Abstract
Purpose
The purpose of this study was to investigate the usefulness of metabolic-volumetric indices of 18F- fluorodeoxy-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) for the evaluation of neoadjuvant chemotherapy outcomes in breast cancer.
Methods
Twenty-four patients with locally advanced breast cancer were enrolled in the study. They underwent baseline 18F-FDG PET/CT scan and received four or six cycles of neoadjuvant chemotherapy, interim 18F-FDG PET/CT was done after second cycle of chemotherapy. Maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary lesions were calculated. Reduction rates of these parameters were obtained between baseline and interim 18F-FDG PET/CT. Chemotherapy outcomes were assessed using tumor size reduction rate and histological grading system (Miller and Payne system). Reduction rates of SUVmax, MTV, and TLG correlated with chemotherapy outcomes.
Results
MTV and TLG reduction rates showed significant correlation with tumor size reduction rate (R = 0.68, P = 0.0004; R = 0.62, P = 0.002, respectively). However, SUVmax reduction rate showed no significant correlation. MTV and TLG reduction rates were significantly higher in responders than nonresponders, as determined by Miller and Payne system (P < 0.0007, P < 0.002). However, SUVmax reduction rate showed no significant difference. On ROC analysis, the area under the MTV and TLG curves was 0.886, and that of SUVmax was 0.743. Sensitivity, specificity, positive predictive value, and negative predictive value to predict histopathologic response were the same for MTV and TLG, and the values were 100 %, 85.7 %, 83.3 %, and 100 %, respectively (at the reduction rate of 93.2 % for MTV, and 95.8 % for TLG).
Conclusion
Changes of metabolic–volumetric indices successfully reflected the neoadjuvant chemotherapy outcomes. MTV and TLG could be robust indices in discriminating pathologic responder as SUVmax, after neoadjuvant chemotherapy.
Keywords: Breast cancer, Neoadjuvant chemotherapy, Metabolic tumor volume, Total lesion glycolysis, 18F-FDG PET/CT
Introduction
Breast cancer is the most common malignancy among women, and locally advanced disease accounts for approximately 5–7 % at diagnosis in United States [1, 2]. Currently, standard treatment for locally advanced breast cancer includes neoadjuvant chemotherapy, due to its several advantages. The main advantage of neoadjuvant chemotherapy is down-staging of the tumor load. As a result, inoperable advanced tumors may become operable, and patients with large operable tumors may be offered breast-conserving surgery. Another advantage of neoadjuvant chemotherapy is the possibility to monitor the response of the primary tumor to the chemotherapy agents that were used [3]. And early clinical response after two cycles of neoadjuvant chemotherapy was found to be a predictor of pathologic complete remission, and might be a predictor of long-term outcome [4]. Thus, early prediction of the response of neoadjuvant chemotherapy is invaluable, because early prediction could guarantee early guidance for proper treatment.
18F-fluorodeoxy-D-glucose (18F-FDG) positron emission tomography (PET) has been evaluated to be a useful tool for predicting the response after chemotherapy in various types of cancer, including breast cancer [5–7]. Especially in breast cancer, several studies showed possible roles of 18F-FDG PET for early prediction of neoadjuvant chemotherapy response [8–11]. In these studies, relative changes in maximum standardized uptake value (SUVmax) after the first or second cycle of chemotherapy are a strong predictor of response. However, the diagnostic power was relatively low and optimal cutoff was highly heterogeneous, so that the role of early 18F-FDG PET/computed tomography (CT) scan during neoadjuvant chemotherapy remains unclear in clinical practice.
We expected that volume–metabolism combinatorial indices, such as metabolic tumor volume (MTV) or total lesion glycolysis (TLG) of 18F-FDG PET/CT, could be more reliable than SUVmax in predicting the chemotherapy response, because SUVmax represents only the most active tumor portions, and may not represent whole tumor status, especially after chemotherapy. MTV and TLG of 18F-FDG PET/CT are suggested to be better indicators of whole tumor burden than SUVmax, and proved to be prognostic factors at diagnosis in a variety of malignancies. [12–17]. Moreover, these indices demonstrated the possibility of predicting chemotherapy effect in osteogenic sarcoma, not only at the end of the neoadjuvant chemotherapy [18, 19], but also after the second cycle [20].
