Abstract
Disruption of blood brain barrier (BBB) is used to enhance chemotherapeutic drug delivery. The purpose of this study was to understand the time course of hemodynamic and metabolic response to intraarterial (IA) mannitol infusions in order to optimize the delivery of drugs for treating brain tumors.
Principal results
We compared hemodynamic response, EEG changes, and mitochondrial function as judged by relative changes in tissue NADH concentrations, after intracarotid (IC) infusion of equal volumes of normal saline and mannitol in our rabbit IC drug delivery model. We observed significantly greater, though transient, hyperemic response to IC infusion of mannitol compared to normal saline. Infusion of mannitol also resulted in a greater increase in tissue NADH concentrations relative to the baseline. These hemodynamic, and metabolic changes returned to baseline within 5 min of mannitol injection.
Conclusion
Significant, though transient, changes in blood flow and brain metabolism occur with IA mannitol infusion. The observed transient hyperemia would suggest that intravenous (IV) chemotherapy should be administered either just before, or concurrent with IA mannitol injections. On the other hand, IA chemotherapy should be delayed until the peak hyperemic response has subsided.
Keywords: Ischemia, Intracarotid, Nicotinamide adenine dinucleotide, Blood brain barrier, Mannitol, Intraarterial chemotherapy, Ultraviolet spectroscopy
1. Introduction
Regional blood flow profoundly affects the delivery of intraarterial (IA) drugs in pharmacokinetic and experimental models (Dedrick, 1988; Joshi et al., 2006, 2008a, 2008b). While an increase in cerebral blood flow (CBF) will improve the deposition of concurrently injected intravenous (IV) drugs to the brain tissue, it will adversely affect the delivery of IA drugs. In theory, any increase in CBF will increase the amount of IV drug delivered due to the proportional increase in CBF. To the contrary, an increase in CBF, will dilute the IA drugs, decrease the transit time, and increase regional clearance, so as to adversely affect the regional deposition of IA drugs. IA mannitol is used for the disruption of the blood brain barrier (BBB) to facilitate delivery of chemotherapeutic drugs (Neuwelt et al., 2008; Riina et al., 2009; Shin et al., 2012). The dose of mannitol for this purpose should be sufficient to displace blood and dehydrate endothelial cells for approximately 30–40 s (Bellavance et al., 2008; Rapoport, 2000), for rabbits it is 8 ml over 30–40 s (Perkins and Strausbaugh, 1983; Wang et al., 2007). Several investigators have reported significant hemodynamic effects such as changes in cardiac output, systemic vascular resistance, hypertension, increased CBF, and increased ICP during BBB disruption (Doolittle et al., 2000; Gumerlock et al., 1994; Hardebo and Nilsson, 1980; Hiesmayr et al., 1987; Marchi et al., 2007). The purpose of this study was to understand the time course of hemodynamic and metabolic response to intraarterial (IA) mannitol infusions in order to help optimize the delivery of drugs for treating brain tumors.
In this report we describe the real-time hemodynamic effects of infusion of 25% mannitol compared to normal saline infusions, in doses that are used for the disruption of BBB in our IA drug delivery model using New Zealand white rabbits. To our best knowledge only a few studies have addressed the temporal hemodynamic and metabolic changes after IA mannitol injections and most of these studies have assessed blood flow or metabolism at specific time points, not continuously (Chi et al., 1991, 2013; Hardebo and Nilsson, 1980; Hiesmayr et al., 1987). To assess changes in mitochondrial function, we monitored tissue nicotinamide adenine dinucleo-tide (NADH) levels using ultraviolet spectroscopy, that assesses tissue redox state in real-time and provides a marker of cerebral ischemia(Mayevsky and Rogatsky, 2007). To rule out that the observed increase in NADH levels during mannitol and saline injections was not due to the displacement of hemoglobin that could unmask tissue fluorescence, we conducted a further dose response study with IA NADH.1
