Abstract
[18F]FDG (fluorine-18 fluoro-2-deoxy-D-glucose) positron emission tomography (PET) is used worldwide for oncologic and neurologic applications. To date, the potential harm caused by [18F]FDG has focused on its radiation exposure effects rather than on its pharmacological effects. While an allergic response in the form of a skin manifestation has been reported after exposure to [18F]FDG, this report describes the first case of hypotension following exposure to this tracer. Here, the development of anaphylaxis after [18F]FDG injection is described.
Keywords: FDG, Anaphylaxis, Allergic
Introduction
[18F]FDG (fluorine-18 fluoro-2-deoxy-D-glucose) is a positron-emitting radiopharmaceutical used in conjunction with positron emission tomography (PET), such as in the diagnosis and treatment monitoring of patients with cancer or neurologic disease. The potential harm caused by [18F]FDG has mainly focused on the damage its radiation causes–not on its potential adverse pharmacologic side effects, which are considered unlikely due to the very small amount of tracer administered [1]. However, recently, Codreanu et al. [2] reported the first case of an allergic reaction caused by [18F]FDG. In this report, we present a case in which a patient suffered a more severe allergic reaction (i.e., anaphylaxis) after injection of [18F]FDG in a PET study.
Case Report
Our local institutional review board (IRB) exempted this case from the informed consent requirement and approved the use of the patient’s medical history and images, as personal information has not been revealed.
A 61-year-old Asian woman with a history of breast-conserving surgery 2 years earlier due to invasive ductal carcinoma of the right breast (T1N1M0) underwent [18F]FDG PET/CT for cancer surveillance. It was her first [18F]FDG PET/CT scan. She had received adjuvant chemotherapy consisting of four cycles of doxorubicin and cyclophosphamide, followed by four cycles of docetaxel. Since then, she had been taking letrozole as hormonal therapy and calcium citrate as a supplement. Her previous history was limited to an appendectomy and right herniorrhaphy 20 years ago. Her most recent blood tests, including a chemistry panel and complete blood count (CBC) blood test, were normal. She had no history of allergy.
Prior to [18F]FDG PET imaging, the patient fasted for 15 h. Twenty-five minutes before radiotracer injection, she was given mebeverin (135 mg, oral tablet) to decrease intestinal [18F]FDG uptake and furosemide (40 mg, oral tablet) to accelerate renal [18F]FDG excretion. Her blood glucose level was 102 mg/dl just before [18F]FDG injection. Minutes after the injection of 12 mCi (444 MBq) of [18F]FDG intravenously into her left hand, she reported itching and exhibited erythematous changes over her whole body, but both were tolerable. Approximately 30 min after the injection, she contacted the medical staff, complaining of progressive itching, erythema, and abdominal pain. Suspecting an allergic reaction, we intravenously injected 2 mg pheniramine and started a 500-ml infusion of normal saline. The patient was alert, but her mental status was drowsy. Her blood pressure (BP) was 70/50 mmHg, but other vital signs were unremarkable. Respiratory distress was not definite and oxygen saturation was 95–100 %, as measured by pulse oximetry. The patient was placed in Trendelenburg’s position and the rate of fluid infusion was accelerated. Ten minutes later, her follow-up BP was 95/70 mmHg, but she still complained of a migrating itching sensation, especially around her ears and legs. We therefore administered 100 mg intravenous steroid mixed with 100 ml normal saline. Twenty minutes later, these symptoms were on the wane but chills and a head tremor newly developed. The patient was covered with a warm blanket without further medication. While the latter symptoms did not resolve, neither was considered indicative of an anaphylactic reaction and the patient therefore underwent PET imaging 105 min after [18F]FDG injection. The duration of the [18F]FDG PET/CT scan was around 15 min, including the CT transmission scan. The patient’s BP immediately after completion of the PET/CT scan was 120/80 mmHg.
