Abstract
Activation of the small guanosine triphosphatase (GTPase) RhoA can promote cell survival in cultured cardiomyocytes and in the heart. Here, we showed that the circulating lysophospholipid sphingosine 1-phosphate (S1P), a G-protein coupled receptor agonist, signaled through RhoA and phospholipase C ε (PLCε) to increase the phosphorylation and activation of protein kinase D 1 (PKD1). Genetic deletion of either PKD1 or its upstream regulator PLCε inhibited S1P-mediated cardioprotection against ischemia/reperfusion injury. Cardioprotection involved PKD1-mediated phosphorylation and inhibition of the cofilin phosphatase Slingshot 1L (SSH1L). Cofilin 2 translocates to mitochondria in response to oxidative stress or ischemia/reperfusion injury, and both S1P pretreatment and SSH1L knockdown attenuated translocation of cofilin 2 to mitochondria. Cofilin 2 associates with the proapoptotic protein Bax, and the mitochondrial translocation of Bax in response to oxidative stress was also attenuated by S1P treatment in isolated hearts or by knockdown of SSH1L or cofilin 2 in cardiomyocytes. Furthermore, SSH1L knockdown, like S1P treatment, increased cardiomyocyte survival and preserved mitochondrial integrity following oxidative stress. These findings reveal a pathway initiated by GPCR agonist-induced RhoA activation, in which PLCε signals to PKD1-mediated phosphorylation of cytoskeletal proteins to prevent the mitochondrial translocation and proapoptotic function of cofilin 2 and Bax and thereby promote cell survival.
Introduction
A subset of G-protein coupled receptors including those for sphingosine 1-phosphate (S1P) couple to the heterotrimeric Gα12/13 protein to activate RhoA (1–5). S1P is released at sites of cell injury, including the ischemic heart (6), and we and others have shown that S1P protects the heart against myocardial ischemia/reperfusion injury (6–8) and protects cardiomyocytes against oxidative stress (9). RhoA expression attenuates the response of cardiomyocytes to apoptotic insults (10) and mice that overexpress RhoA show increased tolerance to ischemia/reperfusion injury whereas RhoA knockout mice demonstrate exaggerated ischemia/reperfusion damage (11).
Phospholipase C ε (PLCε) is the only isoform of PLC that has a GTP-RhoA binding insertion within its catalytic core and that acts as a direct RhoA effector (12, 13). The activation of PLCε generates the second messenger diacylglycerol (DAG), and together DAG and protein kinase C can activate protein kinase D (PKD) (14, 15). Indeed, PKD activation is inhibited by PLCε gene knockout (16, 17). Our previous studies have implicated PKD1 as a downstream mediator of the protective effects of RhoA on ischemia/reperfusion damage (11). The possibility that PLCε or PKD1 mediates cardioprotective signaling in response to S1P and other GPCRs that activate RhoA has not been considered. Although PLCε and PKD have been implicated in cardiac hypertrophy (17, 18) and in the regulation of gene expression (16, 19–21), there is little prior evidence for a role for direct PKD phosphorylation targets in cell survival.
Here we demonstrate a role for PKD in cell protection and identify Slingshot 1L (SSH1L) as the target of PKD1 mediated phosphorylation that regulates this response. SSH1L is a selective phosphatase for the actin-binding protein cofilin (22). Several studies show that cofilin translocates to mitochondria and induces cell death in response to oxidant stimulation (23–25). The work reported here reveals that this process is regulated: SSH1L inhibition, which occurs through PKD1 mediated phosphorylation, abolishes oxidative stress-induced mitochondrial translocation of cofilin 2, preserves mitochondrial membrane integrity and promotes cell survival. Accordingly we delineate a pathway by which S1P, through modulation of the cytoskeletal regulators SSH1L and cofilin 2, couples GPCR activation to mitochondrial events that increase cell survival during oxidative stress.
Results
PKD1 is activated by S1P and mediates S1P cardioprotection in the isolated heart
We used S1P as a physiological stimulus to activate RhoA signaling in the isolated perfused mouse heart. Perfusion with S1P for 10 minutes increased the amount of active (GTP-bound) RhoA (2.1 fold compared to vehicle) in the left ventricle (Fig. 1A). S1P perfusion for 30 minutes increased the phosphorylation of PKD1 at Ser744/748 (3.7 fold compared to vehicle), indicative of its activation (Fig. 1B). To determine whether PKD1 plays a role in S1P induced cardioprotection, PKD1 knockout and wild-type mice were subjected to global ischemia/reperfusion injury. S1P pretreatment significantly attenuated myocardial infarct development in wild-type mice but not in PKD1 knockout mice (Fig. 1 C and D). These findings implicate PKD1 in S1P-mediated cardioprotection.
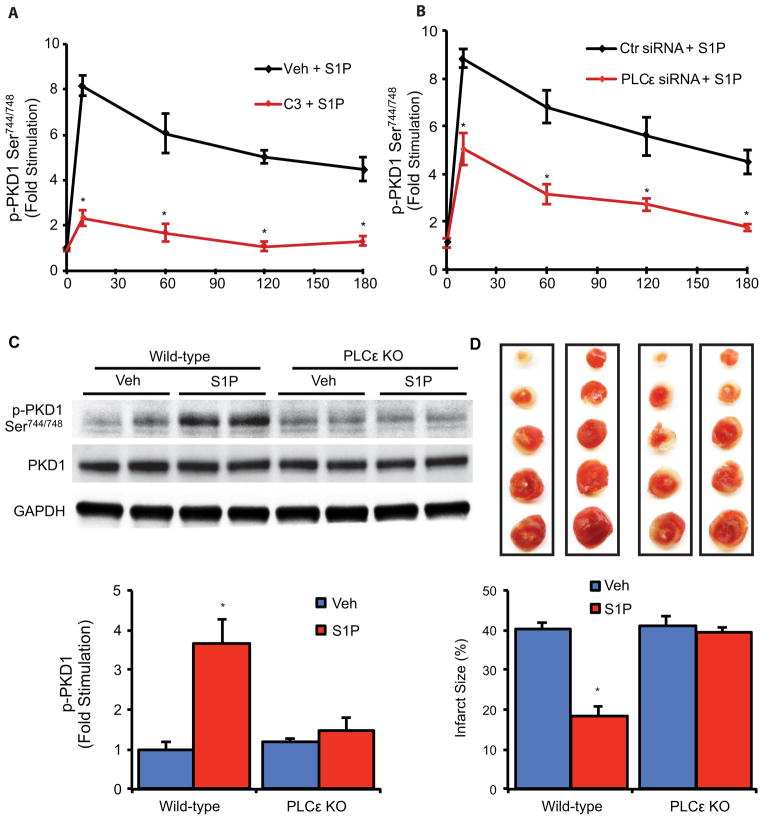
Fig. 1.