2. Results
2.1. Comparison of response to IA saline vs. IA mannitol
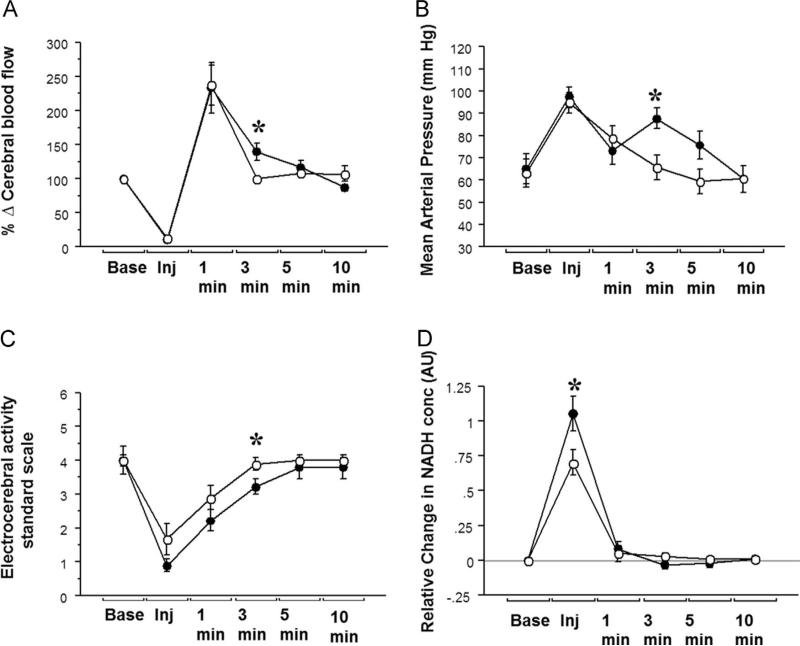
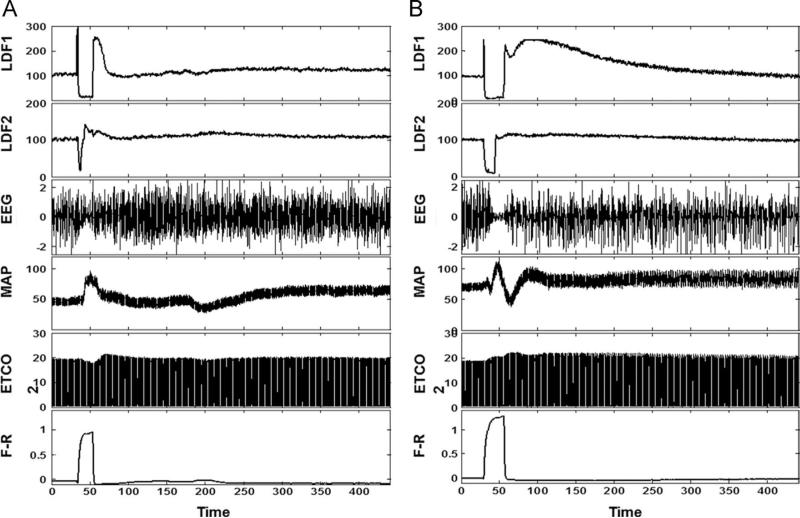
Comparison between saline and mannitol challenges was undertaken in New Zealand white rabbits (n=9). Baseline hemodynamics and end-tidal CO2 were comparable between the two challenges, Table 1. Infusion of both, saline or mannitol, resulted in an initial increase in mean arterial pressure, and decrease in CBF, with rebound increase that was more sustained with mannitol. Greater hemodynamic instability was seen with mannitol as compared to saline, Fig. 2. The increase in mean arterial pressure (MAP) with mannitol was often transient and immediately followed by a decrease and then a second increase in MAP, as shown in Fig. 3A and B. The decrease in MAP coincided with a slight difference in heart rates (262±8 bpm baseline to 246±16 bpm at 1 min, P=0.016), which was significant between the two challenges. However with mannitol there was a secondary increase in MAP at 3 min with a corresponding hyperemic response that was significantly different from saline injections, 88±14 vs. 66±17 mm Hg, P=0.001.
Table 1.
Physiological changes during intracarotid injection of normal saline and mannitol.
| Challenge | Baseline | Peak injection | 1 min | 3 min | 5 min | 10 min | |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Saline | 37.1 ± 0.9 | 37.1 ± 0.9 | 37.1 ± 0.9 | 37.2 ± 0.8 | 37.1 ± 0.8 | 36.9 ± 0.9 |
| Mannitol | 36.7 ± 0.7 | 36.7 ± 0.7 | 36.7 ± 0.7 | 36.8 ± 0.8 | 36.8 ± 0.8 | 36.8 ± 0.5 | |
| Resp. rate (breaths/min) | Saline | 62 ± 8 | 62 ± 8 | 62 ± 8 | 62 ± 8 | 62 ± 8 | 62 ± 8 |
| Mannitol | 62 ± 8 | 62 ± 8 | 62 ± 8 | 62 ± 8 | 62 ± 8 | 62 ± 8 | |
| Heart rate, (beats/min) | Saline | 263 ± 19 | 265 ± 19 | 262 ± 8a | 268 ± 22 | 264 ± 10 | 255 ± 20 |
| Mannitol | 250 ± 21 | 252 ± 36 | 246 ± 16 | 253 ± 10 | 263 ± 29 | 267 ± 27 | |
| MAP (mmHg) | Saline | 63 ± 18 | 95 ± 13b,f | 79 ± 17c | 66 ± 17a,c,d | 59 ± 17c,d,e | 60 ± 19c,d,e |
| Mannitol | 65 ± 21 | 97 ± 13b | 73 ± 19c | 88 ± 14b,d | 75 ± 19d | 60 ± 18c,d,e | |
| % ΔLDI | Saline | 100 ± 0 | 13 ± 5b,f | 237 ± 90b,c | 99 ± 11c,d,e | 109 ± 13c,d,e | 107 ± 34c,d,e |
| Mannitol | 100 ± 0 | 11 ± 6a,f | 234 ± 112b,c | 140 ± 38c,d,e | 118 ± 29c,d,e | 87 ± 17c,d,e | |
| % ΔLDC | Saline | 100 ± 0 | 37 ± 20a,b | 201 ± 82b,c | 120 ± 42c,d | 139 ± 88c | 139 ± 11c |
| Mannitol | 100 ± 0 | 20 ± 11b | 252 ± 207c | 139 ± 44 | 127 ± 46 | 97 ± 41d | |
| EtCO2, (mmHg) | Saline | 21 ± 4 | 22 ± 4 | 21 ± 5 | 22 ± 4 | 21 ± 1 | 21 ± 1 |
| Mannitol | 22 ± 3 | 23 ± 4 | 23 ± 4 | 22 ± 3 | 22 ± 3 | 22 ± 3 | |
| EEG score | Saline | 4 ± 1 | 1 ± 1b,f | 3 ± 1b,c | 4 ± 1a,c,e | 4 ± 1c,e | 4 ± 1c,e |
| Mannitol | 4 ± 1 | 1 ± 1a,f | 2 ± 1b,c | 3 ± 1c,e | 4 ± 1c,e | 4 ± 1c,e | |
| NADH (AU) | Saline | 0 ± 0.00 | 0.7 ± 0.26a,b,f | 0.05 ± 0.16c | 0.03 ± 0.05c | 0.02 ± 0.05c | 0.01 ± 0.05c |
| Mannitol | 0 ± 0.02 | 1.05 ± 0.37b,f | 0.09 ± 0.15c | 0 ± 0.1c | 0 ± 0.1c | 0.01 ± 0.05c |
Abbreviations: EtCO2: end-tidal carbon dioxide tension; MAP: mean arterial pressure; % ΔLDI: %-change in laser doppler blood flow from baseline on the side of mannitol infusion; % ΔLDC: %-change in laser doppler blood flow from baseline on the contralateral side; EEG score: electroencephalographic score 0-5 on standardized scale; NADH: nicotinamide adenine dinucleotide; AU: arbitrary units.
