Abstract
Purpose
11C-Methionine PET/CT (Met-PET/CT) is a useful imaging method for detection of parathyroid adenoma; however, the reported detection rate has been variable. The current study was intended to investigate detection sensitivity and preoperative localization of parathyroid adenoma (PA) or parathyroid hyperplasia (PH) on Met-PET/CT compared with 99mTc-sestamibi (MIBI) scintigraphy in patients with primary hyperparathyroidism (HPT) or suspected PA.
Methods
Met-PET/CT and MIBI scintigraphy images were reviewed by two nuclear medicine physicians unaware of pathologic results. Detection sensitivities and preoperative localization of detected parathyroid tissues into five predefined segments were evaluated by visual assessment and semi-quantitative analysis with ratio of standardized uptake values (SUVR) between parathyroid tissue and normal lung as reference. Linear regression analysis with SUVR and serum parathyroid hormone (sPTH) was performed for characterization of PA or PH. Predicted PTH (pPTH) was calculated and compared with sPTH in PH and PA. Each pPTH was obtained for a calculated SUVR by using linear regression model from the result of previous linear regression analysis between SUVR and sPTH.
Results
In 16 patients, detection sensitivities of Met-PET/CT and MIBI scintigraphy were 91.7 % (11/12) and 41.7 % (5/12) for PA and PH including both biopsy-confirmed and clinically-suspected cases, and 100 % (8/8) and 50 % (4/8) for pathologically confirmed PA and PH cases only, respectively. Met-PET/CT showed higher performance than MIBI scintigraphy in localization of parathyroid tissues; correct localization rate was 87.5 % (7/8) on Met-PET/CT and 50 % (4/8) on MIBI scintigraphy. In semi-quantitative analysis, SUVR was linearly associated with sPTH by linear regression analysis (sPTH = 39.53 × SUVR − 89.84, p = 0.0383). There was a borderline significant difference in pPTH between PH and PA (35.1 vs 204.7 ± 164.0, p = 0.052), while there was no significant difference in sPTH between PH and PA (289 vs 230.4 ± 160.4, p = 0.305).
Conclusions
Met-PET/CT has a potential to be a useful diagnostic modality for preoperative detection and localization of parathyroid tissues with higher sensitivity than MIBI scintigraphy, and for characterization of PA or PH.
Keywords: Primary hyperparathyroidism, Parathyroid adenoma, Parathyroid hyperplasia, 11C-methionine PET/CT, 99mTc-sestamibi scintigraphy, Preoperative localization
Introduction
Primary hyperparathyroidism (HPT), which results in hypercalcemia and hypophosphatemia, is caused by parathyroid adenoma (PA) in 82–87 %, by parathyroid hyperplasia (PH) in 10–15 %, and by parathyroid carcinoma in 2–3 % of patients [1, 2]. Complete surgical removal is the treatment of choice in management of persistent HPT caused by PA or PH. Preoperative localization of suspected parathyroid tissues is crucial for minimally invasive surgical removal, which can increase success rate of surgical treatment, reduce inconvenience of patient during or after operation, and also bring postoperative benefit in aesthetic aspect [3].
Current imaging techniques such as 99mTc-sestamibi (MIBI) or 99mTc-tetrofosmin scintigraphy with single photon emission computed tomography (SPECT) as well as ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI) are not always successful in the localization of parathyroid tissues [4, 5]. Recently, it was reported that MIBI scintigraphy is useful to differentiate benign from malignant parathyroid lesions as well as to detect parathyroid lesions [6]. However, the reported detection sensitivities for MIBI and 99mTc-tetrofosmin scintigraphy were 73–88 % and 95 %, respectively [7–9]. In the case of morphological imaging, such as USG, CT and MRI, the sensitivities were 61–78 %, 46–87 %, and 65–88 %, respectively. In addition, they are less effective when there is a multiglandular disease or an ectopic parathyroid gland [10].
