 | 3 | 21 | |
| T4 | 7 | 7 | |
| Lymph node metastasis | 0.002 | ||
| Positive | 11 | 25 | |
| Negative | 4 | 57 | |
| TNM stage | 0.001 | ||
| I | 3 | 43a | |
| II | 2 | 11 | |
| III | 3 | 21 | |
| IV | 7 | 7 | |
| Operation | 0.681 | ||
| Subtotal gastrectomy | 10 | 63 | |
| Total gastrectomy | 4 | 16 | |
| Exploration | 1 | 3 | |
| SUVmax (mean ± SD) | 10.8 ± 6.6 | 5.7 ± 4.8 | 0.001 |
| MTV (cm3, mean ± SD) | 45.5 ± 56.6b | 27.4 ± 44.6c | 0.248 |
aOne patient with Tis stage was included
b, cResults from 13 of 50 and 37 of 50 patients in MTV measurable group, respectively
Table 3.
Comparison of MTV measurable and non-measurable groups
| Characteristics | MTV measurable | Non-measurable | p value |
|---|---|---|---|
| Age (years, mean ± SD) | 62.0 ± 12.4 | 56.6 ± 16.4 | 0.047 |
| Sex | 0.513 | ||
| Male | 33 | 28 | |
| Female | 17 | 19 | |
| Pathologic type | 0.013 | ||
| Well differentiated | 5 | 15 | |
| Moderately differentiated | 21 | 9 | |
| Poorly differentiated | 18 | 14 | |
| Signet ring cell/mucinous | 6 | 9 | |
| T stage | <0.001 | ||
| T1 | 10 | 36 | |
| T2 | 8 | 5 | |
| T3 | 19 | 5 | |
| T4 | 13 | 1 | |
| Lymph node metastasis | <0.001 | ||
| Positive | 29 | 7 | |
| Negative | 21 | 40 | |
| TNM stage | <0.001 | ||
| I | 12 | 39 a | |
| II | 11 | 5 | |
| III | 20 | 2 | |
| IV | 7 | 1 | |
| Operation | 0.091 | ||
| Subtotal gastrectomy | 33 | 40 | |
| Total gastrectomy | 14 | 6 | |
| Exploration | 3 | 1 | |
| SUVmax (mean ± SD) | 10.1 ± 5.5 | 2.8 ± 1.0 | <0.001 |
| MTV (cm3, mean ± SD) | 33.2 ± 48.5 | Not applicable |
aOne patient with Tis stage was included
Progression-Free Survival for Gastric Cancer
Of 97 patients, 15 (15.5 %) had disease progression during follow-up period. Among these 15 patients, five were pathologically confirmed and ten were verified by follow-up imaging study. Pathologically proven recurrent sites were gastric anastomotic sites (four patients with gastroduodenoscopy) and left supraclavicular lymph node (one patient with fine-needle aspiration cytology). Other recurrent sites verified by follow-up imaging study were peritoneum (four), lymph node (three), liver (two), lung (two), retroperitoneum (two), bone (one), brain (one) and adrenal gland (one). One patients had at least one or more recurrent sites (numbers of the patients were noted in parentheses). Mean follow-up duration of the all patients was 29.6 ± 10.2 months. Optimal cutoff values determined by ROC curves analyses were SUVmax 5.74 and MTV 16.42 cm3. Area under the curve (AUC) of SUVmax and MTV were 0.76 (sensitivity 86.7 %, specificity 67.1 %) and 0.67 (sensitivity 69.2 %, specificity 64.9 %), respectively. Of 97 patients, 57 had SUVmax equal or below 5.74. The remained 40 patients had SUVmax over 5.74. Mean PFS of low SUVmax group (≤5.74) was 30.9 ± 8.0 months and that of high SUVmax group (>5.74) was 24.3 ± 13.6 months. The mean PFS of the low SUVmax group was significantly longer than that of the high SUVmax group (p = 0.008). In univariate analysis, depth of tumor invasion (T1 vs T2, T3, T4), presence of lymph node metastasis, stage (I vs II, III, IV), and SUVmax (>5.74 vs ≤5.74) were significantly associated with recurrence. Figure 3 shows the PFS differences between respective groups. In multivariate analysis, high SUVmax (>5.74) was the only poor prognostic factor for PFS (p = 0.002, HR 11.03, 95 % CI 2.48–49.05) (Table 4). In MTV measurable subgroup analysis (n = 50), high MTV (>16.42) was the only poor prognostic factor for PFS (p = 0.034, HR 3.59, 95 % CI 1.10–11.71) (Table 5).