Significant difference between mannitol and saline (P < 0.05), Significant difference between repeat measure (P < 0.0033).
From base.
From peak.
From 1 min.
From 3 min.
From 5 min.
Fig. 2.
Changes in physiological parameters after IA mannitol and saline: Changes in (A) cerebral blood flow, (B) mean arterial pressure and (C) EEG activity and (D) Brain tissue NADH levels observed during IA mannitol and saline injection in nine New Zealand White rabbits. Open circles – Saline; Close circles – Mannitol; * - significant difference after post-hoc test.
Fig. 3.
Cerebral and systemic response to IA saline and mannitol: The graph depicts monitored cerebral and systemic hemodynamic parameters during IA injection of saline (A) and mannitol (B). LDF 1: ipsilateral to, LDF 2: contralateral to saline and mannitol injection, laser Doppler determined blood flow in arbitrary units (a. u.); EEG: electroencephalogram in a.u.; MAP: mean arterial pressure in mm Hg; ETCO2: end-tidal carbon dioxide tension in mm Hg; relative changes in NADH concentration as determined by subtracting fluorescence and reflectance in a.u.; Time axis is presented in seconds.
Laser Doppler measurements, which quantify red blood cell density and velocity, revealed that there was a similar decrease in CBF during saline and mannitol infusion, reflecting a comparable displacement of the vascular space between the two injections. There was relatively a greater increase in brain tissue NADH concentrations with mannitol compared with normal saline injections, Table 1. All mannitol and saline injections were made at a preset pressure of 10 psi, with a discharge rate of 8 ml of saline or mannitol in 40 s. While the decrease in CBF was comparable for the mannitol and saline challenges, the peak rise in NADH was significantly greater with mannitol infusion, Fig. 2. The attenuation of EEG activity was more significant with mannitol than with saline. The changes in systemic and cerebral hemodynamics, increase in NADH levels, and attenuation of EEG activity all reversed to near baseline values within 5 min of injection, Fig. 2.
2.2. Dose response to IA NADH
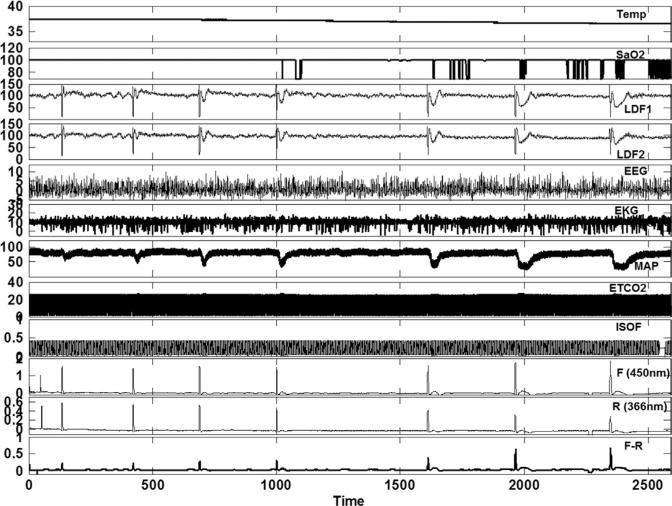
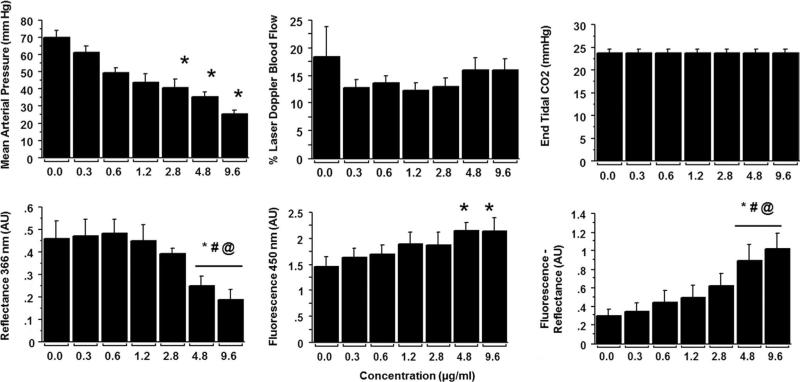
Results of the NADH dose response in an animal are shown in Fig. 4, while Fig. 5 provides the collective data from six animals. As shown in Fig. 5, injection of NADH resulted in a dose dependent increase in brain tissue levels of NADH. The increase in tissue fluorescence demonstrated a rapid rise to near maximum with increasing doses with a less remarkable dose response. On the other hand, there was a dose dependent decrease in 366 nm light reflectance. Compared to the immediate increase in observed NADH levels, a relatively delayed dose-dependent decrease in mean arterial pressure was observed with IA-NADH injections, Figs. 4 and 5.
Fig. 4.
Hemodynamic and fluorescence changes with increasing doses of IA NADH in a given animal: Temp: esophageal temperature (°C); SaO2: pulse oxygen saturation in (%); LDF 1: ipsilateral to, LDF 2: contralateral to saline and mannitol injection, laser Doppler determined blood flow (a.u.); EEG: electroencephalogram (a.u.); EKG: electrocardiogram (a.u.); MAP: mean arterial pressure (mm Hg); ETCO2: end-tidal CO2 (mm Hg); ISOF: end-tidal isoflurane concentrations (%); 450 nm F: fluorescence at 450 nm (a.u.); 366 nm R: reflectance at 366 nm (a.u.); F-R : relative changes in NADH concentration as determined by subtracting fluorescence and reflectance in arbitrary units (a.u.); Time axis is shown in seconds.
Fig. 5.
Dose response with IC NADH injections: Bar graphs representing statistical analysis of mean arterial pressure (A), percent change in cerebral blood flow (B), end-tidal CO2 (C), and 366 nm reflectance (D), 450 nm fluorescence (E) and the increase in NADH as judged by the difference between fluorescence-reflectance. Symbols: significant difference from: *: saline; #: from .3 mg/ml of NADH; and @: from 0.6 mg/ml of NADH.
