Abstract
Image quantification studies in positron emission tomography/computed tomography (PET/CT) are of immense importance in the diagnosis and follow-up of variety of cancers. In this review we have described the current image quantification methodologies employed in 18F-fluorodeoxyglucose (18F-FDG) PET in major oncological conditions with particular emphasis on tumor heterogeneity studies. We have described various quantitative parameters being used in PET image analysis. The main contemporary methodology is to measure tumor metabolic activity; however, analysis of other image-related parameters is also increasing. Primarily, we have identified the existing role of tumor heterogeneity studies in major cancers using 18F-FDG PET. We have also described some newer radiopharmaceuticals other than 18F-FDG being studied/used in the management of these cancers. Tumor heterogeneity studies are being performed in almost all major oncological conditions using 18F-FDG PET. The role of these studies is very promising in the management of these conditions.
Keywords: Positron emission tomography, Quantification, Standard uptake value, Tumor heterogeneity studies, Textural analysis
Introduction
Cancer is a leading cause of death in the world and requires accurate diagnosis, staging and restaging for optimal therapeutic management. Positron emission tomography (PET) with 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG), an analog of glucose, provides important functional information on cancer cells because of the increased glucose uptake and glycolysis and represents metabolic abnormalities before morphological changes occur [1]. PET is increasingly being used for diagnosis, staging, and therapy response evaluation in various tumors [2, 3]. One of the main advantages of PET is its ability to quantitate the metabolic activity of any tumor that cannot be as accurately measured by using other diagnostic modalities. This approach is particularly useful while evaluating prognosis and assessing the response to treatment in routine oncological practice [4]. On the other hand, if the image interpretation is merely done on a visual process, certain useful information is lost as there are features within each image that may not be appreciated readily by the naked eye [5]. In this review, the quantification methods used in PET image interpretation are discussed under volumetric quantification methods and texture-based quantification methods (tumor heterogeneity study). We have also incorporated some of the important non-FDG radiotracers that are being used in clinical practice or are under clinical investigations.
Volumetric Quantification Methods
The most commonly used parameter for semiquantitative analysis of PET images is the standard uptake value (SUV). There are two methods to calculate SUV: one method is to calculate it voxel wise and the other is using the region of interest (ROI). SUV is the ratio of the tissue radioactivity concentration and injected radiotracer dose adjusted by body weight [6]. A commonly used parameter is the maximum SUV (SUVmax), which is obtained for one voxel ROI that corresponds to the maximum voxel value in the tumor. SUVmax is a commonly used parameter as it provides an observer-independent quantity. Another semiquantitative parameter is the mean SUV (SUVmean), which is the mean of metabolic activity in a particular area of tumor mass. An important disadvantage associated with SUVmax is that it does not essentially characterize the total activity for the entire tumor mass. This is because a single voxel might not represent the inhomogeneous overall uptake by the entire tumor [4]. The probable causes of heterogeneity in 18F-FDG uptake, within the tumor mass, are necrosis, cellular proliferation, blood flow, microvessel density, and hypoxia [7]. Intratumoral 18F-FDG heterogeneity may complicate accurate response assessment with PET and is discussed later in this review. Furthermore, SUV is very sensitive to a variety of confounding factors, including technical factors such as the incorrect calibration, biological factors such as the blood glucose level and inflammation, and physical factors such as image reconstruction parameters and the region of interest [2]. As various other quantitative measures can be derived from 18F-FDG PET studies, it is anticipated that the methods other than SUV may be more objective and less influenced by confounding factors and may offer greater assistance in the prognosis assessment [8].
Compartmental modeling (CM) is another quantitative measure in PET imaging, apart from SUV measurements, which is also known as kinetic modeling and is considered the gold standard in PET quantification [9]. Watabe et al. [10] and Gunn et al. [11] have provided in-depth details of the mathematics involved in CM. The radiotracer is supposed to be swapped between the body compartments in CM; thus, each part represents a homogeneous physiological or biochemical unit. The first-order differential equations are used to describe the rates at which the tracer is shifted among these compartments [9]. Advantages of this method are its reliability andindependence of scanning time or plasma clearance in contrast to the SUV. A disadvantage is its complex acquisition protocol that comprises dynamic scanning from the time of injection and, generally, arterial blood sampling used as the input function [9].
The graphical method includes the modified CM and can be used to estimate certain combinations of parameters by properly transforming those estimation equations that are the basis of the compartmental models. Two kinds of graphical methods are the Logan and the Patlak plots, which are applicable to reversible and irreversible tracers, respectively [9, 12].
The spectral analysis (SA) is an additional modification of the CM and was introduced to PET quantification in 1993 by Cunningham and Jones [13]. Similar to compartmental modeling, SA defines the radiotracer kinetics by a series of compartments that represent similar physiological or biochemical states. These four methods are summarized in Table 1. The main objective of these quantification parameters is to measure the metabolic activity within an entire tumor mass and measure the total changes in tumor glycolysis.
Table 1.
Summary of the main characteristics of the quantification methods (modified from [9])
| Is blood sampling required? | Is dynamic scanning required? | Robustness of the method | Applicability in daily clinical practise | |
|---|---|---|---|---|
| SUV | No | No | Low/medium | High |
| Compartmental modeling | Yes | Yes | High | Low |
| Graphical methods | Yes | Yes | Medium/high | Medium |
| Spectral analysis | Yes | Yes | Medium/high | Low |
The other parameters reflecting the metabolic activity of tumors are the metabolic tumor volume (MTV) and total lesion glycolysis (TLG). MTV is a volumetric measurement of the tumor cells with increased DG uptake, thus representing high glycolytic activity, while TLG is defined as the product of the mean SUV and tumor volume [4]. The changes in these parameters between the scans before and after the treatment may be useful in the prediction of therapy response in various types of cancers [14]. These parameters also have the potential to be used as powerful prognostic tools in clinical practice [4]. Thus, patient prognosis and response to a particular therapy can be assessed by using MTV and TLG. However, the clinical significance of these parameters is yet to be established, and more prospective, large-scale studies are required, other than retrospective studies, for the purpose of validating these quantitative parameters [4].
Tumor Heterogeneity Studies
Various factors accounting for heterogeneity within tumors are regional variation in tumor proliferation, cell death, metabolic activity, vascular structure, etc. It is also proposed that the relapse and metastatic behaviors of tumors are due to cancer stem cells, which exist in discrete micro-environmental niches [15]. It has been shown that tumor heterogeneity can be associated with disease progression, response to therapy, and malignant behavior of the tumor. However, information concerning tumor heterogeneity is not properly utilized even in imaging-based studies, and there is no agreement or consensus regarding this aspect [15].
Tumor heterogeneity studies within the solid tumors require either imaging or histological data. The latter provides high spatial resolution and also better biological specificity, but associated weaknesses are difficulty in localization, sampling errors, and basically an inability to repeat. In contrast to histological data, data from imaging studies can not only provide spatial information, but also the procedures are minimal or non-invasive and can be repeated several times [15]. As tumor heterogeneity is associated with various adverse prognostic factors, a measure that can investigate tumor heterogeneity in analysis may also reflect the malignancy potential of tumors, although the biological relationship is still poorly understood [8, 16]. In medical imaging, tumor heterogeneity is recorded as spatial variations in intensity; this type of data can be analyzed by textural analysis.
Textural analysis is a group of computational methods that can extract information about the relation between adjacent pixels (textural features) from the given image. These methods have long been applied to various fields [16–18] and have been applied in other fields of medical imaging like MRI [19], but its introduction to PET imaging was relatively late. Although the amount of data and level of evidence are still not very satisfactory, the results from the limited number of studies show that a group of textural parameters have more predictive and prognostic power compared to the de facto standard SUV [8, 16]. While various measures have been designed for textural analysis, the most frequently used methods employ statistical descriptions of the pixels and the relation between pixels. The textural features can be classified according to the number of pixels involved in calculation of each relation. For example, first order features only consider the statistical distribution of the intensity of each pixel, while second order features consider the relation of two pixels adjacent to each other.
The relations are first summarized into a histogram and a set of matrices. The matrices usually calculated in the analysis are the co-occurrence matrices or spatial gray level dependence matrices (SGLDM), neighborhood gray-tone difference matrices (NGTDM), gray level run length matrices (GLRLM), and gray level size zone matrices (GLSZM) [16]. Each matrix is briefly described in this article, and further information can be found in the works by other authors [8, 16–18, 20, 21].
A co-occurrence matrix is used to extract the second-order information from the given image. It is defined by the number of pixel pairs separated by an offset [17]. Mathematically, a co-occurrence matrix C defined over a n × m gray level matrix I can be expressed as:
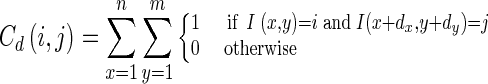 |
Where d is the offset vector. The co-occurrence matrix has the important characteristic that it is invariant under intensity transform [22].
Other matrices are used to extract higher order information. Neighboring gray-tone difference matrices (NGTDM) reflect the amount of local intensity variation and intensity difference relative to the surrounding pixels. An ith entry s(i) in NGTDM is given as the sum of differences between the intensity i and the average intensity Ai over the pixels neighboring the pixel with intensity i:
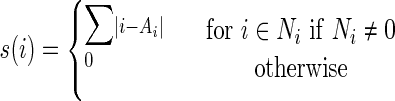 |
Here {Ni} is the set of all pixels with intensity i, and
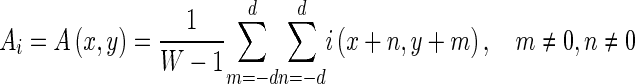 |
where d is the size of the neighborhood and W = (2d + 1)2 [21].
A gray level run length matrix R(α) in some α direction can be expressed as:
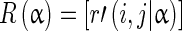 |
Here r'(i,j|α) is the number of runs with intensity i of run length j in the α direction [18, 22].