The purpose of this study is to predict pathologic outcome during neoadjuvant chemotherapy in breast cancer by 18F-FDG PET/CT, and to compare the indices of SUVmax/MTV/TLG.
Materials and Methods
Patients
Twenty-four patients who were recommended to be treated with neoadjuvant chemotherapy for locally advanced breast cancer were retrospectively enrolled. The study was approved by the Institutional Review Board for review of medical records of the patients. Patients with breast cancer larger than 2 cm in diameter and/or lymph node metastasis are recommended to be treated with neoadjuvant chemotherapy in our institute. Among the 27 patients who underwent 18F-FDG PET/CT before and during neoadjuvant chemotherapy in our institute between March 2009 and May 2010, three were excluded because the chemotherapy regimens were switched during neoadjuvant chemotherapy. All breast cancers were initially diagnosed by fine needle aspiration. Core needle biopsy was done to evaluate hormone receptors and human epidermal growth factor receptor 2 (HER2) status. We consider estrogen receptor and progesterone receptor positive when immunoreactive cell nuclei were more than 10 %. HER2 positivity was defined as 3+ by immunohistochemistry, and 1+, 2+ plus positive fluorescence in situ hybridization result. All patients underwent baseline magnetic resonance imaging (MRI) scan before neoadjuvant chemotherapy. Patients received four or six cycles of neoadjuvant chemotherapy before surgery, and interim 18F-FDG PET/CT scan was done after the second cycle of chemotherapy. The age distribution of the patient group was 44 ± 10 years old (range: 22–88). Most of them received anthracycline -based chemotherapy, and the most common type of chemo-regimen was four cycles of adriamycin with cyclophosphamide (AC) in 15 patients, while the second most common chemo-regimen was six cycles of docetaxel with adriamycin (DA) in six patients. The others received six cycles each of Paclitaxel/Avastin, Docetaxel/Herceptin, and Epirubicin/docetaxel. The mean duration of neoadjuvant chemotherapy was 89.6 ± 49.8 days (Table 1), and mean duration between baseline and interim 18F-FDG PET/CT scan was 53.6 ± 25.1. After surgery, tumor specimens were examined to determine responders and nonresponders by the Miller and Payne system [21]. Patients who underwent breast-conserving surgery subsequently received local radiotherapy.
Table 1.
Patient characteristics
| Characteristics | Value | % |
|---|---|---|
| Number of patients | 24 | |
| Age at diagnosis, years | ||
| Median | 44 | |
| Range | 22–88 | |
| cT staging | ||
| T1 | 1 | 4.2 |
| T2 | 12 | 50 |
| T3 | 9 | 37.5 |
| T4 | 2 | 8.3 |
| cN staging | ||
| N1 | 17 | 70.8 |
| N2 | 2 | 8.3 |
| N3 | 5 | 20.8 |
| Tumor histology | ||
| Invasive ductal carcinoma | 24 | 100 |
| Hormone receptor status | ||
| ER/PR positive | 10 | 41.7 |
| ER/PR negative | 14 | 58.3 |
| HER2 status | ||
| Positive | 11 | 45.8 |
| Negative | 13 | 54.2 |
| Type of surgery | ||
| Mastectomy | 16 | 66.7 |
| Breast conserving surgery | 8 | 33.3 |
| Chemotherapy regimen | ||
| AC (adriamycin + cyclophosphamide) | 15 | 62.5 |
| DA (docetaxel with Adriamycin) | 6 | 25 |
| Others (Paclitaxel/Avastin, Docetaxel/Herceptin, Epirubicin/docetaxel) | 3 | 12.5 |
18F-FDG PET/CT Protocol
18F-FDG PET/CT was performed using a PET/CT scanner (Discovery VCT, GE Medical Systems, Milwaukee, WI) in 3D acquisition mode with a 128 × 128 matrix size. After fasting for at least 6 h, appropriate blood sugar level was checked (< 180 mg/dL). Amount of intravenous administration of 18F-FDG was 5.18 MBq/Kg. CT acquisition were 120 kVp, 75 mm (6 × 0.625 mm) slice thickness. PET emission images were obtained 1 hour after injection of 18F-FDG, 5–6 bed position (2.5 min/bed) covering from base of cerebellum to upper thigh, and attenuation correction was done by CT images. Images were reconstructed using an iterative algorithm (ordered-subset expectation maximization, two iterations and eight subsets).