S1P activates RhoA and PKD1, and PKD1 gene deletion prevents S1P protection in the heart. (A and B) Mouse hearts were perfused with S1P or Vehicle (Veh) and RhoA activation and PKD1 phosphorylation in the left ventricle were determined. (A) Quantification of GTP RhoA amount. n = 4 animals per group. (B) Representative blots (top) and quantification (bottom) of phosphorylation of PKD1 (Ser744/748). n = 5 animals per group. (C) Representative blots showing PKD1 protein abundance in WT and PKD1 KO mouse hearts. (D) WT and PKD1 KO mouse hearts were subjected to ischemia/reperfusion injury with Veh or S1P pretreatment. Representative images of TTC stained cross sections of heart following ischemia/reperfusion injury (top) and quantification of the infarct size (bottom). n = 5–6 animals per group.
PLCε mediates PKD1 activation and cardioprotection by S1P
We used isolated cardiomyocytes to explore the mechanism by which PKD1 is activated in response to S1P. Treatment with S1P robustly activated RhoA (fig. S1A) and elicited dose dependent phosphorylation of PKD1 (fig. S1B). PKD1 activation by S1P was sustained (still increased 4 fold at 3 hours) and the response was attenuated by inhibition of RhoA with the C3 exoenzyme (Fig. 2A and fig. S2). Based on our previous work we hypothesized that PKD1 activation by S1P and RhoA occurs through the phospholipase C isoform PLCε (12, 16, 17, 26, 27). Indeed, knockdown of PLCε with siRNA significantly reduced the phosphorylation of PKD1 in response to S1P in cardiomyocytes (Fig. 2B and fig. S3). To determine whether this signaling pathway is also utilized in the intact heart we compared responses to S1P in isolated perfused wild-type and PLCε knockout mouse hearts. S1P treatment failed to stimulate PKD1 phosphorylation in the absence of PLCε (Fig. 2C), confirming a requirement for PLCε in PKD1 activation by S1P in the intact heart. Moreover, the ability of S1P to protect against infarct development was lost in PLCε knockout mice (Fig. 2D), mirroring what was seen in PKD1 knockout mice (Fig. 1D). Thus PLCε transduces agonist induced RhoA activation to PKD1 to confer cardioprotection.
Fig. 2.
S1P activates PKD1 through RhoA and PLCε, and PLCε gene deletion prevents S1P protection in the heart. (A and B) Time course phosphorylation of PKD1 in response to S1P and inhibition by C3 exoenzyme (A) and PLCε knockdown with siRNA (B) in cardiomyocytes. n = 3–5 experiments per time point. (C) WT and PLCε KO mouse hearts were perfused with Veh or S1P for 30 min. Representative blots (top) and quantification (bottom) showing PKD1 phosphorylation. n = 5 animals per group. (D) WT and PLCε KO mouse hearts were subjected to ischemia/reperfusion injury with Veh or S1P pretreatment. Representative images of TTC stained cross sections of heart following ischemia/reperfusion injury (top) and quantification of the infarct size (bottom). n = 5–6 animals per group.
PKD1 substrate SSH1L is an S1P signaling target that is functionally inhibited by S1P
To identify the downstream target through which PKD1 elicits cardioprotection, we searched for proteins that contain a PKD1 consensus sequence (LXRXXS) (20, 28) and are associated with control of cell survival. We focused our attention on Slingshot 1L (SSH1L), a cofilin-specific phosphatase (22) that is phosphorylated at Ser978 within a PKD1 consensus sequence (29, 30). S1P increased phosphorylation of SSH1L at Ser978 in cardiomyocytes in a dose-dependent manner (Fig. 3A). PKD mediated phosphorylation of SSH1L inhibits its ability to dephosphorylate cofilin at Ser3 (29, 30). Accordingly we assessed the functional effects of S1P-mediated SSH1L phosphorylation by examining cofilin phosphorylation. The phosphorylation of Ser3 in cofilin 2, the muscle-specific cofilin isoform, increased in a dose-dependent manner following S1P treatment (Fig. 3B). We also used siRNA-mediated knockdown of SSH1L to simulate SSH1L inhibition by S1P and observed increased phosphorylation of cofilin 2 (Fig. 3C). RhoA inhibition with C3 exoenzyme prevented S1P-induced SSH1L and cofilin 2 phosphorylation (fig. S4). Cardiac-specific RhoA overexpression (11) increased phosphorylation of both SSH1L and cofilin 2 (fig. S5), supporting the conclusion that SSH1L and cofilin 2 are downstream targets of RhoA signaling in the adult mouse heart.
Fig. 3.
PKD1 dependent phosphorylation of SSH1L and cofilin 2 in cardiomyocytes. (A and B) Representative blots (top) and quantification (bottom) of dose-dependent phosphorylation of SSH1L (A) and cofilin 2 (B) by 30 min S1P treatment. n = 4 experiments per time point. (C) Cardiomyocytes were transfected with Ctr siRNA or SSH1L siRNA. Representative blots showing cofilin abundance, phosphorylation, and SSH1L abundance (top) and quantification of cofilin 2 phosphorylation after SSH1L knockdown (bottom). n = 4 experiments per treatment. (D and E) Cardiomyocytes were transfected with Ctr siRNA or PKD1 siRNA then subjected to S1P treatment. Time course of the phosphorylation of SSH1L (D) and cofilin 2 (E) by S1P. n = 4–5 experiments per time point. (F) Hypothetical scheme for S1P-PKD1-SSH1L-cofilin2 signaling.
Phosphorylation of SSH1L and cofilin 2 by S1P was sustained and fully inhibited by PKD1 knockdown with siRNA (fig. S6, Fig. 3 D and E). The p21-activated kinase 4 (PAK4) is another PKD substrate that could regulate phosphorylation of cofilin (31). PAK4 involvement in the phosphorylation of cofilin 2 in cardiomyocytes appeared unlikely, however, because S1P failed to increase phosphorylation of Ser474 in PAK4. In addition, PAK4 knockdown by siRNA did not reduce S1P induced phosphorylation of cofilin 2 (fig. S7). These findings and those presented above suggest that SSH1L, rather than PAK4, is the major endogenous PKD1 target by which S1P transduces cell signals to regulate cofilin 2 (Fig. 3F).