3. Discussion
his study was designed to directly compare the hemodynamic effects of mannitol and saline in doses used to disrupt the BBB. Eight ml of each was injected into the carotid artery using a pneumatic injection pump. Within-animal comparisons were made to avoid variations in drug concentrations due to individual variations in carotid anatomy of rabbits.
Both mannitol and normal saline infusions transiently decreased CBF, caused rebound hyperemia, temporarily impairment of EEG activity and increased the relative tissue NADH concentrations. However, hemodynamic, electrophysiological, and NADH changes with normal saline were transient and recovered rapidly, while mannitol injections had more profound and sustained effects. In the IA-NADH dose response study we observed a dose dependent increase in measured changes in relative NADH concentrations. However, contrary to our expectations, this relative increase was not due to an increase in tissue fluorescence, as would be expected with hemoglobin displacement, but it was due to increased absorption of 366 nm UV light and the decrease in corresponding reflectance values.
During clinical procedures angiographic hyperemia has been reported immediately after intraarterial mannitol injection and is considered to a marker of BBB disruption. However it is not clear from these reports as to how long the hypermia lasted after BBB disruption (Boockvar et al., 2011; Riina et al., 2009; Shin et al., 2012). A critical factor in regional IA delivery of drugs to the brain is the CBF during drug injection. In early studies, a hyperemic response to intracarotid mannitol was reported by Hardebo and Nilsson (1980). The increase was related to osmotic load and was much more profound with urea then with mannitol. In our case of mannitol, a 150% transient increase in blood flow was reported that lasted about 3–5 min during experiments in rats. In other studies, that used CBF measurements at a single time point, such as 12 min after mannitol injection, no increase in CBF was reported, and in some instances even a decrease in CBF was noted.
In the present study, where laser Doppler measurements were used, we saw a biphasic response in which the CBF initially decreased during mannitol and saline infusion, and then immediately afterwards there was a rebound increased in blood flow. The hyperemic response lasted about 5 min. The laser Doppler blood flow measurement technique assesses CBF by quantifying the density and velocity of red cells. Thus, the initial decrease in blood flow most likely reflects the displacement of blood cells during injection. This decreases in hematocrit probably also leads to transient cerebral ischemia that was supported by the rise in tissue NADH levels and a more sustained impairment of EEG activity compared to normal saline injections. Hemodynamic effects of saline are transient and can be explained by the expansion of blood volume. On the other hand, effects of mannitol are more complex, associating with both transient rise and fall of blood pressure and a decrease in heart rate, Fig. 3A and B. Those changes may be due to volume expansion, osmotic loading, or transient electrolyte imbalance, all of which have been reported during rapid mannitol injections (Rosner and Coley, 1987).
Monitoring of NADH during this study was based on measurements of NADH fluorescence and tissue reflectance. This method relies on measuring relative changes in 366 nm reflectance and NADH 450 nm fluorescence, such that the measurement is relatively insensitive to moderate changes in blood volume (Cordeiro et al., 1995). However, with IA injections there are dramatic changes in CBF and tissue hematocrit, and tissue hemoglobin content could be transiently reduced to near zero values. Such dramatic changes in hemoglobin concentrations could artifactually affect NADH values. Blood is a strong quencher of NADH fluorescence that occurs around 460 nm. Extreme changes in hemoglobin that are likely to be seen during and after IA infusions could therefore affect NADH concentration measurements. Consequently, it became imperative to assess whether NADH measurements could be reliably made during IA injections. To address this concern we undertook the dose– response study.
During the NADH dose–response study, a significant dose-dependent transient hypotension also occurred during IC NADH injections. However, the rise in NADH was not associated with a decrease in end-tidal CO2, and the decrease in CBF as measured by laser Doppler probes. There was no significant difference between these parameters with saline or with increasing doses of NADH, Fig. 5. However, as seen in Fig. 4, the decrease in MAP occurred well after the changes of NADH had recovered. It was not associated with a decrease in ETCO2 or CBF. Thus the dose dependent increase in measured NADH was not due to the secondary hypotension that occurred afterwards.
There is prior some evidence that NADH measurement can be reasonably made during hemodilution (Mayevsky and Rogatsky, 2007). In our experiments the injection of NADH was carefully delivered by a pneumatic pump, such that the displacement of hemoglobin was comparable as is evident by the laser Doppler blood flow measurements during saline and NADH injections, Fig. 4. The results from the dose–response study reveal a dose-dependent increase in relative NADH Fig. 5, is similar to the increase reported in-vitro, Fig. 6 (Held, 2007). Therefore, we believe that the relatively greater increase in tissue levels of NADH during mannitol injections compared to saline injections, is a marker due to greater tissue ischemia. Ischemia in the present experiment is also evidenced by the more profound hyperemic response with mannitol and a longer period of EEG suppression compared to equal volumes of saline infusions.
Fig. 6.
In-vitro changes in NADH fluorescence and reflectance in the absence of hemoglobin: Effect of increasing concentrations of NADH on 440 nm fluorescence and 340 nm reflectance. Data adapted with permission from Biotek Inc. (Held, 2007).
However, EEG changes after mannitol infusion have to be interpreted with caution. If the initial suppression of EEG was due to physical displacement of oxygenated blood, it would be similar for saline and mannitol both of which showed a similar reduction in CBF. However, the suppression of in EEG activity were more sustained and lasted for the duration of hyperemia in the case of mannitol. Seizures after mannitol are well-known complications of BBB disruption (Angelov et al., 2009; Marchi et al., 2007; van Vliet et al., 2007) and typically present with initial attenuation of EEG activity followed by seizure activity that can occur hours to days after BBB disruption. The pathogenesis of which seems to be related to the cortical uptake of bile salts and albumin over time across the impaired barrier (Angelov et al., 2009; Janigro, 2007, 2010).
