Abstract
The Stroop effect is considered as a standard attentional measure to study conflict resolution in humans. The response of the brain to conflict is supposed to change over time and it is impaired in certain pathological conditions. Neuropsychological Stroop test measures have been complemented with electroencephalography (EEG) techniques to evaluate the mechanisms in the brain that underlie conflict resolution from the age of 20 to 70. To study the changes in EEG activity during life, we recruited a large sample of healthy subjects of different ages that included 90 healthy individuals, divided by age into decade intervals, which performed the Stroop test while recording a 14 channel EEG. The results highlighted an interaction between age and stimulus that was focused on the prefrontal (Alpha and Theta band) and Occipital (Alpha band) areas. We concluded that behavioural Stroop interference is directly influenced by opposing Alpha and Theta activity and evolves across the decades of life.
Introduction
The classical Stroop test [1] is an executive task used to evaluate prefrontal function that can be applied during the life span of healthy individuals and in neurological pathologies, such as Parkinson's disease [2], Alzheimer's disease [3] and schizophrenia [4]. The test involves the presentation of a series of words (colour names) written in different coloured inks. The ink colour (chromatic information) and the colour's name (semantic information) may be the same (congruent target) or different (incongruent target), demanding the resolution of a cognitive conflict. Accordingly, the subject must respond in function of the ink colour and not the word meaning or the semantic information, and overcoming the automatic response of reading the word produces a delay in the response known as the Stroop interference or Stroop effect.
Some studies suggested that this interference may already occur at the stimulus processing stage [5], an hypothesis that can be verified by measuring evoked response potentials (ERPs) by electroencephalography (EEG), comparing the intensity and signal delays between congruent and incongruent targets [6]. EEG recordings showed that incongruent stimuli have no effect on the amplitude or latency of the P300 component -the cognitive evoked potential-[7], although they induce stronger negativity at around 400 ms than neutral stimuli [8]. This would suggest that interference analysis occurs quite late in time, closer to the response stage than to the stimulus processing stage.
The specific nature of the Stroop effect can also be studied by instantaneous coherence analysis based on a Fast Fourier Transformation (FFT) and in relation to band frequency studies. It was proposed that the 13–20 Hz frequency band was sensitive to discrimination between the congruent and incongruent items, and that higher coherence was observed within the left frontal and left parietal areas [9]–[11]. This is consistent with more recent findings regarding coherence within a time interval of 100–400 ms at 13–18 Hz, which was higher for incongruent situations than for congruent situations in frontal, central and parietal regions without signalling hemisphere. Regarding other bands, increased in the frequency band of 8–10 Hz activity was observed within the prefrontal and parietal areas during the Stroop task, and an interaction was assumed between prefrontal and parietal areas [12].
The location of that effect has also been studied using functional neuroimaging, and the results linked selective attention to activity within the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC). However, the relative contribution of specific regions involved in the Stroop task remains a continuing source of debate [13]. A number of studies have led to the hypotheses that the left DLPFC may be involved in representing and maintaining the attention demands in this task, while response-related activity is associated with the ACC [14], [15].
Regarding the age effect, there is evidence of age-related increases in interference costs. Indeed, ERP studies showed that the peak latency of the P3 wave was delayed in incongruent trials with respect to congruent ones and that this increase was greater for older rather than younger adults. Comparative studies between young and old populations suggested that age differences in the Stroop interference effect can be explained by a general functional slowing down in the older population, which increases Stroop interference [16], [17].
The purpose of this study was to describe the EEG components involved in Stroop interference, the type of band changes and where they occur within the scalp during the lifetime of individuals. We hypothesized that some bands would remain strong and stable throughout life, while others would show significant changes in older rather than younger subjects. The description of this process should improve our understanding of pathological conditions related to ageing in which attention is severally impaired, such as Parkinson's disease.
Materials and Methods
2.1. Subjects
Ninety healthy volunteers took part in this study, divided into five groups according to each age decade (n20–29 = 17, n30–39 = 20, n40–49 = 17, n50–59 = 18, and n60–69 = 18). All volunteers were right-handed, as determined by the Edinburgh Handedness Inventory [18] and they had no clinical history of neurological diseases. The University of Murcia ethics committees approved this study. All participants were informed about the aims of the study and the confidential conditions. They also signed an agreement document, in according with the Ethics Committee of the University of Murcia (Spain), where the EEG tests were carried out.
