As early as the mid 19th century, Virchow taught that necrosis is a recognizable form of cell death, and since then, pathologists have identified necrosis as a cause or consequence of disease. A century later, another form of cell death, apoptosis, was defined, and we now understand that this process is driven by a set of molecular mechanisms that "programs" the cell to die. It has often been assumed that necrosis is distinct from apoptosis; in part, because the former is not programmed by molecular events. In recent years, however, it has become clear that in some settings, necrotic cell death can also be driven by defined molecular pathways. Here, we discuss one such process, a type of necrotic cell death called "necroptosis1–4." and its role in disease. While some investigators have used this term to indicate any form of active necrosis, we will follow recent recommendations5 and use necroptosis to mean "necrotic cell death dependent on receptor-interacting protein kinase-3 (RIPK3)" (Fig. 1). With our understanding of the molecular basis of necroptosis and other forms of regulated necrosis, and the availability of inhibitors, a neglected therapeutic option emerges: It is possible to therapeutically interfere with necrosis.
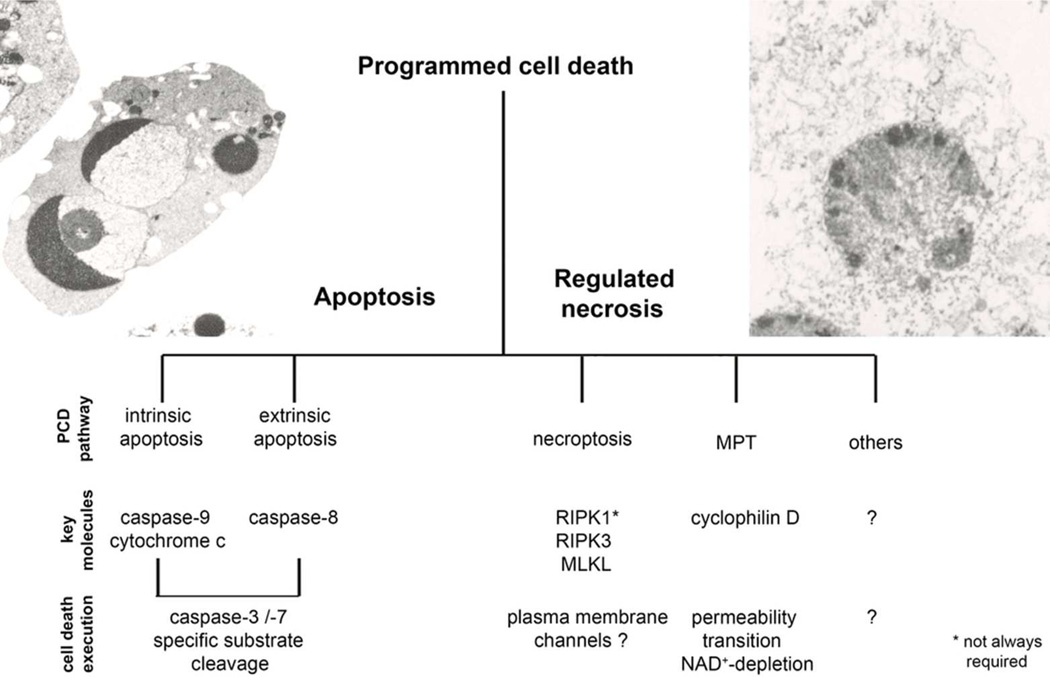
Figure 1. Programmed cell death is mediated by apoptosis or regulated necrosis.
The term programmed cell death was widely used synonymously with apoptosis until necrotic cell death was demonstrated to depend on genetically defined signaling pathways. Whereas the role of caspase-mediated apoptosis in diseases has been revealed in detail over the last three decades, the contribution of regulated necrosis to the pathophysiology of diseases was investigated only recently. Pathways of regulated necrosis include necroptosis, dependent on RIPK3, and regulated necrosis mediated by mitochondrial permeability transition (MPT) which involves cyclophilin D-dependent opening of the mitochondrial permeability transition pore. Whereas MPT and necroptosis have been demonstrated to represent two distinct pathways, other emerging signaling cascades of regulated necrosis have been described, but it remains currently unclear to what extent such pathways may have overlapping mechanisms.
While necroptosis may have evolved as a line of defense against intracellular infection6, 7, recent studies implicate it in a variety of disease states. In myocardial infarction and stroke8, 9, atherosclerosis10, ischemia-reperfusion injury11, 12, pancreatitis2, 4, 13, inflammatory bowel disease14, 15 and a number of other clinically common disorders, necroptosis is of central pathophysiological relevance. At the molecular level, intracellular assembly of a highly regulated complex, the “necrosome”, can be triggered by death receptors (such as TNFR1)16–18, cell surface Toll-like receptors (TLRs) 19–21, DNA-dependent activator of interferon regulatory factors (DAI which may act as a cytoplasmic viral RNA sensor),22, 23 and probably other signals.
How is necroptosis induced, and why?