Cerebral ischemia during mannitol injection could not be due to direct effect as IA mannitol is a cerebral vasodilator (Takayasu and Dacey, 1989). The immediate onset and offset of cerebral ischemia cannot also be explained by rebound vasoconstriction due to decrease in hematocrit as that would have occurred with saline as well (Muizelaar et al., 1983). Furthermore, it is unlikely that mannitol microcrystal embolization contributed to the increase in NADH. We used warm mannitol that was filtered immediately before IA injection. An alternative reason for the increase in NADH during mannitol injection could be attributed to an increase in tissue oxygen consumption that can be attenuated by NMDA receptor blockade (Chi et al., 2013). Although the clinical significance of transient cerebral ischemia can be debated, it could account for some of the neurological complications reported during such procedures (Elkassabany et al., 2008; Marchi et al., 2007).
In the present experiment we observed a significant increase in CBF (≈150%) after intracarotid mannitol injection. Although it was transient lasting <5 min, it could affect regional IA drug delivery if injected immediately after mannitol injection. A variety of drug delivery protocols are used during IA chemotherapy of brain tumors. In most instances drugs are injected intravenously prior to intracarotid mannitol injections, in other instances drugs are injected after BBB disruption (Boockvar et al., 2010; Gumerlock et al., 1992; Heimberger et al., 1986; Hiesiger et al., 1986). There is conflicting data as to how long the BBB remains open after mannitol injection. Some report that the BBB rapidly closes after mannitol injection within 10 min (Bhattacharjee et al., 2001; Rapoport, 2000, 2001; Rapoport), others have reported that the barrier remains open for several hours after injection (Bellavance et al., 2008; Zunkeler et al., 1996). Previously, using Evan's Blue staining as the marker of BBB disruption we found BBB disruption under ketamine propofol anesthesia lasts at least an hour after mannitol injection (Joshi et al., 2005a). However many factors seem to affect the BBB disruption as a net result IA mannitol injection and that it is often hard to anticipate the extent and duration of osmotic BBB disruption (Gumerlock and Neuwelt, 1990; Joshi et al., 2011a). Assuming that the disruption of BBB after mannitol injections lasts only for several minutes after injection, the results of this study would suggest that intravenous chemotherapeutic drugs should best be administered just before or soon after mannitol injection to maximize drug delivery. Increased regional blood flow coincident with maximum BBB disruption after mannitol under these circumstances will increase drug deposition. Conversely, intracarotid drugs are best administered about 5 min after mannitol injection when the peak hyperemic response has subsided. In a recent clinical case report BBB was disrupted with mannitol and hyperemia was observed by angiographic contrast injection during IA chemotherapy. Three-min balloon inflation and 2 min deflation cycles were used over the next 1 h to deliver chemotherapy. A significant tumor response was observed over the next 24 h. Ideally, CBF should be monitored by trans-cranial Doppler or by estimating contrast transit time, so as to decide when to inject intracarotid drugs or whether a balloon occlusion catheter be used to minimize drug washout.
A key limitation of this study is that we did not test for BBBD in individual animals. To address the problem of variability of BBBD in our rabbit models (Joshi et al., 2011a) we have developed a simultaneous permeability drug concentration tracking method (Ergin et al., 2012). In this experiment we were using dual challenges (saline and mannitol) in each animal therefore we did not test the blood brain barrier in each animal due to concerns about interference with optical monitoring of NADH concentrations with dual injection do Evan's blue of indocyanine green (Ergin et al., 2011; Joshi et al., 2011a). In clinical settings, hyperemia after mannitol is taken to be a marker of BBB disruption (Riina et al., 2009, 2012) which occurred after mannitol injection during our experiments suggesting that the BBB was probably disrupted. However, the finding of this study should be limited to the hemodynamic and metabolic response to doses of intracarotid mannitol used for disrupting the BBB.
In summary, we report that the dose of IC mannitol required to disrupt the BBB results in significant alterations in CBF and metabolism that are characterized by a large, though transient hyperemic response, transient cerebral ischemia as evidenced by a rise in relative NADH concentrations. Due to transient hyperemic it would be prudent to wait for the peak hyperemic response to subside before injecting IA chemotherapeutic drugs. Conversely, if the chemotherapeutic drugs are administered intravenously maximum drug deposition is likely to be achieved by injections that are concurrent with mannitol infusion, when the increase in CBF would increase in drug delivery to the brain tissue alternately flow modulation with balloon tipped catheters might be considered.
4. Experimental procedures
4.1. Animal preparation
After approval by the Institution Animal Care and Use Committee, New Zealand white rabbits under propofol and ketamine anesthesia, underwent earlobe vein, femoral arterial and selective internal carotid cannulations, along with tracheotomy. EEG leads were fastened to the skull with 1.5 mm stainless steel screws, and skull shaving was done to the point of bone transparency for laser Doppler probe placement (Joshi et al., 2011b). EEG activity was described on a standardized scale, from 0 (silence) to 5 (mixed high and low amplitude activity) (Joshi et al., 2005b). Laser Doppler probes were mounted onto the thinned skull regions for separate monitoring of ipsi and contralateral CBF (PeriFlux System 5000, Perimed). Anesthesia was administered intravenously by injection of propofol at 20–30 mg/kg/h with mechanical ventilation and supplemental isoflurane 1–2% MAC as needed. Combination anesthetic was used due to the unusually high tolerance of rabbits to propofol while excessive volatile anesthetic agents adversely affect BBB disruption (Gumerlock and Neuwelt, 1990; Remsen et al., 1999). Local anesthetic agent bupivacaine was injected at incision sites to decrease the pain of incision. Craniotomy in the right parietal region was performed in order to allow for placement of the NADH measuring probe in contact with the cerebral cortex in a relatively avascular region of the brain. Details of the preparation are provided in our earlier publications (Mayevsky and Rogatsky, 2007; Wang et al., 2011). The ICA in all the animals was carefully isolated by an experienced operator and was tested by the retinal discoloration test as described earlier (Joshi et al., 2004). Mannitol and saline were injected by a pneumatic pump at 10 psi injection pressure, with a discharge volume of 8 ml in 40 s.
