Abstract
Background
Maternal fish intake during pregnancy may influence risk of child asthma and allergic rhinitis, yet evidence is conflicting on its association with these outcomes.
Methods
We examined associations of maternal fish intake during pregnancy with child asthma and allergic rhinitis. Mothers in the Danish National Birth Cohort (N=28,936) reported their fish intake at 12 and 30 weeks of gestation. Using multivariate logistic regression, we examined associations of fish intake with child wheeze, asthma, and rhinitis assessed at several time points: ever wheeze, recurrent wheeze (>3 episodes), ever asthma and allergic rhinitis, and current asthma, assessed at 18 months (N~22,000) and 7 years (N~17,000) using self-report and registry data on hospitalizations and prescribed medications.
Results
Compared to consistently high fish intake during pregnancy (fish as a sandwich or hot meal >=2-3 times/week), never eating fish was associated with higher risk of child asthma diagnosis at 18 months (1·30, 95%CI: 1·05, 1·63, P=0.02), and ever asthma by hospitalization (1·46, 95%CI: 0·99, 2·13, P=0.05) and medication prescription (1·37, 95%CI: 1·10, 1·71, P=0·01). A dose-response was present for asthma at 18 months only (P for trend: 0·001). We found no associations with wheeze or recurrent wheeze at 18 months or with allergic rhinitis.
Conclusions
Our results suggest that high (vs. no) maternal fish intake during pregnancy is protective against both early and ever asthma in 7 year old children.
Keywords: fish, cohort study, asthma, allergic rhinitis
INTRODUCTION
Asthma is one of the most common chronic childhood diseases in industrialized countries. 1 In Denmark, allergic asthma has increased almost two-fold over the past 15 years. 2 Most children who develop asthma do so by age 7-9 years, suggesting a need to investigate etiologic factors occurring in early life. The identification of these factors is necessary to best guide future preventive programs. More than a decade ago Black and Sharpe proposed that the increase in prevalence of allergic disease may be explained by a shift in the consumption of polyunsaturated fatty acids (PUFA) away from n-3 PUFA and oily fish towards higher intake of n-6 PUFA.3 In support of this hypothesis, animal and in vitro studies have demonstrated the involvement of n-3 PUFA in anti-inflammatory mechanisms by altering membrane phospholipid composition and oxidative balance, influencing cell signaling, cytokine production, and T cell responses (reviewed in 4, 5). N-3 PUFAs have also been shown to increase production of anti-inflammatory eicosanoids (eicosapentaenoic acid) and more recently identified resolvins (both eicosapentaenoic and docosahexaenoic acid).4, 6
Studies in adults and children have provided support for this hypothesis;7, 8 yet, evidence on protective effects of fish intake during pregnancy against child allergic disease has been conflicting. Some observational studies have found lower rates of allergic disease among children whose mothers increased fish consumption or took fish oil supplements during pregnancy,9-11 while others found no associations. 12-15. Maternal fish intake was shown to protect against child eczema in 2-and 5-year old children,9-11 though this was not a consistent finding.14, 15 Three studies that specifically examined risk of child asthma did not find any associations.13, 14, 16 Some of the studies were limited by retrospective assessment of maternal diet up to 5 years post-pregnancy,12-14 which may have increased measurement error and reduced the power to detect an association. Three randomized clinical trials that supplemented women with fish oil during pregnancy found favorable effects on early life sensitization.17-20 However, these studies were limited by the absence of clinical endpoints and supplementation into the lactation period, making it difficult to draw conclusions about exposure timing.
Fish intake during pregnancy could influence the development of allergy and airway inflammation through fatty acid-driven pathways. The available literature is conflicting and would benefit from additional evidence on clinical outcomes relevant to allergic disease development during childhood. The purpose of this study was to examine the associations between maternal fish intake during pregnancy, and early and later allergic disease outcomes in children.