11C-Methionine positron emission tomography/computed tomography (Met-PET/CT) has been increasingly used in oncologic imaging as a complementary method to18F-FDG PET/CT that is limited in the differentiation of a malignant lesion from coexisting peritumoral or post-therapeutic inflammatory change [11–13]. In particular, it has been known that methionine is one of amino acids comprising parathyroid hormone and that it is closely related with biosynthesis of parathyroid hormone [14, 15]. Therefore, Met-PET/CT was introduced and expected to have high detection performance of parathyroid tissues due to specific accumulation of 11C-methionine and high resolution of PET/CT. However, it has been reported that sensitivity of Met-PET/CT in detection of PA is variably 44–90 % [16–20]. Especially, a recent meta-analysis revealed that detection sensitivity and detection rate of Met-PET/CT were 0.81 and 0.70, respectively [18]. As Met-PET/CT being more frequently used, it is necessary to compare detection rate between Met-PET/CT and conventional MIBI scintigraphy to verify their clinical feasibility for surgical treatment.
Here, we tried to investigate the sensitivities of detection and localization of PA as preoperative guidance for minimally invasive surgical removal using Met-PET/CT, compared with conventional MIBI scintigraphy in patients with primary HPT or suspected PA.
Materials and Methods
Patients
From January 1st, 2006 to June 30th, 2011, 18 patients who underwent Met-PET/CT and MIBI scintigraphy for diagnosis of PA or PH were initially enrolled, retrospectively. In most patients with hyperparathyroidism, MIBI scintigraphy was performed initially and was repeated for follow-up to detect and monitor parathyroid tissues. Met-PET/CT was added during follow-ups in case of negative MIBI scintigraphy accompanied with positive or indeterminate anatomical imaging result of USG or neck CT.
Inclusion criteria were primary HPT or at least one PA suspected by other imaging studies such as USG or CT and preoperative imaging studies including Met-PET/CT and MIBI scintigraphy. The exclusion criterion was a time difference longer than 12 months between Met-PET/CT and surgical removal in a patient. Preoperative biochemical findings in all patients were checked for HPT including serum parathyroid hormone (sPTH), serum calcium, and serum phosphorus level. HPT was defined when sPTH >65 ng/ml. Persistent HPT over 6 months without any treatment was considered as clinically-suspected PA or PH even though surgical removal was not performed. The current study was approved by the Institutional Review Board of our hospital (H-0907-076-287).
Acquisition of Images
For each MIBI scintigraphy, a dual head digital gamma camera with LEHR collimator (Forte; Philips Healthcare, Andover, MA, USA) was used. Dual time-point acquisitions were performed on the anterior neck at 15 min and 150 min after intravenous injection of an average of 740 MBq (20 mCi) of MIBI. Dedicated digital cameras with LEHR collimator and matrix size of 256 × 256 were used, and the energy window was 140 ± 21 keV.
For each Met-PET/CT scan, single time PET acquisition with one bed including neck and both upper lungs for 10 min was done 30–40 min after intravenous injection of 370–444 MBq (10–12 mCi) of 11C-methionine, followed by CT scan for attenuation correction. All PET/CT scans were performed by dedicated PET/CT systems (Gemini [Philips Healthcare, Andover, MA, USA] for 7 cases and Biograph mCT [Siemens Healthcare, Erlangen, Germany] for 9 cases).
Image Analysis
In visual analysis of MIBI scintigraphy, a definitely increased or sustained uptake on the 150-min delayed image, compared with the early 15-min image, was considered as a positive finding for a parathyroid tissue. In case of Met-PET/CT, discernibly increased methionine uptake on PET image was considered as positive and its location was determined based on fused Met-PET/CT image.
For visual comparison of localization, five segments as possible locations of parathyroid tissues were intentionally defined as depicted in Fig. 1, including right upper, right lower, left upper, left lower quadrants, and paratracheal area of mediastinum. All Met-PET/CT and MIBI scintigraphy images were reviewed by two nuclear medicine physicians with a consensus, who were blinded to pathologic results. Postoperative pathologic results were used as the “gold standard” for absolute location of parathyroid tissues. Localizations of parathyroid tissues by Met-PET/CT and MIBI scintigraphy were compared with those by pathologic examination in the predefined five segments. Pathology and location of suspected parathyroid tissue were examined and confirmed by experienced pathologists on each postoperative specimen.
Fig. 1.
