Abstract
Purpose
This study aimed to develop a gene expression targeting method for specific imaging and therapy of alpha-fetoprotein (AFP)-producing hepatocellular carcinoma (HCC) cells, using an adenovirus vector containing the human sodium/iodide symporter (hNIS) gene driven by an AFP enhancer/promoter.
Methods
The recombinant adenovirus vector, AdAFPhNIS (containing the hNIS gene driven by human AFP enhancer/promoter) was prepared. After in vitro infection by the adenovirus, hNIS gene expression in AFP-producing cells and in AFP-nonproducing cells was investigated using 125I uptake assay and semi-quantitative reverse transcription polymerase chain reaction (RT-PCR). The killing effect of 131I on AdAFPhNIS-infected HCC cells was studied using an in vitro clonogenic assay. In addition, tumor-bearing mice were intravenously injected with the adenovirus, and scintigraphic images were obtained.
Results
The expression of hNIS was efficiently demonstrated by 125I uptake assay in AFP-producing cells, but not in AFP-nonproducing cells. AFP-producing HCC-targeted gene expression was confirmed at the mRNA level. Furthermore, in vitro clonogenic assay showed that hNIS gene expression induced by AdAFPhNIS infection in AFP-producing cells caused more sensitivity to 131I than that in AFP-nonproducing cells. Injected intravenously in HuH-7 tumor xenografts mice by adenovirus, the functional hNIS gene expression was confirmed in tumor by in vivo scintigraphic imaging.
Conclusions
An AFP-producing HCC was targeted with an adenovirus vector containing the hNIS gene using the AFP enhancer/promoter in vitro and in vivo. These findings demonstrate that AFP-producing HCC-specific molecular imaging and radionuclide gene therapy are feasible using this recombinant adenovirus vector system.
Keywords: Hepatocellular carcinoma (HCC), Alpha-fetoprotein (AFP) promoter, Adenovirus, Tumor-targeted gene expression, Systemic delivery, Radioiodide therapy
Introduction
Hepatocellular carcinoma (HCC) is known as one of the most common cancers in Eastern Asia, and its incidence is also increasing in the West [1, 2]. However, advanced HCC has a poor prognosis in spite of various therapeutic options, whether surgical or non-surgical [3]. Systemic chemotherapy and radiotherapy, which are effective therapies for a wide range of cancers, are also only partially effective against advanced HCC and therefore, new therapeutic strategies are required. The gene therapy, one of many therapeutic alternatives, is being recognized as a promising method on the basis of diverse researches and clinical trials for more than two decades [4–8].
Alpha-fetoprotein (AFP) is a glycoprotein produced in the developing embryo and fetus, but disappears gradually after birth. Unlike rare expression in normal hepatocytes, AFP is re-expressed in about 80 % of HCCs [9]. Therefore, AFP-dependent transcriptional activation is very useful means of tumor-specific gene expression for HCC-targeted gene therapy. Some studies that specifically express a therapeutic gene using the AFP promoter suggested the efficacy of gene therapy as a safe and effective therapeutic strategy to HCC [10–12].
The sodium iodide symporter (NIS) gene is noticeable, along with the herpes simplex virus type 1 thymidine kinase (HSV1-tk) gene, in the way that it can be employed in both imaging and therapy. NIS is an intrinsic transmembrane glycoprotein expressed mainly in the thyroid cells, and can transport iodide within the cells [13, 14]. When the NIS gene was transferred into the specific cell, and radioiodide was injected, only NIS-gene transferred cells accumulate radioiodide. Also, NIS can easily accumulate a variety of radioisotopes including iodide, technetium, rhenium, and astatine instead of complicated radiocompounds [15]. Therefore, expression of the NIS gene, followed by application of radioisotopes, allows noninvasive imaging such as scintigraphy or positron emission tomography (PET), and radionuclide therapy [16–18].
We have previously reported the tumor targeting and therapeutic potential of radioiodine by a plasmid vector expressing the hNIS gene driven by the AFP enhancer/promoter in AFP-producing HCC in vitro and in vivo [19]. These findings suggested that this type of targeted hNIS gene therapy and molecular imaging has the potential to be used in the management of AFP-producing HCC. To increase the possibility of clinical application through in vivo gene delivery, in this study we aimed to develop and demonstrate a gene targeting method for in vitro radioiodide therapy and in vivo scintigraphic imaging of AFP-producing HCC by delivery of an adenovirus vector containing the hNIS gene under control of an AFP enhancer/promoter.
Materials and Methods
Cell Culture
Three human HCC cell lines, HuH-7, HepG2, and SK-Hep-1, were obtained from KCLB (Seoul, Korea). Chang liver cells (human normal liver cells) and C6 (rat glioma cells) were obtained from ATCC (Manassas, VA). Human embryonic kidney cells containing the E1 region of adenovirus (QBI-293A) was purchased from Qbiogene (Carlsbad, CA). Cells were grown in DMEM media supplemented with 10 % fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified 5 % CO2 atmosphere.