Apoptotic cell death involves the engagement of pathways that result in the activation of caspase proteases that ultimately cause the morphological features of this type of cell death. In contrast, necroptosis, the focus of this review, was first recognized as a caspase-independent cell death that can be triggered by treatment with tumor necrosis factor (TNF) only in the presence of a pan-caspase inhibitor such as zVAD-fluoromethyl ketone (zVAD)24. Before that time, we understood that TNF induces apoptosis via the engagement of protein interactions that result in the activation of caspase-8; but necroptosis requires that the function of caspase-8 be inhibited or disrupted. Several of the upstream signaling elements of apoptosis and necroptosis are shared, and the sensitivity for either death-pathway is regulated (although sometimes in opposing ways) by an overlapping cluster of regulatory molecules, such as the FLICE-like inhibitory protein (FLIP)25, the deubiquitinases A20 and cylindromatosis, and the cellular Inhibitors of Apoptosis, cIAP1 and cIAP226, 27. Other death receptors28 and Toll-like receptors29 were shown to induce necroptosis, and subsequently intracellular triggers of necroptosis were also identified, such as DAI23 and protein kinase R (PKR)30 (Fig. 2). TNF receptor-1 (TNFR1)-ligation by TNF induces signaling via the NF-κB pathway that involves the polyubiquitinylation of receptor interacting protein kinase 1 (RIPK1) and the NF-kB essential modulator, NEMO31. Upon deubiquitinylation of the K63 and linear ubiquitin chains of RIPK131 by deubiquitinases32, 33, RIPK1 loses its default pro-survival function to promote cell death. TNFR1 recruits the adapter protein TRADD to associate with the adapter FADD34 which then binds to pro-caspase-8, a protease that autocatalytically activates upon homodimer formation. Conversely, FLIP, a protein that is structurally related to caspase-8 but lacks protease activity, forms a caspase-8-FLIP heterodimer, and this acts to prevent caspase-8-mediated apoptosis.17, 35 The caspase-8-FLIP heterodimer predominates in cells expressing FLIP, which is induced upon NF-kB activation. Upon loss of either caspase-8 or FLIP, or upon interference with the activation or function of caspase-8, RIPK1 forms an intracellular complex with RIPK3 to assemble the necrosome13, 36, 37, an intracellular amyloid-like structure38 that acts as the transducer of the necroptotic signal. Downstream of RIPK3 is another protein, mixed lineage kinase domain-like (MLKL)13, 36, 37, 39, 40, which is a pseudokinase, and functions to cause necroptosis in a manner that has not yet been elucidated.
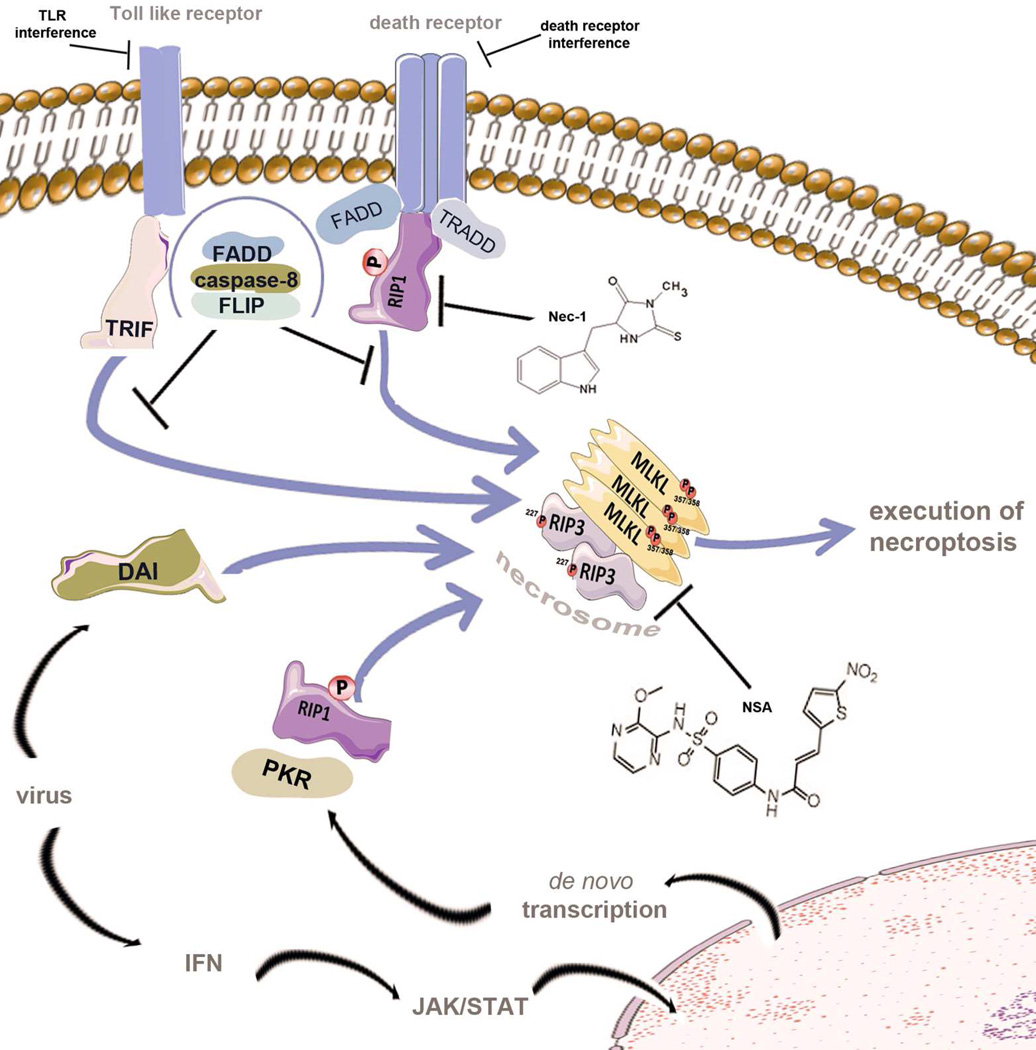
Figure 2. Activators of the necrosome as therapeutic targets.