SSH1L inhibition promotes cell survival and preserves mitochondrial integrity in response to H2O2
S1P treatment reduced cell death in response to H2O2 induced oxidative stress, as indicated by increases in calcein-positive cells and decreases in propidium iodide-positive cells and LDH release. These effects of S1P were abolished in cardiomyocytes transfected with PKD1 siRNA (Fig. 4 A to C). Knockdown of SSH1L, like inhibition of SSH1L by S1P and PKD1, improved cell viability and reduced LDH release in response to H2O2 treatment (Fig. 4 D and E). To demonstrate mitochondrial involvement in the actions of S1P, PKD1, and SSH1L, we first measured H2O2 induced mitochondrial outer membrane permeabilization, as assessed by cytochrome c release to the cytosol. S1P pretreatment and SSH1L knockdown significantly reduced cytochrome c release (Fig. 5 A and B), and the effect of S1P was reversed by siRNA-mediated PKD1 knockdown (fig. S8). We also assessed the mitochondrial inner membrane electrical potential (Δψm) using tetramethyl rhodamine ethyl ester (TMRE). Both S1P pretreatment and SSH1L knockdown significantly reduced the dissipation of TMRE fluorescence in response to H2O2 (Fig. 5 C and D). These findings suggest that the effects of S1P, PKD1, and SSH1L on cell survival result from the maintenance of mitochondrial integrity.
Fig. 4.
PKD1 mediates S1P induced cell survival and SSH1L knockdown mimics S1P protection in response to H2O2 treatment in cardiomyocytes. (A through C) Cardiomyocytes were transfected with Ctr siRNA or PKD1 siRNA. (A) Representative images of cardiomyocytes stained with Calcein (green) and propidium iodide (red) following indicated treatments. Scale bar, 40 μm. Quantification of cardiomyocyte viability (B) and LDH release (C) following the indicated treatments. (D and E) Cardiomyocytes were transfected with Ctr siRNA or SSH1L siRNA. Quantification of cardiomyocyte viability (D) and LDH release (E) following the indicated treatments. n = 6 experiments per treatment for (B) to (E).
Fig. 5.
S1P or SSH1L knockdown decreases cytochrome c release and preserves mitochondrial membrane potential in response to H2O2 treatment in cardiomyocytes. (A and B) Cytochrome c (Cyto c) release in the cytosolic fraction with H2O2. (A) Representative blots (top) and quantification (bottom) of Cyto c release with or without S1P pretreatment. (B) Representative blots (top) and quantification (bottom) of Cyto c release in Ctr siRNA or SSH1L siRNA transfected cells. (C and D) TMRE fluorescence intensity in response to H2O2 treatment. (C) Representative images of TMRE staining (top) and quantification (bottom) of TMRE fluorescence intensity with or without S1P pretreatment. Scale bar, 20 μm. (D) Representative images of TMRE staining (top) and quantification (bottom) of TMRE fluorescence intensity in Ctr siRNA or SSH1L siRNA transfected cells. Scale bar, 20 μm. n = 6 experiments per treatment for (A) to (D).
SSH1L inhibition reduces mitochondrial translocation of cofilin 2 and Bax in response to H2O2
Cofilin 1, the non-muscle isoform, translocates to mitochondria and induces cell death (23–25, 32). We examined the possibility that the muscle specific isoform cofilin 2 also translocates to mitochondria in response to oxidative stress. Following H2O2 treatment, cofilin 2 abundance in the cardiomyocyte mitochondrial fraction was significantly increased (Fig. 6A). Cofilin 1 translocation is driven by oxidation of its cysteine residues and requires dephosphorylation of Ser3 (23, 25, 32). Thus we determined whether S1P or knockdown of SSH1L, which increases phosphorylation of Ser3 in cofilin 2, might reduce its mitochondrial translocation under oxidative stress. Indeed, S1P pretreatment or SSH1L depletion significantly reduced mitochondrial translocation of cofilin 2 (Fig. 6 A and B). When activated by oxidative stress, the Bcl-2 family protein Bax translocates to mitochondria to engage a mitochondrial cell death pathway (33–35). We found that cofilin 2 and Bax formed a protein complex, as demonstrated by co-immunoprecipitation (Fig. 6C) and accordingly explored the possibility that S1P and SSH1L might also affect the mitochondrial translocation of Bax. The physical interaction between Bax and cofilin 2 was not affected by oxidative stress or by S1P (fig. S10). Nevertheless, H2O2 induced increases in mitochondrial Bax were reduced by S1P (Fig. 6D) and by knockdown of SSH1L (Fig. 6E) or cofilin 2 (fig. S9 and Fig. 6F). These findings suggest that the phosphorylation status of cofilin 2 plays a role in Bax translocation under oxidative stress.
Fig. 6.
S1P and SSH1L regulate mitochondrial translocation of cofilin 2 and Bax in H2O2-treated cardiomyocytes. (A) Cardiomyocytes were pretreated with S1P or Veh and then subjected to H2O2 treatment. Representative blots (top) and quantification (bottom) of mitochondrial abundance of cofilin 2. (B) Cardiomyocytes were transfected with Ctr siRNA or SSH1L siRNA then subjected to H2O2 treatment. Representative blots (top) and quantification (bottom) of mitochondrial abundance of cofilin 2. (C) Representative blots showing co-immunoprecipitation of cofilin 2 and Bax. (D) Cardiomyocytes were pretreated with S1P or Veh and then subjected to H2O2 treatment. Representative blots (top) and quantification (bottom) of mitochondrial Bax. (E) Cardiomyocytes were transfected with Ctr siRNA or SSH1L siRNA then subjected to H2O2 treatment. Representative blots (top) and quantification (bottom) of mitochondrial Bax. (F) Cardiomyocytes were transfected with Ctr siRNA or cofilin 2 siRNA then subjected to H2O2 treatment. Representative blots (top) and quantification (bottom) of mitochondrial Bax. n = 5–6 experiments per treatment for (A) to (F).