Gray level size zone matrices describe the flat zones (i.e., connected regions of constant intensity) in the given image. An (sn,gm) th element GS(sn,gm) of GLSZM is given by the number of flat zones of size sn and of gray level gm. GLSZM has the advantage that it does not require calculations in several directions [20].
Textural features can be extracted when the calculation of the histogram and the matrices are done. They can be grouped into global, local, and regional features. Of all the available features, only the commonly used features are described in this article.
Global features are calculated from the histogram. The features include familiar measures such as minimum/mean/maximum intensity, variance/standard deviation, skewness, and kurtosis. Features such as energy, entropy, contrast, correlation, homogeneity, and dissimilarity are calculated from the co-occurrence matrix and NGTDM [17, 23]. They represent the relation between two adjacent pixels, and many of them are analogous to the human perception of texture. The local features calculated from the co-occurrence matrix are summarized in Table 2, while the local features calculated from the NGTDM are summarized in Table 3.
Table 2.
Selected local textural features based on the co-occurrence matrix
| Feature | Definition | Description |
|---|---|---|
| Contrast |
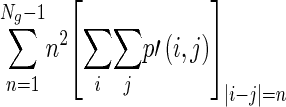
|
Measure of local intensity variation |
| Entropy |
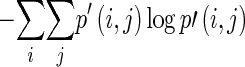
|
Measure of information content |
| Energy |
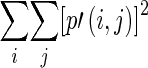
|
Measure of uniformity Also known as second angular moment |
| Homogeneity |
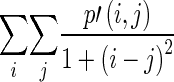
|
Measure of homogeneity Also known as inverse difference moment |
| Correlation |
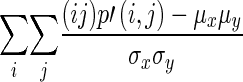
|
Measure of linear relationship |
p ' (i,j) is the normalized co-occurrence matrix p ′ (i,j) = C(i,j)/R; R is the total number of pixels in the given ROI
Table 3.
Selected local features based on the NGTDM
| Feature | Definition | Description |
|---|---|---|
| Coarseness |
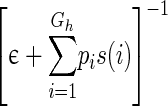
|
The narrow meaning of texture Measure of local uniformity |
| Contrast |
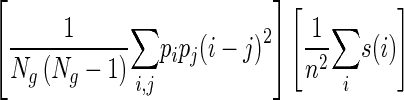
|
Described above |
| Busyness |
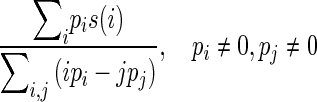
|
Measure of rapid change of intensity |
| Complexity |
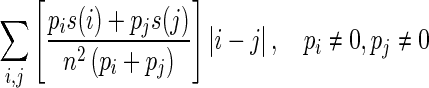
|
Combined measure: rapid change of intensity and small size of primitives |
s(i) is the ith element in the NGTDM defined above; Gh is the highest gray-tone value present in the image; ϵ is a small number to remove singularity; Pi is the probability of occurrence of gray-tone value i given by pi = Ni/n2 where n = N − 2n; Ng is the number of different gray levels
The remaining features are higher order regional features and are calculated from GLRLM and GLSZM. Since GLSZM is an extension of GLRLM into a higher dimension (one-dimensional ‘runs’ into two-dimensional ‘zones’) [20], the two matrices are similar in nature, and Tixier et al. found that the results from the two matrices are very well correlated, thus giving similar results [16]. Because of this similarity, only the textural features calculated from the GLSZM are described below. Corresponding features from GLRLM can be calculated analogously.
Common textural features calculated from GLSZM include size-zone features with various emphases on large-/small-area zones, on high-/low-intensity, and on combinations of these emphases, intensity and size-zone variability, and zone percentage. These regional features are summarized in Table 4 [18, 24]. Galavis et al. [25] studied the variability of textural features in PET images such as entropy-first order, energy, maximal correlation coefficient, low-gray level run emphasis, contrast-NGTD, coarseness, homogeneity, and busyness due to different acquisition modes and reconstruction parameters. They concluded that entropy-first order, energy, maximal correlation coefficient, and low-gray level run emphases were the textural features that showed small variations in cases of different acquisition modes and reconstruction parameters; hence, these could be used for reproducible tumor segmentation, whereas textural features such as homogeneity, contrast-NGTD, busyness, and coarseness were more variable for different parameters.
Table 4.
Selected regional features based on GLSZM
| Feature | Definition |
|---|---|
| High-intensity emphasis |
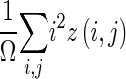
|
| Low-intensity emphasis |
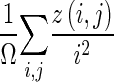
|
| Large-area emphasis |
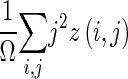
|
| Small-area emphasis |
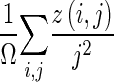
|
| Intensity variability |
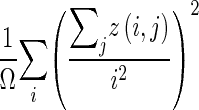
|
| Run-length variability |
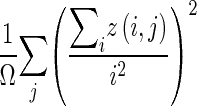
|
| Zone percentage |
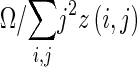
|
z(i,j) is the number of areas with intensity i and size j; Ω is the number of homogeneous areas
PET Heterogeneity Studies in Major Cancers
Breast Cancer
Breast carcinoma is the most common cancer in women in Western Europe and US. Its incidence is highest in the 40-55 age range, and its prevalence is increasing. Breast cancer is the 2nd cause of cancer death in the above-stated countries [26, 27]. Regarding the diagnosis of breast cancer, its detection entails the ability to reveal small (1 cm), nonpalpable, invasive, and in situ malignant lesions, which is beyond the current capability of whole-body 18F-FDG PET. Thus, 18F-FDG PET is infrequently used for the detection of primary breast cancer [28, 29]. However, 18F-FDG PET has been shown to be particularly useful in the restaging of breast cancer and in evaluation of response to therapy. 18F-FDG PET results can also modify therapy options in patients with suspicion of recurrence and/or distant metastasis, mainly by establishing local or distant lymph node involvement that is not seen by other imaging modalities [28, 30].
Winter et al. [31] studied the role of PET in breast carcinoma by performing PET scans on 124 patients with known breast cancer (79 of these patients had a tumor size less than 3 cm in the greatest dimension). All primary tumors were seen clearly by 18F-FDG PET, thus resulting in a sensitivity of 100 %. A study conducted by Samson et al. [29] using 18F-FDG PET resulted in 88 % sensitivity, 80 % specificity for breast cancer, and 61 % sensitivity and 80 % specificity for axillary metastases. Gang et al. [32] conducted a meta-analysis on 18F-FDG PET and other imaging modalities for the evaluation of breast cancer recurrence and metastases. Forty-eight studies were chosen from 1,017 studies. The results showed that ultrasonography and MRI had the highest pooled specificity of 0.962 and 0.929, respectively, whereas PET and MRI showed the highest pooled sensitivity of 0.9530 and 0.9500, respectively. Both MRI and PET had the highest sensitivity, which resulted in a higher cancer detection rate. Mankoff et al. [30] stated that changes in 18F-FDG metabolism are usually ahead of the morphological changes in tumors; thus, FDG PET can show responses earlier than conventional imaging modalities. They also concluded that 18F-FDG PET should be used as an early marker to show therapy resistance. According to Gennari et al. [33], 18F-FDG PET could be particularly useful in evaluating the response of metastatic breast cancer to systemic therapy, since conventional imaging is often challenging in this setting. A decline in 18F-FDG uptake of 50 % or greater indicated a good response to treatment in metastatic disease. Cachin et al. [34] stated that 18F-FDG uptake changes can prognosticate the disease in patients with metastatic breast cancer. They established that the patients showing no 18F-FDG uptake followed by therapy showed better survival than those patients with residual 18F-FDG uptake after therapy in patients with metastatic breast carcinoma.
Currently, SUVmax is considered to be related to several clinicopathological factors of breast cancer and is used most frequently [35, 36]. However, the relationship between SUVmax and clinicopathological factors of breast cancer is diverse and has not been entirely elucidated. Kaida et al. [37] performed studies on PET volumetric parameters in patients with breast carcinoma and concluded that MTV and TLG are related to certain clinicopathological factors of breast carcinoma. By comparing three different quantification parameters, they showed that TLG showed better tumor metabolism for those isolated clinicopathological factors of breast carcinoma than SUVmax or MTV. Kim et al. [38] reported that MTV measured by 18F-FDG PET/CT is helpful in the preoperative evaluation of prognosis in patients having operable breast cancer. In a recent study, Im et al. [39] performed baseline 18F-FDG PET/CT scans of patients with locally advanced breast cancer to calculate the SUVmax, MTV, and TLG of the primary lesions. They concluded that MTV and TLG could successfully describe the outcomes of neoadjuvant chemotherapy.
Tissue heterogeneity is an important factor contributing to the total 18F-FDG uptake in breast tumors. Evaluating heterogeneity in breast cancer is complicated. Schwaiger et al. [40] found only a weak relationship between 18F-FDG uptake and the percentage of tumor cells.
Due to the complex nature of breast cancer, the hallmarks other than glucose metabolism such as angiogenesis, altered proliferation, and evasion of apoptosis are being highlighted in order to understand the nature of breast cancer. Alternative PET tracers are being developed to focus on the specific characteristics of breast cancer [41]. F-18 fluoro-L-thymidine(18 F-FLT) is a tracer that targets DNA replication as a measure of cell proliferation. According to Smyczek-Gargya et al. [42], 18F-FLT provides cell proliferation data rather than merely measuring glucose metabolism. The most promising avenue in the application of 18F-FLT appears to be the follow-up of treatment response. Annexin-V derivatives are tracers that evaluate apoptosis. Initial testing is being performed on F-18 labeled annexin V in various tumors. However, in case of breast cancer, few data are available [30]. Estrogen receptor (ER) tracers such as 16α-F-18 fluoroestradiol-17β (FES) are becoming useful in evaluating treatment response. According to Mortimer et al. [43], FES uptake predicted the response of locally advanced or metastatic breast cancer to tamoxifen therapy. Linden et al. [44] stated that quantitative FES PET can predict the response to hormonal therapy and may help guide treatment selection. They demonstrated a significant relationship between 18F-FES PET uptake and ER expression, which was measured by immunohistochemistry. They concluded that 18F-FES imaging could be useful for assessing the ER status in patients having multiple tumors or for those patients whose biopsy is difficult to perform.