Recombinant Adenovirus Preparation
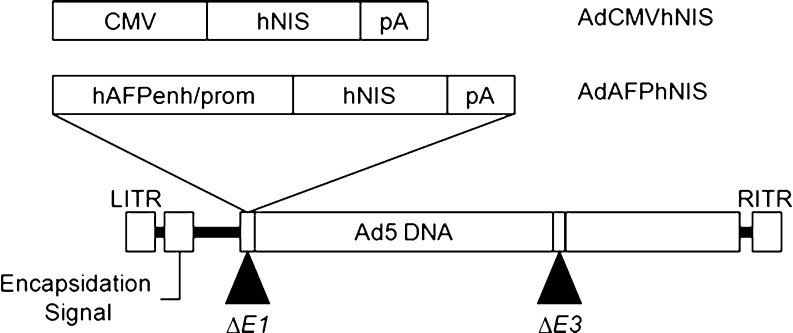
The human NIS gene was cloned by PCR from pCMV-NIS plasmid [20] using 5′-GATCCTCGAGCCACCATGGAGGCCGTGGAGACCGG-3′ (forward primer) 5′-GATCTCTAGATCAGAGGTTTGTCTCCTGCTG-3′ (reverse primer). The hNIS gene was excised by XhoI and XbaI digestion from the above polymerase chain reaction (PCR) products, and inserted into an adenoviral shuttle vector, pShuttle-CMV (Qbiogene). Adenovirus was made by recombining the pShuttle-CMVhNIS construct with an adenoviral backbone using the AdEasyTM vector system (Qbiogene), following the manufacturer’s instructions. Acquired adenoviruses were designated AdCMVhNIS, and used as a control (Fig. 1).
Fig. 1.
Schematic structure of adenoviral vectors. A recombinant replication-defective adenovirus by deletion of the viral E1 and E3 region was used to construct these adenovirus vectors; hAFPenh/prom human AFP enhancer/promoter, hNIS human sodium iodide symporter, pA the SV40 polyadenylation signal
The recombinant adenovirus vector, AdAFPhNIS was prepared as summarized in Fig. 1. An AFP enhancer/promoter DNA fragment was prepared by PCR from pDRIVE-AFP/hAFP (Invivogen, San Diego, CA) using the following primers: 5′-GATCACGCGTGCTTAGAAATATGGGGGTAG-3′ (forward) and 5′-GATCCTCGAGGTTGCTAGTTATTTTGTTATTG-3′ (reverse), and inserted into the MluI/XhoI sites of pGL3-Basic (pGL3-AFPfLuc). The DNA fragment containing the AFP enhancer/promoter and fLuc from pGL3-AFPfLuc was cloned into pShuttle (Qbiogene) and designated pShuttle-AFPfLuc. The fLuc gene of pShuttle-AFPfLuc was replaced with the hNIS gene from pShuttle-CMVhNIS using XhoI/XbaI digestion, and designated pShuttle-AFPhNIS. Obtained plasmids were used for adenovirus production by the same methods as above. Adenovirus titration was performed using the TCID50 method and the value obtained was converted into plaque forming units (pfu)/ml.
Transduction of Reporter Gene by Adenovirus In Vitro
Cells were plated at a density of 2 × 105 cells per well in 24-well culture plates 24 h before adenovirus infection, and immediately before infection, cells were washed and incubated with serum-free medium containing adenovirus for 24 h. The ratio of the number of adenovirus to a cell was expressed as the multiplicity of infection (MOI). Chang and C6 cells showed about a tenfold lower efficiency compared with HCC cells when the cells were infected with AdCMVfLuc (constitutively expressing firefly luciferase) (data not shown). Consequently, in Chang and C6 cells, adenoviruses of tenfold higher titer than those in HCC cells were used. Three HCC cell lines (HuH-7, HepG2, and SK-Hep-1) and two control cell lines (Chang and C6) were infected with AdAFPhNIS at the MOI of 5 and the MOI of 50, respectively.
In Vitro Radioiodide Uptake and Efflux Assay
125I uptake by cells was determined as described by Kim et al. [20]. In brief, iodide uptake levels were determined by incubating the cells with 500 μl Hank’s Balanced Salt Solution (HBSS) with 3.7 KBq (0.1 μCi) of carrier-free Na125I and 10 μM NaI, to yield a specific activity of 740 MBq/mmol (20 mCi/mmol) at 37 °C for 30 min. The cells were detached with 500 μl trypsin after washing twice and radioactivities were measured using a gamma counter (1480 WIZARD; PerkinElmer, Waltham, MA). To evaluate the time course of iodide uptake, the cells were incubated for 5, 30, 60, and 120 min in Na125I solution, and then washed, detached, and counted, as described above.
Iodide efflux studies were performed as described by Lee et al. [21]. After incubating the cells with 500 μl HBSS buffer containing 10 μM NaI and 3.7 KBq of Na125I for 30 min, the cells were washed, and further incubated with HBSS buffer containing 10 μM nonradioactive NaI. The cells were washed once and added to HBSS incubation buffer and further incubated. Every 3 min (0–15 min) or 6 min (15–27 min), and at 45 and 60 min, the buffer was replaced, and the radioactivity in the buffer was counted. After removal of the last medium (at 60 min), the cells were solubilized for counting along with the previously collected medium samples. The total radioactivity at the initiation of the efflux study (100 %) was calculated by adding the radioactivity of final cells to the summation of the radioactivity of each medium.