Measurement of SUVmax, MTV and TLG
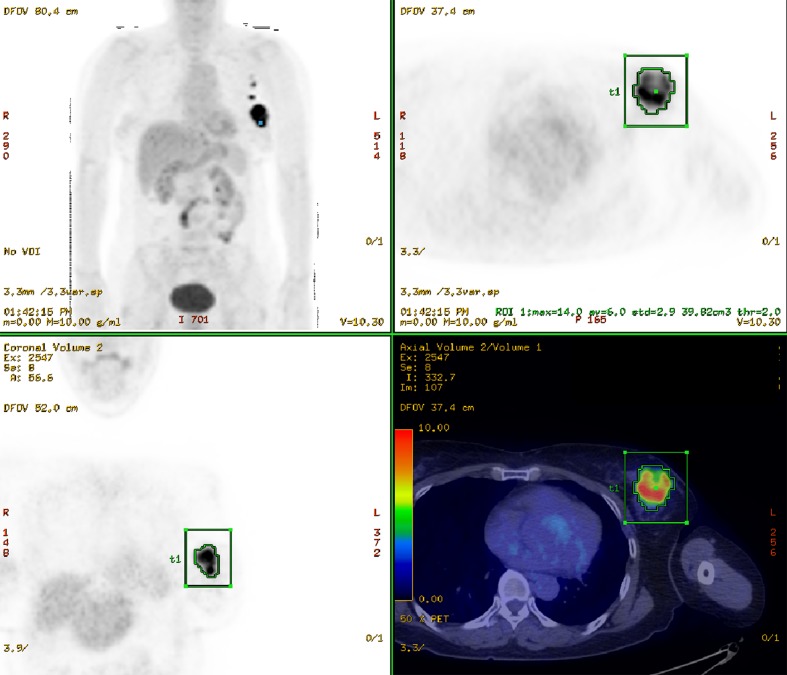
To quantify 18F-FDG uptakes, standardized uptake values (SUV) were calculated as follows: 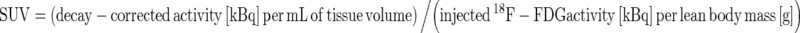 . SUVmax is value of highest SUV in the given volume of interest (VOI). VOI was drawn over the breast cancer lesion that showed increased 18F-FDG uptake on corresponding soft tissue lesion in combined CT. Using PET VCAR application (Advanced workstation 4.4, GE Medical Systems, Milwaukee, WI), we drew a cuboid VOI covering a breast cancer lesion and then VOI was automatically drawn along the margin of the tumor uptake according to the specific SUV threshold. MTV refers to the volume of tumor that has SUV over a certain threshold SUV; in this study, we used 2.0 as threshold SUV [22] (Fig. 1). MTV and TLG were automatically calculated by PET VCAR application (Advanced workstation 4.4, GE Medical Systems, Milwaukee, WI). SUVmax, MTV, and TLG were calculated in baseline and interim PET/CT, and then reduction rates (RR) of these indices were calculated as follows.
. SUVmax is value of highest SUV in the given volume of interest (VOI). VOI was drawn over the breast cancer lesion that showed increased 18F-FDG uptake on corresponding soft tissue lesion in combined CT. Using PET VCAR application (Advanced workstation 4.4, GE Medical Systems, Milwaukee, WI), we drew a cuboid VOI covering a breast cancer lesion and then VOI was automatically drawn along the margin of the tumor uptake according to the specific SUV threshold. MTV refers to the volume of tumor that has SUV over a certain threshold SUV; in this study, we used 2.0 as threshold SUV [22] (Fig. 1). MTV and TLG were automatically calculated by PET VCAR application (Advanced workstation 4.4, GE Medical Systems, Milwaukee, WI). SUVmax, MTV, and TLG were calculated in baseline and interim PET/CT, and then reduction rates (RR) of these indices were calculated as follows.