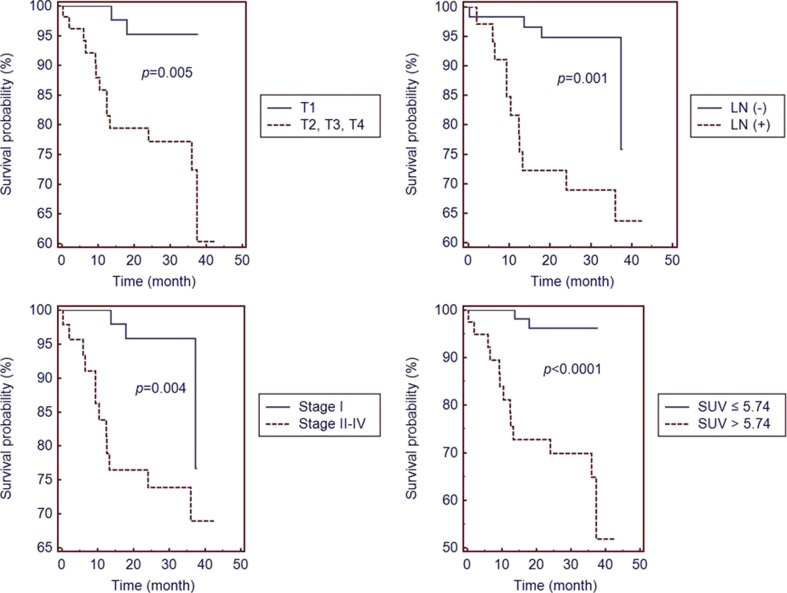
Fig. 3.
Differences in progression-free survival (PFS) in patients with gastric cancer stratified by the depth of tumor invasion (T1 vs T2, T3, T4), absence or presence of lymph node metastasis [LN (−) vs LN (+)], stage (I vs II, III, IV) and SUVmax (>5.74 vs ≤5.74). In patients with T1, absence of lymph node metastasis [LN (−)], stage I and SUV ≤5.74 show significantly better survival than those with T2-T4, presence of lymph node metastasis (LN[+]), stage II-V
Table 4.
Univariate and multivariate analyses for progression-free survival
| Factors | Univariate (n = 97) |
Multivariate (n = 97) |
|
|---|---|---|---|
| p value | HR (95 % CI) | p value | |
| Depth of tumor invasion (T1 vs T2, T3, T4) | 0.005 | 0.249 | |
| Lymph node metastasis (negative vs positive) | 0.001 | 0.148 | |
| Stage (I vs II, III, IV) | 0.004 | 0.349 | |
| SUVmax (≤5.74 vs >5.74) | <0.0001 | 11.03 (2.48–49.05) | 0.002 |
HR hazard ratio, CI confidential interval
Table 5.
Multivariate analysis in MTV measurable subgroup for progression free survival
| Factors | Multivariate (n = 50) | |
|---|---|---|
| HR (95 % CI) | p value | |
| Depth of tumor invasion (T1 vs T2, T3, T4) | 0.266 | |
| Lymph node metastasis (negative vs positive) | 0.138 | |
| Stage (I vs II, III, IV) | 0.389 | |
| SUVmax (≤5.74 vs >5.74) | 0.160 | |
| MTV (≤16.42 vs >16.42) | 3.59 (1.10–11.71) | 0.034 |
HR hazard ratio, b CI confidential interval
Discussion
This study investigated the predictive value of PET parameters (SUVmax and MTV) determined from pretreatment F-18 FDG PET/CT scans for PFS in patients with gastric cancer. The results of this study show that SUVmax and MTV are significant independent prognostic factors for PFS in patients with gastric cancer. Previous studies have shown that F-18 FDG PET/CT has a low detection rate (47–96 %) for primary gastric cancer [22–24], especially for early gastric cancer (below 50 %) and pathologic subtype such as signet ring cell carcinoma [25, 26]. Normally, FDG uptake of gastric wall can exceed SUV 2.5 and physiologic or benign conditions such as gastric mucosal inflammation can show diffuse or focal FDG uptake pattern [26–28]. In this study, SUVmax was measured from all primary lesion including non-detectable cases. Although SUVmax was measured based on a corresponding site seen on gastroduodenoscopy or contrast-enhanced CT, a considerable proportion of the primary lesions may not reflect the true metabolism of the tumor, especially in patients with T1 stage (47.4 %) or signet ring cell carcinoma. In the present study, we assumed that low FDG uptake in gastric wall can represent tumor status (T stage) and so included non-detectable cases in analysis. Fifteen cases with signet ring cell (11 cases) and mucinous adenocarcinoma (four cases) are included (SUVmax, mean 4.0 ± 2.2, range 1.59–9.09). A validation study by pathologic subtypes will be needed.