RNA Extraction and Reverse Transcription-PCR
Total RNA was prepared from cells using the easy-spinTM RNA extraction kit (iNtRON Biotechnology, Seongnam, Korea). Copy DNA preparations were obtained using Superscript III (Invitrogen). Both procedures were carried out according to the respective manufacturers’ protocols. PCR amplifications of individual cDNAs were performed using Taq polymerase (iNtRON Biotechnology) and following specific primers for AFP (NM_001134), hNIS (NM_000453), and β-actin (XM_004814) in a GeneAmp PCR System (Applied Biosystems, Foster City, CA) for 30 cycles of denaturation (95 °C for 30 s), annealing (AFP, 55 °C; hNIS, 52 °C; β-actin, 56 °C for 30 s), and extension (72 °C for 30 s): AFP forward, 5′-GTACGGACATTCAGACTGCT-3′; AFP reverse, 5′-TTGCTGCCTTTGTTTGGAAG-3′, hNIS forward, 5′- TCTCTCAGTCAACGCCTCT-3′; hNIS reverse, 5′-ATCCAGGATGGCCACTTCTT-3′, and β-actin forward, 5′-GTGGGGCGCCCCAGGCACCAGGGC-3′; β-actin reverse, 5′-CTCCTTAATGTCACGCACGATTTC-3′, respectively.
In Vitro Clonogenic Assay
The procedure was performed as described by Mandell et al. [16] with slight modifications. In brief, cells were incubated for 7 h with 0.1 mCi/ml Na131I [total dose 0.8 mCi (29.6 MBq)] in HBSS supplemented with 10 mM NaI and 10 mM HEPES at pH 7.3. Percentage survivals represent the number of cell colonies after 131I treatment expressed as a percentage of the number of cell colonies after mock treatment with HBSS. Results are expressed as mean % survival ± SD and were normalized versus the number of colonies formed by cells infected with adenovirus but not treated with 131I (100 %). Statistical significance was determined using the unpaired t-test. For all analyses, p values of <0.05 were considered statistically significant.
HCC Tumor Xenografts Model in Mice
Tumor xenografts were produced in 6-week-old female NOD/SCID mice (Charles River Laboratories, Wilmington, MA), by subcutaneously injecting 1 × 107 HuH-7 cells suspended in 100 μl PBS into right hind flanks. Adenoviruses were injected after 8 weeks for tumor growth. AdAFPhNIS (5 × 108 pfu) suspended in 100 μl saline was then injected intravenously, 4 days after this injection, scintigraphic images were acquired.
In Vivo Imaging Acquisition
Thirty minutes after injecting 18.5 MBq of [99mTc]-pertechnetate intraperitoneally into animals, they were placed in a spread prone position and scanned with a gamma camera (TRIONICS, Cleveland, OH). Mice were anesthetized with an intraperitoneal injection of ketamine (53 mg/kg) and xylazine (12 mg/kg) at 10 min before imaging acquisition. Acquired images were quantified using the Multi Gauge, version 3.0 image analysis program (Fuji Film, Japan), which determined the optical density (OD) of ROIs on images.
Results
AFP Gene Expression in Different Cell Lines
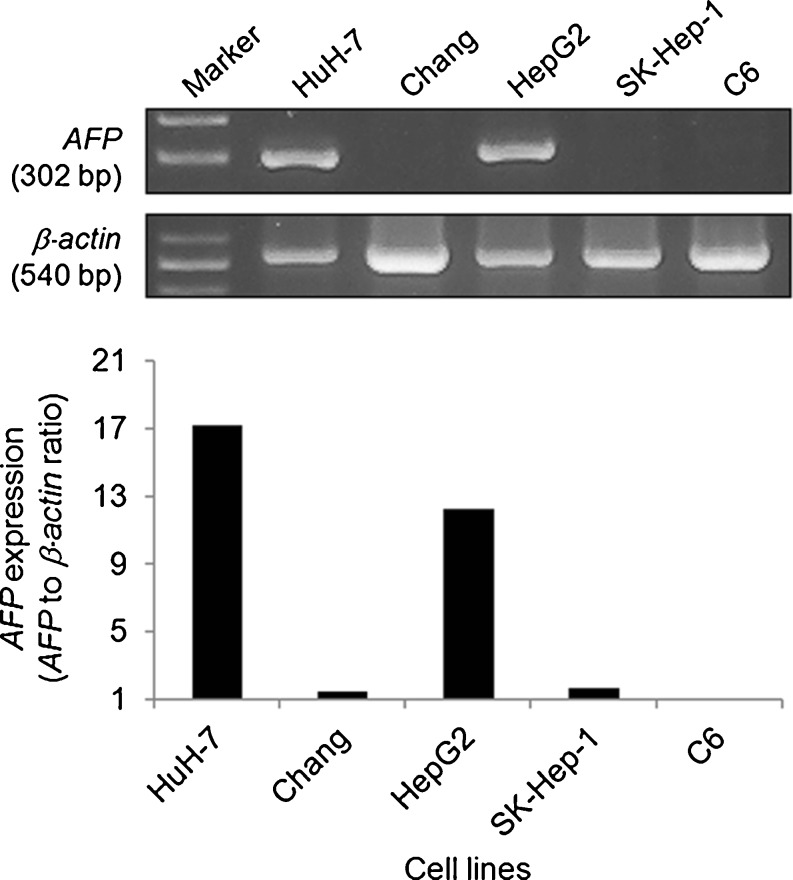
Three human HCC cell lines (HuH-7, HepG2, and SK-Hep-1), human normal liver cells (Chang), and rat glioma cells (C6) were used as target cells for recombinant adenovirus infection. HuH-7 and HepG2 cells were found to express AFP mRNA (Fig. 2). In contrast, AFP expression was almost not observed in SK-Hep-1, Chang, or C6 cell lines. The levels of expressed AFP mRNA in HuH-7 and HepG2 cells were about 13- and 9-fold higher than average in the rest, respectively. HuH-7 cells showed about 1.4-fold higher AFP gene expression than that in HepG2 cells.