Non-small Cell Lung Cancer (NSCLC)
Lung cancer has the highest incidence and mortality in the world. There were 1.61 million new cases and 1.38 million deaths because of lung cancer in 2008 [45]. Lung cancer develops more commonly in people above 50 years of age and those with a history of cigarette smoking. The mortality rate due to lung cancer has been rising in females in recent years, while it has stabilized in men [46]. Various imaging modalities including PET are employed these days for diagnosis, staging, and follow-up of the lung cancer patients [47].
18F-FDG PET is an imaging tool for assessing tumors, nodes, and metastases clinically in NSCLC. Primary tumor SUV has been studied as a potential prognostic factor for survival. In NSCLC, several studies have shown the utility of serial 18F-FDG PET/CT to measure the response to neoadjuvant chemotherapy [48–53], chemoradiotherapy [54, 55], or novel biologic therapies [56, 57]. Meta-analysis suggests that the primary tumor SUV measurement has a prognostic value in NSCLC [58, 59]. There is also more limited evidence that the level of uptake on pretreatment scans, as measured by various standardized uptake value (SUV) parameters, may be predictive [60–63], but the results conflict as to whether high or low SUVs are predictive depending on treatment modality [62, 63]. Some researchers have tried to establish the role of dual time point PET/CT in the management of NSCLC. In an investigation done by Kim et al. [64], it has been shown that dual time point PET/CT can provide improved diagnostic accuracy for the lymph node staging of NSCLC.
PET-based volumetric imaging parameters are also potential prognostic markers of outcome in patients with NSCLC. Several studies have shown that MTV and TLG have prognostic value in non-classified [65, 66], surgical [67, 68], nonsurgical [69–71], and post-surgical [72] patients with NSCLC. In a retrospective study done by Yoo et al. [73], it has been shown that MTV of the primary lung lesion in patients with NSCLC can further stratify them into subgroups of significantly better and worse prognosis. In other recently published data, Hyun et al. [74] suggested that volume-based quantitative PET parameters including MTV and TLG could be used as independent prognostic criteria apart from a pathological staging system. They also recommended these volumetric parameters as more accurate than SUVmax for survival prediction in early stage NSCLC patients.
Some heterogeneity parameters have also been described in the literature using 18F-FDG PET in NSCLC. Van Velden et al. [7] evaluated a cumulative SUV-volume histogram method to parameterize heterogeneous 18F-FDG uptake in NSCLC and concluded that it could be used as a quantitative index of heterogeneity in tracer uptake. In another study, Vaidya et al. [75] investigated a multimodality image-feature approach, based on SUV and Hounsfield units (HU), for predicting post-therapy tumor response in NSCLC. They concluded that multimodality image feature-based modeling could be a predictor of locoregional recurrence after radiotherapy. Cook et al. evaluated 18F-FDG-PET tumor textural features in NSCLC and their association with response and survival after chemoradiotherapy and concluded that the abnormal texture measured by busyness, contrast, and coarseness was related to poor prognosis and no response to chemoradiotharepy [76].
In addition to 18F-FDG, some other radiotracers such as 18F-FLT, 11C-erlotinib, and 49-[methyl-11-C]-thiothymidine (11C-4DST) are being investigated. 18F-FLT PET may be used to evaluate indeterminate pulmonary nodules or to assess resectable NSCLC, and also to assess the response to therapy [77]. Researchers have also shown a potential role of 11C-erlotinib PET/CT in cases of NSCLC [57] particularly in cases where 18F-FDG PET was unable to localize lymph nodes. Another study on lung tumor proliferation showed that 11C-4DST had a higher correlation than for 18F-FDG and that 11C-4DST PET/CT may be used for noninvasive imaging of DNA synthesis in patients with NSCLC [78].
Esophageal Cancer
Esophageal cancer is among the most prevalent cancers and is one of the most difficult cancers to cure [3, 79]. While surgery alone is potentially curative in the early stage, most patients come to the hospital in the later stages and need chemoradiotherapy [16]. Due to these characteristics of esophageal cancer, 18F-FDG PET is important in its management because of the high sensitivity in detecting metastasis and recurrence, and its potential in predicting the response to therapy [3].
Currently, 18F-FDG PET quantification methods that are under routine clinical use are the semiquantitative SUV-based methods [3, 79]. Kato et al. [3] found that SUV in 18F-FDG PET is useful in the differentiation between benign and malignant tumors, in assessing distant metastasis and tumor recurrence, and in monitoring the response to therapy. Other methods based on geometry features (tumor length), geometry-intensity features (MTV), and textural features have been under investigation [80]. Hyun et al. evaluated the importance of MTV measured by 18F-FDG PET in esophageal cancer patients and concluded that MTV can be used as a prognostic tool for the survival of these patients. They also suggested MTV to be the better survival predictor than SUVmax in esophageal cancer patients [81].
Tixier et al. [16] performed a retrospective study with newly diagnosed esophageal cancer patients treated with combined radiochemotherapy and concluded that textural analysis of tumor uptake heterogeneity on baseline 18F-FDG PET scans can predict response to combined chemoradiation treatment in esophageal cancer. Among the 38 textural features they studied, local measures such as coarseness were statistically significant in predicting therapy response and local entropy, and regional measures such as the size variability were statistically significant in the differentiation of all three patient response groups (non-responders, partial responders, and complete responders). In a recently published study, Dong et al. [82] performed a retrospective study on patients with esophageal squamous cell carcinoma and concluded that tumor uptake heterogeneity parameters have significant correlations with SUV and tumor stage. They concluded that the correlations between T stage and SUVmax, energy and entropy were statistically significant; similarly, the correlation between N stage and SUVmax, entropy and energy were also statistically significant. Tan et al. [80] performed a retrospective study on patients with esophageal cancer in order to compare spatial-temporal features available in 18F-FDG PET imaging to each other in predicting pathologic tumor response to neoadjuvant CRT in esophageal cancer and concluded that three textural features (inertia, correlation, and cluster prominence) were significant predictors and that they had the same or moderately higher areas under the receiver-operating characteristic curve (AUCs) compared to traditional SUV-based methods. In a recent study published by Hatt et al. [83], it was proposed that parameters such as entropy, homogeneity, and dissimilarity should be preferred to local heterogeneity characterization and zone percentage should be used for regional characterization for assessing therapy response prediction in esophageal carcinoma. Their selection was based on the high differentiation power in terms of predicting response and a significant robustness that was associated with these methods.
Several radiotracers other than 18F-FDG PET have been developed for use in PET [84]. Among the radiotracers, 18F-FLT and 18F-FAMT(L-[3-18 F]-α-methyltyrosine) have been studied for use in the management of esophageal cancer [85–88].18F-FLT is a thymidine analog with enhanced uptake in S phase and is trapped in the cells after monophosphorylation by thymidine kinase 1(TK1). It was introduced as a PET proliferation tracer [85]. In determining the gross tumor volume, 18F-FLT PET/CT was found to be less favorable compared to 18F-FDG [86, 87]. Also, van Westreenen et al. [85] found that uptake of 18F-FDG or 18F-FLT did not correlate with proliferation. In assessing lymph node metastasis, 18F-FLT PET/CT has significantly lower uptake in regional lymph nodes and has lower sensitivity and higher specificity in detecting regional lymph node metastasis [86]. A potential advantage of 18F-FLT PET was that GTV delineation with 18F-FLT PET/CT could reduce the lung and heart dose in radiotherapy planning [87].18F-FAMT is an amino-acid tracer correlated with the expression of L-type amino acid transporter 1 (LAT1). While 18F-FAMT PET has been found to be effective in other tumors, 18F-FAMT PET was found to have lower sensitivity compared to 18F-FDG PET. Higher specificity of 18F-FAMT PET is potentially beneficial if combined with CT information in the differentiation of malignant tumors [88].
Head and Neck Cancer
Head and neck cancers (HNC) have a higher incidence rate in the developed countries (4.0–6.8 out of 100,000 in males and 0.8–4.5 out of 100,000 in females) [45]. There were around 550,319 fresh HNC cases in the world in 2008 (408,735 in males and 141,584 in females) and the calculated number of deaths due to HNC in 2008 was 229,903 in males and 75,193 in females [89]. The most common site for HNC is the oral cavity in both sexes, which is followed by the larynx in males and pharynx in females. Squamous cell carcinomas (SCC) is the most common tumor in histopathological types, and at least 75 % of these tumors are due to the combination of tobacco and alcohol intake [89]. There is growing evidence that 18F-FDG PET imaging is a valuable imaging tool for the evaluation of patients who have HNC especially for detection of lymph node metastases. 18F-FDG PET study has an advantage over CT and MR imaging regarding local staging and revelation of the malignant characterization of the enlarged cervical lymph nodes [90]. PET has a sensitivity and specificity to detect lymph node metastases of 90 % and 94 %, respectively, which is 82 % and 85 % in case of high-resolution CT [91].
Chung et al. [92] evaluated the relationship between MTV and HNC prognosis. They studied patients with HNC and concluded that the patients with an MTV greater than 40 ml had a 3.4-fold greater risk of recurrence than those with an MTV less than 40 ml. In another study, Choi et al. [93] evaluated the role of MTV in locally advanced head and neck cancer and concluded that MTV could be a significant prognostic indicator for mortality and disease recurrence in patients with locally advanced HNC who were treated with surgery and radiotherapy, with or without chemotherapy. Investigators from Stanford University did 18F-FDG PET scanning in patients who were treated with chemoradiotherapy. They concluded that SUVmax or SUVmean did not show a correlation with overall survival or with disease-free survival. The median MTV was 11.2 ml, while an increase in MTV was associated with recurrence risk and risk of death [94]. Chu et al. [95] evaluated the prognostic impact of MTV velocity, recorded on serial pre-radiotherapy PET/CT scans, and concluded that primary tumor MTV velocity did predict outcome.