METHODS
Population and Study Design
The aim of the Danish National Birth Cohort (DNBC) was to investigate conditions in early life and childhood that may reach into later stages of life. Between 1996 and 2002 we recruited participants at the women's first antenatal visit. Women were eligible to enroll if they planned to carry to term and could speak Danish. The women were interviewed via telephone twice during pregnancy, at 1 and 30 weeks of gestation. Telephone interviews were also administered when the child was 6 and 18 months old. In addition, women completed a 360-item semi-quantitative food frequency questionnaire (FFQ) during the 25th week of gestation. 21 The FFQ asked about intake in the past 4 weeks and has been validated against 7 day food diaries and blood and urine biomarkers for selected nutrient (protein, retinol, folic acid, n-3 PUFAs) and food (fruits and vegetables) intake.22 This study showed significant and acceptable to good correlations for all nutrients and food groups in question with correlation coefficients ranging from 0.32 to 0.66. Furthermore, when the children turned 7 years mothers were asked to complete a mailed questionnaire.
To avoid dependency among correlated observations, out of the 101,045 enrolled pregnancies, we included only the first study pregnancy (n=89,333) (Figure 1). We further limited our analyses to singletons (n= 87,056) and excluded women who took fish oil at any point during pregnancy (n=3,546) or who did not have data on fish intake for both pregnancy interviews (n=58,142), generating a total of 28,936 women who were eligible for the analysis. Sample sizes varied somewhat for the individual outcomes due to differential response rates.
Figure 1.
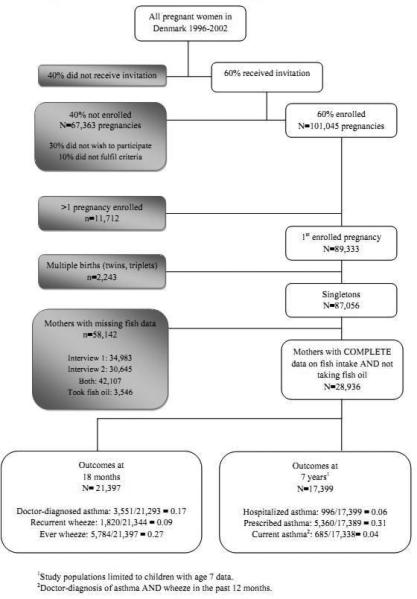
Flow chart of participants in the Danish National Birth Cohort
Mothers provided written informed consent on behalf of their children. The Regional Scientific Ethics Committee for the municipalities of Copenhagen and Frederiksberg approved all study protocols, and all procedures were in accordance with the Declaration of Helsinki.
Exposure Measurements
Fish intake was assessed during both pregnancy interviews. We used a definition of fish intake developed for the DNBC that best differentiated among levels of intake. It assessed fish intake with a sandwich or a hot meal, the most common ways of consuming fish in Denmark, with five categories of intake: 1) never eating fish; 2) hot meal and sandwiches both eaten monthly or less than each month, according to both interviews; 3) hot meal monthly and sandwiches weekly, according to both interviews; 4) sandwiches and hot meals both eaten weekly according to both interviews, at a low frequency (hot meals 1 time/week and sandwiches 1-2 times/week, in at least one interview); 5) sandwiches and hot meals both eaten weekly at a high frequency (hot meals >2 times/week or sandwiches >3 times/week, according to both interviews). We also calculated fish intake in grams per week using data from the mid-pregnancy FFQ.
Outcome Measurements
We asked mothers about doctor diagnosis of child asthma, wheeze symptoms, and the number of wheezing episodes since birth at 6 and 18 months. We defined asthma at 18 months as a doctor diagnosis of asthma, and wheeze at 18 months as reported wheezing or whistling in the chest. We evaluated recurrent wheeze as having >3 episodes in the first 18 months of life compared to having <=3 episodes or no reported wheeze.
We evaluated asthma and allergic rhinitis at age 7 years using standardized questions based on the ISAAC questionnaire. 23 Current asthma was defined as a positive response to ever doctor-diagnosed asthma and wheezing symptoms in the past 12 months. We defined allergic rhinitis as ever reported doctor-diagnosis of hay fever.