Several studies suggested that SUVmax of the primary lesion is a prognostic factor in advanced gastric cancer [29–31]. Hur et al. [29] reported that high SUV of the primary tumor (>5) and positive FDG uptake in local lymph nodes at PET/CT could predict non-curative resection in locally advanced gastric cancer. Chung et al. [30] reported that high FDG uptake of the primary tumor (>8) in patients with metastatic gastric adenocarcinoma is associated with poor overall survival. In a similar study, Park et al. [31] included more patients and undifferentiated histologic type carcinoma, and reported that a high SUVmax of stomach (≥6) showed the strongest association with clinical outcome. In the present study, the determined cutoff value SUVmax 5.74 is similar to previous studies.
The well-established prognostic factors for lower survival rate in patients with gastric cancer are the stage of gastric cancer, depth of tumor invasion and extent of LN metastasis [4, 5]. In 2012, Lee et al. [32] performed a retrospective study on 271 consecutive patients with gastric cancer who underwent F-18 FDG PET/CT and subsequent curative surgery, and found that only depth of invasion, positive F-18 FDG uptake and SUVmax had significance in the prediction of gastric cancer recurrence in multivariate analysis. The current study is similar in that SUVmax is a significant predictor of PFS. In addition, MTV, a volumetric PET parameter is used in this study because several studies suggest that MTV is an independent factor of prognosis in many types of malignancies [12–17]. Consequently MTV is the strongest factor for disease progression. To the best of my knowledge there is no previous study using MTV as prognostic factor in patients with gastric cancer. Use of MTV is limited since MTV could not be measured in almost half of patients (47/97, 48.5 %). Although we used an SUV-based automated contouring program to measure MTV, definite tumor segmentation was difficult in some small or infiltrating cancers due to low or diffuse FDG uptake. Some studies recommended to acquire additional PET image with gastric distension for differentiating primary or recurrent gastric malignancy from physiologic uptake [33, 34]. We did not use this method, but it will be helpful to find additional SUV measurable lesion or delineate more accurate tumor boundary by applying this method.
This study has some limitations. First, this was a retrospective study with variations in treatment protocols that may have biased prognostication. Therefore, a prospective validation study in a more homogenous group will be needed. Second, the number of patients was small, and the median follow-up duration was relatively short. Last, MTV was not applicable in all patients and we did not apply gastric distension method for more precise tumor segmentation.
Conclusion
Although there is a limitation in measuring MTV in all cases, we expect that SUVmax and MTV measured on the pretreatment F-18 FDG PET/CT can be used as useful prognostic factors for predicting PFS in patients with gastric cancer.