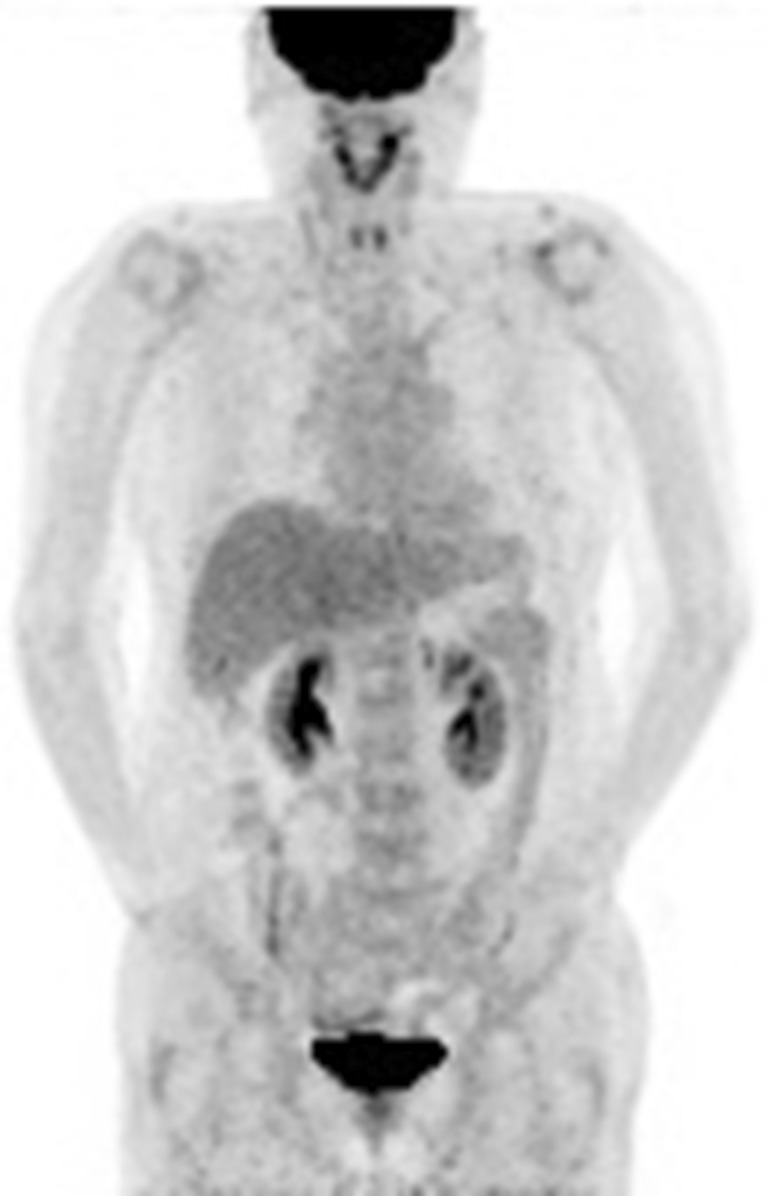
Figure 1 shows the maximal intensity projection image obtained from the [18F]FDG PET scan of this patient. The scanned field shows no abnormal foci of increased [18F]FDG suggestive of local recurrence or distant metastasis. In addition, there was no radiotracer uptake in the skin or in the head and neck muscles, as would have been expected, respectively, from the transient skin erythema and the abnormal head and neck movements after [18F]FDG injection.
Fig. 1.
[18F]FDG PET scan performed after resolution of the anaphylaxis. Tracer uptake is physiologic, with no evidence of abnormalities
One hour after the [18F]FDG PET scan, the patient’s head tremor gradually subsided and she no longer complained of chills. She returned home safely without any medication. Nevertheless, immediately after this event, we examined the [18F]FDG quality control tests and performed a post hoc analysis for endotoxin. Both were unremarkable. Furthermore, among the other 53 patients injected with the same batch of [18F]FDG, none developed similar symptoms. We therefore concluded that this patient had experienced an unexpected adverse reaction of anaphylaxis, probably as an allergic response to the [18F]FDG solution.
Discussion
Allergic responses to radiopharmaceuticals have mainly been reported for 99mTc-labeled agents such as 99mTc-MDP (methylene diphosphonate) [3], 99mTc-nanocolloidal albumin [4], and 99mTc-labeled sestamibi [5, 6]. Allergenic reactions induced by [18F]FDG are very rare, as to the best of our knowledge there has only been one previous case report, by Codreanu et al. [2], of a skin manifestation induced by [18F]FDG. However, there are differences between their case and our case. Firstly, our patient experienced severe hypotension in addition to erythema involving her entire body and itching, which was the only manifestation described in the previous report. Secondly, our patient had no previous allergy history, whereas in the case of Codreanu et al. [2], the patient had a history of allergy to several drugs.