Yu et al. [96] performed texture characterization of HNC with co-registered PET and CT images and concluded that the textural representation of head and neck tissue on PET/CT images could be used to differentiate normal and abnormal tissues. The authors were of the opinion that the textural features established on SGLDM and NGTDM were able to describe the properties of abnormal and normal tissues of the head and neck to an extent to allow ROI classification like a human expert and also that the combined texture features from both PET and CT generated better discrimination results than those using features from one modality alone. In another published study, the same authors proposed that automated segmentation using texture features of PET/CT images had the potential to provide accurate delineation of head and neck cancer [97]. Henriksson et al. [98] investigated the pattern of 18F-FDG uptake in relation to the intratumoral histopathological appearance of head and neck squamous cell carcinoma in an animal model. They concluded that the intratumoral heterogeneity, as shown by histopathology, corresponded to heterogeneous metabolic activity measured with 18F-FDG-PET. Other investigators have also recommended that the capability to measure the spatial distribution of tumor hypoxia might assist radiation oncologists in dose painting schemes [99].
Some investigators have used radiopharmaceuticals other than 18F-FDG in PET imaging for head and neck cancers. Potential tumor hypoxia imaging agents include 18F-FMISO and copper-60-(II)-diacetyl-bis(N4-methylthiosemicarbazone). Under hypoxic conditions, 18F-FMISO has the ability to be reduced and bound to cell constituents and can be used to assess the risk of locoregional failure during the therapy [100]. Similarly 18F-FLT, which is used in proliferation imaging, can be used to discriminate tumor from inflammation. Menda et al. [101] performed serial 18F-FLT scans in HNC patients to measure the effect of radiotherapy on tumor cellular proliferation. The SUV diminished after 10 Gy. They concluded that changes in 18F-FLT uptake could be used as an early metabolic surrogate for treatment response.
Cervical Cancer
Worldwide, cervical cancer is the second most common cause of cancer-related deaths in women. Squamous cell carcinomas represent over 90 % of cervical cancers and originate in the surface epithelium of the cervix. Adenocarcinomas originate in the cervical glandular tissue and represent about 5 % to 9 % of cervical cancers. Adenosquamous carcinoma is comparatively less common and represents about 2 % to 5 % of all cervical cancers. The remainder are cervical sarcomas and small cell carcinoma of the cervix [102]. Cervical cancers differ significantly in their 18F-FDG uptake ability, and cases with lower 18F-FDG uptake may be related to a good prognosis [103].
Kidd et al. [104] tried to establish a relationship between the degree of 18F-FDG uptake and tumor histology type in patients with cervical cancer. They concluded that the mean SUVmax was different in different subtypes of cervical carcinomas. Lin et al. studied dual-phase PET scans in cervical cancer patients and concluded that dual-phase 18F-FDG PET is better than routine 18F-FDG PET or other anatomical imaging modalities for the detection of cervical cancer metastasis [105]. In a recently published study, Mirpour et al. have described SUVmax, MTV, and TLG as emerging predictive and risk stratification markers for the management of cervical cancer patients [106]. In other published data, Yoo et al. [107] described TLG as an independent prognostic factor in the management of patients with uterine cervical cancer and found that TLG might be better than SUV-based parameters and MTV in determining the prognosis of uterine cervical cancer.
El Naqa et al. [108] proposed a systematic pattern recognition approach for analyzing functional imaging data, in particular for 18F-FDG PET in cervical cancer, and suggested that these methodologies can be used to evaluate therapy response. They noticed that the texture-based metrics had the highest predictive ability for failure risk, which was least with SUV. Explicitly, the IVH-based V10–90 and the texture energy were the most significant predictive features. In another study, El-Naqa et al. [109] concluded that certain useful clinical parameters such as overall survival (OS), recurrence-free survival (RFS), and disease-specific survival (DSS) could be better predicted by the pretreatment 18F-FDG PET lymph node status, SUVmax of the cervical tumor, and tumor volume. In another retrospective study by Kidd et al. [110], it was concluded that intratumoral 18F-FDG heterogeneity in cervical cancer in the pretreatment 18F-FDG PET can be used to predict the risk of lymph node metastasis, pelvic recurrence, and also response to therapy.
In a recently published study, Yang et al. [111] analyzed intratumoral metabolic heterogeneity characterized by textural features in cervical cancer and concluded that the temporal changes in the heterogeneity of intratumoral 18F-FDG distribution characterized at a regional scale using image-based textural features may provide an adjunctive or alternative option for understanding tumor response to chemoradiotherapy and interpreting 18F-FDG accumulation dynamics in patients with malignant cervical tumors.
Newer radiopharmaceuticals other than 18F-FDG are being studied in the management of cervical cancer. Schuetz et al. [112] have evaluated the role of 18-F-fluoroazomycin-arabinoside (18 F-FAZA) in the management of advanced cervical cancer. The authors concluded that 18F-FAZAPET imaging is feasible; however, its predictive and prognostic value in cervical cancer remains to be clarified. Another promising agent currently under study is 60Cu-labeled diacetyl-bis (N4-methylthiosemicarbazone) (60Cu-ATSM). In a preliminary study by Dehdashti et al. [113], locally advanced cervical cancers were evaluated before the initiation of definitive chemoradiotherapy. 60Cu-ATSM uptake was evaluated semiquantitatively as the tumor-to-muscle activity ratio (T/M). A log-rang test determined that the T/M cutoff uptake value of >3.5 was significantly associated with worst outcome. Higher uptake of 60Cu-ATSM has been shown to correlate with other biomarkers of tumor hypoxia such as vascular endothelial growth factor receptor (VEGF), epidermal growth factor receptor (EGFR), cycloxygenase-2, and carbonic anhydrase-IV [114].
Lymphomas
PET scanning is being used as a dominant imaging modality in the assessment of patients with both non-Hodgkin’s and Hodgkin’s lymphoma from last 20 years. PET/CT is used in staging, restaging, recognizing possible biopsy sites, and assessing the response to therapy. Because of its ability to image the whole body, the PET scan gives precise information regarding the disease burden within the patient, thus permitting the suitable therapeutic strategy to be chosen.
Schöder et al. [115] reported that 18F-FDG uptake was lower in low-grade NHL than in high-grade lymphomas. Similarly, Ngeow [116] et al. concluded the median SUVmax of high-grade lymphoma was significantly higher than that of the low grade. They concluded that an SUV > 10 indicates a high likelihood of aggressive disease. In another study, Karam et al. [117] were of the opinion that both 5-year overall survival and failure-free survival of patients with pre-treatment SUVmax > 5 were significantly lower than those of patients with pre-treatment SUVmax ≤ 5. Cazaentre et al. [118] reported that pretherapy 18F-FDG PET functional parameters such as SUVmax and TLG may help predict the response to single agent Y-90-based radio-immunotherapy for the management of lymphoma more accurately.
However, there are some limitations of 18F-FDG PET quantification in lymphomas. These are described below:
While interpreting 18F-FDG PET scans, a cutoff SUV used for aggressive lymphoma is more than 13 and for indolent lymphoma is less than 6; hence, a large number of patients (about 45 %) are in the “gray zone.”
- Post-therapy scanning
-
i.Too soon: False-negative result due to temporary stunning of the tumor, not eradication.
-
ii.Too late: False-positive result due to macrophage activity.
-
i.
Diffuse bone marrow uptake is seen in patients who have received granulocyte-stimulating factor (GSF).
Rebound hyperplasia of the thymus usually results in physiologic metabolic activity after chemo- or radiotherapy.
Shinya et al. [119] conducted a study to evaluate the usefulness of 18F-FDG dual-time-point PET/CT with semiquantitative analyses for the initial staging in patients with malignant lymphoma. They reported that this technique could have the potential to provide more accurate diagnoses for the staging of malignant lymphoma and the more important role in predicting the histological grades of malignancy compared with single-time-point 18F-FDG PET scans.
Watabe et al. [120] evaluated the intratumoral metabolic heterogeneity of 18F-FDG uptake on PET to determine whether it might be helpful to discriminate between gastrointestinal stromal tumors (GISTs) and abdominal malignant lymphomas (MLs) on PET/CT and concluded that GISTs exhibited significantly heterogeneous intratumoral tracer uptake compared with the MLs. They also concluded that the evaluation of the intratumoral heterogeneity of 18F-FDG uptake might be helpful in the discrimination between these tumors.
There are some non-FDG radiopharmaceuticals for PET that are being used for diagnosis of lymphomas. These are 18F-FLT and 11C-methionine (MET). 18F-FLT uptake is proportional to tumor proliferation and can accurately discriminate between indolent and aggressive lymphoma; hence, it is used for proliferation imaging [121]. 18F-FLT appears to be more accurate and specific than 18F-FDG, particularly in the setting of interim PET analyses. MET is an amino acid essential for protein synthesis, and its uptake depicts cellular proliferation activity. Its quantification is not affected in hyperglycemic patients. It is superior to 18F-FDG in detecting intermediate- and low-grade lymphomas and is effective in detecting CNS lymphoma [121].