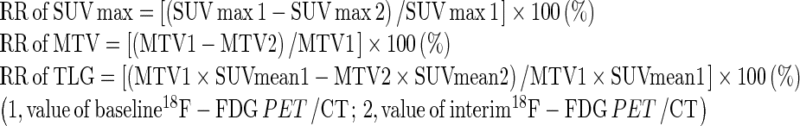 |
Fig. 1.
Automatic calculation of MTV and TLG by PET VCAR application of Advanced workstation 4.4 (GE Medical Systems, Milwaukee, WI). Whole-body 18F-FDG PET MIP image; the transaxial, coronal image, and the 18F-FDG PET/CT fusion transaxial image of patient with left breast cancer and left axillary lymph node metastasis is shown. Inside the green cuboid VOI covering breast cancer lesion, another VOI is automatically delineated on FDG avid portion (SUV threshold > 2.0). And MTV, TLG of the VOI are automatically calculated
** If a lesion is indistinguishable with surrounding tissue on interim PET/CT, RR is considered as 100 %.
MRI
The MRI was performed as previously reported [23], with a 1.5 Tesla machine (GE Medical Systems, Milwaukee, WI) using a dedicated breast coil (GE Medical Systems, Milwaukee, WI). The following is the brief imaging protocol; fat-suppressed T2-weighted fast spin-echo sagittal images were obtained after an axial localizer image. Dynamic contrast-enhanced examinations include one pre-contrast and five post-contrast (76 s, 165 s, 345 s, 434 s, and 583 s after contrast injection). Gadobenate dimeglumine (0.1 mmol/kg Multihance; Bracco Imaging, Milan, Italy) was automatically injected through an indwelling IV catheter. Two dimensional diameters of the tumors were measured in post-contrast images by two experienced radiologists.
Determination of Chemotherapy Response
Response of neoadjuvant chemotherapy was evaluated with two different ways measuring reduction rate of tumor size and cellularity. Surgery was performed 30.5 ± 2.6 days after completion of last cycle of chemotherapy. First, tumor size reduction rate was calculated. Baseline tumor size was estimated as the geometric mean of the largest two diameters (D1, D2) of the tumor in baseline MR images, and the tumor size after neoadjuvant chemotherapy was estimated as the geometric mean of the largest two diameters (d1, d2) of the viable tumor portion in surgical specimen after neoadjuvant chemotherapy [24]. The largest two diameters were obtained from pathologic reports.
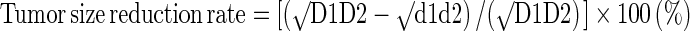 |
- √d1d2
Viable tumor dimension on specimen
- √D1D2
Primary tumor dimension in baseline MR image
Next, tumor specimens were microscopically examined and poor responders and responders were classified by the Miller and Payne system. This grading system evaluates the degree of reduction in tumor cellularity, assessed by comparing the tumor cellularity observed in the residual breast tumor tissue at surgery with a pretreatment core biopsy. The tumors were graded on a scale from 1 to 5 as follows: tumor regression grade (TRG) 1, no response to treatment; TRG 2, < 30 % reduction in cellularity; TRG 3, from 30 % to 90 % reduction in cellularity; TRG 4, > 90 % and < 100 % reduction in cellularity; and TRG 5, a complete response with no residual tumor. Patients were grouped according to prognosis as established by the scale: responder (TGR 4, 5) and nonresponder (TGR 1–3) [21].
Statistical Analysis
All of the statistical analyses were performed using MedCalc for Windows, version 9.4.2.0 (MedCalc Software 10.1, Belgium). Correlation analysis was done between estimated tumor sizes by MR and MTV of 18F-FDG PET/CT, and between tumor size reduction rate and reduction rate of the 18F-FDG PET indices. T-tests were done between reduction rates of the parameters of responders and nonresponders classified by Miller and Payne system. Receiver operating characteristic (ROC) curve analysis was used to compare diagnostic power of the indices and find optimal cutoff. McNemar’s chi square test was done to compare sensitivity and specificity of each index.
Results
Patients and Histopathologic Response
All 24 breast cancers were histologically confirmed as invasive ductal carcinoma. Half of the patients had cT2 and except for one patient, the others had higher T stage. All patients had lymph node metastasis and the majority of them were cN1 (70.8 %). After neoadjuvant chemotherapy, all patients underwent definitive operation; the majority of them (66.7 %) had mastectomy and the others had breast-conserving surgery. By histopathology examination, ten of the 24 classified as responder, and 14 as nonresponder by the Miller and Payne system. Estrogen and progesterone receptor was positive in ten cases, and HER2 was positive in 11 (Table 1).