2.2. Paradigm
During the EEG tests, subjects were asked to resolve a modified version of the Stroop test [19], as used in previous studies [2] and which involved two kinds of stimuli: 1) incongruent targets, colour names printed in incongruently coloured ink (i.e. Rojo [red in English] written in green ink, Verde [green in English] written in blue ink, Azul [blue in English] written in red ink); and 2) congruent targets, animal names always printed in the same colour (e.g. Alce [moose in English] written in blue ink; Rana [frog in English] written in red ink; Visón [mink in English] written in green ink).
2.3. Experimental situation
The experiment was carried out in an electrically-shielded sound-attenuating room. Participants were instructed to answer as soon as possible and to avoid body movement during the recording. Each subject sat on a sofa in the individual sessions, and the stimuli were presented on a plasma TV screen (Samsung LE-32A457, 32 inch, Widescreen, LCD, HD Ready) connected to the main computer and situated 60 cm in front of the sofa. The subject held the experimental keyboard (LUMINA PAD from Cedrus company, model LU430-3B) in his/her right hand and the presentation of the stimuli was carried out using the Transdatix S.L. software, which also allows the responses to be recorded (reaction time and correct/incorrect/missing answers). Subjects used a 3-key keyboard: one red, one blue and one green. The stimuli were presented alternatively as 9 trains of 10 congruent stimuli and 9 trains of 10 incongruent stimuli. Each stimulus lasted 3,000 msec, during which time the subject had to reply by pressing the right key. No feedback was provided.
2.4. EEG recording
EEGs were recorded continuously using a BrainAmp standard EEG amplifier (256 Hz sampling rate; 0.1–39.9 Hz analogue band pass; resolution 0.5 µv: Brainproducts, Munich, Germany) and a BrainCap with 14 electrodes (Fp1, Fp2, Fz, C1, C2, C3, C4, Cz, T3, T4, Pz, O1 and O2) relative to a specific reference electrode within the cap between Afz and Fz. The ground electrode was situated between Fz and Cz. A vertical electrooculogram (VEOG) was recorded from electrodes attached above and below the left eye, and the horizontal electrooculogram (HEOG) was obtained from the outer canthi of both eyes (Lansbergen, Kenemans, 2008). The electrode impedance was kept below 5 kΩ, and the EEG and the EOG signals were online band pass filtered (DC-50 Hz, 50 Hz notch filter).
2.5. Data Analysis
Power spectra were computed across the inter-trial interval. EEG time series were divided into non-overlapping 3,000-ms-long windows, beginning at 0 ms post-response. Power spectra were obtained for each window using the Fast Fourier Transform (FFT) by a cosine windowing method. Spectra for each window were averaged separately for congruent and incongruent trials. Statistical analyses were carried out using long-transform mean power values in each frequency band (from 1 to 32 Hz) for the position of all the electrodes. The data from six subjects were rejected due to technical reasons. Repeated-measures ANOVA (rm-ANOVA) included all 14 electrodes as within-subjects factor and group, band and stimuli (congruent, incongruent and resting) as between-subject factors followed by Bonferroni post hoc analysis. Furthermore, separate one-way ANOVA was used to assess performance in Stroop test: 1) efficiency was measured as ratio of correct responses (number of correct responses/total number of responses), including colour as a within-subject factor and group as the between-subject factor; 2) reaction time (RT) was evaluated including colour as a within-subject factor and group as the between-subject factor (see Figure 1).
Figure 1. Differences in latency time for congruent and incongruent items per decade.
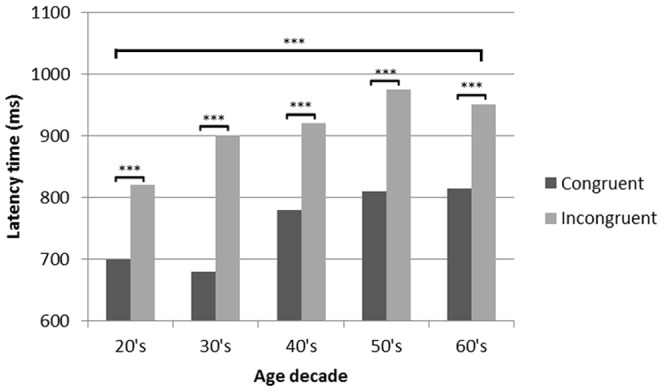
*** stands for p<0.001.
Results
Rm-ANOVA showed no significant effect of the group factor (F<1). There was significant effect of the within-subject factor band [F(4,890) = 1164.463, p<.001] (Table 1), with the Beta rhythm reaching higher values than the rest of the bands (p<.001), and of the electrodes [F(10,890) = 6.611, p<.002] (Table 2). Conversely, there was no significant effect of “stimulus” as a within-subject factor (F<1). A significant interaction effect on EEG activity was apparent between band and group [F(16,316) = 2.882, p<.004], between electrode and group [F(8,148) = 2.552, p<.015], between band and stimuli [F (4,890) = 1164.463, p<.001] and between stimuli, band and group [F(1252,6320) = 1.252, p<.05]. No further interactions were found.