Sarcomas
The sarcomas are derived from mesenchymal tissue elements and as such are diagnosed in myriad clinical presentations. This complex group also has a wide variation in clinical outcomes, ranging from relatively indolent behavior that can be treated with surgical management to some of the most biologically aggressive and treatment-resistant tumors that can be encountered in clinical practice. Commonly, sarcomas are considered in two groups: soft tissue sarcomas and bone tumors. Work in PET imaging in soft tissue sarcomas has focused mainly on the problems in diagnosis and assessment of the biological aggressiveness of the tumor [122]. Many investigators have endeavored to evaluate the use of 18F-FDG for the diagnosis of sarcoma, with particular aims directed toward correlation of 18F-FDG uptake to describe tumor metabolism with tumor histologic grade. Their goal was to improve the diagnostic accuracy. Sarcomas have regional heterogeneity within the tumor mass, and some areas may be less well differentiated than others. These areas are reflected by a regional increase in 18F-FDG uptake, easily noted on a clinical scan. From experience with pathological diagnoses, it is known that the behavior of sarcomas is usually dictated by the most biologically aggressive component. Regional tumor areas with increased uptake have histologic correlates that have been used to support the use of 18F-FDG PET for tumor diagnosis and assessment of tumor grade [122].
Folpe et al. [123] found that an increased tumor SUV was associated with increasing histopathological grade (grades I to III), tissue cellularity, mitotic activity, MIB labeling index, and p53 overexpression. These data suggest that 18F-FDG PET images can be used effectively to guide biopsy. Hain et al. [124] found that areas of the tumor with the highest SUV were the most malignant regions compared to the rest of the tumor. Benign tumors did not show significant 18F-FDG uptake. Ioannidis and Lau [125] performed a meta-analysis of 15 studies with 441 soft tissue lesions analyzed with receiver-operator curves. The authors concluded that 18F-FDG PET might be helpful in assessing soft tissue masses in recurrent and primary tumors, but perform less well in discriminating low-grade tumors from benign ones. In sarcoma, the use of the tumor SUVmax is recommended for reporting and understanding that the areas of the highest 18F-FDG uptake correlate with tumor areas of increased cellularity and mitotic rate, and that these areas are the ones that have the most potential for aggressive behavior. It has long been known that the most biologically aggressive areas of a tumor will control the overall behavior of the tumor, so the use of the 18F-FDG maximum uptake in a tumor has the advantage of describing the biological potential of the tumor most accurately [122].
Similar to findings in soft tissue sarcomas, several authors have found that the level of tumor metabolic activity assessed with 18F-FDG PET in bone tumors indicates tumor grade, and the distinction between benign and malignant tumors can be made[122]. Generally, sarcomas have higher 18F-FDG uptake than benign bone tumors, as described by Schulte et al.[126], but active benign tumors cannot always be distinguished from malignant ones. Patients with the Ewing’s sarcoma family of tumors (ESFT) often present with distant bony metastases and “skip” lesions in an affected extremity, so a whole-body survey with 18F-FDG PET that includes the entire extremities is an important aspect of patient staging [122]. In contrast to the osteosarcomas, the ESFTs typically have a homogenous appearance on whole-body 18F-FDG PET. The tumor 18F-FDG uptake (SUVmax) is generally not as high as that for osteosarcoma, the average range being from 6.0 to 8.0. This is an interesting finding since these are very cellular high-grade tumors according to the microscopic appearance [122].
Eary et al. [127] proposed a heterogeneity analysis method in sarcomas and concluded its validity for the ability to predict patient outcome in a clinical population of patients with sarcoma. They investigated the spatial heterogeneity of 18F-FDG distribution in the primary tumor. Their results showed that heterogeneity analysis was a strong independent predictor of patient outcome using PET imaging. They proposed that this method could also be used in other PET-based imaging modalities. In another study, O'Sullivan et al. [128] evaluated the quantitative assessment of the spatial pattern of PET imaging data of sarcomas and concluded that these patterns had provided improved prognostic information for potential input to treatment decisions for future patients. In another study, O’Sullivan et al. [129] incorporated tumor shape into the assessment of spatial heterogeneity for sarcomas imaged with 18F-FDG PET and concluded that this approach showed some potential improvement in the risk prediction. In a study, Okazumi et al. [130] evaluated malignant behaviors, prognosis, and histological grading of soft tissue sarcomas by performing dynamic 18F-FDG PET studies and concluded that the dynamic 18F-FDG PET imaging could provide important information regarding these parameters.
Several studies are performed in order to evaluate radiopharmaceuticals other than 18F-FDG in PET for the management of sarcomas. Buck et al. [131] studied the role of 18F-FLT PET to differentiate benign from malignant sarcomas and to detect manifestation sites of bone and soft tissue sarcomas. They compared 18F-FLT-PET with 18F-FDG PET/CT, with anatomical imaging modalities such as MR and CT, and also with the results of biopsy. The investigators found a substantial correlation between tumor grade in biopsy and its 18F-FLT uptake. This feature was not seen in case of 18F-FDG; thus they concluded that 18F-FLT PET is an appropriate imaging technique for the assessment of soft tissue and bone tumors. Tateishiet al. [132] investigated the use of choline-PET for staging patients with soft tissue and bone sarcomas compared with bone scintigraphy, chest CT, and MR and concluded that choline-PET was better in assessing the TNM compared to other imaging methodologies. Similarly, fluorine-18 fluoromisonidazole (FMISO) has been used to quantitate the levels of tissue hypoxia in sarcomas [133, 134]. Hypoxia is implied as a result or cause of increased unregulated tumor growth, neovascularization, and upregulation of stress-reactive proteins. A study on sarcomas using FMISO has shown that they are often regionally hypoxic and that these areas of hypoxia do not necessarily relate to areas of increased tumor metabolism determined by 18F-FDG imaging [135].
Current Limitations in PET Heterogeneity Studies
There are some limitations to evaluating tumor heterogeneity with 18F-FDG PET. First, associating each heterogeneity feature with a specific physiologic process within the tumor is not straightforward [24]. Compared to CT or MRI, PET images have a lower spatial resolution. Small tumors below the spatial resolution of PET scans are not suitable for evaluating tumor heterogeneity [5]. In addition, many studies have focused on a limited tumor area, such as the largest cross-sectional area, rather than the whole tumor volume. Intratumoral heterogeneity is likely to be greater in the whole tumor compared to a limited region. With region-of-interest (ROI) delineation around a tumor, this has the potential to introduce inter- and intra-observer variability. If a standardized automated ROI propagation is used, non-tumor areas may be included in the analysis of the pixel values, which may confound the results obtained [5]. Third, in many studies, the number of patients included was relatively small [15, 24, 136, 137]. Results need to be confirmed in a larger data set for which additional validation can be performed. Larger multicenter studies could strengthen the statistical power to detect significant influences of using combinations of multiple PET parameters including heterogeneity on overall diagnostic performance [137].
Summary
Management of cancer mainly depends upon various image findings. 18F-FDG PET imaging has an established role in the management of almost all oncological conditions. There are certain semiquantitative parameters in use for estimation of tumor metabolic activity. Mainly these parameters are SUV based and have certain limitations to their use. There are other parameters that may quantify tumor heterogeneity and thus provide additional information regarding tumor behavior. Image texture analysis is one of these recently studied parameters in 18F-FDG PET imaging, although its role in other imaging modalities such as CT, US, and MRI had been considered previously. Tumor heterogeneity studies can be performed in almost all types of cancers imaged with 18F-FDG PET, and the results can be used to describe potential tumor behavior or to observe the effect of chemoradiotherapy. These studies can also be used to prognosticate the disease. However, to date there have been few tumor heterogeneity studies using 18F-FDG PET. A large number of studies is required to validate these results and to use these as an important clinical tool in 18F-FDG PET imaging. We have also identified some newer radiopharmaceuticals other than 18F-FDG that are being studied or already in use in PET imaging. These radiotracers are mainly used for the assessment of various features of malignancy such as tumor proliferation, hypoxia, etc.
Conclusions
The concept of using heterogeneity studies in 18F-FDG PET imaging is emerging. There are various studies in the literature regarding successful use of textural analysis to measure intratumoral heterogeneity. The comparison made between these studies with other established parameters has shown promising results and has the potential to improve cancer management. However, larger multicenter studies may be required to validate these results.
Acknowledgments
Conflict of Interest
Muhammad Kashif Rahim, Sung Eun Kim, Hyeongryul So, Hyung Jun Kim, Gi Jeong Cheon, Eun Seong Lee, Keon Wook Kang, and Dong Soo Lee declare that they have no conflict of interest.
References
- 1.Almuhaideb A, Papathanasiou N, Bomanji J. 18 F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011;31:3–13. doi: 10.4103/0256-4947.75771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Nakajima M. The efficacy of FDG-PET for the management of esophageal cancer: review article. Ann Thorac Cardiovasc Surg. 2012;18:412–419. doi: 10.5761/atcs.ra.12.01954. [DOI] [PubMed] [Google Scholar]
- 4.Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1–12. doi: 10.3348/kjr.2013.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–589. doi: 10.1007/s13244-012-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 7.van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging. 2011;38:1636–1647. doi: 10.1007/s00259-011-1845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18 F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40:133–140. doi: 10.1007/s00259-012-2247-0. [DOI] [PubMed] [Google Scholar]
- 9.Tomasi G, Turkheimer F, Aboagye E. Importance of quantification for the analysis of PET data in oncology: review of current methods and trends for the future. Mol Imaging Biol. 2012;14:131–146. doi: 10.1007/s11307-011-0514-2. [DOI] [PubMed] [Google Scholar]
- 10.Watabe H, Ikoma Y, Kimura Y, Naganawa M, Shidahara M. PET kinetic analysis–compartmental model. Ann Nucl Med. 2006;20:583–588. doi: 10.1007/BF02984655. [DOI] [PubMed] [Google Scholar]
- 11.Gunn RN, Gunn SR, Cunningham VJ. Positron emission tomography compartmental models. J Cereb Blood Flow Metab. 2001;21:635–652. doi: 10.1097/00004647-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–670. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. doi: 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- 14.Kiyohara S, Nagamachi S, Wakamatsu H, Nishii R, Fujita S. Futami S et al [Usefulness of metabolic volume and total lesion glycolysis for predicting therapeutic response in cancer therapy by 18 F-FDG PET/CT] Kaku Igaku. 2010;47:453–461. [PubMed] [Google Scholar]
- 15.Asselin MCOCJ, Boellaard R, Thacker NA, Jackson A. Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer. 2012;48:447–455. doi: 10.1016/j.ejca.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18 F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369–378. doi: 10.2967/jnumed.110.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haralick RM, Shanmugan K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-3:610–21.