Correlation Between Tumor Size Reduction Rate and Reduction Rate of Interim 18F-FDG PET Indices
All 18F-FDG PET/CT indices reduced significantly after the second cycle of chemotherapy (P < 0.05). Tumor size, which is estimated by MRI (before neoadjuvant chemotherapy) and pathologic specimen (after neoadjuvant chemotherapy), also significantly reduced after neoadjuvant chemotherapy (P < 0.05). SUVmax at baseline was 7.9 ± 6.9 (mean ± SD) and 2.5 ± 1.7 at interim 18F-FDG PET/CT. MTV at baseline was 14.8 ± 13.6 mL and 1.6 ± 2.4 mL at interim 18F-FDG PET/CT. TLG at baseline and at interim 18F-FDG PET/CT was 74.8 ± 95.8 g and 4.6 ± 6.6 g respectively. Tumor size estimated by pretreatment MRI was 4.6 ± 2.6 cm2 and size of viable tumor portion after chemotherapy on surgical specimen was 1.8 ± 1.9 cm2.
MTV and TLG reduction rates showed significant correlation with tumor size reduction rate (r = 0.68, P = 0.0004; r = 0.62, P = 0.002, respectively). However, there was no significant correlation between tumor size reduction rate and reduction rate of SUVmax (r = 0.38, P = 0.07) (Fig. 2).
Fig. 2.
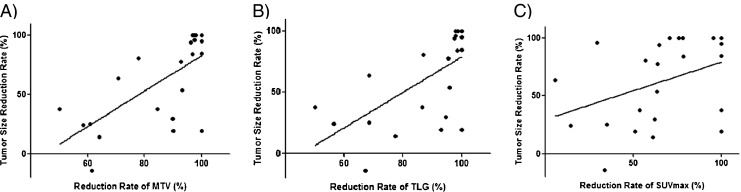
Scatter-gram and linear regression line between tumor size reduction rate and reduction rate (RR) of 18F-FDG PET/CT indices; Significant correlation was found in MTV (a, r = 0.68, P = 0.0004) and TLG (b, r = 0.62, P = 0.002), but not in SUVmax (c, r = 0.38, P = 0.07)
Reduction Rate of Interim 18F-FDG PET/CT Indices According to Histopathologic Tumor Response
Reduction rates of interim 18F-FDG PET/CT indices of responders (TRG 4,5) and nonresponders (TRG 1–3) were compared. Reduction rate of MTV and TLG were significantly higher in responders than in nonresponders (P = 0.0007, P = 0.002). SUVmax reduction rate showed a trend toward difference; however, it did not reach statistical significance (P = 0.08) (Table 2, Fig. 3).
Table 2.
Reduction rate of 18F-FDG PET indices in responders and nonresponders
| Indices | Reduction rate % | P value | ||
|---|---|---|---|---|
| Total (N = 24) | Responders (n = 10) | Nonresponders (n = 14) | ||
| SUVmax | 55.1 ± 23.9 | 72.8 ± 23.2 | 53.6 ± 25.7 | 0.08 |
| MTV | 86.6 ± 15.9 | 98.3 ± 1.4 | 78.1 ± 16.3 | 0.0007 |
| TLG | 88.8 ± 15.0 | 98.9 ± 0.9 | 81.5 ± 16.1 | 0.002 |
18 F-FDG PET 18F- fluorodeoxy-D-glucose positron emission tomography; SUVmax Maximum standardized uptake value; MTV metabolic tumor volume; TLG total lesion glycolysis
Fig. 3.