Table 1. Statistical analysis of the EEG Bands per Group (mean, standard deviation and confidence interval).
| Bands | Groups | Mean | Std. Error | 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||||
| ALPHA | 1 | 5.057 | 0.164 | 4.735 | 5.378 |
| 2 | 5.019 | 0.153 | 4.718 | 5.32 | |
| 3 | 5.133 | 0.164 | 4.811 | 5.454 | |
| 4 | 5.028 | 0.167 | 4.7 | 5.355 | |
| 5 | 4.891 | 0.173 | 4.553 | 5.23 | |
| BETA | 1 | 9.882 | 0.164 | 9.561 | 10.204 |
| 2 | 9.144 | 0.153 | 8.844 | 9.445 | |
| 3 | 9.426 | 0.164 | 9.105 | 9.747 | |
| 4 | 10.946 | 0.167 | 10.618 | 11.274 | |
| 5 | 10.856 | 0.173 | 10.517 | 11.194 | |
| DELTA | 1 | 2.205 | 0.164 | 1.883 | 2.526 |
| 2 | 2.896 | 0.153 | 2.495 | 3.097 | |
| 3 | 2.163 | 0.164 | 1.841 | 2.484 | |
| 4 | 1.993 | 0.167 | 1.665 | 2.321 | |
| 5 | 2.06 | 0.173 | 1.721 | 2.398 | |
| SUB-ALPHA | 1 | 3.69 | 0.164 | 3.368 | 4.011 |
| 2 | 3.595 | 0.153 | 3.294 | 3.896 | |
| 3 | 3.963 | 0.164 | 3.642 | 4.285 | |
| 4 | 3.276 | 0.167 | 2.949 | 3.604 | |
| 5 | 3.267 | 0.173 | 2.928 | 3.606 | |
| THETA | 1 | 2.46 | 0.164 | 2.139 | 2.781 |
| 2 | 2.758 | 0.153 | 2.457 | 3.059 | |
| 3 | 2.512 | 0.164 | 2.19 | 2.833 | |
| 4 | 2.078 | 0.167 | 1.75 | 2.406 | |
| 5 | 2.099 | 0.173 | 1.761 | 2.438 | |
Table 2. Statistical analysis of the electrodes in function of the stimuli (mean, standard deviation and confidence interval).
| Stimuli | electrode | Mean | Std. Error | 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||||
| 1 | 4.598 | 0.074 | 4.452 | 4.743 | |
| 2 | 4.604 | 0.101 | 4.405 | 4.803 | |
| 3 | 4.674 | 0.075 | 4.528 | 4.82 | |
| Incongruent | 4 | 4.643 | 0.075 | 4.497 | 4.789 |
| Items | 5 | 4.659 | 0.067 | 4.527 | 4.89 |
| 6 | 4.689 | 0.069 | 4.543 | 4.815 | |
| 7 | 4.686 | 0.074 | 4.54 | 4.832 | |
| 8 | 4.659 | 0.076 | 4.51 | 4.809 | |
| 9 | 4.652 | 0.07 | 4.515 | 4.89 | |
| 10 | 4.647 | 0.074 | 4.502 | 4.892 | |
| 11 | 4.692 | 0.075 | 4.544 | 4.84 | |
| 1 | 4.577 | 0.074 | 4.431 | 4.722 | |
| 2 | 4.555 | 0.101 | 4.356 | 4.754 | |
| 3 | 4.668 | 0.075 | 4.521 | 4.814 | |
| Congruent | 4 | 4.637 | 0.075 | 4.491 | 4.783 |
| items | 5 | 4.655 | 0.067 | 4.524 | 4.787 |
| 6 | 4.682 | 0.069 | 4.546 | 4.818 | |
| 7 | 4.681 | 0.074 | 4.535 | 4.827 | |
| 8 | 4.685 | 0.076 | 4.536 | 4.834 | |
| 9 | 4.666 | 0.07 | 4.528 | 4.804 | |
| 10 | 4.648 | 0.074 | 4.503 | 4.893 | |
| 11 | 4.692 | 0.075 | 4.544 | 4.839 | |
| 1 | 4.665 | 0.075 | 4.518 | 4.812 | |
| 2 | 4.273 | 0.103 | 4.072 | 4.475 | |
| 3 | 4.675 | 0.075 | 4.527 | 4.824 | |
| Resting | 4 | 4.688 | 0.075 | 4.54 | 4.836 |
| state | 5 | 4.699 | 0.068 | 4.566 | 4.832 |
| 6 | 4.698 | 0.07 | 4.56 | 4.835 | |
| 7 | 4.704 | 0.075 | 4.557 | 4.852 | |
| 8 | 4.652 | 0.077 | 4.501 | 4.803 | |
| 9 | 4.696 | 0.071 | 4.557 | 4.836 | |
| 10 | 4.703 | 0.075 | 4.556 | 4.85 | |
| 11 | 4.718 | 0.076 | 4.568 | 4.867 | |
3.1. Band results
The post hoc analysis indicated the significant effects indicated below.