Predefined location of parathyroid tissues. Perithyroidal space is divided into four quadrants and paratracheal space is defined as the fifth location
For semi-quantitative analysis, the ratio of the standardized uptake value (SUVR) was calculated in each parathyroid tissue lesion on Met-PET/CT images as follows:
 |
SUVmax in parathyroid tissue was obtained by taking the maximum value of SUVmax in a region of interest (ROI) drawn on each of all the axial slices covering a suspected parathyroid tissue. SUVmean in normal bilateral lungs was calculated by averaging SUVmean values from ellipsoid ROIs on three different axial slices in bilateral normal lungs. Each ellipsoid ROI did not contain any abnormal lung lesion such as pneumonia, bronchiectasis, emphysema, consolidation and other inflammatory conditions, and it was checked based on the corresponding CT image under the lung setting.
After SUVR was obtained, linear correlation between any two variables of SUVR, sPTH and size of postoperative parathyroid tissue was carried out and then linear regression analysis was performed for further characterization of two variables with linear correlation. For differentiation of PH from PA, predicted PTH (pPTH) was calculated and compared with sPTH in PH and PA. Each pPTH was obtained for a calculated SUVR by using the linear regression model from the result of the previous linear regression analysis between SUVR and sPTH.
Statistical Analysis
Spearman correlation analysis and linear regression analysis were used for linearity of two variables and one sample t-test in sPTH and pPTH was tried to figure out if PH has the same characteristic of 11C-methionine uptake as PA.
PASW Statistics 18 release 18.0.0 (WinWrap Basic; IBM, New York, NY, USA) and GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA, USA) were used for analysis and generation of graph, and p < 0.05 was considered as statistically significant.
Results
Patients’ Characteristics
A total of 18 patients with primary HPT or with suspicious abnormal finding suggesting PA on neck USG and/or neck CT were initially enrolled. Two patients were excluded due to long time difference between Met-PET/CT and operation (74 and 28 months). Finally, 16 patients (age, 58.4 ± 10.0; male:female, 8:8) remained for analysis. However, only 8 patients underwent surgical removal and the others were medically followed up without operation. All eight patients were pathologically confirmed as positive parathyroid tissues: seven were PAs and one was PH. All sPTH levels decreased after surgical removal of suspected parathyroid tissues. Of eight patients without operation, four patients were categorized into clinically suspected PA or PH based on persistent HPT for over 6 months. Therefore, total 12 patients were finally considered as PA or PH and four patients were considered as negative of PA or PH. Patients’ characteristics are summarized in Table 1.
Table 1.
Basic characteristics of patients
| Patient no. | Sex | Age | sPTH (ng/ml) | Ca (mg/dl) | P (mg/dl) | USG | CT | MIBI | Met-PET/CT | Operation | Pathology | Final diagnosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop. | Postop. | ||||||||||||