Acknowledgments
Conflict of Interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward D, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:60–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14:31–36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 4.Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255–261. doi: 10.1002/1097-0142(20000715)89:2<255::AID-CNCR8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Wan F, Pan J, Yu GZ, Chen Y, Wang JJ. Tumor size: a non-neglectable independent prognostic factor for gastric cancer. J Surg Oncol. 2008;97:236–240. doi: 10.1002/jso.20951. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F] fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005;23:1136–1143. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- 7.Davies A, Tan C, Paschalides C, Barrington SF, O’Doherty M, Utley M, et al. FDG-PET maximum standardised uptake value is associated with variation in survival: analysis of 498 lung cancer patients. Lung Cancer. 2007;55:75–78. doi: 10.1016/j.lungcan.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Minn H, Lapela M, Klemi PJ, Grenman R, Leskinen S, Lindholm P, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997;38:1907–1911. [PubMed] [Google Scholar]
- 9.Konski A, Doss M, Milestone B, Haluszka O, Hanlon A, Freedman G, et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1123–1128. doi: 10.1016/j.ijrobp.2004.07.717. [DOI] [PubMed] [Google Scholar]
- 10.Greco C, Rosenzweig K, Cascini GL, Tamburrini O. Current status of PET/CT for tumour volume definition in radiotherapy treatment planning for non-small cell lung cancer (NSCLC) Lung Cancer. 2007;57:125–134. doi: 10.1016/j.lungcan.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, el El-Ghazi A, Lehmann W, et al. Standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398–1404. doi: 10.1200/JCO.20.5.1398. [DOI] [PubMed] [Google Scholar]
- 12.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–274. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Yoo SW, Kim JH, Chong A, Kwon SY, Min JJ, Song HC, et al. Metabolic tumor volume measured by F-18 FDG PET/CT can further stratify the prognosis of patients with stage IV non-small cell lung cancer. Nucl Med Mol Imaging. 2012;46:286–293. doi: 10.1007/s13139-012-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–5868. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 15.Choi KH, Yoo IR, Han EJ, Kim YS, Kim GW, Na SJ, et al. Prognostic value of metabolic tumor volume measured by F-18 FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging. 2011;45:43–51. doi: 10.1007/s13139-010-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Yoo SW, Kang SR, Cho SG, Oh JR, Min JJ, et al. Prognostic significance of metabolic tumor volume measured by F-18 FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–285. doi: 10.1007/s13139-012-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–122. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 18.De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002;29:525–529. doi: 10.1007/s00259-001-0743-8. [DOI] [PubMed] [Google Scholar]
- 19.Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JF, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35:449–455. doi: 10.1016/j.ejso.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using 18F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–1588. [PubMed] [Google Scholar]
- 21.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 22.Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–253. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148–155. doi: 10.1007/s00259-005-1887-8. [DOI] [PubMed] [Google Scholar]
- 24.Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, et al. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol. 2011;104:530–533. doi: 10.1002/jso.21985. [DOI] [PubMed] [Google Scholar]
- 25.Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–196. doi: 10.1007/s10120-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 26.Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–295. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 27.Koga H, Sasaki M, Kuwabara Y, Hiraka K, Nakagawa M, Abe K, et al. An analysis of the physiological FDG uptake pattern in the stomach. Ann Nucl Med. 2003;17:733–738. doi: 10.1007/BF02984984. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi H, Ukawa K, Ohkawa N, Kato K, Hayashi Y, Yoshimoto K, et al. Significance of 18F-2-deoxy-2-fluoro-glucose accumulation in the stomach on positron emission tomography. Ann Nucl Med. 2009;23:391–397. doi: 10.1007/s12149-009-0255-3. [DOI] [PubMed] [Google Scholar]
- 29.Hur H, Kim SH, Kim W, Song KY, Park CH, Jeon HM. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol. 2010;8:86. doi: 10.1186/1477-7819-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung HW, Lee EJ, Cho Y, Yoon SY, So Y, Kim S, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136:1929–1935. doi: 10.1007/s00432-010-0852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JC, Lee JH, Cheoi K, Chung H, Yun MJ, Lee H, et al. Predictive value of pretreatment metabolic activity measured by fluorodeoxyglucose positron emission tomography in patients with metastatic advanced gastric cancer: the maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med Mol Imaging. 2012;39:1107–1116. doi: 10.1007/s00259-012-2116-x. [DOI] [PubMed] [Google Scholar]
- 32.Lee JW, Lee SM, Lee MS, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012;39:1425–1434. doi: 10.1007/s00259-012-2164-2. [DOI] [PubMed] [Google Scholar]
- 33.Kamimura K, Nagamachi S, Wakamatsu H, Fujita S, Nishii R, Umemura Y, et al. Role of gastric distension with additional water in differentiating locally advanced gastric carcinomas from physiologic uptake in the stomach on 18F-fluoro-2-deoxy-D-glucose PET. Nucl Med Commun. 2009;30:431–439. doi: 10.1097/MNM.0b013e3283299a2f. [DOI] [PubMed] [Google Scholar]
- 34.Yun M, Choi HS, Yoo E, Bong JK, Ryu YH, Lee JD. The role of gastric distention in differentiating recurrent tumor from physiologic uptake in the remnant stomach on 18F-FDG PET. J Nucl Med. 2005;46:953–957. [PubMed] [Google Scholar]