Abstract
Purpose
We evaluated whether the maximum standardized uptake values (SUVmax) of primary tumor from the initial staging by 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) of patients with breast cancer could identify patients at risk for early recurrence within 2 years, particularly in comparison to the American Joint Committee on Cancer (AJCC) stage.
Methods
We reviewed the staging 18F-FDG PET/CT images of patients with primary breast cancer and their medical records. The SUVmax of the primary tumor was measured. The presence or absence of FDG uptake in the axillary lymph node (ALN) was also assessed. The patient’s pathologic primary tumor stage (pT), pathologic regional lymph node stage (pN), stage grouping, age, estrogen receptor (ER) and progesterone receptor (PR) status, and neoadjuvant chemotherapy history were evaluated with the FDG uptake parameters for recurrence within 2 years following the end of first-line therapy.
Results
Recurrence within 2 years was present in 9.1 % (n = 40) out of the 441 patients assessed. The FDG uptake in ALN, pT, pN, stage grouping and neoadjuvant chemotherapy history were prognostic for early recurrence, while primary tumor SUVmax, age, and ER or PR status were not significant on logistic regression. On multivariate analysis, only the stage grouping (odds ratio 2.79; 95 % CI 1.73, 4.48; p < 0.0001) and neoadjuvant chemotherapy history (odds ratio 2.70; 95 % CI 1.22, 5.98; p = 0.0141) could identify patients at increased risk for recurrence within 2 years.
Conclusions
Primary tumor FDG uptake measured by SUVmax, and visual assessment of FDG uptake in the ALN in the initial staging PET/CT of patients with breast cancer may not have additional prognostic value compared with the AJCC stage grouping for early recurrence.
Keywords: 18F-FDG PET/CT, Breast cancer, Prognosis, Staging
Introduction
Reports have demonstrated the prognostic value of FDG uptake in various tumors, including head and neck cancer, lung cancer and cervical cancer [1–3]. Several papers have demonstrated the prognostic and predictive values of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in breast cancer as well [4–8]. Correlation between FDG uptake in breast tumor or axillary lymph node and known various clinical or pathologic markers of prognosis was noted in multiple other studies [9–12], but the data appear insufficient to form a conclusion on the association between FDG uptake and prognosis in breast cancer [13–15].
We evaluated the staging 18F-FDG PET/CT images of patients with breast cancer who underwent definitive surgical therapy to find out the prognostic value of FDG uptake for identifying those at risk for early recurrence within 2 years, especially in comparison to the pathologic stage.
Patients and Methods
The study retrospectively reviewed the 18F-FDG PET/CT images and medical charts of patients with breast cancer from a single center who underwent staging by 18F-FDG PET/CT from November 2003 to June 2009 prior to any treatment for breast cancer. Patients who were treated with intent to cure through definitive surgical therapy were included. The patient received either mastectomy or lumpectomy followed by adjuvant radiation therapy. Patients with non-operable stage IV (distant metastasis) at the time of initial diagnosis were excluded. Patients whose primary breast tumor was completely excised before the staging 18F-FDG PET/CT, for example through excision biopsy or mammotome, were also excluded. Patients enrolled in therapeutic intervention clinical trials were also excluded. Patients without regular follow-up examinations for at least 2 years after the end of first line therapy were excluded. Patients visited the hospital every 2–3 months for the first 2 years of follow-up. Routine examination included mammography and breast US every 6 months. Magnetic resonance imaging (MRI) and FDG PET/CT were not part of routine follow-up during the study period.
The date when recurrence was first detected was recorded. The hospital’s institutional review board approved the study.
18F-FDG PET/CT Image Acquisition
Patients were imaged with a dedicated PET/CT scanner with two-slice CT (Siemens Biograph Classic, Germany) before 2008, and with a PET/CT scanner with 40-slice CT (Siemens Biograph TruePoint, Germany) afterwards. The 18F-FDG uptake in primary breast tumor was quantified by maximum standardized uptake value (SUVmax) measurement. The tumors with no focal FDG uptake greater than the surrounding normal breast tissue on visual analysis were considered ‘not quantifiable’ for SUVmax. The FDG uptake in axillary lymph node station was considered positive when the 18F-FDG activity in ALN was greater than surrounding fat tissue by visual assessment. The FDG uptake in the ALN was recorded on patient basis, and not per nodal station. Two nuclear medicine physicians reviewed the images, and a third nuclear medicine physician reviewed the cases with discordant interpretation to reach a consensus.