| 1 | F | 45 | 270 | 8.6 | 1.5 | FP | FP | FP | TN | ND | ND | N | |
| 2 | F | 54 | 289 | 8 | 11.3 | 3.4 | TP | TP | FN | TP | DO | PH | P |
| 3 | F | 58 | 291 | 102 | 12.6 | 2.5 | ND | FN | TP | TP | DO | PA | P |
| 4 | M | 68 | 304 | <5 | 11.4 | 2.3 | TP | TP | TP | TP | DO | PA | P |
| 5 | F | 52 | 251 | 11.3 | 3.1 | TP | TP | FN | FN | ND | ND | P | |
| 6 | M | 57 | 73 | 11.5 | 2.2 | FP | ND | TN | TN | ND | ND | N | |
| 7 | F | 52 | 206 | 12.1 | 2.9 | FN | TP | TP | TP | ND | ND | P | |
| 8 | M | 79 | 83 | 5 | 10.6 | 1.1 | TP | TP | TP | TP | DO | PA | P |
| 9 | F | 55 | 65 | 10.8 | 2.8 | TN | ND | TN | TN | ND | ND | N | |
| 10 | F | 74 | 122 | 33 | 13.8 | 2.7 | TP | TP | TP | TP | DO | PA | P |
| 11 | M | 61 | 37 | 10.9 | 2.5 | FP | ND | TN | TN | ND | ND | N | |
| 12 | M | 59 | 142 | 11.4 | 2.2 | TP | ND | FN | TP | ND | ND | P | |
| 13 | F | 47 | 594 | 12.0 | 2.6 | TP | TP | FN | TP | ND | ND | P | |
| 14 | M | 68 | ND | 34 | 7.8 | 4.0 | TP | TP | FN | TP | DO | PA | P |
| 15 | M | 61 | 81 | 10.8 | 2.1 | TP | TP | FN | TP | DO | PA | P | |
| 16 | M | 44 | 61,600a | <5 | 11.1 | 1.8 | TP | TP | FN | TP | DO | PA | P |
P positive, N negative, TP true positive, TN true negative, FP false positive, FN false negative, NC not checked due to no pathologic result, DO done, ND not done
aPTH from aspirate of PA
Detection and Localization of Parathyroid Tissues
Among all the included patients with primary or suspected PA, 11 of 16 (68.8 %) were positive on Met-PET/CT, whereas 6 of 16 (37.5 %) were positive on MIBI scintigraphy. The sensitivity and specificity of Met-PET/CT were 91.7 % (11/12) and 100.0 % (4/4), and those of MIBI scintigraphy were 41.7 % (5/12) and 75.0 % (3/4), respectively, for those patients with final diagnosis of both biopsy-confirmed and clinically suspected PA or PH (Table 2). When considering the pathologic results only, on the other hand, the sensitivities of Met-PET/CT and MIBI scintigraphy were 100 % (8/8) and 50 % (4/8), respectively. Specificity cannot be evaluated because there was no biopsy-proven negative PA or PH. Figures 2 and 3 depict representative cases of positive Met-PET/CT and positive MIBI, and positive Met-PET/CT and negative MIBI, respectively.
Table 2.
Summary of diagnosis in different methodologies
| Pathologic diagnosis | Final diagnosis | ||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Met-PET/CT | Positive | 8 | 0 | 11 | 0 |
| Negative | 0 | 0 | 1 | 4 | |
| MIBI scintigraphy | Positive | 4 | 0 | 5 | 1 |
| Negative | 4 | 0 | 7 | 3 | |
Fig. 2.
Representative case of parathyroid adenoma with positive 99mTc-sestamibi scintigraphy and positive 11C-methionine PET/CT. Parathyroid adenoma is visualized on scintigraphic images at 15 min (a) and 2 h 30 min (b) after intravenous injection of 99mTc-sestamibi, and fused axial PET/CT image (c) and axial PET image (d) after intravenous injection of 11C-methioinine. The arrow indicates suspicious parathyroid tissue in the right lower quadrant of thyroid gland
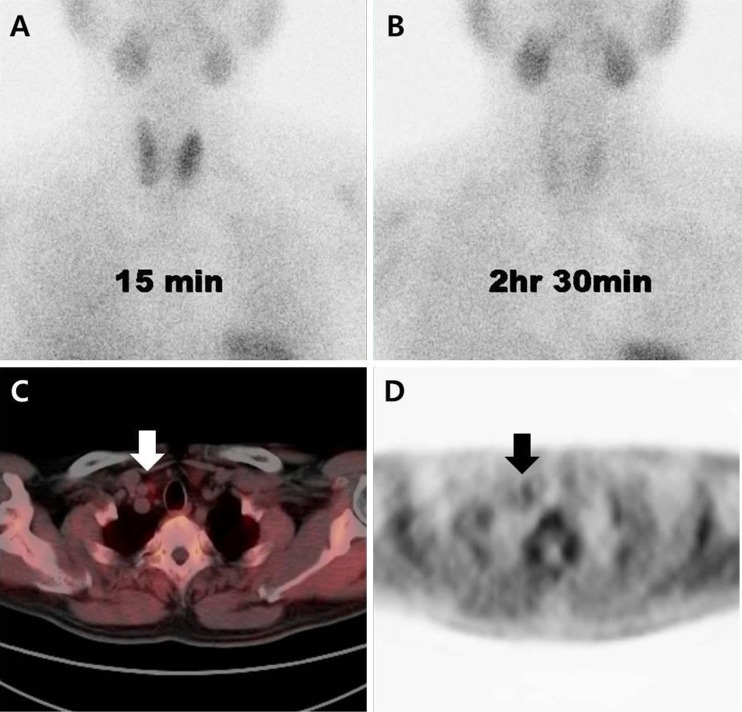
Fig. 3.