We also followed up on the children through national registries, linked using the Central Person Registry (CPR) number, a unique identification number provided to all Danish citizens. We had access to two national registries, the Danish National Patient Registry (DNPR) and the Register of Medicinal Product Statistics (RMPS).
The DNPR collects data on all hospital admissions, emergency room, and outpatient contacts. The registry has been well validated against asthma diagnosis from hospital records. 24 Data from the DNPR was extracted in Aug 2010 and linked to our data using the CPR number. We used the International Classification of Disease 10 (ICD-10) for asthma (J45, J45.0, J45.1, J45.8, J45.9, J46, and J46.9) to classify first registered diagnosis of admitted asthma for every child. Allergic rhinitis diagnosis was not examined due to low number of hospitalization (226/47,677= 0.005).
The RMPS contains detailed individual-level information on prescriptions (with the exclusion of over-the-counter) filled at all pharmacies. We used a definition from a previous validation study to classify ever prescribed asthma cases as individuals with at least two prescriptions of any combination of drugs for obstructive airway disease, except for beta-2-agonists as liquid. 25. Classification of ever prescribed allergic rhinitis cases was based on at least two prescriptions of any combinations of anti-allergic drugs.
Covariates
Covariates suspected to be confounders, intermediates in the causal pathway or effect modifiers were considered. Variables that were evaluated for inclusion in the multivariate model included socioeconomic status by parental education level and occupation (SES: high level proficiency, medium level proficiency, skilled, unskilled, student, unemployment), maternal age at birth of child (<=20 years, 21-39 years, >=40 years), parity (nulli- and multiparous), maternal prepregnancy Body Mass Index (BMI in kg/m2) (<=18.5, 18.6-24.9, 25.0-29.9, 30-34.9, >=35.0), maternal smoking during pregnancy (nonsmoker, occasional smoker, <15 cigarettes/day, >=15 cigarettes/day), maternal exercise during pregnancy (yes/no), gestational weight gain (g/week), breastfeeding duration (none, 0-1, 2-3, 4-6, 7-9, >=10), birth weight (in grams), gestational age (in days since the last menstrual period), child sex, and parental history of asthma and allergies. We also evaluated intakes of vitamins A, D, E, selenium, and zinc from dietary sources and supplements, and intake of essential fatty acids, fruits, vegetables, and alcohol. We energy-adjusted all nutrient intakes from diet and alcohol using the residual method. 26
Statistical Analysis
We evaluated the distribution of covariates across fish intake categories to identify potential confounding variables. There were significant differences in fish intake across maternal age categories with ‘never’ consumers being younger compared to consistently high fish consumers; we therefore age-standardized the covariate distributions. Final set of covariates was determined by chi-square and partial F-tests with a P<0.10, and prior literature. Covariates suspected to be intermediates (gestational weight gain, birth weight, gestational age) on the causal pathways were excluded from the model to avoid over-adjustment. Fish intake was entered as an indicator variable into the multivariate logistic regression model and individual exposure categories compared to the highest, reference, category. We report here the estimated odds ratios (ORs) and 95% CIs. We examined models with several levels of confounder adjustment. We adjusted first for sociodemographic variables and then further adjusted for dietary covariates. As further adjustment for dietary factors did not change the results, we report results for the sociodemographic model only. In all adjusted models, breastfeeding accounted for the largest attenuation of ORs. Ordinal values for the exposure categories were entered separately into the models as a continuous variable to evaluate the P-value for trend. All tests were two-sided and statistical significance was considered at P<0.05. The analyses were performed using the Statistical Analyses System software (release 9.3; SAS Institute, Cary, NC).
RESULTS
Study cohort
A total of 28,936 women had information on fish intake based on the two pregnancy interviews. Most women in the study cohort were between the ages 21 and 39 years (98%), of higher socioeconomic position (high or medium level proficiencies: 55%), and multiparous (53%) (Table 1). Close to a quarter of participants reported any smoking during pregnancy with 12% daily smokers. The prevalence of maternal history of asthma and allergies was 9% and 31% respectively. Paternal asthma and allergies were reported for 8% and 24% of children respectively.