Fig. 2.
AFP gene expression in different cell lines. The transcript of AFP was quantified by RT-PCR with β-actin as an internal control. In HuH-7 and HepG2 cells, high AFP expression was observed unlike in the other cell lines
Adenovirus-Mediated hNIS Gene Transfer in Different Cell Lines
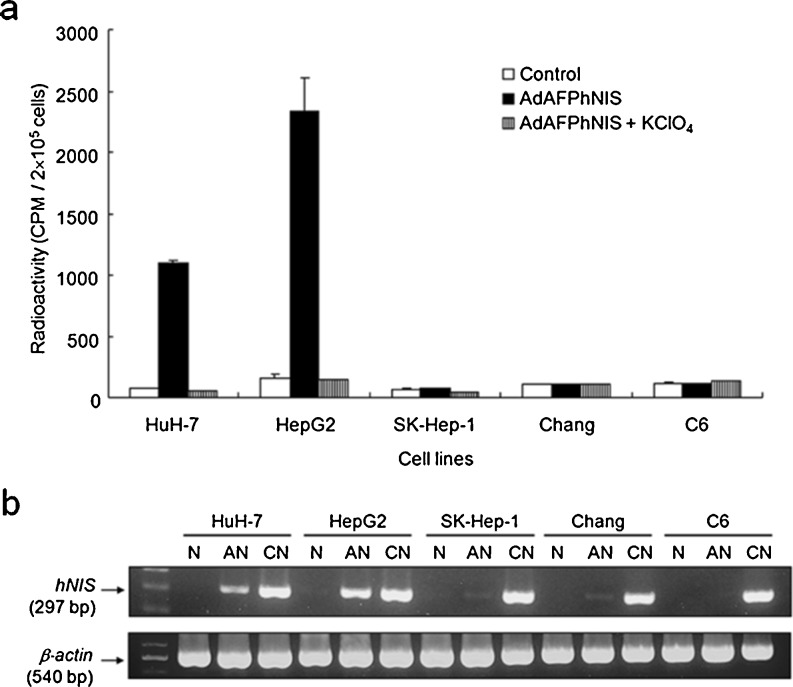
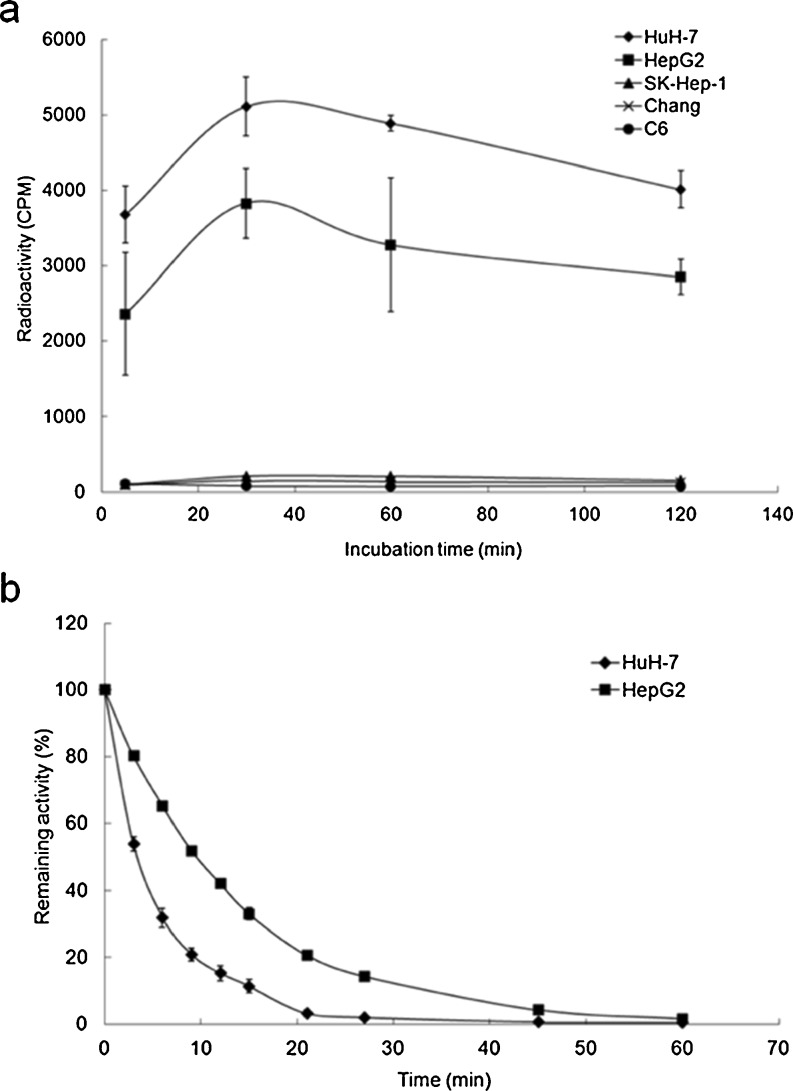
Increased radioiodide uptake was observed in two AFP-producing cell lines, HuH-7 and HepG2, after infection of AdAFPhNIS (Fig. 3a). This uptake was inhibited by potassium perchlorate, a potent inhibitor of active iodide transport, confirming that iodide uptake is essentially dependent on NIS expression. In contrast, any significant increase of radioiodide uptake was not observed at all in the AFP-nonproducing cells, SK-Hep-1, Chang, and C6. 125I accumulation reached a steady status within 30 minutes after iodide was added in HuH-7 and HepG2 cells, but SK-Hep-1, Chang, and C6 cells did not show any significant change (Fig. 4a). Figure 4b shows the amount of 125I present within the cells as a function of time. Up to 70 % of the cellular radioactivity was released into the medium during the first 6 min (HuH-7 curves), showing that efflux is very rapid in HuH-7 cells (T1/2 = 4 min). The efflux rate in HepG2 cells was approximately twice as slow as that observed in HuH-7 cells.