Representative case of parathyroid adenoma with negative 99mTc-sestamibi scintigraphy and positive 11C-methionine PET/CT. Scintigraphic images at 15 min (a) and 2 h 30 min (b) after intravenous injection of 99mTc-sestamibi, and fused axial PET/CT image (c) and axial PET image (d) after intravenous injection of 11C-Methioinine. The arrow indicates suspicious parathyroid tissue in the right upper quadrant of thyroid gland
In preoperative localization of parathyroid tissues, all locations of parathyroid lesions detected by either Met-PET/CT or MIBI scintigraphy were consistent with postoperative pathologic results. The correct localization rate was 87.5 % (seven of eight) on Met-PET/CT and 50 % (four of eight) on MIBI scintigraphy. In case of PH, Met-PET/CT detected only one lesion at the right lower quadrant and MIBI scintigraphy failed to detect any lesion, even though multiple lesions at both lower quadrants were confirmed by pathologic examination (Table 3).
Table 3.
Comparison of localization of parathyroid tissues among methodologies
| Patient no. | Preoperative | Postoperative | Pathology | |
|---|---|---|---|---|
| Met-PET/CT | MIBI | |||
| 2 | LL | UD | RL & LL | PH |
| 3 | PT | PT | PT | PA |
| 4 | RU | RU | RU | PA |
| 8 | RU | RU | RU | PA |
| 10 | RL | RL | RL | PA |
| 14 | RL | UD | RL | PA |
| 15 | RL | UD | RL | PA |
| 16 | RU | UD | RU | PA |
UD undetected, RU right upper, RL right lower, LU left upper, LL left lower, PT paratracheal, PH parathyroid hyperplasia, PA parathyroid adenoma
Semi-Quantitative Analysis
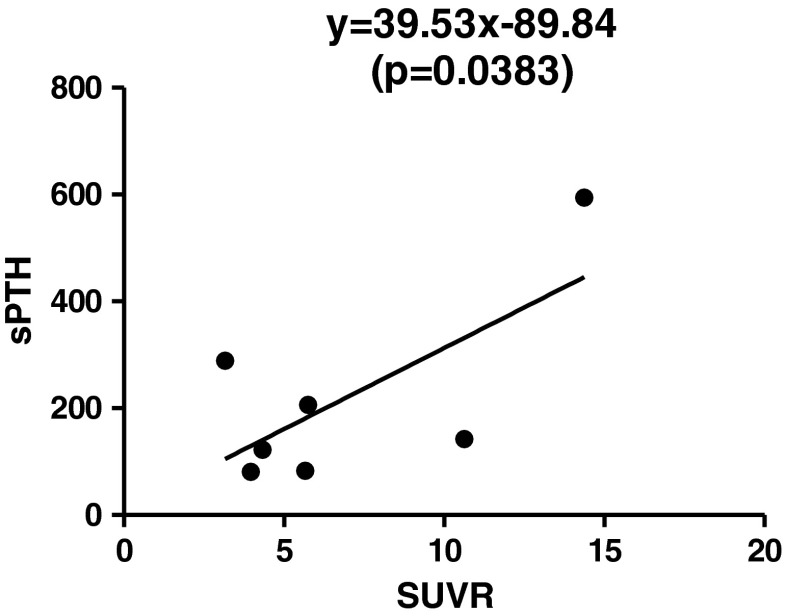
In semi-quantitative analysis, SUVR of PA showed statistically significant linear correlation with sPTH (Spearman correlation coefficient = 0.886, p = 0.019), but there was no significant linear association between size of parathyroid tissue and either sPTH or SUVR (Tables 4 and 5). By linear regression analysis, a linear regression model, sPTH = 39.53 × SUVR − 89.84 was deduced (Table 5, Fig. 4).