Anaphylaxis is a severe immediate-type generalized hypersensitivity reaction affecting multiple organ systems [7]. Although a consensus definition of anaphylaxis is still lacking [8], it is generally considered to consist of the following [9]: (1) acute onset of an illness involving the skin and/or mucosal tissue with at least one of the following: (a) respiratory compromise and (b) reduced blood pressure or associated symptoms of end-organ dysfunction; (2) two or more of the following rapidly occurring symptoms after the patient’s exposure to a likely allergen: involvement of the skin-mucosal tissue, respiratory compromise, reduced BP or associated symptoms, and persistent gastrointestinal symptoms; (3) reduced BP after exposure to a known allergen; for infants and children, a low systolic BP (age specific) or a >30 % decrease in systolic BP; for adults, a systolic BP of 90 mmHg. Anaphylaxis was diagnosed in our patient based on the skin involvement (erythema and itching sensation) and hypotension (70/50 mmHg). The probability of an allergic reaction was subsequently determined using the Naranjo nomogram for adverse drug reaction assessment [10]. Her total score was 6, thus classifying this case as ‘probable’ among the categories ‘definite’, ‘probable’, ‘possible’, and ‘doubtful’.
Anaphylaxis induced by a radiopharmaceutical has been reported for [131I]iodohippurate sodium, 99mTc-albumin colloid, 99mTc-exametazime, 99mTc-macroaggregated albumin, 99mTc-medronate, 99mTc-pentetate, 99mTc-sodium pertechnetate, and 99mTc-sulfur colloid [11]; however, the present report is the first to describe an anaphylactic reaction to [18F]FDG.
It is unlikely that anaphylaxis in our patient was caused by an inappropriate [18F]FDG preparation. In our institution, [18F] fluoride is produced in a PETtrace 10 cyclotron (GE Healthcare) from [18O] water, and [18F]FDG by nucleophilic substitution using the Fastlab module (GE Healthcare). After [18F]FDG production, quality control is performed according to the standards of the Korea Pharmaceutical Codex. The radiochemistry department of our institution has never had a significant problem in terms of radiopharmaceutical production and all of its products have passed quality control inspections. Furthermore, the other 53 patients who underwent [18F]FDG PET/CT on the same day expressed no complaints after being injected with [18F]FDG obtained from the same chemistry module. Non-tracer-related impurities, including phase-transfer catalyst, residual solvent, or any unreacted 18F-fluoride, must be removed during radiotracer production [12]. Allergy to another radionuclide is unlikely given the very high (>99.99 %) radionuclide purity of the [18F]FDG solution [13]. 2-Deoxy-2-chloro-D-glucose (ClDG) may arise from hydrolysis with HCl, but in our radiochemistry department Cl- is routinely removed on QMA using a K2CO3 solution before the synthesis and hydrolysis is carried out with NaOH. Although there was a possibility of 2-[18F]fluoro-2-deoxy-mannose (FDM) formation as an impurity because of the hydrolysis under basic conditions, this was unlikely [14]. Therefore, FDM or ClDG as the cause of the anaphylaxis can be ruled out. Nonetheless, there is still probability of presence of other allergen that we have not considered even though very little.
At the time of the study, our patient was being treated with letrozole and calcium citrate, both of which can cause an anaphylactic reaction, as noted by the respective manufacturers. However, these drugs were prescribed 2 years earlier, and the patient had not experienced any prior adverse events related to this regimen. The two drugs used as premedication, furosemide and mebeverine, can cause a skin rash or itching according to their manufacturers, and indeed there is one case report of anaphylaxis caused by oral furosemide [15]. Unfortunately, our patient did not undergo an allergen skin test, which could have identified the anaphylactic agent. However, considering that she had no complaints prior to the [18F]FDG injection and that anaphylaxis developed immediately afterwards, its onset was likely caused by [18F]FDG and not by premedications. However, a delayed adverse reaction to the oral agents cannot be completely excluded. Another limitation of this case report is the lack of laboratory tests such as those for serum histamine and tryptase levels. Although anaphylaxis is a clinical condition, an elevation of these two markers in the acute setting of anaphylaxis could help to confirm the diagnosis [16].