Alpha Band
Congruent stimuli
At electrode Fp2, group 2 showed significantly less Alpha activity than group 5 (t89 = −1.944, p<.055).
Resting state
At electrodes Fp2, (t89 = 2.211, p<.038) and O2 (t89 = 1.865, p<.05) group 3 showed significantly stronger Alpha activity than group 5.
Beta Band
Incongruent stimuli
At electrodes Fp1 (t89 = −2.248, p<.027), Fp2 (t89 = −1.773, p<.05), C3 (t89 = −2.6961.944, p<.009), Cz (t89 = −2.405, p<.019), C4 (t89 = −3.042, p<.003), T4 (t89 = −2.906, p<.005) and O2 (t = −3.707, p<.001) groups 2 and 3 showed significantly weaker Beta activity than group 5.
Congruent stimuli
At electrodes Fp1 (t89 = −2.835, p<.006), Fp2 (t89 = −2.413, p<.018), C3 (t89 = −3.129, p<.002), Cz (t89 = −3.027, p<.003), C4 (t89 = −2.618, p<.011), T4 (t89 = −2.339, p<.022) and O2 (t89 = −3.055, p<.003) groups 2 and 3 showed significantly weaker Beta activity than group 5.
Resting state
At the electrode Fp1 (t89 = −2.258, p<.027), Fp2 (t89 = −2.244, p<.028), C4 (t89 = −2.656, p<.010), T4 (t89 = −2.656, p<.010) and O2 (t89 = −2.099, p<.039) group 2 showed significantly weaker Beta activity than group 5. Furthermore, at Fp2, group 4 displayed significantly weaker Beta activity than group 5.
Delta Band
Incongruent stimuli
At electrode Pz (t89 = 1.988, p<05) group 2 showed significantly stronger Delta activity than group 5. Furthermore, at electrode Fp2, group 1 (t89 = 2.238, p<.05), group 2 (t89 = 3.277, p<.002), group 3 (t89 = 1.942, p<.05) and group 4 (t89 = 1.900 p<.05) displayed significantly stronger Delta activity than group 5.
Congruent stimuli
At the Fp2 electrodes (t89 = 3.440, p<.028), T3 (t89 = 2.068, p<.042), the T4 (t89 = 2.150, p<.035) and O2 (t89 = 2.609, p<.011) groups 2 showed significantly stronger Delta activity than group 5. Furthermore, at electrode Fp2. group 1 (t89 = 2.238, p<.028), group 3 (t89 = 2.596, p<.011) and group 4 (t89 = −2.996 p<.004) displayed significantly stronger Delta activity than group 5.
Resting state
Within electrode Fp1 (t89 = −2.377, p<.02) group 4 showed significantly less Delta activity than group 5. At the Fp2 electrode (t89 = 2.370, p<.02) group 1 showed significantly stronger Delta activity than group 5. Furthermore, at electrode T3 (t89 = 3.58, p<.003), C4 (t89 = 2.526, p<.014), Cz (t89 = 2.012, p<.048), C4 (t89 = 2.703, p<.008), T4 (t89 = 2.274, p<.025) and Pz (t89 = 2.546, p<.013) group 2 showed significantly stronger Delta activity than group 5.
Sub-Alpha Band
Incongruent stimuli
At electrode C3 (t89 = 3.580, p<.003), Cz (t89 = 2.767, p<.007), Cz (t89 = 2.587, p<.012) and C4 (t89 = 2.200, p<.029), group 3 showed significantly stronger Sub-Alpha activity than group 5.
Congruent stimuli
At the Fp1 (t89 = 2.316, p<.023) and Fpz (t89 = 2.473, p<.016) electrodes, group 1 showed significantly stronger Sub-Alpha activity than group 5. Furthermore, at Fpz, group 3 (t89 = 2.955, p<.004) displayed significantly stronger Sub-Alpha activity than group 5.