Statistical Analysis
The patient’s age, tumor histology subtype (invasive ductal carcinoma, invasive lobular carcinoma, invasive carcinoma not otherwise specified, mucinous carcinoma and others), pathologic primary tumor stage (pT: in situ, T1, T2, T3 or T4), pathologic regional lymph node stage (pN: N0, N1, N2 or N3), stage grouping (I, II or III), estrogen receptor (ER) and progesterone receptor (PR) status, and history of neoadjuvant chemotherapy were analyzed with the FDG uptake parameters to see if the FDG uptake parameters could identify patients at risk of early recurrence within 2 years from the end of first-line therapy. Chi-square or Fisher exact test and t-test were done to compare the FDG uptake and clinicopathologic parameters with the recurrence positive and recurrence negative groups. Logistic regression was performed to see which parameters could identify the patients at higher risk for recurrence. Multiple logistic regression was done by backward selection to decide on the parameters with the greatest prognostic value. SAS system for Windows version 9.2 was used for statistical analysis.
Results
Of 529 included patients, 67 patients who did not have curative surgery and 21 patients with incomplete medical history were excluded. A total of 441 breast cancer patients who had surgery and received treatment with curative intent were evaluated. There were 55 patients who received neoadjuvant chemotherapy prior to surgery, and 98 patients who received adjuvant radiation therapy following surgery. Of the 441 patients assessed, 401(90.9 %) patients remained disease-free for at least 2 years from therapy. The other 40 (9.1 %) patients had recurrence within 2 years from the end of initial therapy. The patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Recurrence within 2 years | ||||
|---|---|---|---|---|
| Parameter | Total | (−) | (+) | p valuea |
| Number of patients | 441 | 401 | 40 | |
| Median age | 50.6 | 50.7 | 48.8 | 0.2210 |
| Pathologic T stage | ||||
| In situ/1 2 3 4 |
260 164 14 3 |
247 142 10 2 |
13 22 4 1 |
0.0012 |
| Pathologic N stage | ||||
| 0 1 2 3 |
285 84 50 22 |
274 69 42 16 |
11 15 8 6 |
<0.0001 |
| Stage grouping | ||||
| 0/I II III |
199 167 75 |
196 145 60 |
3 22 15 |
<0.0001 |
| Histology | ||||
| Invasive ductal carcinoma Invasive lobular carcinoma Invasive carcinoma NOS Mucinous carcinoma and others |
366 10 34 31 |
329 10 33 29 |
37 0 1 2 |
|
| Neoadjuvant therapy | ||||
| No Yes |
386 55 |
360 41 |
26 14 |
<0.0001 |
| ER | ||||
| − + |
153 288 |
134 267 |
19 21 |
0.070 |
| PR | ||||
| − + |
184 257 |
162 239 |
22 18 |
0.065 |
| Primary tumor SUVmax | ||||
| Focal FDG uptake (−) Focal FDG uptake (+) Mean SUVmax ± SD |
38 403 4.2 ± 3.1 |
38 363 4.1 ± 3.1 |
0 40 4.9 ± 3.0 |
0.1578 |
| Axillary LN FDG uptake | ||||
| Imperceptible Perceptible |
271 170 |
253 148 |
18 22 |
0.0250 |
aValues in italics are statistically significant
On PET/CT, 38 (8.6 %) of patients had 18F-FDG uptake in the primary tumor no greater than the physiologic breast parenchyma, and the tumor could not be clearly delineated in the PET images. The other 403 patients had mean SUVmax of 4.2 (range 0.7–22.6) in the primary tumor. And 170 (38.5 %) patients had visually perceptible 18F-FDG uptake in axillary lymph node (ALN) that could be delineated from the surrounding fat tissue.
Comparing the recurrence negative and recurrence positive groups, there was a significant difference between the two groups for the following parameters: pathologic T stage, pathologic N stage, stage grouping, neoadjuvant chemotherapy history, and FDG uptake in ALN. No difference was seen between the recurrence negative and positive groups for ER and PR status, age and SUVmax of the primary tumor.
On logistic regression, pathologic T stage, pathologic N stage, stage grouping, neoadjuvant chemotherapy history and FDG uptake in ALN were predictive of recurrence within 2 years (Table 2). However, on multivariate analysis, only the stage grouping and neoadjuvant chemotherapy history were significantly associated with early recurrence.
Table 2.