During concurrent studies with IA drug delivery, we observed that the hemodynamic effects of mannitol and saline injections were transient, and blood flow rapidly returned to baseline within 5 min and we also observed that there were inconsistent effects of mannitol on CBF and BBB disruption (Joshi et al., 2011a). We believe that variations in mannitol response could be in part due to subtle variations in distal carotid anatomy across individual animals. Several variations in carotid anatomy of rabbits have been described in the literature (Lee et al., 1994). Since the object of this experiment was to compare hemodynamic responses of mannitol and saline, we therefore elected to use saline and mannitol challenges in the same animals rather than in two separate groups. There was a period of rest, approximately 30-min, between saline and mannitol challenges to ensure a return to near baseline values of physiological parameters.
4.2. Optical measurement of NADH
To determine the changes in mitochondrial function we assessed the tissue redox state by optically measuring tissue NADH levels. The absorbance and emission spectra for NADH are provided in Fig. 1 (Held, 2007). When excited with ultraviolet light, NADH fluoresces with an emission peak at 460 nm (Mayevsky et al., 1988, 1992). Absorbance methods alone are seldom used for NADH quantification in blood-perfused tissues due to its spectral overlap with hemoglobin. In addition, hemoglobin considerably absorbs wavelengths in the range of NADH light-emission (300–466 nm). Previous publications provide a detailed description and application of our NADH measuring device in our drug delivery model (Wang et al., 2011). Briefly, ultraviolet light (366 nm central wavelength) is delivered to a sampling region via optical fibers in the contact probe. The backscattered and emitted light from the tissue is gathered through a collection fiber. The backscattered beam is then split and band pass-filtered at 366 nm and 450 nm to isolate reflectance and fluorescence components, respectively. Photomulti-pliers and pre-amplifiers are then used to obtain analog signals compatible with an A/D converter, allowing for real-time monitoring on a computer. Jobsis et al. (1971) showed that artifacts caused by hemodynamic changes could be minimized by incorporating changes in diffused reflectance of excitation light into the fluorescence analysis. Although the brain is an exceedingly well perfused organ, it is also rich in mitochondrial content which enables detection of ischemia in brain tissue using ultraviolet assessment of relative changes in tissue NADH level. What happens when the blood is transiently displaced from the brain tissue, such as during IA injections, has not been thoroughly investigated; although limited data suggests that reliable NADH measurements are still possible despite hemodilution (Mayevsky and Rogatsky, 2007).
Fig. 1.
Optical properties of hemoglobin and NADH: (A) Absorption spectra of reduced hemoglobin (HHb) and oxyhemoglobin (HbO) shows extensive light absorption under 500 nm overlapping with the peak absorption and emission of NADH. (B) Absorption and emission spectra of NADH in solution.
4.3. Dose response study with IA NADH
The increase in NADH seen with mannitol injection raised the possibility that due to quenching of NADH fluorescence at baseline by hemoglobin, IA injections were causing artifactual increases in relative NADH concentrations. Hence, we needed to test the reliability of NADH measurements when there were profound changes in CBF during IA injections. To test the reliability of optically measured NADH in our experimental setting, we conducted a dose response study in six rabbits. We reconfigured our system to record the fluorescence (F) and reflectance (R) values in separate channels along with the net difference between the two (F – R). Six doubling doses in the range of 0.3–9.6 mg/ml of NADH were injected IA to obtain a dose response curve, with the NADH (Sigma Aldridge Inc., St Louis MO, Cat # N4505, di-potassium ß NADH) dissolved in 1 ml of normal saline and injected by a pneumatic pump at 40 psi pressure to discharge the volume in about 2 s to replicate bolus injections. Di-potassium ß NADH is a large and complex molecule with a molecular weight 741.62 Da; consequently, when injected IA it is likely to remain in the vascular compartment. Thus, in the tissue volume being interrogated the measured fluorescence signal will be a function of the proportional volumes of blood and extravascular tissue. For cerebral tissue, the tissue blood volume is about 2% of the total tissue volume at baseline but could double with hemodilution (Todd et al., 1992). During peak injection, the rate of NADH infusion will be sufficient to displace blood and the NADH solution will most likely be located essentially in the vascular compartment.
We therefore estimate that the sampled, tissue averaged NADH signal will have tissue contributions approximately <5% of the injected concentrations. IA doses of 0.3–9.6 mg/ml (or 0.5–1.5 μmol/ml) used in this study were simulating the concentrations likely to be encountered in animal cells (1 μmol/gm) (Reiss et al., 1984). If we assume that the signal of injected NADH solution was attenuated by extravascular tissue volume, then the dose response study of IA injections generated effective molar concentrations in increments that were only a fraction of the background NADH signal arising from the tissues. Thus, the dose range of NADH was sufficient to test if the relative changes in NADH concentrations could be assessed during IA injections.
4.4. Data analysis
Data during mannitol and saline injections were compared at baseline, peak decrease, peak increase or 1 min, and three, 5and 10 min after the start of infusion. In the dose response study the effects were exceedingly rapid and comparisons were made at baseline and at peak changes with NADH dose injections. Unless stated, the data has been presented as mean and standard deviation. They were analyzed by factorial (mannitol vs. saline) or repeated-measure ANOVA. To correct for multiple time point comparisons of physiologic data, post-hoc Bonferroni Dunn test was used to determine statistical significance. These analyses were done using the Statview 5.2 software, SAS Institute Inc., Cary, NC.
Acknowledgments
This work was supported by the National Cancer Institute, NIH R01 Grants CA-127500, and R01-CA-138643, SJ.
Footnotes
IC: intracarotid, IA: intra-arterial; BBB: blood brain barrier; NADH: nicotinamide adenine dinucleotide; CBF: cerebral blood flow; MAP: mean arterial pressure (MAP); A/D: analog-to-digital.