- 18.Loh HH, Leu JG, Luo RC. Analysis of natural textures using run length features. IEEE Trans Ind Electron. 1988;35:323–8.
- 19.Schad LR, Bluml S, Zuna I. MR tissue characterization of intracranial tumors by means of texture analysis. Magn Reson Imaging. 1993;11:889–896. doi: 10.1016/0730-725x(93)90206-s. [DOI] [PubMed] [Google Scholar]
- 20.Thibault G, Fertil B, Navarro C, Pereira S, Cau P, Levy N, et al. Texture indexes and gray level size zone matrix: application to cell nuclei classification. Pattern Recognition Inf Process. 2009:140-5.
- 21.Amadasun M, King R. Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern. 1989;19:1264–74.
- 22.Mohamed SS, Youssef AM, El-Saadany EF, Salama MMA. Prostate tissue characterization using TRUS image spectral features. Image Anal Recog. 2006;4142:589–601. [Google Scholar]
- 23.Clausi DA. An analysis of co-occurrence texture statistics as a function of grey level quantization. Can J Remote Sens. 2002;28:45–62. [Google Scholar]
- 24.Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18 F-FDG PET. J Nucl Med. 2012;53:693–700. doi: 10.2967/jnumed.111.099127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galavis PE, Hollensen C, Jallow N, Paliwal B, Jeraj R. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol. 2010;49:1012–1016. doi: 10.3109/0284186X.2010.498437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 27.von Fournier D, Anton HW, Junkermann H, Bastert G. van Kaick G [Breast cancer screening. State of the art and introduction to preventive measures] Radiologe. 1993;33:227–235. [PubMed] [Google Scholar]
- 28.Phelps ME. PET: Molecular imaging and its biological applications. Springer; 2004.
- 29.Samson DJ, Flamm CR, Pisano ED, Aronson N. Should FDG PET be used to decide whether a patient with an abnormal mammogram or breast finding at physical examination should undergo biopsy? Acad Radiol. 2002;9:773–783. doi: 10.1016/s1076-6332(03)80347-1. [DOI] [PubMed] [Google Scholar]
- 30.Rosen EL, Eubank WB, Mankoff DAFDGPET. PET/CT, and breast cancer imaging. Radiographics. 2007;27(Suppl 1):S215–S229. doi: 10.1148/rg.27si075517. [DOI] [PubMed] [Google Scholar]
- 31.Utech CI, Young CS, Winter PF. Prospective evaluation of fluorine-18 fluorodeoxyclucose positron emission tomography in breast cancer for staging of the axilla related to surgery and immunocytochemistry. Eur J Nucl Med. 1996;23:1588–1593. doi: 10.1007/BF01249621. [DOI] [PubMed] [Google Scholar]
- 32.Pan L, Han Y, Sun X, Liu J, Gang H. FDG-PET and other imaging modalities for the evaluation of breast cancer recurrence and metastases: a meta-analysis. J Cancer Res Clin Oncol. 2010;136:1007–1022. doi: 10.1007/s00432-009-0746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gennari A, Donati S, Salvadori B, Giorgetti A, Salvadori PA, Sorace O, et al. Role of 2-[18 F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin Breast Cancer. 2000;1:156–161. doi: 10.3816/cbc.2000.n.014. [DOI] [PubMed] [Google Scholar]
- 34.Cachin F, Prince HM, Hogg A, Ware RE, Hicks RJ. Powerful prognostic stratification by [18 F]fluorodeoxyglucose positron emission tomography in patients with metastatic breast cancer treated with high-dose chemotherapy. J Clin Oncol. 2006;24:3026–3031. doi: 10.1200/JCO.2005.04.6326. [DOI] [PubMed] [Google Scholar]
- 35.Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S80–S87. doi: 10.1007/s00259-004-1529-6. [DOI] [PubMed] [Google Scholar]
- 36.Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18 F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48:1266–1272. doi: 10.2967/jnumed.106.037440. [DOI] [PubMed] [Google Scholar]
- 37.Kaida H, Toh U, Hayakawa M, Hattori S, Fujii T, Kurata S, et al. The relationship between 18 F-FDG metabolic volumetric parameters and clinicopathological factors of breast cancer. Nucl Med Commun. 2013;34:562–570. doi: 10.1097/MNM.0b013e328360d945. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Yoo SW, Kang SR, Cho SG, Oh JR, Chon R, Min JJ, et al. Prognostic significance of metabolic tumor volume measured by 18 F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–85. [DOI] [PMC free article] [PubMed]
- 39.Im HJ, Kim YK, Kim YI, Lee JJ, Lee WW, Kim SE. Usefulness of combined metabolic–volumetric indices of 18 F-FDG PET/CT for the early prediction of neoadjuvant chemotherapy outcomes in breast cancer. Nucl Med Mol Imaging. 2013;47:36–43. [DOI] [PMC free article] [PubMed]
- 40.Avril N, Menzel M, Dose J, Schelling M, Weber W, Janicke F, et al. Glucose metabolism of breast cancer assessed by 18 F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- 41.Tomasi G, Rosso L. PET imaging: implications for the future of therapy monitoring with PET/CT in oncology. Curr Opin Pharmacol. 2012;12:569–575. doi: 10.1016/j.coph.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Smyczek-Gargya B, Fersis N, Dittmann H, Vogel U, Reischl G, Machulla HJ, et al. PET with [18 F]fluorothymidine for imaging of primary breast cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2004;31:720–724. doi: 10.1007/s00259-004-1462-8. [DOI] [PubMed] [Google Scholar]
- 43.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 44.Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 45.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 46.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 47.Schrevens L, Lorent N, Dooms C, Vansteenkiste J. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist. 2004;9:633–643. doi: 10.1634/theoncologist.9-6-633. [DOI] [PubMed] [Google Scholar]
- 48.Yoon DH, Baek S, Choi CM, Lee DH, Suh C, Ryu JS, et al. FDG-PET as a potential tool for selecting patients with advanced non-small cell lung cancer who may be spared maintenance therapy after first-line chemotherapy. Clin Cancer Res. 2011;17:5093–5100. doi: 10.1158/1078-0432.CCR-10-2791. [DOI] [PubMed] [Google Scholar]
- 49.Shiraishi K, Nomori H, Ohba Y, Kaji M, Mori T, Shibata H, et al. Repeat FDG-PET for predicting pathological tumor response and prognosis after neoadjuvant treatment in nonsmall cell lung cancer: comparison with computed tomography. Ann Thorac Cardiovasc Surg. 2010;16:394–400. [PubMed] [Google Scholar]
- 50.Rebollo-Aguirre AC, Ramos-Font C, Villegas Portero R, Cook GJ, Llamas Elvira JM, Romero TA. Is FDG-PET suitable for evaluating neoadjuvant therapy in non-small cell lung cancer? Evidence with systematic review of the literature. J Surg Oncol. 2010;101:486–494. doi: 10.1002/jso.21525. [DOI] [PubMed] [Google Scholar]
- 51.Lee DH, Kim SK, Lee HY, Lee SY, Park SH, Kim HY, et al. Early prediction of response to first-line therapy using integrated 18 F-FDG PET/CT for patients with advanced/metastatic non-small cell lung cancer. J Thorac Oncol. 2009;4:816–821. doi: 10.1097/JTO.0b013e3181a99fde. [DOI] [PubMed] [Google Scholar]
- 52.Dooms C, Verbeken E, Stroobants S, Nackaerts K, De Leyn P, Vansteenkiste J. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–1134. doi: 10.1200/JCO.2007.13.9550. [DOI] [PubMed] [Google Scholar]
- 53.de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18 F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–1598. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 54.Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of (1)(8)F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38:1628–1635. doi: 10.1007/s00259-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 55.Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer. 2005;49:95–108. doi: 10.1016/j.lungcan.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien ME, Myerson JS, Coward JI, Puglisi M, Trani L, Wotherspoon A, et al. A phase II study of (1)(8)F-fluorodeoxyglucose PET-CT in non-small cell lung cancer patients receiving erlotinib (Tarceva); objective and symptomatic responses at 6 and 12 weeks. Eur J Cancer. 2012;48:68–74. doi: 10.1016/j.ejca.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Memon AA, Weber B, Winterdahl M, Jakobsen S, Meldgaard P, Madsen HH, et al. PET imaging of patients with non-small cell lung cancer employing an EGF receptor targeting drug as tracer. Br J Cancer. 2011;105:1850–1855. doi: 10.1038/bjc.2011.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 59.Al-Sarraf N, Gately K, Lucey J, Aziz R, Doddakula K, Wilson L, et al. Clinical implication and prognostic significance of standardised uptake value of primary non-small cell lung cancer on positron emission tomography: analysis of 176 cases. Eur J Cardiothorac Surg. 2008;34:892–897. doi: 10.1016/j.ejcts.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, et al. Diffusion-weighted MRI versus 18 F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol. 2012;198:75–82. doi: 10.2214/AJR.11.6525. [DOI] [PubMed] [Google Scholar]