Resting state
At electrode Fp1, group 1 (t89 = 2.248, p<.027) and group 2 (t89 = 2.009, p<.048) showed significantly stronger Sub-Alpha activity than group 5.
Theta Band
Incongruent stimuli
At electrodes Fp1 (t89 = 1.758, p<.05), T3 (t89 = 2.843, p<.006), C3 (t89 = 3.142, p<.002), Cz (t89 = 1.950, p<.05), C4 (t89 = 2.536, p<.013), T4 (t89 = 2.301, p<.024) and O2 (t89 = 2.276, p<.026), group 2 showed significantly stronger Theta activity than group 5. Furthermore, at electrode Fp2, group 3 achieved stronger Theta activity than group 5 (t89 = 2.613, p<.05).
Congruent stimuli
At electrodes Fp2 (t89 = 2.613, p<.011), C3 (t89 = 2.401, p<.019), C4 (t89 = 1.91, p<.05), groups 2 and 3 showed significantly stronger Theta activity than group 5. Furthermore, at electrode C3, group 1 displayed significantly stronger Theta activity than group 5 (t89 = 1.980, p<05).
Resting State
Among the Fp2 (t89 = 3.166, p<.002), T3 (t89 = 2.974, p<.004), C3 (t89 = 1.842, p<.05), Cz (t89 = 4.049, p<.044), C4 (t89 = 3.674, p<.001), T4 (t89 = 1.866, p<.05) and Pz (t89 = 2.627, p<.01) electrodes, group 2 showed significantly stronger Theta activity than group 5. Furthermore, at electrode Fp2 (t89 = 2.774, p<.007) and C3 (t89 = 2.451, p<.016) group 3 displayed stronger Theta activity than group 5. Finally, at electrode T3 (t89 = 2.084, p<.04) the Theta activity in group 1 was enhanced with respect to group 5.
3.2 Stroop test results
The conflict effect in this Stroop task was verified for each age group (p<.001, Figure 1). In terms of the ratio of correct responses, rm-ANOVA showed a significant effect of the group factor [F(4,53) = 12.258, p<.001] but no effect of colour (p>.005). A Post hoc analysis indicated significant differences in groups 1, 2, 3 and 4 with respect to group 5 (p>.001). With regards reaction time, there was a significant effect of the group factor [F(16,890) = 5.985, p<.001], the post hoc t-test analysis indicating significant differences of groups 1 (t89 = 6.332, p<.001), 2 (t89 = 6.334, p<.001), 3 (t89 = 6.298, p<.001) and 4 (t89 = 5.813, p<.001) with respect to group 5.
Discussion
In this study we have analysed the changes in Stroop interference at different stages in the life of individuals, analysing the responses within specific bands at electrodes placed at different locations during Stroop task performance. Our results suggest that a complex combination of changes in the Alpha and Theta bands evolve between ages in the 20's until the 70's together with a progressive increase in latency of response between congruent and incongruent items.
In general terms, alertness is characterized by reduced Alpha activity and increases in the rest of the frequencies [20]. In particular, increase in Theta activity is related to information processing and contributes to cognitive function such as memory encoding engagement [21], [22], learning [23] and creativity processing [24]. During conflict resolution, the reduction in Alpha activity corresponded to diffuse electrical inhibition over the scalp that was required to resolve any demanding cognitive tasks. This process is essential to guarantee a correct analysis of the information and correct processing, mainly within parietal areas. In our study, conflict solving produced a reduction of Alpha waves in the right occipital lobe (O2), particularly in older groups (those in their 60's) and an increase in the Theta frequency at Fp2. The location O2 corresponds to the parietal homotypical isocortex from the parieto-occipital region, which is involved in resuming and processing visual information. Simultaneously, Theta activity increased within the prefrontal lobes (Fp1 and Fp2), frontal lobes (F3 and C3), frontal sagittal line (Fz) and central sagittal line (Cz), being higher at Fp2. The Right Frontopolar cortex (Fp2) is essential for the processing of information received from the associative cortex, and is in continuous exchange with memory areas [25]. Petrides [26] described the implication of prefrontal areas during the Stroop test: the anterior fronto-basal region (area 11) is involved in novelty flagging (this requires memory connexions, [27]) and the posterior fronto-basal region (area 13) contributes to the meaning analysis of the stimulus; in case of incongruence, signal analysis would require the activation of areas 11 and 13, which are connected to area 25 (Subgenual), the amygdala entorhinal and the perirhinal cortex for meaning elaboration [28]. The peak of Theta activity under Fp2 resembles the confluence of lateral prefrontal and anterior fronto-basal electrical fields, which respond to data comparison and memory processing. Theta activity increase was also found under electrode Fz, which corresponds to the confluence of area 13 (posterior fronto-basal cortex) and areas 32a and 24 (anterior pregenual cortex, limbic system) electrical fields. Cz electrode activity corresponds to areas 32b and 24b (anterior cingulate cortex), as described for both congruent and incongruent items using fMRI and PET techniques [2], [29], [30]. Further areas, such as the left premotor area (F3) and the left motor area (C3), would stand for the motor response, performed by pressing a button with the right hand. Such a complex and long process, particularly in incongruent analysis, originates longer reaction time and more errors, leading to the so-called “Stroop effect”.