S1P prevents mitochondrial cofilin 2 and Bax translocation during ischemia/reperfusion injury in the isolated heart
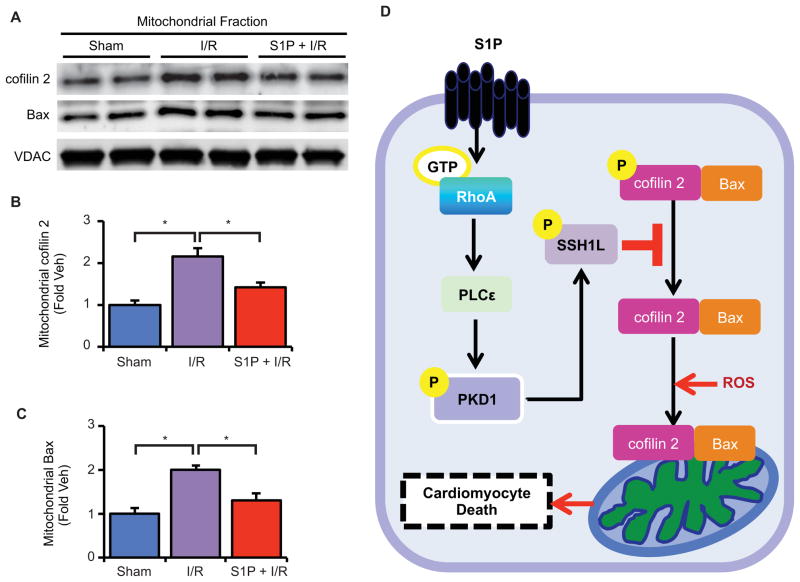
Finally we asked whether cofilin 2 translocates to mitochondria in the isolated perfused heart subjected to oxidative stress. Analysis of mitochondria from hearts subjected to ischemia/reperfusion revealed marked increases in mitochondrial cofilin 2 as well as Bax protein. Pretreatment with S1P for 10 minutes significantly reduced the appearance of both proteins in the mitochondrial fraction (Fig. 7 A–C). These data, coupled with our demonstration of a requirement for PLCε and PKD1 in protection against ischemia/reperfusion injury (Fig. 1D and 2D), suggest that S1P signals through the pathway delineated here and protects the heart from ischemia/reperfusion injury by increasing the phosphorylation of cofilin 2 and limiting its mitochondrial translocation as well as that of Bax (Fig. 7D).
Fig. 7.
S1P attenuates mitochondrial translocation of cofilin 2 and Bax by ischemia/reperfusion injury in the isolated heart. Isolated mouse hearts were perfused with Veh or S1P prior to 20 min ischemia followed by 30 min reperfusion. (A) Representative blots showing mitochondrial cofilin 2 and Bax abundance. (B) Quantification of mitochondrial cofilin 2 and (C) mitochondrial Bax. n = 4–6 animals per group. (D) Hypothetical schema showing the role of the S1P-RhoA-PKD1-SSH1L signaling pathway in oxidative stress induced cofilin 2 and Bax translocation to mitochondria and cardiomyocyte cell death.
Discussion
The activation of RhoA by S1P and other GPCR agonists that couple to Gα12/13 to regulate RhoA guanine nucleotide exchange factors has been well established (1–5). We have demonstrated that in vivo expression of RhoA in the heart elicits PKD activation, and furthermore that RhoA signaling affords protection against ischemic injury through PKD (11). The data presented here use this physiologically relevant system to demonstrate that S1P activates this pathway, determine how this occurs, and provide a molecular link between PKD and cardioprotection.
RhoA regulates various proteins that control the actin cytoskeleton to modulate cell shape, migration, and adhesion (36–38). In addition, transcriptional responses can be initiated through RhoA, contributing to cell proliferation and inflammation (16, 39, 40). Because RhoA plays a role in regulating cell survival (10, 11, 41), elucidating downstream RhoA targets responsible for these additional pleiotropic effects of RhoA is critical to our understanding of the actions of the broad subset of GPCRs that signal primarily through Gα12/13 mediated RhoA activation.
Active (GTP-liganded) RhoA binds to various target proteins including Rho kinase, the mammalian diaphanous (mDia), and PKN (PRK1/2) to stimulate their activity (5). RhoA signaling leads to PKD activation (42, 43) but the effector mechanism through which this occurs has not been elucidated. PLCε is not activated through the actions of Gαq, the G-protein that couples canonical PLC linked receptors to PLCβ, but rather through binding of low molecular weight G-proteins including RhoA and Rap (13, 27, 44). In addition PLCε appears to be compartmentalized within the cell, which allows for its continued activation and sustained signaling (17, 45, 46). Our studies using primary astrocytes demonstrated that GPCR agonist mediated RhoA activation elicits DAG generation as well as sustained PKD activation through PLCε (16, 26). We demonstrate here that PLCε mediates S1P-RhoA signaling to PKD in the intact heart. As evidenced by the results discussed below this signaling pathway plays a prominent role in mitochondrial regulation and cell survival.
S1P is released in the heart following ischemia/reperfusion and treatment with S1P can protect against ischemia/reperfusion damage (6, 7). We show here that the ability of S1P to attenuate infarct development in response to ischemia/reperfusion is significantly attenuated in the absence of PKD1 or PLCε. Previously we demonstrated that genetic loss of RhoA exacerbates ischemia/reperfusion injury (11). Thus S1P signaling to RhoA, PLCε and PKD1 participates in cardioprotective signaling. There are other protective signaling pathways activated in response to reperfusion, including the pro-survival effects of Akt and ERK activation (47, 48). Indeed S1P has been suggested by our group and by the Karliner lab to mediate cardioprotection through Akt or ERK activation (7, 49). These pathways are, however, regulated through signaling of S1P receptors to Gi mediated pathways, which are distinct from, although potentially complementary to the RhoA and PKD mediated protective pathway revealed here.
The involvement of PLCε and PKD activation in acute cardioprotective response to GPCR agonists can be contrasted with the role these mediators play in cardiac hypertrophy, a more chronic response involving alterations in cardiac gene expression. Hypertrophy induced by in vivo pressure overload or by chronic adrenergic and angiotensin II signaling depends on PKD1 because hypertrophic responses are attenuated in PKD1 knockout mice (18). PKD phosphorylates class II HDACs, leading to their nuclear-cytoplasmic shuttling and de-repression of MEF-2 mediated gene expression. PLCε may also play a role in activating PKD in a perinuclear compartment, where PKD contributes to pressure overload induced hypertrophic gene expression (17).
Although nuclear signaling underlies the chronic, transcription dependent changes in cardiomyocyte hypertrophy, the acute onset of cell death that accompanies reperfusion of the ischemic heart, is more closely associated with signals affecting mitochondria. Although little is known regarding mechanisms by which PKD elicits signals that affect mitochondrial function and cell survival, it is noteworthy that DAG, which targets and is required for activation of PKD, is increased in the mitochondrial compartment of HeLa cells following oxidative stress (50). Furthermore, PKD activates the transcription factor NF-κB to increase the expression of the gene encoding the antioxidant enzyme MnSOD, which in turn reduces oxidative damage to mitochondria and increases cell survival (51, 52). Although this potential mechanism is of considerable interest, the protection we observe occurs within one hour, a time frame during which transcriptional responses are unlikely to be of primary importance. Accordingly we focused on posttranslational modifications, in particular phosphorylation events, mediated through PKD that could be responsible for more acute effects on cell survival.