- 61.Zhang HQ, Yu JM, Meng X, Yue JB, Feng R, Ma L. Prognostic value of serial [18 F]fluorodeoxyglucose PET-CT uptake in stage III patients with non-small cell lung cancer treated by concurrent chemoradiotherapy. Eur J Radiol. 2011;77:92–96. doi: 10.1016/j.ejrad.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Borst GR, Belderbos JS, Boellaard R, Comans EF, De Jaeger K, Lammertsma AA, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer. 2005;41:1533–1541. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 63.Lee KH, Lee SH, Kim DW, Kang WJ, Chung JK, Im SA, et al. High fluorodeoxyglucose uptake on positron emission tomography in patients with advanced non-small cell lung cancer on platinum-based combination chemotherapy. Clin Cancer Res. 2006;12:4232–4236. doi: 10.1158/1078-0432.CCR-05-2710. [DOI] [PubMed] [Google Scholar]
- 64.Kim DW, Kim WH, Kim CG. Dual-time-point FDG PET/CT: Is it useful for lymph node staging in patients with non-small-cell lung cancer? Nucl Med Mol Imaging. 2012;46:196–200. [DOI] [PMC free article] [PubMed]
- 65.Davison J, Mercier G, Russo G, Subramaniam RM. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol. 2013;200:635–640. doi: 10.2214/AJR.12.9138. [DOI] [PubMed] [Google Scholar]
- 66.Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–566. doi: 10.1148/radiol.12111148. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Wroblewski K, Liao S, Kampalath R, Penney BC, Zhang Y, et al. Prognostic value of metabolic tumor burden from (18)F-FDG PET in surgical patients with non-small-cell lung cancer. Acad Radiol. 2013;20:32–40. doi: 10.1016/j.acra.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Lin Y, Lin WY, Kao CH, Yen KY, Chen SW, Yeh JJ. Prognostic value of preoperative metabolic tumor volumes on PET-CT in predicting disease-free survival of patients with stage I non-small cell lung cancer. Anticancer Res. 2012;32:5087–5091. [PubMed] [Google Scholar]
- 69.Zaizen Y, Azuma K, Kurata S, Sadashima E, Hattori S, Sasada T, et al. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur J Radiol. 2012;81:4179–4184. doi: 10.1016/j.ejrad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 70.Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18 F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38. doi: 10.1007/s00259-011-1934-6. [DOI] [PubMed] [Google Scholar]
- 71.Liao S, Penney BC, Zhang H, Suzuki K, Pu Y. Prognostic value of the quantitative metabolic volumetric measurement on 18 F-FDG PET/CT in Stage IV nonsurgical small-cell lung cancer. Acad Radiol. 2012;19:69–77. doi: 10.1016/j.acra.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 72.Kim K, Kim SJ, Kim IJ, Kim YS, Pak K, Kim H. Prognostic value of volumetric parameters measured by F-18 FDG PET/CT in surgically resected non-small-cell lung cancer. Nucl Med Commun. 2012;33:613–620. doi: 10.1097/MNM.0b013e328351d4f5. [DOI] [PubMed] [Google Scholar]
- 73.Yoo SW, Kim J, Chong A, Kwon SY, Min JJ, Song HC, et al. Metabolic tumor volume measured by F-18 FDG PET/CT can further stratify the prognosis of patients with stage IV non-small cell lung cancer. Nucl Med Mol Imaging. 2012;46:286–93. [DOI] [PMC free article] [PubMed]
- 74.Hyun SH, Choi JY, Kim K, Kim J, Shim YM, Um SW, et al. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg. 2013;257:364–370. doi: 10.1097/SLA.0b013e318262a6ec. [DOI] [PubMed] [Google Scholar]
- 75.Vaidya M, Creach KM, Frye J, Dehdashti F, Bradley JD, El Naqa I. Combined PET/CT image characteristics for radiotherapy tumor response in lung cancer. Radiother Oncol. 2012;102:239–245. doi: 10.1016/j.radonc.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18 F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54:19–26. doi: 10.2967/jnumed.112.107375. [DOI] [PubMed] [Google Scholar]
- 77.Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo validation of 3′deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–3323. [PubMed] [Google Scholar]
- 78.Minamimoto R, Toyohara J, Seike A, Ito H, Endo H, Morooka M, et al. 4′-[Methyl-11C]-thiothymidine PET/CT for proliferation imaging in non-small cell lung cancer. J Nucl Med. 2012;53:199–206. doi: 10.2967/jnumed.111.095539. [DOI] [PubMed] [Google Scholar]
- 79.Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology. 2010;254:707–17. [DOI] [PubMed]
- 80.Tan S, Kligerman S, Chen WG, Lu M, Kim G, Feigenberg S, et al. Spatial-temporal [F-18]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1375–1382. doi: 10.1016/j.ijrobp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18 F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–122. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 82.Dong XZ, Xing LG, Wu PP, Fu Z, Wan HL, Li DW, et al. Three-dimensional positron emission tomography image texture analysis of esophageal squamous cell carcinoma: relationship between tumor F-18-fluorodeoxyglucose uptake heterogeneity, maximum standardized uptake value, and tumor stage. Nucl Med Commun. 2013;34:40–46. doi: 10.1097/MNM.0b013e32835ae50c. [DOI] [PubMed] [Google Scholar]
- 83.Hatt M, Tixier F, Cheze Le Rest C, Pradier O, Visvikis D. Robustness of intratumour F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur J Nucl Med Mol Imaging. 2013 doi: 10.1007/s00259-013-2486-8. [DOI] [PubMed] [Google Scholar]
- 84.MacManus M, Nestle U, Rosenzweig KE, Carrio I, Messa C, Belohlavek O, et al. Use of PET and PET/CT for Radiation Therapy Planning: IAEA expert report 2006-2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 85.van Westreenen HL, Cobben DC, Jager PL, van Dullemen HM, Wesseling J, Elsinga PH, et al. Comparison of 18 F-FLT PET and 18 F-FDG PET in esophageal cancer. J Nucl Med. 2005;46:400–404. [PubMed] [Google Scholar]
- 86.Han D, Yu J, Yu Y, Zhang G, Zhong X, Lu J, et al. Comparison of (18)F-fluorothymidine and (18)F-fluorodeoxyglucose PET/CT in delineating gross tumor volume by optimal threshold in patients with squamous cell carcinoma of thoracic esophagus. Int J Radiat Oncol Biol Phys. 2010;76:1235–1241. doi: 10.1016/j.ijrobp.2009.07.1681. [DOI] [PubMed] [Google Scholar]
- 87.Han D, Yu J, Zhong X, Fu Z, Mu D, Zhang B, et al. Comparison of the diagnostic value of 3-deoxy-3-18 F-fluorothymidine and 18 F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of regional lymph node in thoracic esophageal squamous cell carcinoma: a pilot study. Dis Esophagus. 2012;25:416–426. doi: 10.1111/j.1442-2050.2011.01259.x. [DOI] [PubMed] [Google Scholar]
- 88.Sohda M, Kato H, Suzuki S, Tanaka N, Sano A, Sakai M, et al. 18 F-FAMT-PET is useful for the diagnosis of lymph node metastasis in operable esophageal squamous cell carcinoma. Ann Surg Oncol. 2010;17:3181–3186. doi: 10.1245/s10434-010-1177-y. [DOI] [PubMed] [Google Scholar]
- 89.Curado MP, Boyle P. Epidemiology of head and neck squamous cell carcinoma not related to tobacco or alcohol. Curr Opin Oncol. 2013;25:229–234. doi: 10.1097/CCO.0b013e32835ff48c. [DOI] [PubMed] [Google Scholar]
- 90.Shah GV KK, Gandhi D, Parmar H, Mukherji SK. Squamous cell carcinoma: Initial diagnosis and staging with PET/CT. PET Clinics: Elsevier; 2008. [DOI] [PubMed]
- 91.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 92.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [F-18]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–5868. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 93.Choi KH, Yoo IR, Han EJ, Kim YS, Na SJ, Sun DI, et al. Prognostic value of metabolic tumor volume measure by 18 F-FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging. 2011;45:43–51. [DOI] [PMC free article] [PubMed]
- 94.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–1341. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chu KP, Murphy JD, La TH, Krakow TE, Iagaru A, Graves EE, et al. Prognostic value of metabolic tumor volume and velocity in predicting head-and-neck cancer outcomes. Int J Radiat Oncol Biol Phys. 2012;83:1521–1527. doi: 10.1016/j.ijrobp.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu H, Caldwell C, Mah K, Mozeg D. Coregistered FDG PET/CT-based textural characterization of head and neck cancer for radiation treatment planning. IEEE Trans Med Imaging. 2009;28:374–383. doi: 10.1109/TMI.2008.2004425. [DOI] [PubMed] [Google Scholar]
- 97.Yu H, Caldwell C, Mah K, Poon I, Balogh J, MacKenzie R, et al. Automated radiation targeting in head-and-neck cancer using region-based texture analysis of PET and CT images. Int J Radiat Oncol Biol Phys. 2009;75:618–625. doi: 10.1016/j.ijrobp.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 98.Henriksson E, Kjellen E, Wahlberg P, Ohlsson T, Wennerberg J, Brun E. 2-Deoxy-2-[18 F] fluoro-D-glucose uptake and correlation to intratumoral heterogeneity. Anticancer Res. 2007;27:2155–2159. [PubMed] [Google Scholar]
- 99.Brizel DM. Head and neck cancer as a model for advances in imaging prognosis, early assessment, and posttherapy evaluation. Cancer J. 2011;17:159–165. doi: 10.1097/PPO.0b013e31821e8a09. [DOI] [PubMed] [Google Scholar]