Fig. 3.
Reporter gene activity assay and analysis of hNIS gene expression. Cultured cells were infected with adenovirus. At 24 h post infection, radioiodide uptake assay and RT-PCR analysis were performed. (a) 125I uptake after infection of AdAFPhNIS. Two AFP-producing cell lines (HuH-7 and HepG2) concentrated 125I. In contrast, radioiodide uptake was not observed at all in the AFP-nonproducing cells, SK-Hep-1, Chang, and C6. In all cell lines, 125I uptake was completely inhibited by 100 μM potassium perchlorate, a competitive inhibitor of NIS. Data are expressed as mean ± SD (n = 4). (b) Human NIS mRNA expressions after infection of adenovirus. The mRNA expression of reporter genes correlated well with induced reporter activities. N no infection, AN AdAFPhNIS infection, CN AdCMVhNIS infection
Fig. 4.
Time course of iodide uptake and efflux assay after infection of AdAFPhNIS in different cell lines. Radioiodide uptake was rapidly increased and maximized within 30 min in HuH-7 and HepG2 cells (a), and effluxes of 125I from HuH-7 and HepG2 cells were rapid, with activity half lives (T1/2) of 4 min and 10 min, respectively (b). Data are expressed as mean ± SD (n = 4)
In two AFP-producing cells, increased radioiodide uptake correlated well with mRNA expression of hNIS gene (Fig. 3b). When the cells were infected with AdCMVhNIS, the expression of hNIS mRNA was detected in all the HCC cells (HuH-7, HepG2, and SK-Hep-1) and control cells (Chang and C6) by RT-PCR.
In Vitro Therapeutic Effects of AdAFPhNIS and 131I Treatment
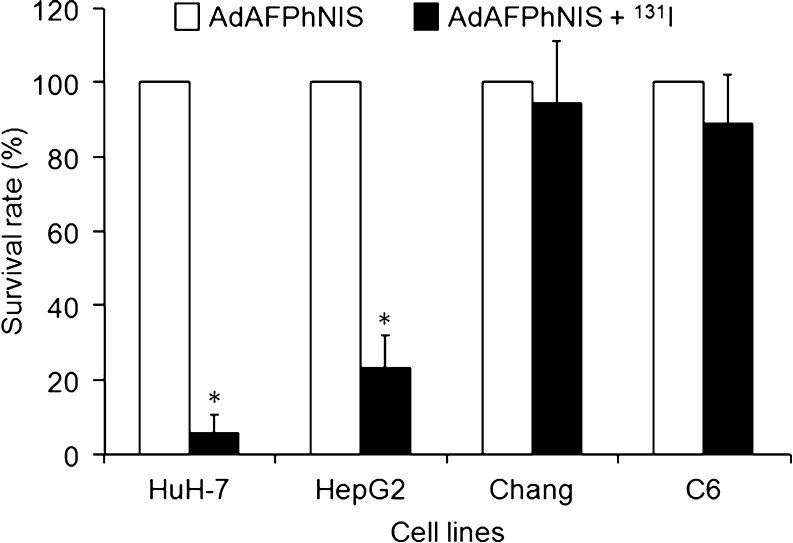
To evaluate therapeutic effects of AdAFPhNIS and 131I treatment, cells were infected with AdAFPhNIS and then treated with 0.8 mCi (29.6 MBq) of 131I. The survival rates of AdAFPhNIS infected Chang and C6 cells were unchanged by radionuclide treatment, but the survival rates of AdAFPhNIS infected HuH-7 and HepG2 cells were only 5.67 ± 5.46 % and 23.33 ± 8.70 %, respectively, which were significant reductions versus virus only infected cells (p < 0.05; Fig. 5).
Fig. 5.