Table 1.
Age-standardized covariate distribution across categories of maternal fish intake during pregnancy in the Danish National Birth Cohort, N= 28,9351
| Frequency of fish intake | N (%) | Warm meal and sandwich each week, high frequency N=3,521 % or means (SD) | Warm meal and sandwich each week, low frequency N=6,931 % or means (SD) | Warm meal each month, sandwich each week N=10,486 % or means (SD) | Warm meal and sandwich each month / less than each month N=6,548 % or means (SD) | Zero intake N=1,449 % or means (SD) |
|---|---|---|---|---|---|---|
| Maternal age (years)* | ||||||
| ≤ 20 | 206 (1) | 0 | 0 | 0 | 1 | 4 |
| 21 – 39 | 28,381 (98) | 98 | 98 | 99 | 98 | 95 |
| ≥ 40 | 349 (1) | 2 | 2 | 1 | 1 | 1 |
| Prepregnancy BMI (kg/m2) | ||||||
| ≤ 18.5 | 1,254 (5) | 6 | 5 | 4 | 4 | 6 |
| 18.6 – 24.9 | 19,372 (69) | 74 | 74 | 69 | 64 | 62 |
| 25.0 – 29.9 | 5,262 (19) | 14 | 16 | 20 | 22 | 20 |
| 30.0 – 34.9 | 1,601 (6) | 4 | 4 | 6 | 7 | 8 |
| ≥ 35.0 | 581 (2) | 2 | 1 | 2 | 3 | 4 |
| Physical activity | ||||||
| Yes | 11,289 (39) | 47 | 45 | 38 | 33 | 30 |
| Smoking in pregnancy | ||||||
| Nonsmoker | 21,812 (75) | 76 | 78 | 76 | 74 | 60 |
| Occasional smoker | 3,670 (13) | 13 | 13 | 13 | 12 | 16 |
| <15 cigarettes/day | 2,946 (10) | 10 | 8 | 10 | 11 | 20 |
| ≥15 cigarettes/day | 508 (2) | 1 | 1 | 1 | 3 | 5 |
| Parity | ||||||
| Nullipara | 13,520 (47) | 42 | 46 | 44 | 52 | 56 |
| Socioeconomic position | ||||||
| High level proficiencies | 6,627 (24) | 31 | 31 | 22 | 19 | 13 |
| Medium level proficiencies | 8,508 (31) | 31 | 34 | 33 | 28 | 19 |
| Skilled | 7,100 (26) | 19 | 20 | 27 | 32 | 38 |
| Unskilled | 3,069 (11) | 10 | 8 | 11 | 14 | 21 |
| Students | 1,155 (4) | 6 | 5 | 4 | 4 | 3 |
| Unemployed | 712 (3) | 3 | 2 | 2 | 3 | 5 |
| Breastfeeding duration | ||||||
| No breastfeeding | 355 (2) | 2 | 1 | 1 | 2 | 4 |
| 0-1 months | 1,661 (8) | 6 | 6 | 7 | 11 | 17 |
| 2-3 months | 2,025 (10) | 7 | 8 | 9 | 12 | 18 |
| 4-6 months | 3,649 (18) | 15 | 16 | 17 | 21 | 20 |
| 7-9 months | 5,508 (27) | 26 | 28 | 29 | 25 | 17 |
| ≥10 months | 7,303 (36) | 44 | 40 | 36 | 28 | 23 |
| Maternal asthma | ||||||
| Yes | 2,464 (9) | 9 | 8 | 8 | 9 | 14 |
| Maternal allergies | ||||||
| Yes | 9,083 (31) | 33 | 32 | 31 | 32 | 34 |
| Paternal asthma | ||||||
| Yes | 2,230 (8) | 8 | 8 | 7 | 7 | 10 |
| Paternal allergies | ||||||
| Yes | 6,829 (24) | 25 | 26 | 24 | 22 | 22 |
| Child sex | ||||||
| Male | 14,707 (51) | 51 | 51 | 51 | 51 | 51 |
| Gestational weight gain (g/week) | 21,265 | 462 (218) | 459 (204) | 464 (207) | 465 (217) | 461 (260) |
| Birth weight (g) | 27,800 | 3,599 (540) | 3,623 (545) | 3,637 (539) | 3,577 (559) | 3,521 (583) |
| Gestational age (days) | 28,929 | 281 (12) | 281 (11) | 281 (11) | 281 (11) | 280 (13) |
| Dietary intake | ||||||
| Energy intake (kJ/day) | 21,854 | 10,863 (2,824) | 10,341 (2,589) | 10,239 (2,494) | 9,631 (2,617) | 9,679 (3,085) |