Table 4.
Size of parathyroid tissue, serum PTH and SUVR data for linear regression analysis
| Patient no. | Size (cm) | sPTH | pPTH | ΔPTH(%) | SUVR |
|---|---|---|---|---|---|
| 2 | NC | 289 | 105.3 | −63.6 | 3.16 |
| 3 | 1.5 | 291 | ND | ND | NA |
| 4 | 2.8 | 304 | ND | ND | NA |
| 7 | UK | 206 | 184.0 | −10.7 | 5.75 |
| 8 | 1.3 | 83 | 181.3 | 118.4 | 5.66 |
| 10 | 0.3 | 122 | 140.8 | 15.4 | 4.33 |
| 12 | UK | 142 | 332.4 | 134.1 | 10.63 |
| 13 | UK | 594 | 446.0 | −24.9 | 14.37 |
| 15 | 0.6 | 81 | 129.6 | 60.0 | 3.96 |
Patient 14 was excluded because preoperative sPTH was not checked and patient 16 was excluded because preoperative sPTH was obtained from aspirate of parathyroid tissue
sPTH serum parathyroid hormone, pPTH parathyroid hormone predicted by linear regression model (sPTH = 39.53 × SUVR − 89.84), ΔPTH(%) (pPTH − sPTH)/sPTH × 100, ND not done, NA not accessible due to loss of DICOM data, NC not checked on pathologic examination due to ill-defined margin, UK unknown because of no surgical removal
Table 5.
Result of correlation and linear regression analyses
| R by Correlation | Slope by Linear Regression | |
|---|---|---|
| SUVR vs sPTH | 0.886 (p = 0.019) | 39.53 ± 12.99 (p = 0.0383) |
| Size vs sPTH | 0.700 (p = 0.188) | 88.76 ± 42.44 (p = 0.1276) |
| SUVR vs size | 0.800 (p = 0.200) | 0.7243 ± 0.1775 (p = 0.0551) |
Fig. 4.
Correlation between SUVR and sPTH
By one-sample t-test, preoperative sPTH of PH was not significantly different from that of PA (289 vs 230.4 ± 160.4, p = 0.305). However, there was a borderline significant difference in pPTH between PH and PA (35.1 vs 204.7 ± 164.0, p = 0.052, Fig. 5), which suggests that SUVR, the uptake ratio of 11C-methionine, may be different between PH and PA, although there was no statistical significance.
Fig. 5.
Change of sPTH level by application of linear regression model, sPTH = 39.53 × SUVR-89.84. Preoperative sPTH level of PH is not significantly different from that of PA; however, pPTH level of PH is significantly lower than that of PA. It suggests that PH can have lower methionine uptake than PA
Discussion
In this current study, Met-PET/CT showed higher detection sensitivity of PA or PH compared with MIBI scintigraphy as expected. Our results were generally agreed with the previous reports [9, 10, 16–18] and showed even higher performance compared with the results of meta-analysis reported by Caldarella et al. [21]. In quantitative analysis, SUVR showed correlation with sPTH level in patients with PA. This result agreed with the correlation between maximum standardized uptake value (SUVmax) of Met-PET/CT and sPTH reported by Otto et al. [16] even though SUVR was used instead of SUVmax in the current study. In addition, Met-PET/CT could detect and localize parathyroid tissues in the lesions of negative on MIBI scintigraphy, which was also consistent with the previous reports [22, 23]. From our data and other reports, it seems to be apparent that Met-PET/CT is more useful for detection and localization of abnormal parathyroid tissue than MIBI scintigraphy.
In case of PH, accurate localization can be difficult because most patients may have multiple glandular lesions and all four parathyroid glands would have elevated excretion of PTH [2]. Even though an individual parathyroid gland has not so high PTH-excretion activity, their accumulated sPTH from all four glands may result in high level of sPTH, which can be similar to or higher than sPTH level of single PA. Therefore, it is less possible to differentiate PH from PA with preoperative sPTH only.