Therapeutic effects of radioiodide on the survivals after infection of AdAFPhNIS in AFP-producing and AFP-nonproducing cells. Therapeutic effects of 131I on survival rates of AdAFPhNIS infected cells treated with 0.8 mCi (29.6 MBq) of 131I. In HuH-7 and HepG2 cells, the adjusted p values (*) between AdAFPhNIS + 131I versus AdAFPhNIS alone were all <0.05
HCC-Targeted Reporter Gene Expression of Adenovirus In Vivo
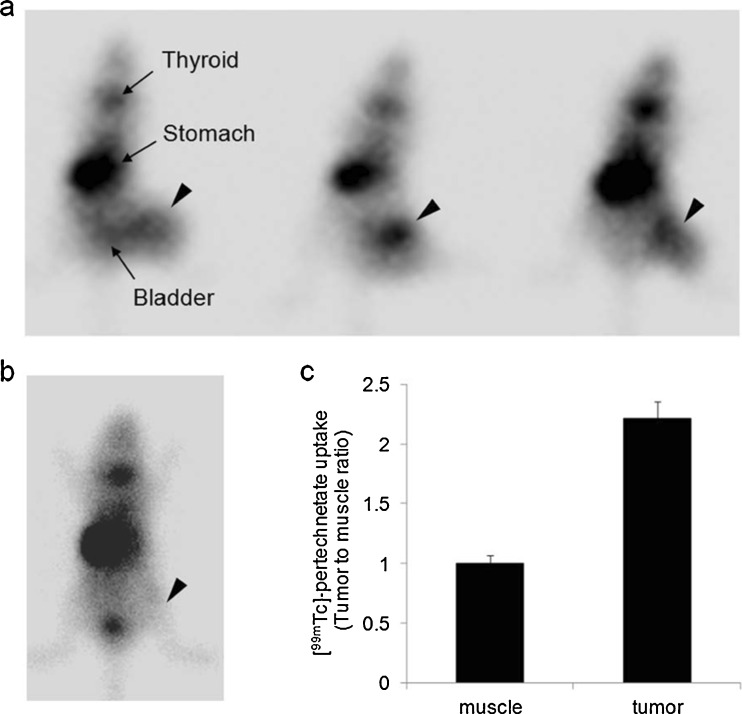
The targeted reporter gene expression from recombinant adenovirus in AFP-producing HCCs was evaluated in mice bearing HuH-7 tumor xenografts. To evaluate the in vivo specificity of AdAFPhNIS, NOD/SCID mice bearing HuH-7 xenografts were established. Animals harboring 500 mm3 HuH-7 xenografts were injected with AdAFPhNIS (5 × 108 pfu) via the tail vein. Four days after this injection, HCC-specific hNIS expression was demonstrated in scintigraphic images (Fig. 6a). HuH-7 xenografts showed significant [99mTc]-pertechnetate uptake, with the exception of normal physiological uptake in thyroid, stomach, and bladder. Tumor-to-muscle intensity ratio was about 2.2-fold (Fig. 6c). Just background activity was observed in tumor of HuH-7-xenografted mouse without AdAFPhNIS (Fig. 6b).
Fig. 6.
HCC-targeted in vivo scintigraphic images. HuH-7 tumor-bearing mice were injected with AdAFPhNIS (5 × 108 pfu) via the tail vein (n = 3) (a) and with saline (b). Scintigraphic images were acquired after intraperitoneal injection of [99mTc]-pertechnetate with γ-camera. Arrowheads indicate HuH-7 tumor. (c) Image based analysis of [99mTc]-pertechnetate uptake
Discussion
It was the aim of this study to develop and demonstrate a gene targeting method for in vitro radioiodide therapy and in vivo scintigraphic imaging of AFP-producing HCCs by delivery of recombinant adenoviral vector. HuH-7 cells and HepG2 cells infected with AdAFPhNIS showed approximately 15-fold higher 125I uptakes than non-infected cells, whereas no significant uptake was observed in AFP-nonproducing cells (Fig. 3a). In spite of rapid efflux resulting from the lack of iodide organification (Fig. 4b) [22], the amount of accumulated 131I has been demonstrated to be sufficiently high to selectively kill hNIS-transduced HCC cells in a clonogenic assay (Fig. 5). In HuH-7 tumor xenografts model (Fig. 6), scintigraphic images with AdAFPhNIS was shown to be significantly specific except for normal physiological uptake of [99mTc]-pertechnetate in thyroid, stomach and bladder [23].
Until recently, diverse gene therapy strategies for HCC have been examined, such as gene augmentation therapy for tumor suppressor gene [5], drug sensitization therapy including the HSV1-tk/ganciclovir [6, 24] or cytosine deaminase/5-fluorocytosine [25], and genetic immunotherapy using a cytokine gene [4]. Also, since the NIS gene was first cloned in 1996 [26], many studies have demonstrated the efficacy of molecular imaging or radioiodide gene therapy using NIS in vitro and in vivo [16, 17, 27–29]. NIS has a number of obvious advantages, such as, its potential use as a diagnostic gene for noninvasive imaging, the bystander effect due to crossfire by β-emitters, the lack of immune response due to its endogenous origin, its well-known physiological effects, and others [23]. Furthermore, cytoreductive gene therapy based on NIS gene transfer followed by radioiodide application has also been found to offer promise for treatment of HCC [30, 31].