Various stimuli lead to the activation of the supramolecular necroptosis-inducing complex, referred to as the necrosome. Initially, studies of necroptosis employed models of death receptor stimulation in the presence of caspase-inhibition (not shown). The intracellular adapter molecules FADD and TRADD recruit RIPK1, which subsequently undergoes an incompletely understood series of ubiquitylation, deubiquitinylation, and phosphorylation events before exposing its rip homotypic interaction motif (RHIM-domain) to recruit RIPK3. RIPK1, RIPK3 and MLKL are phosphorylated during the assembly of the necrosome. Within the human genome, RIPK1, RIPK3 and two other proteins exhibit RHIM-domains. One of these is TRIF, an intracellular signal transducer that is capable of activating the necrosome downstream of Toll-like receptors which are triggered by microbial molecules. The fourth RHIM-domain protein, DAI, integrates signals from viral RNA sensors into the necrosome. Finally, viral infection is accompanied by production of interferon, which triggers the JAK/STAT-dependent de novo synthesis of protein kinase R (PKR) which phosphorylates FADD to directly interact with RIPK1 and induce necrosome formation.
Death receptor-mediated necroptosis involves deubiquitinylation of RIPK1, the kinase domain of which is targeted by necrostatin-1 (Nec-1). Second generation RIP1-kinase inhibitors, such as Nec-1s, a stable version of Nec-1 which is more potent at lower concentrations, might reduce observed side effects. Necrosulfonamide (NSA) inhibits MLKL and prevents the activity of the necrosome in human cells. In addition, RIPK3-inhibitors, death receptor antagonists or plasma membrane channel blockers might be attractive therapeutical targets.
Deletion of FADD41, FLIP42 or caspase-843 in mice results in early embryonic lethality at day e10.5 which is fully rescued if the deletion is applied to mice that are already deficient in RIPK3. 35, 44. Tissue-specific deletion of FADD or caspase-8 also causes disease (dependent upon the tissue) involving cell death, and again this is prevented by ablation of RIPK314, 15. Therefore, a critical function of the FADD-caspase-8-FLIP complex appears to be the prevention of RIPK3-mediated necrotic cell death (although alternative interpretations are discussed below). It is on this basis that we have provided our definition of necroptosis as a form of regulated necrosis that follows an intracellular signaling cascade via RIPK3.
The downstream mediators in the necroptosis-pathway are currently incompletely understood, but it is tempting to speculate that plasma membrane channels are involved in the rapid swelling of necroptotic cells that results in plasma membrane rupture. Unlike in apoptosis, in which several of the highly immunogenic intracellular proteins are sequestered in the dead cell corpse, necroptosis is a strong trigger of innate and adaptive immune responses45. But why would higher organisms preserve immunogenic cell death? The answer may involve the recognition and response to microbes. RIPK3 can interact with other proteins through a Rip-Homotypic Interacting Motif (RHIM) present in both RIPK1 and RIPK3. To date, only four known RHIM-containing proteins, RIPK1, RIPK3, DAI and TRIF (TIR-domain-containing adapter-inducing interferon-β) have been identified in the human genome (although this may be a function of our ability to recognize this motif). TRIF is capable of triggering necroptosis following ligation of Toll-like receptors 3 and 421, and DAI integrates viral signals into the necroptosis pathway7. Indeed, infection with vaccinia virus, which expresses a viral caspase inhibitor, was lethal in RIPK3-deficient but not wild-type mice1, 46. Further, both apoptosis and necroptosis can be induced by Type I and Type II interferons, which promote the death and removal of virally-infected cells30. Several viruses and intracellular bacteria express proteins that interfere with the activation of caspase-8 and therefore can sensitize cells for necroptosis47. We can therefore hypothesize that necroptosis provides higher vertebrates with a defense mechanism for such intracellular invaders, and this is further supported by the identification of viral inhibitors of necroptosis7, 35. Similarly, loss of FLIP will also promote cell death by both apoptosis and necroptosis. Because FLIP undergoes rapid protein turnover and is expressed through NF-κB activation, anything that blocks de novo protein synthesis or interferes with NF-kB might therefore sensitize cells to die. Regulation of the necroptotic pathway by proteins such as the deacetylase SIRT248, acid sphingomyelinase49 and the mitochondrial phosphatase phosphoglycerate mutase 5 40 may also contribute to the control of this process under a variety of conditions, although further evidence for their involvement is needed. While the necroptotic pathway may be beneficial for humans to defend against some infections, this process may turn against us in a number of pathologic states.
The contribution of necroptosis to pathophysiology
The development of the RIP1 kinase inhibitor necrostatin-1 (Nec-1, a compound that was later found to be identical to a previously reported indoleamine 2,3-dioxygenase [IDO]-inhibitor50) has stimulated necroptosis research. The use of this agent was taken as evidence to support a role of necroptosis in neurologic disorders (Table S1), and the first disease model investigated for its contribution of necroptosis was the ischemic brain51. In addition to ischemic injury, neuronal tissue is associated with Nec-1-inhibitable cell death in clinically related models of brain damage, including controlled cortical impact52 and neonatal hypoxia-ischemia models in which Nec-1 not only protected from oxidative damage but also prevented the subsequent debilitating immune cell infiltration53, 54. Microglia that are treated with caspase-inhibitors were reported to exhibit significant necroptosis55 and this mechanism was interpreted as a protective strategy to save neurons, but detailed mechanisms in vivo are lacking. Necrotic cell death is also a hallmark of retinal detachment56 and retinal ischemic cell death57, and it has been suggested that in this specialized compartment, both apoptosis and necroptosis are triggered simultaneously56. Later, it was proposed that cones, but not rods, undergo necroptosis in a genetic model of retinitis pigmentosa58. These findings provided preliminary support for the concept that apoptosis and necroptosis are not mutually exclusive programs and may occur in the same organ.