PKD1 substrates have been identified through approaches combining in vitro biochemical and in silico screening with a PKD1 substrate phospho-MOTIF antibody based on a consensus PKD1 substrate motif (28, 53, 54). The cofilin-specific phosphatase SSH1L has two PKD1 phosphorylation consensus motifs; PKD1 directly phosphorylates SSH1L to inhibit its function, and constitutively active PKD acts as an inhibitor of SSH1L (20, 29, 30, 55). We demonstrate here that this pathway is physiologically regulated, in that endogenous SSH1L is a S1P and PKD1 signaling target in cardiomyocytes. Our studies further demonstrate that S1P actions on PKD1 lead to inhibition of SSH1L function, evidenced by increased phosphorylation of the muscle-specific cofilin, cofilin 2. SSH1L has been implicated in the regulation of the actin cytoskeleton, and affects cell migration, protrusions, and actin dynamics (22, 29, 30, 56). Cofilin 1 is involved in oxidative stress induced cell death (23–25, 32). Oxidized cofilin translocates to mitochondria, a process that has been suggested to require dephosphorylation of cofilin1 at Ser3 (23, 25, 32). Here we extend the observation of cofilin 1 translocation to mitochondria to also include the muscle specific cofilin 2. Our findings suggest that physiological regulation of cofilin phosphorylation, mediated through agonist induced PKD1 activation and SSH1L inhibition, affects the mitochondrial translocation of cofilin 2.
Cardiomyocyte viability and mitochondrial membrane integrity are diminished by oxidative stress in a manner that is ameliorated by S1P treatment or SSH1L knockdown. The association of improved mitochondrial integrity and cell survival with cofilin 2 phosphorylation and diminished mitochondrial translocation implicates mitochondrial cofilin 2 in a cell death pathway. How mitochondria associated cofilin promotes cell death is largely unknown. Cofilin 1 has been proposed to directly affect mitochondria and to induce swelling and cytochrome c release by directly regulating the mitochondrial permeability transition pore (mPTP) (23). A role for the pro-apoptotic Bcl-2 family protein Bax in cofilin mediated cell death has also been suggested by studies using cancer cell lines and primary rat neurons (24, 57). Although a mechanistic connection between cofilin and Bax remains unclear, we provide new evidence that endogenous cofilin 2 and Bax form a protein complex in cardiomyocytes. Furthermore translocation of Bax to mitochondria in response to oxidative stress is significantly inhibited by S1P treatment or by SSH1L or cofilin 2 knockdown. Bax translocates to mitochondria and regulates outer mitochondrial membrane permeability or mPTP induced cell death (58, 59). Thus it is possible that cofilin 2 functions as a Bax transporter and chaperones Bax to mitochondria. Because the physical interaction between Bax and cofilin 2 we observed was not affected by oxidative stress or by S1P induced changes in phosphorylation of cofilin 2, this protein interaction may be constitutive rather than regulated. Further studies to demonstrate the mechanic relationship between SSH1L, cofilin 2, and Bax are currently in progress.
In summary, the findings reported here reveal a cardioprotective signaling cascade initiated by S1P, mediated through RhoA and its target PLCε, leading to activation of PKD1 and functional inhibition of its substrate SSH1L, and ultimately promoting cell survival (Fig. 7D). This pathway may provide new therapeutic targets for limiting tissue injury induced by oxidative stress. Future studies will be directed at elucidating the precise mechanism by which cell death is induced through regulation of cofilin 2, and determining whether the actin severing function of cofilin 2 plays a role in cell death and mitochondrial integrity.
Materials and Methods
Cell culture and reagents
Neonatal rat ventricular myocytes (cardiomyocytes) were isolated from cardiac ventricles of 1–2-day-old Sprague-Dawley rat pups, digested with collagenase, and cardiomyocytes were purified through a Percoll gradient (60). Cardiomyocytes were plated at density of 3.5×104/cm2 on gelatin-coated dishes and maintained overnight in 4:1 Dulbecco’s modified Eagle’s medium (DMEM)/medium199, supplemented with 10% horse serum, 5% fetal calf serum, and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) at 37°C with 10% CO2. After an overnight culture, cells were either serum starved with DMEM (4.5 g/L D-Glucose) for 24 hours or transfected with siRNA for further analysis. The RhoA inhibitor C3 exoenzyme was obtained from Cytoskeleton (CT04) and used at 1.5 μg/ml overnight. S1P was obtained from Avanti Polar Lipid.
Transfection of Cardiomyocytes with siRNA
Pre-designed PLCε, PKD1, SSH1L, PAK4, and cofilin 2 siRNA for rat and scrambled control siRNA were purchased from Qiagen. Cardiomyocytes were transfected with siRNA using DharmaFECT-I transfection reagent (Thermo Scientific) based on manufacture’s instruction. 3μg siRNA were transfected into 1×106 cells. siRNA and DharmaFECT-1 (1:3 ratio) were individually incubated in conical tubes containing OPTI-MEM media (GIBCO) at room temperature for 10 min, mixed and incubated at room temperature for 20 min. Media in culture dishes were replaced with fresh media and siRNA/DharmaFECT-I mixtures were added to culture dishes. Following overnight incubation, cells were washed and cultured for another 24 hours in serum free DMEM supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin before further treatment.
GTP-RhoA pull-down assay
RhoA activation was determined by affinity pull-down assay using a glutathione S-transferase (GST) fusion protein of the RhoA binding domain of the RhoA effector rhotekin, as described previously by our group (61). Briefly, following 24 hours of serum starvation, cardiomyoyctes were treated with S1P (0.3 μM), phenylephrine (PE) (50 μM) or vehicle (DMEM) for 5 min and then rinsed with ice-cold Tris-buffered saline, lysed in buffer containing 50 mM Tris, pH 7.4, 0.1% Triton X-100, 150 mM, NaCl, 5 mM MgCl2, and 10% glycerol, supplemented with protease and phosphatase inhibitors (Sigma), clarified by brief centrifugation, and then incubated with the sepharose-bound GST-rhotekin-RhoA binding domain for 45 min at 4°C. The beads and precipitated proteins were washed, boiled, and separated by SDS-PAGE. The precipitated GTP-bound RhoA was normalized to total RhoA present in the whole cell lysate.
Western blotting and immunoprecipitation
Cardiomyocytes cell lysates or left ventricular lysates were prepared with various lysis buffers for various purposes. Western blot analysis was performed according to protocols described previously (60). The antibodies used for immunoblotting were from the following sources: RhoA from Santa Cruz Biotechnology, Inc.; GAPDH, p-PKD1 (Ser744/748), PKD1, and Bax (for Western blot and immunoprecipitation of total Bax) from Cell Signaling Technology; SSH1L and p-SSH1L (Ser978) from ECM Biosciences Laboratories; Bax (6A7 for immunoprecipitation of activated Bax), VDAC, cofilin 2, and p-cofilin 2 (Ser3) from Millipore. Peroxidase conjugated secondary antibodies are used at dilution of 1:2500 (Sigma) and the enhanced chemiluminescent substrate from Thermo Scientific. Total Bax, cofilin 2 and activated Bax were immunoprecipitated by using antibody to total Bax (Cell Signaling Technology), total cofilin 2 (Millipore), and 6A7 Bax (Millipore) respectively. Lysates were precleared with Protein A/G PLUS-agarose beads for 30 min at 4°C and 300 μg of total protein was then incubated with antibodies (1 μg for total Bax and cofilin 2, 4 μg for activated Bax) and protein A/G PLUS agarose (Santa Cruz Biotechnology) (40 μl of 50% slurry) at 4°C overnight. Immunocomplexes were washed with ice-cold lysis buffer for 4 times, and beads were boiled in 2x LDS buffer to elute captured protein and subjected to Western blotting analysis as described above.