Transcriptional activation of the therapeutic gene by a tumor-specific promoter is considered as indispensable option for maximization of tumor-specific cytotoxicity with minimal effects on normal tissues in cancer gene therapy. However, in vivo transcriptional activity of currently being used tumor-specific promoters including the AFP promoter is not strong. Combination with different enhancers [32] or use of a transcriptional amplification system [33] have been attempted to improve weak transcriptional activity of tumor-specific promoter by several researchers. But more studies must be done to achieve a meaningful therapeutic response in clinical application.
Safe and effective delivery of therapeutic gene to target is a critical factor for successful cancer gene therapy. In the current study, we have used serotype 5 human adenovirus vector because it can infect various human cells easily without any severe side effects, and localize preferentially to the liver when administered intravenously, which suggests that it is suitable for selective HCC treatment [34]. Most studies of targeted NIS gene therapy using tumor specific promoter have been conducted under limited gene delivery condition including the use of stable cell lines established with a retrovirus or plasmid, or local injection of adenovirus vector [30, 35, 36]. The present study shows the possibility of non-invasive in vivo imaging of tumor-targeted gene expression after intravenous administration of adenovirus.
This study demonstrates the AFP-producing HCC-targeted reporter gene expression following systemic adenoviral delivery through nuclear imaging modalities in xenografted mouse models, and also shows that the transfer of a therapeutic gene using this adenovirus caused the cell type specific cytoreduction of human HCC cell lines. Further studies into systemic administration of tumor specific adenoviral vector could help us verify the applicability of targeted NIS gene therapy for distant metastatic HCC as well as disseminated HCC tumors throughout the liver.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant (grant no. 2012-013480) and the Basic Research Program of the KOSEF (grant no. R01-2006-000-10249-0) funded by the Korean government (MEST).
Conflicts of interest
None.
Contributor Information
June-Key Chung, Phone: +82-2-20723376, FAX: +82-2-7457690, Email: jkchung@snu.ac.kr.
Joo Hyun Kang, Phone: +82-2-9701339, FAX: +82-2-9701341, Email: kang2325@kirams.re.kr.
References
- 1.Levin B, Amos C. Therapy of unresectable hepatocellular carcinoma. N Engl J Med. 1995;332(19):1294–1296. doi: 10.1056/NEJM199505113321910. [DOI] [PubMed] [Google Scholar]
- 2.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54(2):463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004;127(5 Suppl 1):S218–S224. doi: 10.1053/j.gastro.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez MM, Ryu SM, Qian C, Geissler M, Grimm C, Prieto J, et al. Immunotherapy of murine hepatocellular carcinoma by alpha-fetoprotein DNA vaccination combined with adenovirus-mediated chemokine and cytokine expression. Hum Gene Ther. 2008;19(7):753–759. doi: 10.1089/hum.2007.130. [DOI] [PubMed] [Google Scholar]
- 5.Okimoto T, Yahata H, Itou H, Shinozaki K, Tanji H, Sakaguchi T, et al. Safety and growth suppressive effect of intra-hepatic arterial injection of AdCMV-p53 combined with CDDP to rat liver metastatic tumors. J Exp Clin Cancer Res. 2003;22(3):399–406. [PubMed] [Google Scholar]
- 6.Bilbao R, Gerolami R, Bralet MP, Qian C, Tran PL, Tennant B, et al. Transduction efficacy, antitumoral effect, and toxicity of adenovirus-mediated herpes simplex virus thymidine kinase/ ganciclovir therapy of hepatocellular carcinoma: the woodchuck animal model. Cancer Gene Ther. 2000;7(5):657–662. doi: 10.1038/sj.cgt.7700175. [DOI] [PubMed] [Google Scholar]
- 7.Havlik R, Jiao LR, Nicholls J, Jensen SL, Habib NA. Gene therapy for liver metastases. Semin Oncol. 2002;29(2):202–208. doi: 10.1053/sonc.2002.31678. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007—an update. J Gene Med. 2007;9(10):833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 9.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31(4):302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Sa Cunha A, Bonte E, Dubois S, Chretien Y, Eraiser T, Degott C, et al. Inhibition of rat hepatocellular carcinoma tumor growth after multiple infusions of recombinant Ad.AFPtk followed by ganciclovir treatment. J Hepatol. 2002;37(2):222–230. doi: 10.1016/S0168-8278(02)00111-3. [DOI] [PubMed] [Google Scholar]
- 11.Miao J, Chen GG, Chun SY, Yun JP, Chak EC, Ho RL, et al. Adenovirus-mediated tBid overexpression results in therapeutic effects on p53-resistant hepatocellular carcinoma. Int J Cancer. 2006;119(8):1985–1993. doi: 10.1002/ijc.22040. [DOI] [PubMed] [Google Scholar]
- 12.Willhauck MJ, Sharif Samani BR, Klutz K, Cengic N, Wolf I, Mohr L, et al. Alpha-fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther. 2008;15(3):214–223. doi: 10.1038/sj.gt.3303057. [DOI] [PubMed] [Google Scholar]