| Fruit intake (g/day) | 21,950 | 378 (269) | 354 (256) | 316 (236) | 291 (269) | 316 (347) |
| Vegetable intake (g/day) | 21,950 | 182 (134) | 158 (109) | 124 (92) | 104 (87) | 113 (104) |
| ALA intake (g/day) | 21,854 | 2.1 (0.5) | 2.0 (0.4) | 2.1 (0.5) | 2.0 (0.7) | 1.9 (0.5) |
| LA intake (g/day) | 21,854 | 9.5 (1.9) | 9.3 (1.8) | 9.5 (2.0) | 9.3 (2.3) | 8.9 (2.2) |
| Total vitamin A (RE/day) | 21,854 | 3,264 (74,013) | 1,933 (45,7015) | 1,699 (37,345) | 1,474 (13,532) | 1,265 (670) |
| Total vitamin D (μg/day) | 21,854 | 10.5 (5.9) | 9.8 (5.5) | 8.7 (5.1) | 8.3 (5.4) | 7.4 (5.2) |
| Total vitamin E (α-TE/day) | 21,854 | 17.4 (25.2) | 15.9 (14.1) | 15.2 (13.6) | 14.6 (12.1) | 14.4 (15.8) |
| Total selenium (μg/day) | 21,854 | 77.6 (31.4) | 74.9 (26.9) | 71.7 (26.8) | 66.4 (26.5) | 60.9 (28.0) |
| Total zinc (mg/day) | 21,854 | 19.4 (7.7) | 20.0 (8.1) | 19.9 (7.4) | 19.5 (7.5) | 18.8 (7.8) |
Values are standardized to the age distribution of the study population.
Value not age-adjusted.
One participant excluded because of extreme vitamin D intake (>100,000 μg/day.)
ALA: α-linolenic acid
At 18 months, compared to the 7,643 eligible participants excluded from our analysis due to missing outcome data, the 21,293 included participants were more likely to be multiparous (54% vs. 51%), and less likely to smoke during pregnancy (11% vs. 13%). Comparing the 11,598 participants without data on current asthma at 7 years, the 17,338 included mothers were of higher sociodemographic status (high and medium proficiency: 58% vs. 53%), they reported lower prepregnancy BMI (>=30 kg/m2: 7% vs. 9%) and lower pregnancy smoking rates (10% vs.14%); and they were more likely to have breastfed past 7 months (65% vs.58%). There were no substantial differences in gestational weight gain, birth weight, and intake of fruits, vegetables, micronutrients, and essential fatty acids.
A total of 5% of women reported no fish intake at both interviews, while 12% of women reported eating fish at a high frequency at both time points. Fish intake estimated by the FFQ increased monotonously across categories: women in the lowest category reported consuming 21(±49) grams of fish per week, while intake was 292(±147) g/week among high consumers, confirming successful separation of the exposure categories.
Predictors of fish intake
We examined fish intake across age-standardized participant characteristics (Table 1). Compared to never consumers, high frequency fish consumers tended to be of high/medium level proficiency. They also tended to have a lower prepregnancy BMI and more previous pregnancies. They smoked less during pregnancy and breastfed for >=7 months. They reported an overall healthier diet with higher intake of fruit, vegetables, vitamins and minerals. Birth weight was generally higher for children whose mothers consumed more fish.