REFERENCES
- Angelov L, et al. Blood–brain barrier disruption and intraarterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J. Clin. Oncol. 2009;27:3503–3509. doi: 10.1200/JCO.2008.19.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance MA, Blanchette M, Fortin D. Recent advances in blood–brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10:166–177. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee AK, et al. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res.—Brain Res. Protoc. 2001;8:126–131. doi: 10.1016/s1385-299x(01)00094-0. [DOI] [PubMed] [Google Scholar]
- Boockvar JA, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clin. Artic. J. Neurosurg. 2010;114:624–632. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boockvar JA, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clin. Artic. J. Neurosurg. 2011;114:624–632. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi OZ, et al. Effects of MK-801 on cerebral regional oxygen consumption in focal cerebral ischemia in rats. Circ. Res. 1991;69:414–420. doi: 10.1161/01.res.69.2.414. [DOI] [PubMed] [Google Scholar]
- Chi OZ, et al. Effects of blockade of NMDA receptors on cerebral oxygen consumption during hyperosmolar BBB disruption in rats. J. Neurol. Sci. 2013;326:29–34. doi: 10.1016/j.jns.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Cordeiro PG, et al. Ultraviolet excitation fluorescence spectroscopy: a noninvasive method for the measurement of redox changes in ischemic myocutaneous flaps. Plastic Reconstr. Surg. 1995;96:673–680. [PubMed] [Google Scholar]
- Dedrick RL. Arterial drug infusion: pharmacokinetic problems and pitfalls. J. Natl. Cancer Inst. 1988;80:84–89. doi: 10.1093/jnci/80.2.84. [DOI] [PubMed] [Google Scholar]
- Doolittle ND, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Elkassabany NM, et al. Perioperative complications of blood brain barrier disruption under general anesthesia: a retrospective review. J. Neurosurg. Anesthesiol. 2008;20:45–48. doi: 10.1097/ANA.0b013e31815d5f1f. [DOI] [PubMed] [Google Scholar]
- Ergin A, et al. Retention of indocyanine green as a potential marker for optical detection of blood brain barrier disruption. SPIE. 2011:79070L. [Google Scholar]
- Ergin A, et al. Noninvasive in vivo optical assessment of blood brain barrier permeability and brain tissue drug deposition in rabbits. J. Biomed. Opt. 2012;17:057008. doi: 10.1117/1.JBO.17.5.057008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumerlock MK, Neuwelt EA. The effects of anesthesia on osmotic blood–brain barrier disruption. Neurosurgery. 1990;26:268–277. doi: 10.1097/00006123-199002000-00014. [DOI] [PubMed] [Google Scholar]
- Gumerlock MK, et al. Osmotic blood–brain barrier disruption and chemotherapy in the treatment of high grade malignant glioma: patient series and literature review. J. Neurooncol. 1992;12:33–46. doi: 10.1007/BF00172455. [DOI] [PubMed] [Google Scholar]
- Gumerlock MK, York D, Durkis D. Visual evoked responses as a monitor of intracranial pressure during hyperosmolar blood–brain barrier disruption. Acta Neurochir. Suppl. (Wien) 1994;60:132–135. doi: 10.1007/978-3-7091-9334-1_35. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Nilsson B. Hemodynamic changes in brain caused by local infusion of hyperosomolar solutions, in particular relation to blood–brain barrier opening. Brain Res. 1980;181:49–59. doi: 10.1016/0006-8993(80)91258-5. [DOI] [PubMed] [Google Scholar]
- Heimberger K, et al. Blood–brain barrier disruption: Interventional radiology in brain tumor therapy. Ann. Radiol. 1986;29:230–232. [PubMed] [Google Scholar]
- Held P. Determination of NADH concentrations with SynergyTM 2 Multi-Mode Plate Readermulti-mode plate reader using Fluorescencefluorescence and Absorbance. 2007 http://www.biotek.com/resources/docs/NADH_App_Note.pdf.
- Hiesiger EM, et al. Opening the blood-brain and blood-tumor barriers in experimental rat brain tumors: the effect of intracarotid hyperosmolar mannitol on capillary permeability and blood flow. Ann. Neurol. 1986;19:50–59. doi: 10.1002/ana.410190110. [DOI] [PubMed] [Google Scholar]
- Hiesmayr M, et al. [Hemodynamic effects of an osmotic bolus for the reversible opening of the blood–brain barrier]. Schweiz Med. Wochenschr. 1987;117:450–454. [PubMed] [Google Scholar]
- Janigro D. Does leakage of the blood–brain barrier mediate epileptogenesis?. Epilepsy Curr. 2007;7:105–107. doi: 10.1111/j.1535-7511.2007.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D. Not again! The role of blood–brain barrier failure in epileptogenesis: a molecular update. Epilepsy Curr. 2010;10:67–69. doi: 10.1111/j.1535-7511.2010.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis FF, et al. Intracellular redox changes in functioning cerebral cortex. I. Metabolic effects of epileptiform activity. J. Neurophysiol. 1971;34:735–749. doi: 10.1152/jn.1971.34.5.735. [DOI] [PubMed] [Google Scholar]
- Joshi S, Wang M, Hartl R. Retinal discoloration test. J. Cereb. Blood Flow Metab. 2004;24:305–308. doi: 10.1097/01.WCB.0000107731.66603.18. [DOI] [PubMed] [Google Scholar]
- Joshi S, Wang M, Etu J. Cerebral hypoperfusion enhances effectiveness of intracarotid mannitol (Abstract). J. Neurosurg. Anesthesiol. 2005a;17:242. doi: 10.1097/ANA.0b013e3181453851. [DOI] [PubMed] [Google Scholar]
- Joshi S, et al. Reducing cerebral blood flow increases the duration of electroencephalographic silence by intracarotid thiopental. Anesth. Analg. 2005b;101:851–858. doi: 10.1213/01.ANE.0000160583.42078.B2. [DOI] [PubMed] [Google Scholar]
- Joshi S, et al. Cerebral blood flow affects dose requirements of intracarotid propofol for electrocerebral silence. Anesthesiology. 2006;104:290–298. doi: 10.1097/00000542-200602000-00014. [DOI] [PubMed] [Google Scholar]