Several independent studies related the effects of Nec-1 to genetic ablation of RIPK3, and confirmed effects of either in ischemic organ damage and ischemia-reperfusion injury in the heart and the kidney in mice (Table S1). Whereas Nec-1 strikingly protected in brain ischemia models51, prevention of cardiac remodelling after myocardial infarction12 and myocardial9 and renal ischemia-reperfusion injury11 was marked, but not excessive. Application of Nec-1 30 minutes after reperfusion failed to protect from kidney ischemia-reperfusion injury11, pointing to off-target effects of Nec-1, a rapid assembly of the necrosome, or another role for RIPK1 in endothelial cells of peritubular capillaries. The latter might be of particular interest, as necroptosis has not been demonstrated in primary kidney cells, neurons, retinal cells or cardiomyocytes. Nevertheless, RIPK3-deficient mice are protected from ischemia-reperfusion injury, and treatment of RIPK3-deficient mice with Nec-1 does not provide further protection59. Changes in parenchymal blood flow might explain the benefit of blocking necroptosis in models of ischemia-reperfusion injury as Nec-1 may influence the capillary diameters60. It was demonstrated that glomerular endothelial cells, in contrast to renal tubular cells, mesangial cells or podocytes, express high levels of RIPK311, which can correlate with the likelihood of cells to undergo necroptosis upon death receptor ligation2. Therefore, it will be of interest to see if MLKL-deficient mice13 confirm the involvement of the necroptosis pathway in IRI. Ultimately, it will be important to develop tissue-specific RIPK3 and/or MLKL deletion models to investigate the tissues most relevant to the role of necroptosis in ischemia-reperfusion injury.
Necroptosis may also be associated with disorders in the skin and intestinal epithelium. A RIPK3-dependent dermal chronic inflammatory phenotype results from conditional deletion of FADD61 or caspase-862 from keratinocytes, a phenomenon that is partly reversed on a TNFR1-deficient background61. While other dermatological disorders have not yet been linked with necroptosis, it is tempting to speculate that regulated necrosis triggers skin infections, such as atopic dermatitis. Apart from atopic dermatitis, chronic proliferative dermatitis was described for mice deficient in the RIPK1-regulator SHARPIN63, a component of the linear ubiquitin chain assembly complex, and this was reversed by combined deficiency of TNFR131. However, it is not yet clear how RIPK3 -dependent necroptosis is involved in this inflammatory reaction.
As with epithelial cells of the skin, specific depletion of caspase-8 or FADD from intestinal epithelium results in spontaneous necroptosis and pathologies that are morphologically similar to those seen in inflammatory bowel diseases, especially Crohn´s disease14, 15(Table S1). Crossing the conditionally deleted FADD- or caspase-8 mice to a RIPK3-deficient background completely prevented this pathology. Similarly, ablation of the RIPK1-deubiquitinase A20 sensitizes for lethal colitis, and this effect is caused by TUNEL-positive TNF-mediated cell death of the intestinal epithelial cells14, 15. While this was interpreted as apoptosis, it remains possible that this cell death may involve necroptosis. Mechanistically, these inflammatory conditions may be due to the high immunogenicity of necrotic cells and/or the loss of barrier function that occurs upon such cell death45. In contrast to the chronic inflammation in Crohn´s disease, necrotizing pancreatitis is clinically characterized by acute appearance. The preclinical model of cerulein-induced pancreatitis was the first description of necroptosis in the gastrointestinal tract2, 4. Because necrotizing pancreatitis is a devastating disorder in which conventional treatment is limited to intensive care, application of fluids and anesthetics, the potential interference with necroptosis has raised significant hopes for therapy. However, whereas RIPK3−/− mice showed marked protection from cerulein-induced pancreatitis 2, 4, Nec-1 administration increased serum lipase and amylase levels as well as histological damage scores64. Recently, MLKL−/− mice were reported to be protected from cerulein-induced pancreatitis 13, yet another finding that keeps the debate ongoing. A possible explanation might be the short half-life of Nec-1, and second generation RIP1-kinase inhibitors or MLKL inhibitors are expected to clarify this issue. However, regardless of whether or not it will someday be possible to interfere with disease-promoting necroptosis in ischemic events, sepsis, inflammatory bowel diseases or pancreatitis, in which it will be critical to assess the narrow therapeutic window due to the rapid assembly of the necrosome, an obvious clinical situation in which necroptosis is predictable is solid organ transplantation.