In this current study, we performed the quantitative analysis of 11C-methionine uptake of the parathyroid lesions as SUVR. Our novel approach was to acquire pPTH from a linear regression model obtained from SUVR and preoperative sPTH. From the regression, pPTH of PH was shown to be lower than that of PA, which means that PH has the character of low 11C-methionine uptake compared with PA. Therefore, Met-PET/CT has a potential to be used for differentiation of PH from PA, as well as for localization of parathyroid tissue. SUVR can be a useful parameter for the differentiation of PA from PH.
There are several limitations in the current study. A relatively small number of patients in a single center may not be enough to draw an objectively acceptable conclusion. Multi-centered studies would be expected to make up for this drawback. Because of the retrospective nature of this study, the enrolled patients’ data were inhomogeneous and therefore some data loss was inevitable. In addition, only half of the patients were pathologically confirmed, which might result in incorrect data, although it was compensated by clinical follow-up results. A well-organized prospective study in the future is expected to come over this limitation.
In conclusion, Met-PET/CT showed higher detection sensitivity of PA or PH than MIBI scintigraphy in patients with primary HPT or suspected PA, and could even predict precise location of PA or PH, which can be helpful in planning the minimally invasive surgical removal of suspicious parathyroid tissues. In addition, semi-quantitative analysis with Met-PET/CT may help to distinguish PA from PH.
Acknowledgments
This study was partly supported by Seoul National University Hospital Intramural Research Grants. The authors have no conflicts of interests.
References
- 1.Taillefer R. Tc-99m sestamibi parathyroid scintigraphy. In: Freeman LM, editor. Nuclear Medicine Annual 1995. New York: Raven Press; 1995. p. 51–79.
- 2.Vestergaard P, Thomsen SS. Medical treatment of primary, secondary, and tertiary hyperparathyroidism. Current Drug Safety. 2011;6:108–13. doi: 10.2174/157488611795684703. [DOI] [PubMed] [Google Scholar]
- 3.Hindie E, Ugur O, Fuster D, O’Doherty M, Grassetto G, et al. EANM parathyroid guidelines. Eur Nucl Med Mol Imaging. 2009;36:1201–16. doi: 10.1007/s00259-009-1131-z. [DOI] [PubMed] [Google Scholar]
- 4.Weber T, Cammerer G, Schick C, Solbach C, Hillenbrand A, et al. C-11 methionine positron emission tomography/computed tomography localizes parathyroid adenomas in primary hyperparathyroidism. Horm Metab Res. 2010;42:209–14. doi: 10.1055/s-0029-1243185. [DOI] [PubMed] [Google Scholar]
- 5.Pallan S, Rahman MO, Khan AA. Diagnosis and management of primary hyperparathyroidism. BMJ. 2012;344:e1013. doi: 10.1136/bmj.e1013. [DOI] [PubMed] [Google Scholar]
- 6.Cheon M, Choi JY, Chung J, Lee JY, Cho SK, et al. Differential findings of Tc-99m sestamibi dual-phase parathyroid scintigraphy between benign and malignant parathyroid lesions in patients with primary hyperparathyroidism. Nucl Med Mol Imaing. 2011;45:276–84. doi: 10.1007/s13139-011-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant CS, Thompson G, Farley D, Heerden J. Primary hyperparathyroidism surgical management since the introduction of minimally invasive parathyroidectomy. Arch Surg. 2005;140:472–9. doi: 10.1001/archsurg.140.5.472. [DOI] [PubMed] [Google Scholar]
- 8.Hiromatsu Y, Ishibashi M, Hishida H, Okuda S, Miyake I. Technetium-99m tetrofosmin parathyroid imaging in patients with primary hyperparathyroidism. Internal Medicine. 2000;39(2):101–6. doi: 10.2169/internalmedicine.39.101. [DOI] [PubMed] [Google Scholar]