- Joshi S, et al. Transient cerebral hypoperfusion enhances intraarterial carmustine deposition into brain tissue. J. Neurooncol. 2008a;86:123–132. doi: 10.1007/s11060-007-9450-z. [DOI] [PubMed] [Google Scholar]
- Joshi S, Meyers PM, Ornstein E. Intracarotid delivery of drugs: the potential and the pitfalls. Anesthesiology. 2008b;109:543–564. doi: 10.1097/ALN.0b013e318182c81b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, et al. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: implications for chemotherapy. J. Neurooncol. 2011a;104:11–19. doi: 10.1007/s11060-010-0466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, et al. Intra-arterial mitoxantrone delivery in rabbits: an optical pharmacokinetic study. Neurosurgery. 2011b;69:706–712. doi: 10.1227/NEU.0b013e3182181b67. discussion 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hamilton MG, Zabramski JM. Variations in the anatomy of the rabbit cervical carotid artery. Stroke. 1994;25:501–503. doi: 10.1161/01.str.25.2.501. [DOI] [PubMed] [Google Scholar]
- Marchi N, et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayevsky A, et al. Brain oxidative metabolism of the newborn dog: correlation between 31P NMR spectroscopy and pyridione nucleotide redox state. J. Cereb. Blood Flow Metab. 1988;8:201–207. doi: 10.1038/jcbfm.1988.50. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, et al. Multiparametric evaluation of brain functions in the Mongolian gerbil in vivo. J. Basic Clin. Physiol. Pharmacol. 1992;3:323–342. doi: 10.1515/jbcpp.1992.3.4.323. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Rogatsky GG. Mitochondrial function in vivo evaluated by NADH fluorescence: from animal models to human studies. Am J. Physiol. Cell Physiol. 2007;292:C615–C640. doi: 10.1152/ajpcell.00249.2006. [DOI] [PubMed] [Google Scholar]
- Muizelaar JP, et al. Mannitol causes compensatory cerebral vasoconstriction and vasodilation in response to blood viscosity changes. J. Neurosurg. 1983;59:822–828. doi: 10.3171/jns.1983.59.5.0822. [DOI] [PubMed] [Google Scholar]
- Neuwelt E, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Perkins BA, Strausbaugh LJ. Effect of mannitol infusions into the internal carotid artery on entry of two antibiotics into the cerebrospinal fluid and brains of normal rabbits. Antimicrob. Agents Chemother. 1983;24:339–342. doi: 10.1128/aac.24.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol. Neurobiol. 2000;20:217–230. doi: 10.1023/a:1007049806660. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Advances in osmotic opening of the blood–brain barrier to enhance CNS chemotherapy. Expert Opin. Investig. Drugs. 2001;10:1809–1818. doi: 10.1517/13543784.10.10.1809. [DOI] [PubMed] [Google Scholar]
- Reiss PD, Zuurendonk PF, Veech RL. Measurement of tissue purine, pyrimidine, and other nucleotides by radial compression high-performance liquid chromatography. Anal. Biochem. 1984;140:162–171. doi: 10.1016/0003-2697(84)90148-9. [DOI] [PubMed] [Google Scholar]
- Remsen LG, et al. The influence of anesthetic choice, PaCO2, and other factors on osmotic blood–brain barrier disruption in rats with brain tumor xenografts. Anesth. Analg. 1999;88:559–567. doi: 10.1097/00000539-199903000-00018. [DOI] [PubMed] [Google Scholar]
- Riina HA, et al. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neurooncology for malignant glioma. J. Exp. Ther. Oncol. 2009;8:145–150. [PubMed] [Google Scholar]
- Riina HA, et al. Short-term clinico-radiographic response to super-selective intra-arterial cerebral infusion of Bevacizumab for the treatment of vestibular schwannomas in neurofibromatosis type 2. Interv. Neuroradiol. 2012;18:127–132. doi: 10.1177/159101991201800201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner MJ, Coley I. Cerebral perfusion pressure: a hemodynamic mechanism of mannitol and the postmannitol hemogram. Neurosurgery. 1987;21:147–156. doi: 10.1227/00006123-198708000-00003. [DOI] [PubMed] [Google Scholar]
- Shin BJ, et al. Superselective intra-arterial cerebral infusion of novel agents after blood–brain disruption for the treatment of recurrent glioblastoma multiforme: a technical case series. Neurosurg. Clin N. Am. 2012;23:323–329. ix–x. doi: 10.1016/j.nec.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Takayasu N, Dacey RG., Jr. Effects of mannitol on intracerebral arteriolar diameter in vitro: extraluminal and intraluminal application. Neurosurgery. 1989;25:747–751. doi: 10.1097/00006123-198911000-00009. (discussion 751-2) [DOI] [PubMed] [Google Scholar]
- Todd MM, Weeks JB, Warner DS. Cerebral blood flow, blood volume, and brain tissue hematocrit during isovolemic hemodilution with hetastarch in rats. Am J. Physiol. 1992;263:H75–H82. doi: 10.1152/ajpheart.1992.263.1.H75. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, et al. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Optically measured NADH concentrations are unaffected by propofol induced EEG silence during transient cerebral hypoperfusion in anesthetized rabbits. Brain. Res. 2011;1396:69–76. doi: 10.1016/j.brainres.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Etu J, Joshi S. Enhanced disruption of the blood brain barrier by intracarotid mannitol injection during transient cerebral hypoperfusion in rabbits. J. Neurosurg. Anesthesiol. 2007;19:249–256. doi: 10.1097/ANA.0b013e3181453851. [DOI] [PubMed] [Google Scholar]
- Zunkeler B, et al. Quantification and pharmacokinetics of blood–brain barrier disruption in humans. J. Neurosurg. 1996;85:1056–1065. doi: 10.3171/jns.1996.85.6.1056. [DOI] [PubMed] [Google Scholar]