Cell damage-associated molecular patterns (DAMPs) are released from necroptotic cells and are heavy triggers of the immune system45, 65. It is conceivable that DAMPs, by their ability to activate both innate and adaptive immunity, promote many of the harmful immunologic responses observed in solid organ transplants and interference with necroptosis might be beneficial because the prevention of necrotic cell death i) minimizes the loss of functional parenchymal cells in the transplant and ii) the total amount of DAMPs would reduce pro-inflammatory responses that activate rejection pathways. Following this path, protection of RIPK3-deficient kidneys in a mouse model of allotransplantation with a strong survival benefit was recently reported66. In that model, inhibition of caspase-8 by siRNA upregulated necroptosis and reduced renal allograft survival whereas in comparison with kidneys from wild-type mice, RIPK3-deficient allografts had better renal function and longer rejection-free survival 66. Those experiments might impact on clinical transplantation, as it should be sufficient to saturate donor organs with necroptosis-inhibiting drugs prior to implantation by machine perfusion systems. However, a protective effect of Nec-1 or its derivatives has not been analyzed thus far in a transplantation model, and it must be taken into consideration that blockade of necroptosis may have side effects in transplanted patients who are on immunosuppression and commonly suffer from viral infection, e.g. cytomegalovirus infection67, even without blockade of necroptosis. Nevertheless, the potential to inhibit necroptosis will likely impact upon organ transplantation68. Further investigations are prerequisite to begin clinical trials to specifically target necroptosis and to elucidate the mechanisms by which RIPK3-deficiency benefits transplanted organs. Given the protection from ischemia reperfusion injury, it will be of importance to clearly separate primary cell death from secondary organ damage mediated by infiltrating immune cells45. Current everyday clinical strategies are based exclusively on the latter.
TNF-shock models suggested that RIPK1 and RIPK3 may be involved in TNF-induced signaling in endothelial cells (Table S1). In vivo intravenous injection of TNF causes acute TNFR1–dependent apoptotic detachment of enterocytes and kills mice within 48 hours64, 69. Addition of the pan-caspase inhibitor zVAD-fmk does not protect but rather accelerates time to death in this model, referred to as hyperacute TNF shock, which leads to death of all mice within 24 hours. RIPK3-deficiency partially protects from hyperacute TNF-shock64, 70. It should be noted that the hyperacute TNF-shock model does not accurately mimic sepsis, and that the more widely used sepsis model is that of cecal ligation and puncture (CLP)71. However, whereas one study found that Nec-1 was protective in the CLP-model 70, others reported that Nec-1 can further accelerate the time to death in the hyperacute TNF-shock model64, 72, and RIPK3-and MLKL-deficient mice appear to exhibit no benefit in the CLP-model 13, 64. The role of necroptosis in sepsis therefore remains an open question.
It is important to note that the involvement of RIPK3 in pathophysiology is not an unequivocal demonstration of a role for necroptosis, even as defined by us. RIPK3 activation, and its regulation by RIPK1 and the FADD-caspase-8-FLIP complex may directly cause inflammatory effects independently of necroptotic cell death. The activation of RIPK3 has been suggested to directly participate in inflammation mediated by the DNA sensor RIG-I73 and by the NLRP3 inflammasome39. At present, it is not possible to formally separate such putative effects from those of necroptosis and the release of DAMPs, especially since in at least one case, the pro-inflammatory effect was observed to also depend on MLKL39. Ultimately, the direct contribution of RIPK3 to inflammation versus its indirect contribution via necroptosis may have to await elucidation of the final effector mechanism of necroptosis. Therefore, while our admitted bias is that the effects of RIPK1 inhibition and/or RIPK3 ablation in pathophysiological settings is most likely due to effects on necroptosis, we recognize that alternatives exist. Nevertheless, this distinction may ultimately be irrelevant to clinicians, since interference with RIPK3 activation and/or function will likely have therapeutic benefits irrespective of the ultimate pathological mechanism. It is in that context that we continue our discussion of therapeutic intervention.
Therapeutic strategies for the prevention of necroptotic diseases
Theoretically, interference with necroptosis is possible at the levels of the receptor, RIPK1, RIPK3, MLKL, the assembly of the necrosome and undefined intermediate and downstream mechanisms (Fig. 2) that may ultimately lead to cellular swelling and plasma membrane rupture. With these targets only recently in hand, the major focus has been on Nec-1. Due to the outstanding specificity of Nec-1 to the RIP1 kinase74, some authors have referred to the inhibition by Nec-1 as a definition of necroptosis21. As the structural interaction of Nec-1 with the RIP1 kinase domain has been unraveled75, novel interpretations of the inhibition of necroptosis by Nec-1 have emerged. RIPK1-deficient mice die perinatally76, and it remains formally possible that RIPK1 acts as an inhibitor of necroptosis unless its kinase activity is engaged, and Nec-1 might stabilize RIPK1 in its inhibitory state.
Apparently, there are effects of Nec-1 that are not related to cell death60, and drawbacks regarding the clinical applicability of Nec-1 have been reported as the drug appears to accelerate death in some models in which RIPK3-ablation is beneficial (discussed above). Fortunately, second generation RIPK1-kinase inhibitors have been identified with higher affinity and specificity (Nec-1s)72, and the acceleration of disease was not observed for Nec-1s treatment for TNF shock72. Further studies in other models are expected to clarify the mechanisms of this dichotomy.
Another attractive treatment modality emerged from a direct inhibitor of human MLKL, necrosulfonamide (NSA)36. Although the potential application of NSA will depend on further analysis of its specificity and pharmacokinetics, it provides evidence that MLKL may serve as a drug target in principle. Further emerging concepts may include RIP3-kinase inhibitors, but it should be noted that besides phosphorylation of MLKL, relevant targets of the RIP3-kinase remain unknown. Identification of the putative necroptosis-mediating plasma membrane channels downstream of the necrosome might define promising targets. Besides small-molecules and inhibition of plasma membrane channels, successful strategies in clinical use include specific inhibition of an event-triggering receptor. Whereas in vitro data have often referred to TNFR1 as the primary inducer of necroptosis, this could not be confirmed in vivo in studies that investigated TNFR1-deficient77 or TNFR1/2-double-deficient mice78 in the renal ischemia reperfusion injury model. Therefore, receptors or other signals, other than TNFR1, are actively being explored for their ability to induce necroptosis. Promising candidates for the latter include other death receptors, Toll-like receptors and intracellular receptors.