Isolated perfused heart (Langendorff) ischemia/reperfusion
Hearts from age-matched (8–12 week old) male mice were removed quickly and perfused retrogradely with modified Krebs–Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 0.5 mM EDTA, 1.2 mM MgSO4, 11 mM Glucose, 1.5 mM Na-Pyruvate and 2 mM CaCl2) in a Langendorff apparatus (Radnoti) at constant pressure (80 mmHg). After stabilization, hearts were subjected to a period of global ischemia, followed by reperfusion. To measure infarct size, hearts were subjected to 30-min (PLCε WT and knockout mice) global ischemia and 1-hour reperfusion. S1P was perfused at either 10-min before ischemia or right at the onset of reperfusion at 0.1 μM. For PKD1 wild-type and knockout mice the ischemia time was adjusted to 20 min. At the end of the ischemia/reperfusion protocols, ventricles were frozen and cut transversely into five slices of equal thickness. The slices were then incubated in 1% TTC/PBS and fixed in 10% formalin-phosphate buffered saline for 24 hours. Fixed slices were scanned and Image J was used to measure and calculate the size of the infarct area and the total area.
Gene targeting and generation of global PKD1-deficient mice
The PKD1 mouse genomic DNA clone was isolated from a 129SVJ mouse genomic library (Stratagene) and used to construct the PKD1 targeting vector by standard techniques as described previously (62). Briefly, 2 fragments of PKD1 gene were cloned into a targeting vector that contained a neomycin selection cassette flanked by two FRT sites that further flanked by two LoxP sites. A 971-bp fragment containing exon14 of PKD1 (107 bp) was inserted between the first LoxP and the first FRT sites. Targeting vector was linearized with NsiI and subsequently electroporated into R1 embryonic stem (ES) cells. G418-resistant ES clones were screened for homologous recombination by DNA blot analysis, as described below. Two independent homologous recombinant ES clones were microinjected into blastocysts from C57BL/6J mice to generate male chimeras. Male chimeras were bred with female Black Swiss mice to generate germline transmitted floxed heterozygous mice (PKD1+/flox-neo), which were subsequently crossed with Pro-Cre mice (63) to generate homozygous knockout mice (PKD1-/-) as previously described (62). Offspring from intercrosses were genotyped by PCR analysis using mouse tail DNA. All animal procedures were approved by the UCSD Animal Care and Use Committee.
DNA analysis for PKD1-deficient mice
Genomic DNA was extracted from G418-resistant ES cell clones and mouse tails, as previously described (64). ES cell DNA was digested using NsiI, and DNAs were electrophoresed on a 1% (wt/vol) agarose gel, and subsequently blotted onto a nitrocellulose membrane. A 396-bp fragment was generated by PCR using mouse genomic DNA and specific PKD1 primers (forward, CCCTATACATCATATATCATCAAG; reverse, CATAGACCCTTTGTGGCTTGAT AA). The PCR product was subsequently radiolabeled using γ-[32P]dATP by random priming (Invitrogen). DNA blots were hybridized with the radiolabeled probe and visualized by autoradiography. Offspring from intercrosses were genotyped by PCR analysis using mouse tail DNA and the following PKD1 gene specific primers (forward, ATGAGGGCAGTGTATCAG AGGT; reverse, TCTTGCATCCTGTTCTCACTGT), mutant allele (forward, ATGAGGGCA GTGTATCAGAGGT; reverse, CAAAGCAGCAATCAGAAAAATG). PCR products were visualized by ethidium bromide staining.
Cell viability and lactate dehydrogenase (LDH) activity assay
Following 24 hours of serum starvation, Cardiomyoyctes were exposed to H2O2 (150 μM, 2 hours) with or without S1P (0.3 μM, 10 min pretreatment), culture medium was then collected for LDH assay and replaced with Hank’s Balanced Salt Solution (HBSS, GIBCO) with 3 μM calcein, AM, 5 μM Propidium Iodide (PI), and Hoechst (1: 800) from Invitrogen and incubated at 37°C for 30 min. The fluorescence was then measured by a TECAN microplate reader. LDH release in the culture medium following H2O2 treatment was measured using a LDH activity assay kit (MBL International Corp.) as per manufacturer’s instruction.
Mitochondrial fractionation
A mitochondrial fractionation kit was used to isolate mitochondria from Cardiomyocytes (EMD Millipore, Billerica, MA). Briefly, cells were serum starved for 24 hrs before stimulated by H2O2 (100 μM, 2 hours) or S1P (0.3 μM, 15 min pretreatment) followed by H2O2, collected in ice cold PBS, spun down at 600 × g for 5 min. PBS was carefully aspirated off and cells were resuspended in the isotonic mitochondrial buffer, briefly vortexed and incubated on ice for 10 minutes. Samples were centrifuged at 700 × g for 10 minutes to spin down nuclei and cell debris. Supernatants were transferred to new tubes and spun at 12,000 × g for 15 min to precipitate mitochondria. The pellet was washed once and resuspended in mitochondrial lysis buffer as the mitochondrial fraction. Mitochondria were isolated from adult mouse hearts as previously described (60). Briefly, left ventricle of the mouse hearts was homogenized by hand in isolation buffer containing 70 mM sucrose, 190 mM mannitol, 20 mM Hepes and 0.2 mM EDTA, 1 μM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.5 mM PNPP and 0.5 mM PMSF. The homogenate was centrifuged at 600 × g for 10 min to remove nuclei and debris. The resulting supernatant was then centrifuged at 5000 × g for 15 min. The resulting mitochondrial pellet was washed by isolation buffer and re-centrifuged twice. After final spin, mitochondrial pellet was resuspended in RIPA buffer and subjected to Western blotting.
Mitochondrial Membrane Potential (Δψm)
Loss of Δψm in response to H2O2 was measured using tetramethylrhodamine ethyl ester (TMRE, Invitrogen). Assessment of Δψm was performed utilizing a TECAN microplate reader to assess a large population of cells in an unbiased manner as previously described (65). Briefly, cardiomyocytes were plated at a density of 6.5×104/cm2 in gelatin-coated 24-well plates, and following 24-hour serum starvation, cardiomyocytes were treated with 50 μM H2O2 with or without S1P pretreatment (0.3 μM, 10 min) for 90 min. Cardiomyocytes were loaded with 50 nM TMRE and Hoechst (1:800) for 30 minutes prior to washing twice in HBSS. TMRE and Hoechst fluorescence was measured using a microplate reader and TMRE fluorescence intensity was normalized to the Hoechst staining.