Univariate predictors of the outcomes
We found that younger age, higher parity, higher prepregnancy BMI, smoking and lack of physical activity during pregnancy, shorter breastfeeding duration, parental history of either asthma or allergy, and male sex were directly associated with doctor-diagnosed asthma at 18 months (Table 2). Similar results were present for ever admitted and prescribed asthma, and current asthma at 7 year.
Table 2.
Univariate predictors of asthma at 18 months, current asthma at 7 years, and ever asthma by hospitalization or prescription medication in the Danish National Birth Cohort
| Sociodemographic characteristics | Doctor-diagnosed asthma (18 months) N=21,293 OR (95 % CI) | P | Ever admitted asthma1 N=17,399 OR (95 % CI) | P | Ever prescribed asthma2 N=17,389 OR (95 % CI) | P | Current asthma3 (7 years) N=17,338 OR (95 % CI) |
|---|---|---|---|---|---|---|---|
| Maternal age (years) | |||||||
| ≤ 20 | 2.19 (1·42, 3·40) | 0·0004 | 2·82 (1·48, 5·36) | 0·0002 | 1·70 (1·39, 2·08) | <0·0001 | 2·10 (0·91, 4·86) |
| 21 – 39 | 1·00 | 1·00 | 1·00 | 1·00 | |||
| ≥ 40 | 0·58 (0·39, 0,86) | 0·01 | 0·15 (0·04, 0·61) | 0·01 | 0·68 (0·55, 0·83) | 0·0002 | 0·23 (0·06, 0·91) |
| Socioeconomic position | |||||||
| High level proficiencies | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Medium level proficiencies | 1·19 (1·07, 1·32) | 0·001 | 1·19 (0·99, 1·43) | 0·07 | 1·15 (1·08, 1·21) | <0·0001 | 1·20 (0·96, 1·50) |
| Skilled | 1·37 (1·23, 1·52) | <0·0001 | 1·21 (0·99, 1·47) | 0·06 | 1·44 (1·36, 1·53) | <0·0001 | 1·16 (0·92, 1·47) |
| Unskilled | 1·58 (1·39, 1·80) | <0·0001 | 1·64 (1·30, 2·07) | <0·0001 | 1·61 (1·50, 1·73) | <0·0001 | 1·58 (1·20, 2·09) |
| Students | 0·96 (0·76, 1·19) | 0·68 | 1·34 (0·95, 1·88) | 0·09 | 1·15 (1·03, 1·28) | 0·01 | 0·93 (0·58, 1·49) |
| Unemployed | 1·86 (1·47, 2·36) | <0·0001 | 1·75 (1·18, 2·58) | 0·01 | 1·92 (1·70, 2·17) | <0·0001 | 1·98 (1·28, 3·08) |
| Prepregnancy BMI (kg/m2) | |||||||
| ≤ 18·5 | 0·83 (0·69, 1·01) | 0·07 | 1·18 (0·86, 1·62) | 0·30 | 1·02 (0·92, 1·13) | 0·72 | 0.49 (0·28, 0·85) |
| 18·6 – 24·9 | 1·00 | 1·00 | 1·00 | 1·00 | |||
| 25·0 – 29·9 | 1·13 (1·03, 1·24) | 0·01 | 1·23 (1·05, 1·45) | 0·001 | 1·21 (1·15, 1·27) | <0·0001 | 1·28 (1·06, 1·56) |
| 30·0 – 34·9 | 1·41 (1·22, 1·63) | <0·0001 | 1·51 (1·17, 1·96) | 0·002 | 1·37 (1·26, 1·50) | <0·0001 | 1·74 (1·30, 2·33) |
| ≥ 35·0 | 1·35 (1·06, 1·71) | 0·02 | 1·38 (0·87, 2·18) | 0·18 | 1·58 (1·38, 1·83) | <0·0001 | 1·08 (0·59, 1·99) |
| Physical activity | |||||||
| Yes | 1·00 | 1·00 | 1·00 | 1·00 | |||