- 13.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24(1):48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 14.Chung JK, Youn HW, Kang JH, Lee HY, Kang KW. Sodium iodide symporter and the radioiodine treatment of thyroid carcinoma. Nucl Med Mol Imaging. 2010;44:4–14. doi: 10.1007/s13139-009-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dingli D, Russell SJ, Morris JC., 3rd In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003;90(6):1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- 16.Mandell RB, Mandell LZ, Link CJ., Jr Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res. 1999;59(3):661–668. [PubMed] [Google Scholar]
- 17.Spitzweg C, Dietz AB, O’Connor MK, Bergert ER, Tindall DJ, Young CY, et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001;8(20):1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer RM, Bergert ER, O’Connor MK, Gendler SJ, Morris JC. Sodium iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Ther. 2006;13(1):60–66. doi: 10.1038/sj.gt.3302599. [DOI] [PubMed] [Google Scholar]
- 19.Jin YN, Chung HK, Kang JH, Lee YJ, Kimm KI, Kim YJ, et al. Radioiodine gene therapy of hepatocellular carcinoma targeted human alpha fetoprotein. Cancer Biother Radiopharm. 2008;23(5):551–560. doi: 10.1089/cbr.2008.0467. [DOI] [PubMed] [Google Scholar]
- 20.Kim KI, Kang JH, Chung JK, Lee YJ, Jeong JM, Lee DS, et al. Doxorubicin enhances the expression of transgene under control of the CMV promoter in anaplastic thyroid carcinoma cells. J Nucl Med. 2007;48(9):1553–1561. doi: 10.2967/jnumed.106.038612. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Chung JK, Shin JH, Kang JH, Jeong JM, Lee DS, et al. In vitro and in vivo properties of a human anaplastic thyroid carcinoma cell line transfected with the sodium iodide symporter gene. Thyroid. 2004;14(11):889–895. doi: 10.1089/thy.2004.14.889. [DOI] [PubMed] [Google Scholar]
- 22.Spitzweg C, O’Connor MK, Bergert ER, Tindall DJ, Young CY, Morris JC. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60(22):6526–6530. [PubMed] [Google Scholar]
- 23.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43(9):1188–1200. [PubMed] [Google Scholar]
- 24.Li HJ, Everts M, Yamamoto M, Curiel DT, Herschman HR. Combined transductional untargeting/retargeting and transcriptional restriction enhances adenovirus gene targeting and therapy for hepatic colorectal cancer tumors. Cancer Res. 2009;69(2):554–564. doi: 10.1158/0008-5472.CAN-08-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sia KC, Huynh H, Chinnasamy N, Hui KM, Lam PY. Suicidal gene therapy in the effective control of primary human hepatocellular carcinoma as monitored by noninvasive bioimaging. Gene Ther. 2012;19(5):532–42. [DOI] [PubMed]
- 26.Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, et al. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun. 1996;226(2):339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- 27.Dadachova E, Bouzahzah B, Zuckier LS, Pestell RG. Rhenium-188 as an alternative to Iodine-131 for treatment of breast tumors expressing the sodium/iodide symporter (NIS) Nucl Med Biol. 2002;29(1):13–18. doi: 10.1016/S0969-8051(01)00279-7. [DOI] [PubMed] [Google Scholar]
- 28.Dwyer RM, Bergert ER, O’Connor MK, Gendler SJ, Morris JC. Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors. Hum Gene Ther. 2006;17(6):661–668. doi: 10.1089/hum.2006.17.661. [DOI] [PubMed] [Google Scholar]
- 29.Jeon YH, Choi Y, Lee JT, Kim CW, Chung JK. CpG oligodeoxynucleotides enhance the activities of CD8+ cytotoxic T-lymphocytes generated by combined hMUC1 vaccination and hNIS radioiodine gene therapy. Nucl Med Mol Imaging. 2010;44:199–206. doi: 10.1007/s13139-010-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Altmann A, Mier W, Eskerski H, Leotta K, Guo L, et al. Radioiodine therapy of hepatoma using targeted transfer of the human sodium/iodide symporter gene. J Nucl Med. 2006;47(5):854–862. [PubMed] [Google Scholar]
- 31.Klutz K, Willhauck MJ, Wunderlich N, Zach C, Anton M, Senekowitsch-Schmidtke R, et al. Sodium iodide symporter (NIS)-mediated radionuclide ((131)I, (188)Re) therapy of liver cancer after transcriptionally targeted intratumoral in vivo NIS gene delivery. Hum Gene Ther. 2011;22(11):1403–1412. doi: 10.1089/hum.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh YJ, Chen FD, Ke CC, Wang HE, Huang CJ, Hou MF et al. The EIIAPA chimeric promoter for tumor specific gene therapy of hepatoma. Mol Imaging Biol. doi:10.1007/s11307-011-0509-z. [DOI] [PubMed]
- 33.Huyn ST, Burton JB, Sato M, Carey M, Gambhir SS, Wu L. A potent, imaging adenoviral vector driven by the cancer-selective mucin-1 promoter that targets breast cancer metastasis. Clin Cancer Res. 2009;15(9):3126–3134. doi: 10.1158/1078-0432.CCR-08-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2(2):107–115. [PubMed] [Google Scholar]
- 35.Faivre J, Clerc J, Gerolami R, Herve J, Longuet M, Liu B, et al. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats. Cancer Res. 2004;64(21):8045–8051. doi: 10.1158/0008-5472.CAN-04-0893. [DOI] [PubMed] [Google Scholar]
- 36.Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Ther. 2005;12(3):272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]