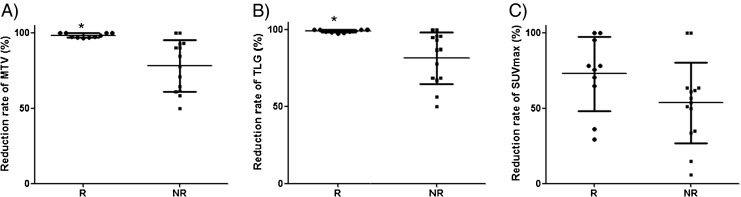
Reduction rates of the indices (a, MTV; b, TLG; c, SUVmax) in responders and nonresponders classified by the Miller and Payne system. Reduction rate (RR) of MTV and TLG were significantly higher in responders than in nonresponders (P = 0.0007, P = 0.002) but SUVmax reduction rate was not (P = 0.08); R = responders; NR = nonresponders; * = P < 0.05
Determination of the Optimal Cutoff Value of the Reduction Rate of Interim 18F-FDG PET/CT Indices to Predict Tumor Response
Receiver operating characteristic (ROC) curve analysis was done to determine the optimal cutoff value of the reduction rate of interim 18F-FDG PET/CT indices to predict tumor response. ROC curves of reduction rate of MTV and TLG to predict histopathologic response (Miller and Payne system) were equally drawn with an area under curve of 0.886 (95%CI 0.690 to 0.977, P = 0.0001). AUC by reduction rate of SUVmax was 0.743 (95 % CI, 0.525–0.897, P = 0.02) (Fig. 4). With the cutoff value to differentiate responder from nonresponder at the reduction rate of MTV 93.2 %, and TLG 95.8 %, sensitivity, specificity, positive predictive value, and negative predictive value were 100 %, 85.7 %, 83.3 %, and 100 % respectively (same values between MTV and TLG). By the reduction rate of SUVmax, at the cutoff value of 63.6 %, sensitivity, and negative predictive value were lower than those of MTV and TLG, and specificity and positive predictive value were comparable with those of MTV and TLG (Table 3). However, differences of sensitivity and specificity between indices were not statistically significant (P = n.s.).
Fig. 4.
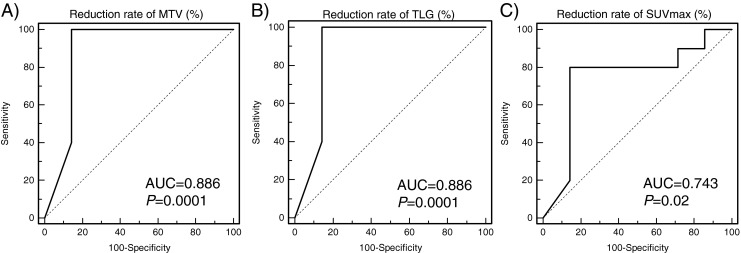
ROC curve analysis of response prediction by reduction rate of 18F-FDG PET/CT indices (a, reduction rate of MTV; b, reduction rate of TLG; c, reduction rate of SUVmax). AUCs of reduction rate of MTV, TLG, SUVmax were 0.886, 0.886 and 0.743
Table 3.
Diagnostic accuracy of reduction rate of PET/CT indices
| Cutoff (%) | AUC | Sen (%) | Spe (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|---|
| RR of SUVmax | 63.6 | 0.743 | 80 | 85.7 | 80 | 85.7 | 83.3 |
| RR of MTV | 93.2 | 0.886 | 100 | 85.7 | 83.3 | 100 | 91.7 |
| RR of TLG | 95.8 | 0.886 | 100 | 85.7 | 83.3 | 100 | 91.7 |
RR Reduction rate; AUC Area under Receiver Operative Characteristics Curve; Sen sensitivity; Spe specificity; PPV positive predictive value; NPV negative predictive value; SUVmax Maximum standardized uptake value; MTV metabolic tumor volume; TLG total lesion glycolysis
Discussion
18F-FDG PET/CT has been used in predicting early response of chemotherapy in various types of malignancy. Particularly, change in 18F-FDG PET after one or two cycles of chemotherapy reflects the response to chemotherapy, and is related to prognosis [4, 25]. The present study was conducted to show usefulness of MTV and TLG over SUVmax in predicting tumor response, and the present study showed that firstly, MTV and TLG correlated more precisely with tumor size reduction rate than SUVmax. This result is probably due to the help of volumetric information of MTV and TLG. MTV and tumor size estimated by MRI at baseline showed moderate correlation (r = 0.62, P = 0.002). Secondly, MTV and TLG showed better ability to predict responder, as classified by the Miller and Payne system, than SUVmax. The Miller and Payne system reflects the decrease in viable cancer cellularity, not tumor size. In previous studies, 18F-FDG PET has been shown to discriminate responders according to the Miller and Payne system with SUVmax, which reflects metabolic status of viable cancer [11–14]. Similarly, in the present study, SUVmax decreased more in responders (72.8 ± 23.2 %) than in nonresponders (53.6 ± 25.7 %) the second cycle of chemotherapy. Also, the level of decrement in responders and nonresponder