Is all necrosis regulated?
Apart from necroptosis, other pathways of regulated necrosis have been identified, but only a few of these have been mechanistically separated from the necroptotic core machinery. Mitochondrial permeability transition is a process that induces necrotic cell death dependent on the mitochondrial matrix protein cyclophilin D, an intracellular target of cyclosporine A (CsA). Cyclophilin D-deficient mice are partially protected from ischemia reperfusion injury in various organs and CsA was demonstrated to prevent ischemic myocardial organ damage in humans79. Although it was recently concluded from studies in the zebra fish, Danio, that cyclophilin D might be a downstream target of the necrosome49, mitochondrial permeability transition and necroptosis are now clearly understood to be separate pathways, for example, as demonstrated by ischemia reperfusion injury experiments in cyclophilin D-RIPK3-double-deficient mice59. Therapeutically targeting these two pathways of regulated necrosis by combination therapy (Fig. 3) has shown strong additive protection from ischemia reperfusion injury in initial experiments59, and future strategies to inhibit regulated necrosis might best be based on interference with multiple pathways.
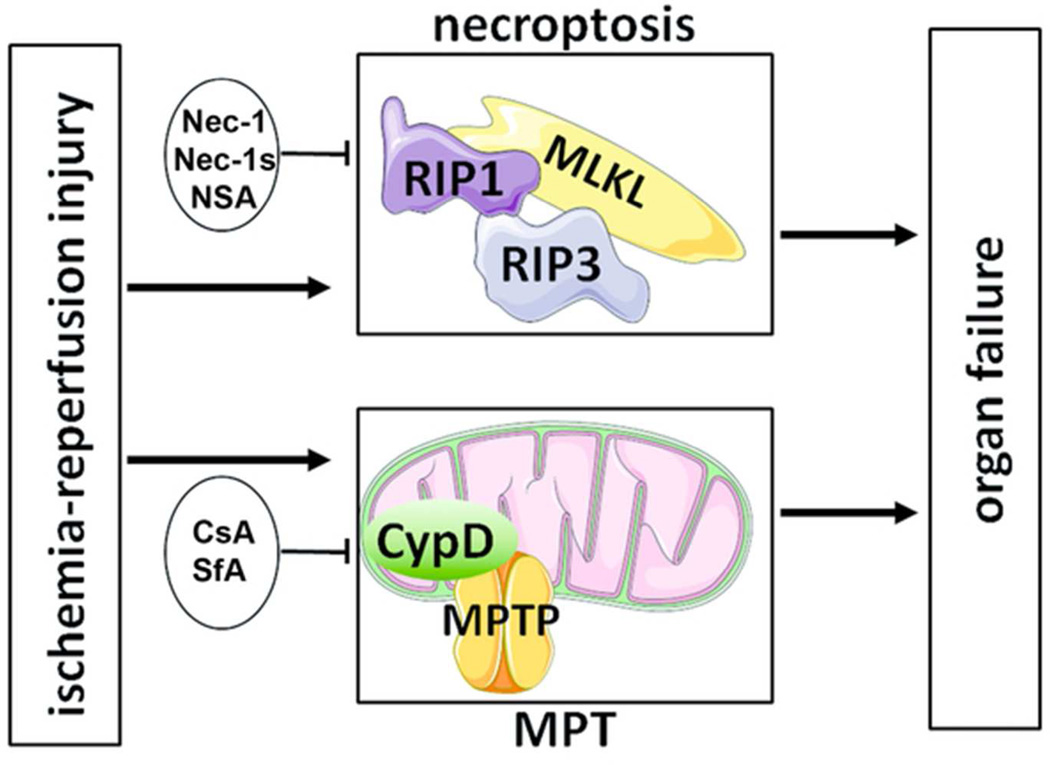
Figure 3. Combination therapy to target regulated necrosis.
In ischemia-reperfusion injury, independent pathways of regulated necrosis contribute additively to overall organ damage. Whereas formation of the necroptotic pathway involves RIPK1-triggered assembly of the necrosome (MLKL-RIPK3), mitochondrial permeability transition (MPT) independently contributes in a cyclophilin D dependent manner. Necroptosis may be blocked by necrostatin-1 (Nec-1), second generation “stable” Nec-1 (Nec-1s) or the MLKL-inhibitor necrosulfonamide (NSA). MPT is inhibited by cyclosporine A (CsA) or sanglifehrin A (SfA). Combination therapy with Nec-1 and SfA exhibited significantly stronger protection compared to each monotherapy.
Undoubtedly, CsA has revolutionized solid organ transplantation, widely accepted because of its immunosuppressive properties. However, data from isolated mitochondria clearly demonstrate the prevention of mitochondrial permeability transition by CsA59, 80–82. The immunosuppressive capacity of CsA, when applied after reperfusion, is clearly less potent in direct comparison with other immunosuppressants such as tacrolimus, rapamycin or mycophenolate-mofetil83. However, with the exception of tacrolimus84, none of these compounds prevented graft loss as effectively as CsA in clinical trials85. One might therefore speculate that clinicians have already been exploiting the mitochondrial permeability transition-blocking potential of CsA with the result of a reduced inflammatory response, reduced regulated necrosis, and better overall graft survival. Taken together, regulated necrotic cell death, i.e. necroptosis, mitochondrial permeability transition, or both, may be anticipated in solid organ transplantation, rendering this clinical field an ideal application for further investigations on the clinical potential of preventing necrosis.