In conclusion, we present a case of anaphylaxis that most likely occurred in response to the injected [18F]FDG solution. Our study showed that [18F]FDG allergy is possible in a patient with no history of allergy. It is therefore important that physicians be aware of the possibility of an anaphylactic reaction to [18F]FDG, although the risk is minimal. Nonetheless, we recommend that PET centers be prepared to treat anaphylaxis.
Acknowledgments
Disclosures
All authors have no conflicts of interest or financial ties to disclose.
References
- 1.Silberstein EB. Prevalence of adverse reactions to positron emitting radiopharmaceuticals in nuclear medicine. Pharmacopeia Committee of the Society of Nuclear Medicine. J Nucl Med. 1998;39(12):2190–2192. [PubMed] [Google Scholar]
- 2.Codreanu I, Dasanu CA, Weinstein GS, Divgi C. Fluorodeoxyglucose-induced allergic reaction: A case report. J Oncol Pharm Pract. 2013;19(1):86–88. doi: 10.1177/1078155211436023. [DOI] [PubMed] [Google Scholar]
- 3.Balan KK, Choudhary AK, Balan A, Wishart G. Severe systemic reaction to (99 m)Tc-methylene diphosphonate: a case report. J Nucl Med Technol. 2003;31(2):76–78. [PubMed] [Google Scholar]
- 4.Chicken DW, Mansouri R, Ell PJ, Keshtgar MR. Allergy to technetium-labelled nanocolloidal albumin for sentinel node identification. Ann R Coll Surg Engl. 2007;89(2):W12–W13. doi: 10.1308/147870807X160443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mujtaba B, Adenaike M, Yaganti V, Mujtaba N, Jain D. Anaphylactic reaction to Tc-99 m sestamibi (Cardiolite) during pharmacologic myocardial perfusion imaging. J Nucl Cardiol. 2007;14(2):256–258. doi: 10.1016/j.nuclcard.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Thomson LE, Allman KC. Erythema multiforme reaction to sestamibi. J Nucl Med. 2001;42(3):534. [PubMed] [Google Scholar]
- 7.Brown SG, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Med J Aust. 2006;185(5):283–289. doi: 10.5694/j.1326-5377.2006.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman P. Epidemiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):316–320. doi: 10.1097/ACI.0b013e3283036a69. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Vadas P. Anaphylaxis: mechanisms and management. Clin Exp Allergy. 2011;41(7):923–938. doi: 10.1111/j.1365-2222.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- 10.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 11.Silberstein EB, Ryan J. Prevalence of adverse reactions in nuclear medicine. Pharmacopeia Committee of the Society of Nuclear Medicine. J Nucl Med. 1996;37(1):185–192. [PubMed] [Google Scholar]
- 12.Hjelstuen OK, Svadberg A, Olberg DE, Rosser M. Standardization of fluorine-18 manufacturing processes: new scientific challenges for PET. Eur J Pharm Biopharm. 2011;78(3):307–313. doi: 10.1016/j.ejpb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Marengo M, Lodi F, Magi S, Cicoria G, Pancaldi D, Boschi S. Assessment of radionuclidic impurities in 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) routine production. Appl Radiat Isot. 2008;66(3):295–302. doi: 10.1016/j.apradiso.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Mosdzianowsk C, Lemaire C, Simoens F, Aerts J, Morelle JL, Luxen A. Epimerization study on [18f]FDG produced by an alkaline hydrolysis on solid support under stringent conditions. Appl Radiat Isot. 2002;56(6):871–875. doi: 10.1016/S0969-8043(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez-Ortega J, Martinez-Alonso JC, Dominguez-Ortega C, Fuentes MJ, Frades A, Fernandez-Colino T. Anaphylaxis to oral furosemide. Allergol Immunopathol (Madr) 2003;31(6):345–347. doi: 10.1016/s0301-0546(03)79210-6. [DOI] [PubMed] [Google Scholar]
- 16.Arnold JJ, Williams PM. Anaphylaxis: recognition and management. Am Fam Physician. 2011;84(10):1111–1118. [PubMed] [Google Scholar]