is similar to previous studies [4, 8]. However, the difference was not statistically significant, probably due to the small number of patients in the present study. On the other hand, MTV and TLG discriminate responders from nonresponder with statistical significance, despite the small number of patients (P = 0.0007, 0.002). SUVmax represent only one spot that has the highest SUV; however, pathologic examination may not be done at the point that showed highest SUV, but is done at several points of the tumor bed to assess whole tumor status. Thus, we could assume that metabolic status of whole tumor can be more correctly reflected by MTV and TLG than SUVmax. For example, SUVmax of one patient modestly decreased after neoadjuvant chemotherapy (from 3.4 to 2.4, reduction rate = 29.4 %), but that patient was found to be a responder in histopathologic examination. On the contrary, change of MTV (from 4 to 0.1, reduction rate = 97.5 %) and TLG (from 9.2 to 0.2, reduction rate = 97.9 %) of the patient correctly predicted the tumor response. This result is in accordance with the ressult of a previous study done in an osteosarcoma cohort [20].
After testing various thresholds, we used threshold SUV 2.0 for calculating MTV and TLG. Although previously, SUV 2.5 has been used most frequently as the threshold in head and neck cancer and esophageal cancer [12–14], SUV 2.5 neglects a considerable portion of tumor, especially after chemotherapy in breast cancer. Further, one previous report also showed that MTV with threshold SUV 2.0 was the most robust predictor of outcome in head and neck cancer among MTV with various thresholds of SUV 2.0 to ~ 4.0 [22]. In addition, using lower threshold could be suggested in breast cancer for sensitive tumor detection, because there is almost no FDG avid area with more than SUV 2.0 in breast and chest wall under normal conditions [26]. Finally, MTV with a threshold of SUV 2.0 are well correlated with MRI-based tumor size in our study.
Response prediction capability of reduction rate of MTV and TLG were compared when different thresholds of SUV were used. MTV with lower SUV threshold (1.5 and 1.0) or another suggested method, fixed percentage of SUVmax (25 %. 50 %, 75 %), were tested, but showed major discordance in lesion delineation between 18F-FDG PET image and combined CT/corresponding MRI. On comparison of MTV reduction rate with threshold SUV 2.5 and SUV 2.0, there were two more false positives and one more false negative case with SUV 2.5 threshold, which might be caused by underestimation of residual MTV or underestimation of primary tumor before chemotherapy. And on comparison of TLG reduction rate, there were four more false positives with threshold SUV 2.5 than with threshold SUV 2.0.
The present study has a few limitations. The first is the retrospective design with inhomogeneous chemo-regimen of the study. However, duration of chemotherapy and interval between baseline and interim 18F-FDG PET/CT were relatively homogenous. Secondly, we could not demonstrate the statistical difference between diagnostic performances to differentiate responders between SUVmax, MTL and TLG. However, considering that SUVmax is the only used 18F-FDG PET/CT parameter to assess response to chemotherapy in breast cancer despite of several limitations, showing non-inferiority of MTV and TLG in this study could be important information. Finally, the small number of patients is a limitation of the study. However, as far as we know, no study has been performed to assess the ability of MTV or TLG in predicting the chemotherapy response in breast cancer. Thus, the promising results of the present study could give rise to more studies with prospective design and large populations, to assess MTV and TLG as a predictor during neoadjuvant chemotherapy in breast cancer.