| No | 1·17 (1·09, 1·26) | <0·0001 | 1·03 (0·90, 1v17) | 0·65 | 1·08 (1·03, 1·12) | 0·0004 | 1·11 (0·95, 1·30) |
| Smoking in pregnancy | |||||||
| Nonsmoker | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Occasional smoker | 1·04 (0·93, 1·16) | 0·51 | 1·30 (1·08, 1·56) | 0·01 | 1·22 (1·16, 1·30) | <0·0001 | 1·17 (0·93, 1·46) |
| <15 cigarettes/day | 1·61 (1·44, 1·79) | <0·0001 | 1·68 (1·38, 2·04) | <0·0001 | 1·71 (1·61, 1·82) | <0·0001 | 1·34 (1·05, 1·71) |
| ≥15 cigarettes/day | 2·00 (1·57, 2·56) | <0·0001 | 1·73 (1·08, 1·56) | 0·02 | 2·01 (1·75, 2·30) | <0·0001 | 1·54 (0·87, 2·72) |
| Parity | |||||||
| Nullipara | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Multipara | 1·22 (1·13, 1·31) | <0·0001 | 1·06 (0·93, 1·20) | 0·33 | 0·87 (0·83, 0·90) | <0·0001 | 1·11 (0·95, 1·29) |
| Breast-feeding duration | |||||||
| No breastfeeding | 1·00 | 1·00 | 1·00 | 1·00 | |||
| 0-1 months | 1·08 (0·78, 1·49) | 0·64 | 0·93 (0·53, 1·64) | 0·80 | 0·88 (0·74, 1·04) | 0·14 | 0·961 (0·33, 1·12) |
| 2-3 months | 1·16 (0·84, 1·59) | 0·37 | 1·17 (0·68, 2·02) | 0·58 | 0·88 (0·74, 1·04) | 0·13 | 0·72 (0·40, 1·30) |
| 4-6 months | 0·96 (0·70, 1·30) | 0·79 | 0·72 (0·42, 1·23) | 0·23 | 0·71 (0·61, 0·84) | <0·0001 | 0·46 (0·26, 0·81) |
| 7-9 months | 0·70 (0·52, 0·95) | 0·02 | 0·61 (0·36, 1·04) | 0·07 | 0·55 (0·47, 0·64) | <0·0001 | 0·42 (0·24, 0·73) |
| ≥10 months | 0·61 (0·45, 0·83) | 0·001 | 0·62 (0·37, 1·06) | <0·08 | 0·49 (0·42, 0·57) | <0·0001 | 0·52 (0·30, 0·89) |
| Maternal asthma | |||||||
| Yes | 1·86 (1·66, 2·08) | <0·0001 | 2·01 (1·67, 2·42) | <0·0001 | 1·76 (1·65, 1·88) | <0·0001 | 2·77 (2·27, 3·39) |
| No | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Maternal allergies | |||||||
| Yes | 1·27 (1·18, 1·37) | <0·0001 | 1·36 (1·19, 1·55) | <0·0001 | 1·21 (1·16, 1·26) | <0·0001 | 1·79 (1·53, 2·09) |
| No | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Paternal asthma | |||||||
| Yes | 1·68 (1·49, 1·89) | <0·0001 | 2·31 (1·92, 2·77) | <0·0001 | 1·55 (1·44, 1·66) | <0·0001 | 2·89 (2·35, 3·54) |
| No | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Paternal allergies | |||||||
| Yes | 1·19 (1·09, 1·29) | <0·0001 | 1·41 (1·22, 1·62) | <0·0001 | 1·09 (1·04, 1·14) | 0·0003 | 1·53 (1·30, 1·81) |
| No | 1·00 | 1·00 | 1·00 | 1·00 | |||
| Child sex | |||||||
| Male | 1·60 (1·49, 1·72) | <0·0001 | 1·83 (1·60, 2·09) | <0·0001 | 1·37 (1·32, 1·42) | <0·0001 | 1·96 (1·67, 2·30) |
| Female | 1·00 | 1·00 | 1·00 | 1·00 |
Ever admitted asthma: first registered asthma diagnosis in the Danish National Patient Registry.