Statistical analysis
All results are reported as mean ± S.E. Comparison of two groups with one characteristic was accomplished using an unpaired Student’s t test. Data from two groups with multiple characteristics were compared with two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Data from experiments with more than two groups with one characteristic were compared by one-way ANOVA followed by the Tukey’s multiple comparison test. D’Agostino & Pearson omnibus normality test was used to determine whether the data points were normally distributed. Probability values of < 0.05 were considered significant and are indicated by * in all figures.
Supplementary Material
fig. S1. S1P induces robust RhoA and PKD1 activation in cardiomyocytes.
fig. S2. S1P-induced phosphorylation of PKD1 is RhoA-dependent.
fig. S3. S1P-induced phosphorylation of PKD1 is PLCε-dependent.
fig. S4. RhoA is responsible for S1P-induced phosphorylation of SSH1L and cofilin 2 in cardiomyocytes.
fig. S5. Phosphorylated SSH1L and cofilin 2 are increased in the RhoA transgenic (TG) mouse heart.
fig. S6. PKD1 knockdown with siRNA in cardiomyocytes.
fig. S7. Phosphorylation of PAK4 is not increased following S1P treatment and PAK4 knockdown does not reduce S1P-induced phosphorylation of cofilin 2 in cardiomyocytes.
fig. S8. The S1P-induced reduction in cytochrome c release in cardiomyocytes is PKD1-dependent.
fig. S9. Cofilin 2 knockdown by siRNA in cardiomyocytes.
fig. S10. The interaction of cofilin 2 and Bax in cardiomyocytes is not disrupted by S1P or H2O2.
Acknowledgments
We thank Jeffery Smith and Melisa Ridout (UC San Diego) for technical support and animal care.
Funding: Supported by NIH grant HL28143, J.H.B. and R01HL112388, J.C.; S.Y.X. is supported by a postdoctoral fellowship from the American Heart Association (AHA11POST7580130).
Footnotes
Data and materials availability: The global PKD1 knockout mice require an MTA from UC San Diego
Competing interests: The authors declare that they have no competing interests.
Author contributions: S.Y.X. designed and performed all experiments, proposed directions to investigate; analyzed data, and wrote the paper; K.O. made the PKD1 knockout mice; B.S.Y. contributed performed the PAK4 and RhoA TG mice experiment presented in the supplementary materials. S.M. provided technical support and advice; J.C. and A.V.S. provided the knockout mice; J.H.B. supervised the project, directed the study, and revised the paper.
References and Notes
- 1.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998 Jun 26;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 2.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998 Jun 26;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 3.Siehler S, Manning DR. Pathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptors. Biochim Biophys Acta. 2002 May 23;1582:94–99. doi: 10.1016/s1388-1981(02)00142-7. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003 Mar;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang SY, Dusaban SS, Brown JH. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochim Biophys Acta. 2013 Jan;1831:213–222. doi: 10.1016/j.bbalip.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009 Oct;297:H1429–1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007 Jun;292:H2944–2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 8.Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre- and postcondition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol. 2008 Mar-Apr;22:113–118. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 9.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001 Sep;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 10.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008 Dec 19;283:35622–35629. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang SY, Vanhoutte D, Del Re DP, Purcell NH, Ling H, Banerjee I, Bossuyt J, Lang RA, Zheng Y, Matkovich SJ, Miyamoto S, Molkentin JD, Dorn GW, 2nd, Brown JH. RhoA protects the mouse heart against ischemia/reperfusion injury. J Clin Invest. 2011 Aug;121:3269–3276. doi: 10.1172/JCI44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing MR, Bourdon DM, Harden TK. PLC-epsilon: a shared effector protein in Ras-, Rho-, and G alpha beta gamma-mediated signaling. Mol Interv. 2003 Aug;3:273–280. doi: 10.1124/mi.3.5.273. [DOI] [PubMed] [Google Scholar]
- 13.Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-epsilon by Rho. J Biol Chem. 2003 Oct 17;278:41253–41258. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
- 14.Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem. 2003 Jan 3;278:154–163. doi: 10.1074/jbc.M208075200. [DOI] [PubMed] [Google Scholar]
- 15.Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006 Jun;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Dusaban SS, Purcell NH, Rockenstein E, Masliah E, Cho MK, Smrcka AV, Brown JH. Phospholipase C epsilon links G protein-coupled receptor activation to inflammatory astrocytic responses. Proc Natl Acad Sci U S A. 2013 Feb 26;110:3609–3614. doi: 10.1073/pnas.1217355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, Blaxall BC, Smrcka AV. Phospholipase cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013 Mar 28;153:216–227. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci U S A. 2008 Feb 26;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 2011 Feb;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Y, Rubin CS. Protein kinase D: coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep. 2011 Aug;12:785–796. doi: 10.1038/embor.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004 Oct;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002 Jan 25;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 23.Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, Yu LR, Veenstra TD, Shacter E. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat Cell Biol. 2009 Oct;11:1241–1246. doi: 10.1038/ncb1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posadas I, Perez-Martinez FC, Guerra J, Sanchez-Verdu P, Cena V. Cofilin activation mediates Bax translocation to mitochondria during excitotoxic neuronal death. J Neurochem. 2012 Feb;120:515–527. doi: 10.1111/j.1471-4159.2011.07599.x. [DOI] [PubMed] [Google Scholar]
- 25.Wabnitz GH, Goursot C, Jahraus B, Kirchgessner H, Hellwig A, Klemke M, Konstandin MH, Samstag Y. Mitochondrial translocation of oxidized cofilin induces caspase-independent necrotic-like programmed cell death of T cells. Cell Death Dis. 2010;1:e58. doi: 10.1038/cddis.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Citro S, Malik S, Oestreich EA, Radeff-Huang J, Kelley GG, Smrcka AV, Brown JH. Phospholipase Cepsilon is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc Natl Acad Sci U S A. 2007 Sep 25;104:15543–15548. doi: 10.1073/pnas.0702943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): a novel Ras effector. EMBO J. 2001 Feb 15;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutti JE, Jarrell ET, Chang JD, Abbott DW, Storz P, Toker A, Cantley LC, Turk BE. A rapid method for determining protein kinase phosphorylation specificity. Nat Methods. 2004 Oct;1:27–29. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]