Statistical analysis
| Logistic regression | |||
|---|---|---|---|
| Parameter | Odds ratio | 95 % confidence limits | p valuea |
| T stageb | 2.11 | 1.21, 3.81 | 0.0078 |
| N stage | |||
| N1 vs N0 | 5.42 | 2.38, 12.31 | 0.2446 |
| N2 vs N0 | 4.75 | 1.80, 12.48 | 0.5714 |
| N3 vs N0 | 9.34 | 3.06, 28.50 | 0.0252 |
| Stage groupingb | 2.93 | 1.74, 5.13 | <0.0001 |
| Neoadjuvant chemotherapy done | 4.73 | 2.29, 9.77 | <0.0001 |
| FDG uptake present in ALN | 2.09 | 1.09, 4.02 | 0.0275 |
| Multiple logistic regression | |||
| Stage groupingb | 2.79 | 1.73, 4.48 | <0.0001 |
| Neoadjuvant chemotherapy done | 2.70 | 1.22, 5.98 | 0.0141 |
aValues in italics are statistically significant
bOdds ratio shown per one categorical unit increase to the next advanced stage
Patients who had surgery without neoadjuvant chemotherapy were assessed separately, and only the negative PR status (odds ratio 3.18; 95 % CI 1.35, 7.52; p = 0.0083) and stage grouping (odds ratio 3.05; 95 % CI 1.74, 5.34; p < 0.001) were predictive of early recurrence.
Discussion
In different cancers, the patients within the same TNM group have different prognosis depending on FDG uptake findings or quantification values. One prospective cohort study demonstrated FDG PET’s striking ability to stratify cervical cancer patients further within the same TNM stage group [16]. This is plausible as increased FDG uptake represents increased glucose metabolism, a hallmark of cancer cells. Even for two patients who share the same staging based on anatomical features, the disease entities at the molecular level could be different, and further stratification of the patient according to the biological behavior of the cancer cells seems reasonable.
For breast cancer, there have been studies that linked the FDG uptake in tumor with various known prognostic markers such as tumor size, histologic grade, hormone receptor status, Ki-67 index, Her2 expression, and nuclear grade [9–12]. Studies showed peak SUV in breast tumor correlating to the actual overall survival and disease-free survival [5] or computer program-derived simulated prognosis [8]. Another study with 44 patients suggested a new index of SUVmax/ADC (apparent diffusion coefficient) could predict worse prognosis [17]. Other studies showed FDG PET findings could identify patients at risk of recurrence with more accuracy than conventional TNM staging [7] or clinical tumor characteristics [18].
However, it is also known that FDG PET can have low overall sensitivity for breast cancer, especially in small tumors less than 1 cm in size or in situ cancers, and has been suggested for use in only a subset of patients. Neither can FDG PET replace surgical axillary lymph node staging due to the low sensitivity [19]. Though a number of studies showed that increased FDG uptake means unfavorable outcome, routine use of FDG PET as a prognostic biomarker is argued against [13, 20]. We wanted to find out how our institution’s data acquired in routine clinical practice compare with the previous reports either suggesting or discouraging the use of FDG PET as a prognostic marker.
Contrary to the reference mentioned above, in our study the SUVmax did not vary meaningfully between the recurrence positive and negative groups, and the higher SUVmax did not have statistically meaningful risk for poor prognosis (Fig. 1). However, this study has the limitation that we combined the SUVmax measurements acquired from two different PET/CT scanners for assessment. Though we made our best efforts to control other biological and technical factors that could influence the SUV measurement, difference in quantification would be inherent in this study population. Regarding the axillary LN, we did not include quantitative measurement of FDG uptake because we thought the very low SUV in many of the axillary LNs could not be accurately and reliably reproduced. We also did not measure all possible parameters from the FDG PET images. Documented parameters such as metabolic tumor volume (MTV) and tumor lesion glycolysis (TLG) were not included for analysis in this paper because the readers showed very high inter- and intra-reader variability. If measured after the optimal threshold and delineation method are defined in detail, MTV and TLG could provide more important clinical data.
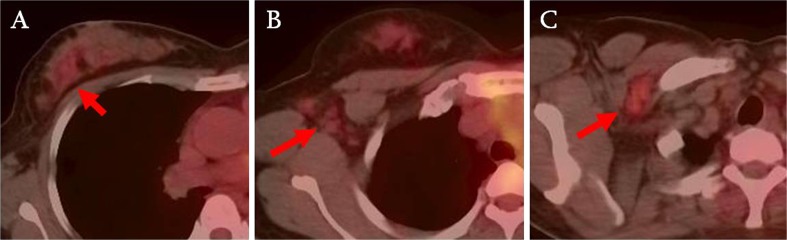
Fig. 1.
A 24-year-old patient was diagnosed invasive ductal carcinoma. The FDG uptake in breast cancer (a) and axillary lymph nodes (b) is faint and difficult to define. T2 primary tumor and 3 axillary lymph nodes with metastases were confirmed on surgery. The patient had follow-up PET/CT 14 months later, which showed newly developed moderate focal FDG uptake in right axilla (c), and biopsy confirmed metastatic carcinoma
As patients with bulkier tumors were candidates for neoadjuvant chemotherapy, we separately evaluated patients who had surgery without the neoadjuvant therapy to eliminate the possible bias. Similar results showed no further prognostic information provided by FDG PET/CT in addition to the stage grouping.