Semantically, it is important to understand regulated necrosis as an umbrella term that encompasses mitochondrial permeability transition, necroptosis (Fig. 1), and pathways which have recently emerged such as ferroptosis86, pyroptosis87, PARP-1-mediated regulated necrosis88, NADPH-oxidase-mediated regulated necrosis89, lysosomal membrane permeabilization90 and others. However, it is not clear to what extent these pathways represent distinct, non-overlapping cell death programs. To carefully unravel these pathways, major efforts will hopefully identify reliable biomarkers for the specific detection of defined necrosis pathways. The continuing elucidation of the molecular subroutines of different forms of regulated necrosis, including necroptosis, and the efficient design of combination therapies hold promise for our ability to control regulated necrosis in clinical settings.
Supplementary Material
Glossary
- CIP
Cerulein-induced pancreatitis
- CsA
cyclosporine A. CsA is best known for its immunosuppressive quality. Besides conferring immunosuppression, CsA is a potent inhibitor of MPT
- DAI
DNA-dependent activator of interferon regulatory factors
- DAMPs
Cell damage-associated molecular patterns. DAMPs are released from necrotic cells, presumably cells that succumb from any necrotic type cell death. Therefore, CDAMP-release is not restricted to necroptosis
- FLIP
FLICE-like inhibitory protein
- IFN
Interferon
- JAK
Janus kinase
- MLKL
Mixed lineage kinase domain like
- MPT
Mitochondrial permeability transition. MPT is defined as a common increase in permeability of both the inner and outer mitochondrial membrane which may result in mitochondrial swelling, production of reactive oxygen species, NAD+-depletion and subsequent necrotic cell death.
- Nec-1
Necrostatin-1 (first in class compound)
- Nec-1s
Nec-1 stable (second generation RIP1-kinase inhibitor)
- Necroptosis
RIPK3-dependent regulated necrosis
- Necrosome
Supramolecular complex that consists of RIPK3 and other cell death mediating molecules, like RIPK1, dependent on the necroptotic trigger
- NEMO
NF-κB essential modulator
- NF-κB
nuclear factor 'kappa-light-chain-enhancer' of activated B-cells
- NSA
Necrosulfonamide
- PKR
protein kinase R
- RHIM
RIP homotypic interacting motif
- RIPK1
Receptor interacting protein kinase 1
- RIPK3
Receptor interacting protein kinase 3. RIPK3 is the key molecule in necroptotic cell death
- ROS
Reactive oxygen species
- SfA
Sanglifehrin A
- SIRS
Systemic inflammatory response syndrome
- STAT
Signal transducer and activator of transcription
- TNF
Tumor necrosis factor
- TNFR
TNF-receptor
- TLR
Toll-like receptor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Disclosure:
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 4.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2011;19(1):107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho Y, McQuade T, Zhang H, Zhang J, Chan FK. RIP1-dependent and independent effects of necrostatin-1 in necrosis and T cell activation. PLoS One. 2011;6(8):e23209. doi: 10.1371/journal.pone.0023209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser WJ, Upton JW, Mocarski ES. Viral modulation of programmed necrosis. Curr Opin Virol. 2013;3(3):296–306. doi: 10.1016/j.coviro.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21(4):227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 10.Lin J, Li H, Yang M, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3(1):200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Linkermann A, Brasen JH, Himmerkus N, et al. Rip1 (Receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81(8):751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 12.Oerlemans MI, Liu J, Arslan F, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107(4):270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Huang Z, Ren J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23(8):994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477(7364):335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welz PS, Wullaert A, Vlantis K, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477(7364):330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 16.Challa S, Chan FK. Going up in flames: necrotic cell injury and inflammatory diseases. Cell Mol Life Sci. 2010;67(19):3241–3253. doi: 10.1007/s00018-010-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12(11):757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinlich R, Dillon CP, Green DR. Ripped to death. Trends Cell Biol. 2011;21(11):630–637. doi: 10.1016/j.tcb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013;4:e716. doi: 10.1038/cddis.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seya T, Shime H, Takaki H, Azuma M, Oshiumi H, Matsumoto M. TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. Oncoimmunology. 2012;1(6):917–923. doi: 10.4161/onci.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser WJ, Upton JW, Long AB, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welz PS, Pasparakis M. A way to DAI. Cell Host Microbe. 2012;11(3):223–225. doi: 10.1016/j.chom.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vercammen D, Beyaert R, Denecker G, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silke J, Strasser A. The FLIP Side of Life. Sci Signal. 2013;6(258):e2. doi: 10.1126/scisignal.2003845. [DOI] [PubMed] [Google Scholar]
- 26.Vanlangenakker N, Vanden Berghe T, Bogaert P, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18(4):656–665. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2:e230. doi: 10.1038/cddis.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 29.Kim SO, Ono K, Han J. Apoptosis by pan-caspase inhibitors in lipopolysaccharide-activated macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1095–L1105. doi: 10.1152/ajplung.2001.281.5.L1095. [DOI] [PubMed] [Google Scholar]
- 30.Thapa RJ, Nogusa S, Chen P, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 32.Mevissen TE, Hospenthal MK, Geurink PP, et al. OTU Deubiquitinases Reveal Mechanisms of Linkage Specificity and Enable Ubiquitin Chain Restriction Analysis. Cell. 2013;154(1):169–184. doi: 10.1016/j.cell.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Donnell MA, Perez-Jimenez E, Oberst A, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10(4):348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 35.Oberst A, Dillon CP, Weinlich R, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L, Wang H, Wang Z, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Jitkaew S, Cai Z, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, McQuade T, Siemer AB, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012;38(1):27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1–2):228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Yeh WC, Pompa JL, McCurrach ME, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 42.Yeh WC, Itie A, Elia AJ, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12(6):633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 43.Varfolomeev EE, Schuchmann M, Luria V, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 44.Dillon CP, Oberst A, Weinlich R, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1(5):401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35(8):434–41. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, Zhang L, Yao Q, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013 doi: 10.1038/nature12436. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Narayan N, Lee IH, Borenstein R, et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature. 2012;492(7428):199–204. doi: 10.1038/nature11700. [DOI] [PubMed] [Google Scholar]