- 29.Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009 May;11:545–556. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterburs P, Heering J, Link G, Pfizenmaier K, Olayioye MA, Hausser A. Protein kinase D regulates cell migration by direct phosphorylation of the cofilin phosphatase slingshot 1 like. Cancer Res. 2009 Jul 15;69:5634–5638. doi: 10.1158/0008-5472.CAN-09-0718. [DOI] [PubMed] [Google Scholar]
- 31.Spratley SJ, Bastea LI, Doppler H, Mizuno K, Storz P. Protein kinase D regulates cofilin activity through p21-activated kinase 4. J Biol Chem. 2011 Sep 30;286:34254–34261. doi: 10.1074/jbc.M111.259424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003 Dec;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 33.Nie C, Tian C, Zhao L, Petit PX, Mehrpour M, Chen Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J Biol Chem. 2008 May 30;283:15359–15369. doi: 10.1074/jbc.M800847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 35.Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci U S A. 1996 Dec 10;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000 Jun 1;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 37.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008 Sep;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 38.Ridley AJ, Allen WE, Peppelenbosch M, Jones GE. Rho family proteins and cell migration. Biochem Soc Symp. 1999;65:111–123. [PubMed] [Google Scholar]
- 39.Cen B, Selvaraj A, Prywes R. Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J Cell Biochem. 2004 Sep 1;93:74–82. doi: 10.1002/jcb.20199. [DOI] [PubMed] [Google Scholar]
- 40.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997 Feb 15;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto S, Del Re DP, Xiang SY, Zhao X, Florholmen G, Brown JH. Revisited and revised: is RhoA always a villain in cardiac pathophysiology? J Cardiovasc Transl Res. 2010 Aug;3:330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowell CF, Yan IK, Eiseler T, Leightner AC, Doppler H, Storz P. Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. J Cell Biochem. 2009 Mar 1;106:714–728. doi: 10.1002/jcb.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan J, Slice LW, Rozengurt E. Activation of protein kinase D by signaling through Rho and the alpha subunit of the heterotrimeric G protein G13. J Biol Chem. 2001 Oct 19;276:38619–38627. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- 44.Bunney TD, Katan M. Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol. 2006 Dec;16:640–648. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Kelley GG, Kaproth-Joslin KA, Reks SE, Smrcka AV, Wojcikiewicz RJ. G-protein-coupled receptor agonists activate endogenous phospholipase Cepsilon and phospholipase Cbeta3 in a temporally distinct manner. J Biol Chem. 2006 Feb 3;281:2639–2648. doi: 10.1074/jbc.M507681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smrcka AV, Brown JH, Holz GG. Role of phospholipase Cepsilon in physiological phosphoinositide signaling networks. Cell Signal. 2012 Jun;24:1333–1343. doi: 10.1016/j.cellsig.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008 Apr;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009 Jul;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Tao R, Hoover HE, Zhang J, Honbo N, Alano CC, Karliner JS. Cardiomyocyte S1P1 receptor-mediated extracellular signal-related kinase signaling and desensitization. J Cardiovasc Pharmacol. 2009 Jun;53:486–494. doi: 10.1097/FJC.0b013e3181a7b58a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cowell CF, Doppler H, Yan IK, Hausser A, Umezawa Y, Storz P. Mitochondrial diacylglycerol initiates protein-kinase D1-mediated ROS signaling. J Cell Sci. 2009 Apr 1;122:919–928. doi: 10.1242/jcs.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005 Oct;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003 Jan 2;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem. 2005 Apr 15;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 54.Nishikawa K, Toker A, Wong K, Marignani PA, Johannes FJ, Cantley LC. Association of protein kinase Cmu with type II phosphatidylinositol 4-kinase and type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1998 Sep 4;273:23126–23133. doi: 10.1074/jbc.273.36.23126. [DOI] [PubMed] [Google Scholar]
- 55.Nagel AC, Schmid J, Auer JS, Preiss A, Maier D. Constitutively active protein kinase D acts as negative regulator of the Slingshot-phosphatase in Drosophila. Hereditas. 2010 Oct;147:237–242. doi: 10.1111/j.1601-5223.2010.02200.x. [DOI] [PubMed] [Google Scholar]
- 56.Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006 Feb;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh YC, Rao YK, Wu CC, Huang CY, Geethangili M, Hsu SL, Tzeng YM. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem Res Toxicol. 2010 Jul 19;23:1256–1267. doi: 10.1021/tx100116a. [DOI] [PubMed] [Google Scholar]
- 58.Walensky LD, Gavathiotis E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem Sci. 2011 Dec;36:642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, 2nd, O’Rourke B, Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012 Apr 24;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, Castillo R, Glembotski CC, Sussman MA, Newton AC, Brown JH. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ Res. 2010 Aug 20;107:476–484. doi: 10.1161/CIRCRESAHA.109.215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sagi SA, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. Physical and functional interactions of Galphaq with Rho and its exchange factors. J Biol Chem. 2001 May 4;276:15445–15452. doi: 10.1074/jbc.M008961200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng H, Kimura K, Peter AK, Cui L, Ouyang K, Shen T, Liu Y, Gu Y, Dalton ND, Evans SM, Knowlton KU, Peterson KL, Chen J. Loss of enigma homolog protein results in dilated cardiomyopathy. Circ Res. 2010 Aug 6;107:348–356. doi: 10.1161/CIRCRESAHA.110.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997 Dec 23;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lange S, Ouyang K, Meyer G, Cui L, Cheng H, Lieber RL, Chen J. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J Cell Sci. 2009 Aug 1;122:2640–2650. doi: 10.1242/jcs.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation. 2008 Mar 18;117:1378–1387. doi: 10.1161/CIRCULATIONAHA.107.752618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fig. S1. S1P induces robust RhoA and PKD1 activation in cardiomyocytes.
fig. S2. S1P-induced phosphorylation of PKD1 is RhoA-dependent.
fig. S3. S1P-induced phosphorylation of PKD1 is PLCε-dependent.
fig. S4. RhoA is responsible for S1P-induced phosphorylation of SSH1L and cofilin 2 in cardiomyocytes.
fig. S5. Phosphorylated SSH1L and cofilin 2 are increased in the RhoA transgenic (TG) mouse heart.
fig. S6. PKD1 knockdown with siRNA in cardiomyocytes.
fig. S7. Phosphorylation of PAK4 is not increased following S1P treatment and PAK4 knockdown does not reduce S1P-induced phosphorylation of cofilin 2 in cardiomyocytes.
fig. S8. The S1P-induced reduction in cytochrome c release in cardiomyocytes is PKD1-dependent.
fig. S9. Cofilin 2 knockdown by siRNA in cardiomyocytes.
fig. S10. The interaction of cofilin 2 and Bax in cardiomyocytes is not disrupted by S1P or H2O2.