- 9.Eslamy HK, Ziessman HA. Parathyroid scintigraphy in patients with primary hyperparathyroidism: 99mTc Sestamibi SPECT and SPECT/CT. Radiographics. 2008;28:1461–76. doi: 10.1148/rg.285075055. [DOI] [PubMed] [Google Scholar]
- 10.Phillips CD, Shatzkes DR. Imaging of the parathyroid glands, semin in ultrasound. CT and MRI. 2012;33:123–9. doi: 10.1053/j.sult.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhu A, Shim H. Current molecular imaging positron emitting radiotracers in oncology. Nucl Med Mol Imaging. 2011;45:1–14. doi: 10.1007/s13139-011-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi M, Sharma R, Varshney R, Jaimini A, Jain J, et al. Comparison of F-18 FDG and C-11 methionine PET/CT for the evaluation of recurrent primary brain tumors. Clin Nucl Med. 2012;37(2):158–63. doi: 10.1097/RLU.0b013e318238f51a. [DOI] [PubMed] [Google Scholar]
- 13.Shiiba M, Ishihara K, Kimura G, Kuwako T, Yoshihara H, et al. Evaluation of primary prostate cancer using 11C-methionine-PET/CT and 18F-FDG-PET/CT. Ann Nucl Med. 2012;26(2):138–45. doi: 10.1007/s12149-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 14.Frelinger AL, III, Zull JE. The role of the methionine residues in the structure and function of parathyroid hormone. Arch Biochem Biophys. 1986;244(2):641–9. doi: 10.1016/0003-9861(86)90632-6. [DOI] [PubMed] [Google Scholar]
- 15.Potts JT, Murray TM, Peacock M, Niall HD, Tregear GW, et al. Parathyroid hormone: Sequence, synthesis, immunoassay studies. Am J Med. 1971;50:639–49. doi: 10.1016/0002-9343(71)90119-7. [DOI] [PubMed] [Google Scholar]
- 16.Otto D, Boerner AR, Hofmann M, Brunkhorst T, Meyer GJ, et al. Pre-operative localisation of hyperfunctional parathyroid tissue with 11C-methionine PET. Eur J Nucl Med Mol Imaging. 2004;31:1405–12. doi: 10.1007/s00259-004-1610-1. [DOI] [PubMed] [Google Scholar]
- 17.Beggs AD, Hain SF. Localization of parathyroid adenomas using 11C-methioninepositron emission tomography. Nucl Med Commun. 2005;26:133–6. doi: 10.1097/00006231-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Tang BN, Moreno-Reyes R, Blocklet D, Corvilain B, Cappello M, et al. Accurate pre-operative localization of pathological parathyroid glands using 11C-methionine PET/CT. Contrast Media Mol Imaging. 2008;3:157–63. doi: 10.1002/cmmi.243. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann K, Takei T, Kanegae K, Shiga T, Buck AK, et al. Clinical value and limitations of [(11)C]-Methionine PET for detection and localization of suspected parathyroid adenomas. Mol Imaging Biol. 2009;11:356–63. doi: 10.1007/s11307-009-0205-4. [DOI] [PubMed] [Google Scholar]
- 20.Tublin ME, Pryma DA, Yim JH, Ogilvie JB, Mountz JM, Bencherif B, et al. Localization of PAs by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: Are both studies really needed? J Ultrasound Med. Feb 2009;28(2):183–90. [DOI] [PubMed]
- 21.Caldarella C, Treglia G, Isgro MA, Giordano A. Diagnostic performance of positron emission tomography using 11C-methionine in patients with suspected parathyroid adenoma: A meta-analysis. Endocrine. 2013;43(1):78–83. doi: 10.1007/s12020-012-9746-4. [DOI] [PubMed] [Google Scholar]
- 22.Rubello D, Fanti S, Nanni C, Farsad M, Castellucci P, et al. 11C-methionine PET/CT in 99mTc-sestamibi-negative hyperparathyroidism in patients with renal failure on chronic haemodialysis. Eur J Nucl Med Mol Imaging. 2006;33:453–9. doi: 10.1007/s00259-005-0008-z. [DOI] [PubMed] [Google Scholar]
- 23.Oksuz MO, Dittmann H, Wicke C, Mussig K, Bares R, et al. Accuracy of parathyroid imaging: A comparison of planar scintigraphy, SPECT, SPECT-CT, and C-11 methionine PET for the detection of parathyroid adenomas and glandular hyperplasia. Diagn Interv Radiol. 2011;17:297–307. doi: 10.4261/1305-3825.DIR.3486-10.1. [DOI] [PubMed] [Google Scholar]