- 49.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153(3):521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20(2):185–187. doi: 10.1038/cdd.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 52.You Z, Savitz SI, Yang J, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28(9):1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chavez-Valdez R, Martin LJ, Flock DL, Northington FJ. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31(1):178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fricker M, Vilalta A, Tolkovsky AM, Brown GC. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J Biol Chem. 2013;288(13):9145–9152. doi: 10.1074/jbc.M112.427880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trichonas G, Murakami Y, Thanos A, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107(50):21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee YS, Dayma Y, Park MY, Kim KI, Yoo SE, Kim E. Daxx is a key downstream component of receptor interacting protein kinase 3 mediating retinal ischemic cell death. FEBS Lett. 2013;587(3):266–271. doi: 10.1016/j.febslet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Murakami Y, Matsumoto H, Roh M, et al. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc Natl Acad Sci U S A. 2012;109(36):14598–14603. doi: 10.1073/pnas.1206937109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linkermann A, Brasen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110(29):12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linkermann A, Heller JO, Prokai A, et al. The RIP1-Kinase Inhibitor Necrostatin-1 Prevents Osmotic Nephrosis and Contrast-Induced AKI in Mice. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012121169. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonnet MC, Preukschat D, Welz PS, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35(4):572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Lasse S, Lee P, et al. Development of atopic dermatitis-like skin disease from the chronic loss of epidermal caspase-8. Proc Natl Acad Sci U S A. 2010;107(51):22249–22254. doi: 10.1073/pnas.1009751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seymour RE, Hasham MG, Cox GA, et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8(5):416–421. doi: 10.1038/sj.gene.6364403. [DOI] [PubMed] [Google Scholar]
- 64.Linkermann A, Brasen JH, De ZF, et al. Dichotomy between RIP1- and RIP3-mediated Necroptosis in Tumor Necrosis Factor alpha-induced Shock. Mol Med. 2012;18:577–86. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ladoire S, Hannani D, Vetizou M, et al. Cell-Death-Associated Molecular Patterns As Determinants of Cancer Immunogenicity. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.5133. [DOI] [PubMed] [Google Scholar]
- 66.Lau A, Wang S, Jiang J, et al. RIPK3 mediated necroptosis promotes donor kindey inflammatory injury and reduces allograft survival. Am J Trans. 2013 doi: 10.1111/ajt.12447. In press. [DOI] [PubMed] [Google Scholar]
- 67.De KK, Van LS, Peeters P, Vanholder R. Human cytomegalovirus and kidney transplantation: a clinician's update. Am J Kidney Dis. 2011;58(1):118–126. doi: 10.1053/j.ajkd.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Linkermann A, Hackl MJ, Kunzendorf U, et al. Necroptosis in Immunity and Ischemia-Reperfusion Injury. Am J Trans. 2013 doi: 10.1111/ajt.12448. In press. [DOI] [PubMed] [Google Scholar]
- 69.Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28(11):3499–3505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 70.Duprez L, Takahashi N, Van HF, et al. RIP Kinase-Dependent Necrosis Drives Lethal Systemic Inflammatory Response Syndrome. Immunity. 2011;35(6):908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi N, Duprez L, Grootjans S, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou J, Kawai T, Tsuchida T, et al. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity. 2013;38(4):717–728. doi: 10.1016/j.immuni.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145(1):92–103. doi: 10.1016/j.cell.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 75.Xie T, Peng W, Liu Y, et al. Structural Basis of RIP1 Inhibition by Necrostatins. Structure. 2013;21(3):493–499. doi: 10.1016/j.str.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 76.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 77.Burne MJ, Elghandour A, Haq M, et al. IL-1 and TNF independent pathways mediate ICAM-1/VCAM-1 up-regulation in ischemia reperfusion injury. J Leukoc Biol. 2001;70(2):192–198. [PubMed] [Google Scholar]
- 78.Ko GJ, Jang HR, Huang Y, et al. Blocking Fas Ligand on Leukocytes Attenuates Kidney Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2011;22(4):732–42. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 80.Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 81.Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 82.Schinzel AC, Takeuchi O, Huang Z, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102(34):12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang B, Jain S, Pawluczyk IZ, et al. Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney Int. 2005;68(5):2050–2067. doi: 10.1111/j.1523-1755.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- 84.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 85.Merion RM, White DJ, Thiru S, Evans DB, Calne RY. Cyclosporine: five years' experience in cadaveric renal transplantation. N Engl J Med. 1984;310(3):148–154. doi: 10.1056/NEJM198401193100303. [DOI] [PubMed] [Google Scholar]
- 86.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 88.Sosna J, Voigt S, Mathieu S, et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1381-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yazdanpanah B, Wiegmann K, Tchikov V, et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460(7259):1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 90.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.