Conclusion
Changes of combined metabolic–volumetric indices, MTV and TLG, between baseline and interim 18F-FDG PET/CT after the second cycle of neoadjuvant chemotherapy successfully predicted the pathologic outcomes of neoadjuvant chemotherapy in breast cancer. Larger case studies are needed to determine the most useful 18F-FDG PET/CT index for evaluating neoadjuvant chemotherapy outcomes in breast cancer.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korea government (MEST) (No. 2011-0018636).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. [DOI] [PubMed]
- 2.Giordano SH. Update on locally advanced breast cancer. Oncologist. 2003;8:521–530. doi: 10.1634/theoncologist.8-6-521. [DOI] [PubMed] [Google Scholar]
- 3.Groheux D, Giacchetti S, Espie M, Rubello D, Moretti JL, Hindie E. Early monitoring of response to neoadjuvant chemotherapy in breast cancer with (18)F-FDG PET/CT: defining a clinical aim. Eur J Nucl Med Mol Imaging. 2011;38:419–25. [DOI] [PubMed]
- 4.Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–5372. doi: 10.1200/JCO.2006.05.7406. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp M, van Westreenen HL, Reitsma JB, Hoekstra OS, Stoker J, Fockens P, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology. 2005;236:841–851. doi: 10.1148/radiol.2363041042. [DOI] [PubMed] [Google Scholar]
- 6.Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47:14–22. [PubMed] [Google Scholar]
- 7.Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101–2111. doi: 10.1200/JCO.1993.11.11.2101. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Kumar R, Seenu V, Gupta SD, Chawla M, Malhotra A, et al. The role of 18F-FDG PET/CT in evaluation of early response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Eur Radiol. 2009;19:1347–1357. doi: 10.1007/s00330-009-1303-z. [DOI] [PubMed] [Google Scholar]
- 10.Duch J, Fuster D, Munoz M, Fernandez PL, Paredes P, Fontanillas M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–1557. doi: 10.1007/s00259-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 11.Martoni AA, Zamagni C, Quercia S, Rosati M, Cacciari N, Bernardi A et al. Early (18)F-2-fluoro-2-deoxy-d-glucose positron emission tomography may identify a subset of patients with estrogen receptor-positive breast cancer who will not respond optimally to preoperative chemotherapy. Cancer. 2010;116:805–13. [DOI] [PubMed]
- 12.Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–8. [DOI] [PubMed]
- 13.Roedl JB, Halpern EF, Colen RR, Sahani DV, Fischman AJ, Blake MA. Metabolic tumor width parameters as determined on PET/CT predict disease-free survival and treatment response in squamous cell carcinoma of the esophagus. Mol Imaging Biol. 2009;11:54–60. doi: 10.1007/s11307-008-0169-9. [DOI] [PubMed] [Google Scholar]
- 14.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–5868. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 15.Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73:332–337. doi: 10.1016/j.lungcan.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Choi K-H, Yoo IR, Han EJ, Kim YS, Kim GW, Na SJ, et al. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging. 2011;45:43–51. doi: 10.1007/s13139-010-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BS, Kim IJ, Kim S-J, Nam H-Y, Pak KJ, Kim K, et al. The prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011;45:36–42. doi: 10.1007/s13139-010-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–347. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 19.Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009;50:1435–1440. doi: 10.2967/jnumed.109.063602. [DOI] [PubMed] [Google Scholar]
- 20.Im HJ, Kim TS, Park SY, Min HS, Kim JH, Kang HG, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012;39:39–49. doi: 10.1007/s00259-011-1936-4. [DOI] [PubMed] [Google Scholar]
- 21.Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–327. doi: 10.1016/S0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 22.Murphy JD, La TH, Chu K, Quon A, Fischbein NJ, Maxim PG, et al. Postradiation metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:514–521. doi: 10.1016/j.ijrobp.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyou CY, Cho N, Kim SM, Jang M, Park JS, Baek SY et al. Computer-aided evaluation of breast MRI for the residual tumor extent and response monitoring in breast cancer patients receiving neoadjuvant chemotherapy. Korean J Radiol. 2011;12:34–43. [DOI] [PMC free article] [PubMed]
- 24.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 25.McDermott GM, Welch A, Staff RT, Gilbert FJ, Schweiger L, Semple SI, et al. Monitoring primary breast cancer throughout chemotherapy using FDG-PET. Breast Cancer Res Treat. 2007;102:75–84. doi: 10.1007/s10549-006-9316-7. [DOI] [PubMed] [Google Scholar]
- 26.Vranjesevic D, Schiepers C, Silverman DH, Quon A, Villalpando J, Dahlbom M, et al. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med. 2003;44:1238–1242. [PubMed] [Google Scholar]