- 100.Srinivasan A, Mohan S, Mukherji SK. Biologic imaging of head and neck cancer: the present and the future. AJNR Am J Neuroradiol. 2012;33:586–594. doi: 10.3174/ajnr.A2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Menda Y, Ponto LLB, Dornfeld KJ, Tewson TJ, Watkins GL, Schultz MK, et al. Kinetic analysis of 3 ′-deoxy-3 ′-(18)F-fluorothymidine ((18)F-FLT) in head and neck cancer patients before and early after initiation of chemoradiation therapy. J Nucl Med. 2009;50:1028–1035. doi: 10.2967/jnumed.108.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grigsby PW. Cervical and Uterine Cancer. In: Wahl RL, editor. Principles and Practice of PET and PET/CT. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 348–354. [Google Scholar]
- 103.Kwee TC, Basu S, Saboury B, Ambrosini V, Torigian DA, Alavi A. A new dimension of FDG-PET interpretation: assessment of tumor biology. Eur J Nucl Med Mol Imaging. 2011;38:1158–1170. doi: 10.1007/s00259-010-1713-9. [DOI] [PubMed] [Google Scholar]
- 104.Kidd EA, Spencer CR, Huettner PC, Siegel BA, Dehdashti F, Rader JS, et al. Cervical cancer histology and tumor differentiation affect 18 F-fluorodeoxyglucose uptake. Cancer. 2009;115:3548–3554. doi: 10.1002/cncr.24400. [DOI] [PubMed] [Google Scholar]
- 105.Lin CT, Yen TC, Chang TC, Ng KK, Tsai CS, Ho KC, et al. Role of [18 F]fluoro-2-deoxy-D-glucose positron emission tomography in re-recurrent cervical cancer. Int J Gynecol Cancer. 2006;16:1994–2003. doi: 10.1111/j.1525-1438.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 106.Mirpour S, Mhlanga JC, Logeswaran P, Russo G, Mercier G, Subramaniam RM. The role of PET/CT in the management of cervical cancer. Am J Roentgenol. 2013;201:W192–W205. doi: 10.2214/AJR.12.9830. [DOI] [PubMed] [Google Scholar]
- 107.Yoo J, Choi JY, Moon SH, Bae DS, Park SB, Choe YS, et al. Prognostic significance of volume-based metabolic parameters in uterine cervical cancer determined using 18 F-fluorodeoxyglucose positron emission tomography. Int J Gynecol Cancer. 2012;22:1226–1233. doi: 10.1097/IGC.0b013e318260a905. [DOI] [PubMed] [Google Scholar]
- 108.El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009;42:1162–1171. doi: 10.1016/j.patcog.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kidd EA, El Naqa I, Siegel BA, Dehdashti F, Grigsby PW. FDG-PET-based prognostic nomograms for locally advanced cervical cancer. Gynecol Oncol. 2012;127:136–140. doi: 10.1016/j.ygyno.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14:5236–5241. doi: 10.1158/1078-0432.CCR-07-5252. [DOI] [PubMed] [Google Scholar]
- 111.Yang F, Thomas MA, Dehdashti F, Grigsby PW. Temporal analysis of intratumoral metabolic heterogeneity characterized by textural features in cervical cancer. Eur J Nucl Med Mol Imaging. 2013;40:716–727. doi: 10.1007/s00259-012-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuetz M, Schmid MP, Potter R, Kommata S, Georg D, Lukic D, et al. Evaluating repetitive 18 F-fluoroazomycin-arabinoside (18FAZA) PET in the setting of MRI guided adaptive radiotherapy in cervical cancer. Acta Oncol. 2010;49:941–947. doi: 10.3109/0284186X.2010.510145. [DOI] [PubMed] [Google Scholar]
- 113.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2008;49:201–205. doi: 10.2967/jnumed.107.048520. [DOI] [PubMed] [Google Scholar]
- 114.Grigsby PW, Malyapa RS, Higashikubo R, Schwarz JK, Welch MJ, Huettner PC, et al. Comparison of molecular markers of hypoxia and imaging with (60)Cu-ATSM in cancer of the uterine cervix. Mol Imaging Biol. 2007;9:278–283. doi: 10.1007/s11307-007-0095-2. [DOI] [PubMed] [Google Scholar]
- 115.Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:4643–4651. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 116.Ngeow JY, Quek RH, Ng DC, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20:1543–1547. doi: 10.1093/annonc/mdp030. [DOI] [PubMed] [Google Scholar]
- 117.Karam M, Ata A, Irish K, Feustel PJ, Mottaghy FM, Stroobants SG, et al. FDG positron emission tomography/computed tomography scan may identify mantle cell lymphoma patients with unusually favorable outcome. Nucl Med Commun. 2009;30:770–778. doi: 10.1097/MNM.0b013e32832e0c13. [DOI] [PubMed] [Google Scholar]
- 118.Cazaentre T, Morschhauser F, Vermandel M, Betrouni N, Prangere T, Steinling M, et al. Pre-therapy 18 F-FDG PET quantitative parameters help in predicting the response to radioimmunotherapy in non-Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:494–504. doi: 10.1007/s00259-009-1275-x. [DOI] [PubMed] [Google Scholar]
- 119.Shinya T, Fujii S, Asakura S, Taniguchi T, Yoshio K, Alafate A, et al. Dual-time-point F-18 FDG PET/CT for evaluation in patients with malignant lymphoma. Ann Nucl Med. 2012;26:616–621. doi: 10.1007/s12149-012-0619-y. [DOI] [PubMed] [Google Scholar]
- 120.Watabe T, Tatsumi M, Watabe H, Isohashi K, Kato H, Yanagawa M, et al. Intratumoral heterogeneity of F-18 FDG uptake differentiates between gastrointestinal stromal tumors and abdominal malignant lymphomas on PET/CT. Ann Nucl Med. 2012;26:222–227. doi: 10.1007/s12149-011-0562-3. [DOI] [PubMed] [Google Scholar]
- 121.Kong FL, Ford RJ, Yang DJ. Managing lymphoma with non-FDG radiotracers: current clinical and preclinical applications. Biomed Res Int. 2013;2013:626910. doi: 10.1155/2013/626910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eary J. Sarcomas. In: Wahl RL, editor. Principles and Practice of PET and PET/CT. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 392–401. [Google Scholar]
- 123.Folpe AL, Lyles RH, Sprouse JT, Conrad EU, III, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res. 2000;6:1279–1287. [PubMed] [Google Scholar]
- 124.Hain SF, O’Doherty MJ, Bingham J, Chinyama C, Smith MA. Can FDG PET be used to successfully direct preoperative biopsy of soft tissue tumours? Nucl Med Commun. 2003;24:1139–1143. doi: 10.1097/00006231-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Ioannidis JPA, Lau J. F-18-FDG PET for the diagnosis and grading of soft-tissue sarcoma: A meta-analysis. J Nucl Med. 2003;44:717–724. [PubMed] [Google Scholar]
- 126.Schulte M, Brecht-Krauss D, Heymer B, Guhlmann A, Hartwig E, Sarkar MR, et al. Grading of tumors and tumorlike lesions of bone: Evaluation by FDG PET. J Nucl Med. 2000;41:1695–1701. [PubMed] [Google Scholar]
- 127.Eary JF, O’Sullivan F, O’Sullivan J, Conrad EU. Spatial heterogeneity in sarcoma (18)F-FDG uptake as a predictor of patient outcome. J Nucl Med. 2008;49:1973–1979. doi: 10.2967/jnumed.108.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Sullivan F, Wolsztynski E, O’Sullivan J, Richards T, Conrad EU, Eary JF. A statistical modeling approach to the analysis of spatial patterns of FDG-PET uptake in human sarcoma. Med Imaging IEEE Trans. 2011;30:2059–2071. doi: 10.1109/TMI.2011.2160984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Sullivan F, Roy S, O’Sullivan J, Vernon C, Eary J. Incorporation of tumor shape into an assessment of spatial heterogeneity for human sarcomas imaged with FDG-PET. Biostatistics. 2005;6:293–301. doi: 10.1093/biostatistics/kxi010. [DOI] [PubMed] [Google Scholar]
- 130.Okazumi S, Dimitrakopoulou-Strauss A, Schwarzbach MHM, Strauss LG. Quantitative, dynamic 18 F-FDG-PET for the evaluation of soft tissue sarcomas: Relation to differential diagnosis, tumor grading and prediction of prognosis. Hell J Nucl Med. 2009;12:223–228. [PubMed] [Google Scholar]
- 131.Buck AK, Herrmann K. zum Bueschenfelde CM, Juweid ME, Bischoff M, Glatting G et al. Imaging bone and soft tissue tumors with the proliferation marker [F-18]fluorodeoxythymidine. Clin Cancer Res. 2008;14:2970–2977. doi: 10.1158/1078-0432.CCR-07-4294. [DOI] [PubMed] [Google Scholar]
- 132.Tateishi U, Yamaguchi U, Maeda T, Seki K, Terauchi T, Kawai A, et al. Staging performance of carbon-11 choline positron emission tomography/computed tomography in patients with bone and soft tissue sarcoma: Comparison with conventional imaging. Cancer Sci. 2006;97:1125–1128. doi: 10.1111/j.1349-7006.2006.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [F-18]fluoromisonidazole: A pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36:417–428. doi: 10.1016/s0360-3016(96)00325-2. [DOI] [PubMed] [Google Scholar]
- 134.Rajendran JG, Krohn KA. Imaging hypoxia and angiogenesis in tumors. Radiol Clin North Am. 2005;43:169–187. doi: 10.1016/j.rcl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 135.Rajendran JG, Wilson DC, Conrad EU, Peterson LM, Bruckner JD, Rasey JS, et al. [F-18]FMISO and [F-18]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 136.Willaime JM, Turkheimer FE, Kenny LM, Aboagye EO. Quantification of intra-tumour cell proliferation heterogeneity using imaging descriptors of 18 F fluorothymidine-positron emission tomography. Phys Med Biol. 2013;58:187–203. doi: 10.1088/0031-9155/58/2/187. [DOI] [PubMed] [Google Scholar]
- 137.Salamon J, Derlin T, Bannas P, Busch JD, Herrmann J, Bockhorn M, et al. Evaluation of intratumoural heterogeneity on (1)(8)F-FDG PET/CT for characterization of peripheral nerve sheath tumours in neurofibromatosis type 1. Eur J Nucl Med Mol Imaging. 2013;40:685–692. doi: 10.1007/s00259-012-2314-6. [DOI] [PubMed] [Google Scholar]