Current research on genetic and molecular markers of breast cancer may be able to accurately stratify breast cancer patients according to prognosis and allow fine tuned therapeutic planning [21, 22]. Our results suggest that the tumor SUVmax, which is the most commonly measured metric from the FDG PET/CT images, may not provide further prognostic value compared with the classic cancer stage grouping.
Conclusion
In patients with operable breast cancer, the SUVmax of primary tumor measured from staging 18F-FDG PET/CT may not have additional prognostic value beyond the classic TNM staging group.
Acknowledgments
Disclosure
The authors report nothing to disclose.
Contributor Information
Joo Hyun O, Phone: +82-2-22581547, Email: ojoohyun@gmail.com.
Sung Hoon Kim, Phone: +82-2-22581550, Email: sghnk@catholic.ac.kr.
References
- 1.Liao C, Chang JT, Wang H, Ng S, Hsueh C, Lee L, et al. Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys. 2009;73(3):764–771. doi: 10.1016/j.ijrobp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Casali C, Cucca M, Rossi G, Barbieri F, Iacuzio L, Bagni B, et al. The variation of prognostic significance of Maximum Standardized Uptake Value of [18F]-fluoro-2-deoxy-glucose positron emission tomography in different histological subtypes and pathological stages of surgically resected Non-Small Cell Lung Carcinoma. Lung Cancer. 2010;69(2):187–193. doi: 10.1016/j.lungcan.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer. 2010;116(6):1469–1475. doi: 10.1002/cncr.24972. [DOI] [PubMed] [Google Scholar]
- 4.Song BI, Lee SW, Jeong SY, Chae YS, Lee WK, Ahn BC, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med. 2012;53(9):1337–1344. doi: 10.2967/jnumed.111.098640. [DOI] [PubMed] [Google Scholar]
- 5.Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998;82(11):2227–2234. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2227::AID-CNCR18>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Alberini J, Lerebours F, Wartski M, Fourme E, Le Stanc E, Gontier E, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer. 2009;115(21):5038–5047. doi: 10.1002/cncr.24534. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [(18)F]2-Deoxy-2-fluoro-D-glucose-positron emission tomography. J Cancer Res Clin Oncol. 2004;130(5):273–278. doi: 10.1007/s00432-003-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38(4):250–258. doi: 10.1093/jjco/hyn019. [DOI] [PubMed] [Google Scholar]
- 9.Koolen BB, Vrancken Peeters MJ, Wesseling J, Lips EH, Vogel WV, Aukema TS, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012. [DOI] [PubMed]
- 10.Gil Rendo A, Martnez-Regueira F, Zornoza G, Garca-Velloso MJ, Beorlegui C, Rodriguez Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96(2):166–170. doi: 10.1002/bjs.6459. [DOI] [PubMed] [Google Scholar]
- 11.Groheux D, Giacchetti S, Moretti J, Porcher R, Espi M, Lehmann Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38(3):426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 12.Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15(6):588–593. doi: 10.1007/s10147-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 13.Hodgson NC, Gulenchyn KY. Is there a role for positron emission tomography in breast cancer staging? J Clin Oncol. 2008;26(5):712–720. doi: 10.1200/JCO.2007.13.8412. [DOI] [PubMed] [Google Scholar]
- 14.Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M. The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast cancer. 2007;14(3):260–268. doi: 10.2325/jbcs.14.260. [DOI] [PubMed] [Google Scholar]
- 15.Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S80–S87. doi: 10.1007/s00259-004-1529-6. [DOI] [PubMed] [Google Scholar]
- 16.Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010;28(12):2108–2113. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
- 17.Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37(11):2011–2020. doi: 10.1007/s00259-010-1529-7. [DOI] [PubMed] [Google Scholar]
- 18.Dunnwald LK, Gralow JR, Ellis GK, Livingston RB, Linden HM, Specht JM, et al. Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 2008;26(27):4449–4457. doi: 10.1200/JCO.2007.15.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahl RL, Siegel BA, Coleman RE, Gatsonis CG, Group PETS. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22(2):277–285. doi: 10.1200/JCO.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer N NCCN guideline update: breast cancer version 1.2004. J Natl Compr Cancer Netw JNCCN. 2004;2(3):183–184. [PubMed] [Google Scholar]
- 21.Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378(9805):1812–1823. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 22.Dedeurwaerder S, Fumagalli D, Fuks F. Unravelling the epigenomic dimension of breast cancers. Curr Opin Oncol. 2011;23(6):559–565. doi: 10.1097/CCO.0b013e32834bd481. [DOI] [PubMed] [Google Scholar]