These results are in agreement with the relationships previously identified between central executive, working memory processes and fronto-parietal electrode coupling [31]. Moreover, the general functional scheme used here matches that applied in a previous study where similar relationships between central executive and working memory processes, and fronto-parietal electrode coupling were described [32]. Regarding the specific location of the changes in EEG signal, our results confirm that Stroop interference involves the right frontal cortex (lateral and basal prefrontal areas –Fp2-), and posterior fronto-sagittal ones, Fz, as proposed from previous fMRI clinical studies in healthy controls and patients with schizophrenia [33].
Considering the whole pool of data by decades across the sample and irrespective of age, reaction time was significantly shorter for congruent than for incongruent items. Regarding the effect of age in relation with the Stroop incongruence, our results indicated that older adults have a longer reaction time for both congruent and incongruent items. However, response time was significantly shorter for the younger participants than for the older participants on congruent and incongruent items (younger: 700 ms for congruent and 825 ms for incongruent; older: 725 ms for congruent and 1250 ms for incongruent). These data suggest an increase of 25 ms/decade for congruent and 85 ms/decade for incongruent items, probably due to the contribution of different aging processes that may start from age 20. These is in agreement with previous studies: the Stroop test in different age groups reported decreases in reaction times to incongruent stimuli from 30 to 20 years of age (−0.5 z scores) and these times start to increase from 40 years of age onwards, at a rate of 0.2 z-scores/decade [34].
Consistent with well-described anatomical changes, Stroop interference reaches adaptive levels relatively early in childhood (6–7 years), although control interference continues to develop into late adolescence [35]. In fact, 10–12 year-old subjects are still more susceptible to interference errors than adults [36]. Likewise, previous studies revealed age-related differences within Correct Response Negativity (CRN) amplitude and CRN amplitude was larger after incongruent than congruent Stroop stimuli in young adults, whereas older adults showed greater amplitude of CRN in both incompatible as well as compatible trials. Hence, there appeared to be an age-related impairment in (post-)response conflict [37]–[39]. These effects are connected with other age-related anatomical changes in the brain associated with age, such as the increased of ventricle volume (10–15%) from the 40th to the 80th decade in the healthy population [40]. Standard ageing processes may start age 20 [24], although some ageing parameters such as myelinisation increase through age 40 and in some cases until age 60, especially in the intracortical horizontal plexuses [41]. Within the central nervous system, standard ageing changes include microcirculation decrease [42], [43], ventricles enlargement [44], white matter reduction [45]–[47] and encephalic weight loss [48]. Slowing in responses with age may be well attributed to standard ageing changes in the central nervous system.
In summary, our results highlight the cortical areas involved in conflict resolution, implicating the right posterior parietal or occipito-parietal, the fronto-basal and the left ACC. The EEG frequency that best defines the engagement of these areas is represented by the appearance of 4–6 Hz Theta activity in Fp2 (some peaks of which may reach 9 Hz), and the simultaneous reduction of Alpha and Beta rhythms. The brain's ability to swap these EEG activity bands is crucial to achieve efficient performance [31]. Such a key combination seems not to be optimal in the 20's but rather in the 30's, and from then on this starts to decline until the 60th decade as healthy aging occurs. We reckon that our results enhance our current knowledge on the EEG changes that take place under cognitive demanding conditions during the life span of an individual. However, further studies should consider to increase the number of electrodes [49] and apply basal interpolation software to identify the contribution of each EEG component. These data are being used in the EXOLEGS project, which aims to improve the capacity of autonomy of elderly and impaired people by the application of user interface techniques, focusing mainly in the chapter of Brain Computer Interface.
Acknowledgments
We would like to thank all the volunteers who agreed to participate in this study.
Funding Statement
This work was supported by BIO-MED 07/01/0006 (IRENE project, D.G. de Investigación, Region of Murcia, Spain) to CN, PHARMACOG-European Community's Seventh Framework Program (FP7/2007–2013) for the Innovative Medicine Initiative under Grant Agreement No. 115009 and CIBERNED (Spanish Network of Research on Neurodegeneratives Diseases) to MTH and AAL actions from the Spanish Ministry of Industry (EXOLEGS project) to JLC and TG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stroop JR (1935) Studies of interference in serial verbal reactions. Journal of Experimental Psychology 18: 643–662. [Google Scholar]
- 2. Nombela C, Bustillo PJ, Castell PF, Sanchez L, Medina V, et al. (2011) Cognitive rehabilitation in Parkinson's disease: evidence from neuroimaging. Front Neurol 2: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bondi MW, Serody AB, Chan AS, Eberson-Shumate SC, Delis DC, et al. (2002) Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer's disease. Neuropsychology 16: 335–343. [DOI] [PubMed] [Google Scholar]
- 4. Gigaux J, Le Gall D, Jollant F, Lhuillier JP, Richard-Devantoy S (2013) Cognitive inhibition and quality of life in schizophrenia: a pilot study. Schizophr Res 143: 297–300. [DOI] [PubMed] [Google Scholar]
- 5. Glaser MO, Glaser WR (1982) Time course analysis of the Stroop phenomenon. J Exp Psychol Hum Percept Perform 8: 875–894. [DOI] [PubMed] [Google Scholar]
- 6. Markela-Lerenc J, Schmidt-Kraepelin C, Roesch-Ely D, Mundt C, Weisbrod M, et al. (2009) Stroop interference effect in schizophrenic patients: an electrophysiological approach. Int J Psychophysiol 71: 248–257. [DOI] [PubMed] [Google Scholar]
- 7. Rosenfeld JP, Skogsberg KR (2006) P300-based Stroop study with low probability and target Stroop oddballs: the evidence still favors the response selection hypothesis. Int J Psychophysiol 60: 240–250. [DOI] [PubMed] [Google Scholar]
- 8. Markela-Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, et al. (2004) Prefrontal-cingulate activation during executive control: which comes first? Brain Res Cogn Brain Res 18: 278–287. [DOI] [PubMed] [Google Scholar]
- 9. Schack B, Chen AC, Mescha S, Witte H (1999a) Instantaneous EEG coherence analysis during the Stroop task. Clin Neurophysiol 110: 1410–1426. [DOI] [PubMed] [Google Scholar]
- 10. Schack B, Grieszbach G, Krause W (1999b) The sensitivity of instantaneous coherence for considering elementary comparison processing. Part I: The relationship between mental activities and instantaneous EEG coherence. Int J Psychophysiol 31: 219–240. [DOI] [PubMed] [Google Scholar]
- 11. Schack B, Rappelsberger P, Weiss S, Moller E (1999c) Adaptive phase estimation and its application in EEG analysis of word processing. J Neurosci Methods 93: 49–59. [DOI] [PubMed] [Google Scholar]
- 12. West R, Bell MA (1997) Stroop color-word interference and electroencephalogram activation: evidence for age-related decline of the anterior attention system. Neuropsychology 11: 421–427. [DOI] [PubMed] [Google Scholar]
- 13. Heflin LH, Laluz V, Jang J, Ketelle R, Miller BL, et al. (2011) Let's inhibit our excitement: the relationships between Stroop, behavioral disinhibition, and the frontal lobes. Neuropsychology 25: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milham MP, Banich MT, Claus ED, Cohen NJ (2003) Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- 15. Kim C, Chung C, Kim J (2010) Multiple cognitive control mechanisms associated with the nature of conflict. Neurosci Lett 476: 156–160. [DOI] [PubMed] [Google Scholar]
- 16. Davidson DJ, Zacks RT, Williams CC (2003) Stroop interference, practice, and aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 10: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dulaney CL, Rogers WA (1994) Mechanisms underlying reduction in Stroop interference with practice for young and old adults. J Exp Psychol Learn Mem Cogn 20: 470–484. [DOI] [PubMed] [Google Scholar]
- 18. Oldfield R (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 19. Scholes KE, Harrison BJ, O'Neill BV, Leung S, Croft RJ, et al. (2007) Acute serotonin and dopamine depletion improves attentional control: findings from the stroop task. Neuropsychopharmacology 32: 1600–1610. [DOI] [PubMed] [Google Scholar]
- 20. Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, et al. (2006) Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol 95: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 21. Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, et al. (2009) Brain oscillatory substrates of visual short-term memory capacity. Curr Biol 19: 1846–1852. [DOI] [PubMed] [Google Scholar]
- 22. Baddeley A (2003) Working memory and language: an overview. J Commun Disord 36: 189–208. [DOI] [PubMed] [Google Scholar]
- 23. Tsujimoto T, Shimazu H, Isomura Y (2006) Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol 95: 2987–3000. [DOI] [PubMed] [Google Scholar]
- 24.Restak R (2012) Brain. The complete mind. Washington D. C.: National Geografic. [Google Scholar]
- 25. Barbas H (2007) Specialized elements of orbitofrontal cortex in primates. Ann N Y Acad Sci 1121: 10–32. [DOI] [PubMed] [Google Scholar]
- 26. Petrides M (2007) The orbitofrontal cortex: novelty, deviation from expectation, and memory. Ann N Y Acad Sci 1121: 33–53. [DOI] [PubMed] [Google Scholar]
- 27.Price JL (2007) Defition of the orbital cortex in relation to specific connexions with limbic and visceral structures and other cortical regions. In: cols Sa, editor. Linkin affects to action. New York: Annals of New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- 28. Pribram KH, Maclean PD (1953) Neuronographic analysis of medial and basal cerebral cortex. II. Monkey. J Neurophysiol 16: 324–340. [DOI] [PubMed] [Google Scholar]
- 29. Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, et al. (1998) The counting Stroop: an interference task specialized for functional neuroimaging–validation study with functional MRI. Hum Brain Mapp 6: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, et al. (1998) The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 31. Kawasaki M, Kitajo K, Yamaguchi Y (2010) Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. Eur J Neurosci 31: 1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palva S, Kulashekhar S, Hamalainen M, Palva JM (2011) Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. J Neurosci 31: 5013–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pompei F, Dima D, Rubia K, Kumari V, Frangou S (2011) Dissociable functional connectivity changes during the Stroop task relating to risk, resilience and disease expression in bipolar disorder. Neuroimage 57: 576–582. [DOI] [PubMed] [Google Scholar]
- 34. Bugg JM, DeLosh EL, Davalos DB, Davis HP (2007) Age differences in Stroop interference: contributions of general slowing and task-specific deficits. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14: 155–167. [DOI] [PubMed] [Google Scholar]
- 35. Wright BC, Wanley A (2003) Adults' versus children's performance on the Stroop task: interference and facilitation. Br J Psychol 94: 475–485. [DOI] [PubMed] [Google Scholar]
- 36. Comalli PE Jr, Wapner S, Werner H (1962) Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol 100: 47–53. [DOI] [PubMed] [Google Scholar]
- 37. Vidal F, Hasbroucq T, Grapperon J, Bonnet M (2000) Is the ‘error negativity’ specific to errors? Biol Psychol 51: 109–128. [DOI] [PubMed] [Google Scholar]
- 38. Vidal F, Burle B, Bonnet M, Grapperon J, Hasbroucq T (2003) Error negativity on correct trials: a reexamination of available data. Biol Psychol 64: 265–282. [DOI] [PubMed] [Google Scholar]
- 39. Eppinger B, Kray J, Mecklinger A, John O (2007) Age differences in task switching and response monitoring: evidence from ERPs. Biol Psychol 75: 52–67. [DOI] [PubMed] [Google Scholar]
- 40. Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, et al. (2012) Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord 26: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yakovlev PI (1962) Morphological criteria of growth and maturation of the nervous system in man. Res Publ Assoc Res Nerv Ment Dis 39: 3–46. [PubMed] [Google Scholar]
- 42. Joseph JA, Berger RE, Engel BT, Roth GS (1978) Age-related changes in the nigrostriatum: a behavioral and biochemical analysis. J Gerontol 33: 643–649. [DOI] [PubMed] [Google Scholar]
- 43. Carlsson A (1978) Age-dependent changes in central dopaminergic and other monoaminergic systems. Adv Exp Med Biol 113: 1–13. [DOI] [PubMed] [Google Scholar]
- 44. Barron SA, Jacobs L, Kinkel WR (1976) Changes in size of normal lateral ventricles during aging determined by computerized tomography. Neurology 26: 1011–1013. [DOI] [PubMed] [Google Scholar]
- 45. Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, et al. (2000) Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216: 672–682. [DOI] [PubMed] [Google Scholar]
- 46. Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, et al. (2008) Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 39: 566–577. [DOI] [PubMed] [Google Scholar]
- 47. Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, et al. (2005) Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26: 1215–1227. [DOI] [PubMed] [Google Scholar]
- 48. Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, et al. (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14: 721–730. [DOI] [PubMed] [Google Scholar]
- 49. Carp J, Compton RJ (2009) Alpha power is influenced by performance errors. Psychophysiology 46: 336–343. [DOI] [PubMed] [Google Scholar]