Ever prescribed asthma: >=2 asthma prescription in the Register of Medicinal Product Statistics except for beta-2-agonists as liquid, inhaled beta-2-agonists only once, or inhaled steroid only once.
Current asthma: self-reported doctor diagnosis of asthma plus wheeze in the past 12 months.
Multivariate analysis
Child asthma, wheeze, and recurrent wheeze at 18 months
A total of 17% (3,551/21,293) of children were classified with doctor-diagnosed asthma, 27% (5,784/21,397) with wheeze symptoms, and 9% (1,820/21,344) with recurrent wheeze in the first 18 months of life. After adjusting for potential confounders, we found a direct association for asthma (zero vs. high frequency intake: OR: 1·30, 95% CI: 1·05, 1·63, P=0·02; P for trend: 0·001), and a suggestive association for recurrent wheeze (zero vs. high frequency intake: OR: 1·24, 95% CI: 0·94, 1·64, P=0·13; P for trend: 0·28) (Table 3).
Table 3.
Association between maternal fish intake during pregnancy and child asthma diagnosis and wheeze symptoms at 18 months in the Danish National Birth Cohort
| Categories of fish intake | Cases/N(%) | Doctor-diagnosed asthma N=21,293/16,867 OR (95% CI) | P for trend1 | Cases/N(%) | Recurrent wheeze2 N=21,344/16,905 OR (95% CI) | P for trend1 | Cases/N(%) | Ever wheeze N=21,397/16,950 OR (95% CI) | P for trend1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Warm meal and sandwich each week, high frequency | crude | 360/2,448 (15) | <0·0001 | 199/2,456 (8) | 0·0002 | 634/2,457 (26) | <0·0001 | |||
| adjusted* | 293/1,992 (15) | 1·00 (ref.) | 0·001 | 167/1,997 (8) | 1·00 (ref.) | 0·28 | 531/2001 (27) | 1·00 (ref.) | 0·20 | |
| Warm meal and sandwich each week, low frequency | crude | 760/5,074 (15) | 1·02 (0·89, 1·17) | 393/5,098 (8) | 0·95 (0·79, 1·13) | 1,280/5,110 (25) | 0·96 (0·86, 1·07) | |||
| adjusted* | 600/4,095 (15) | 1·00 (0·86, 1·17) | 314/4,113 (24) | 0·91 (0·75, 1·11) | 1,017/4,124 (25) | 0·91 (0·81, 1·03) | ||||
| Warm meal each month, sandwich each week | crude | 1,297/7,850 (17) | 1·15 (1·01, 1·30) | 665/7,860 (8) | 1·05 (0·89, 1·24) | 2,130/7,883 (27) | 1·07 (0·96, 1·18) | |||
| adjusted* | 995/6,214 (16) | 1·06 (0·92, 1·22) | 488/6,223 (8) | 0·88 (0·73, 1·06) | 1,617/6,243 (26) | 0·95 (0·84, 1·06) | ||||
| Warm meal and sandwich each month / less than each month | crude | 905/4,904 (18) | 1·31 (1·15, 1·50) | 438/4,912 (9) | 1·11 (0·93, 1·32) | 1,427/4,925 (29) | 1·17 (1·05, 1·31) | |||
| adjusted* | 698/3,803 (18) | 1·19 (1·02, 1·38) | 346/3,808 (9) | 0·98 (0·80, 1·20) | 1,087/3,816 (28) | 1·04 (0·92, 1·18) | ||||
| Zero intake | crude | 229/1,017 (23) | 1·69 (1·40, 2·03) | 125/1,018 (12) | 1·59 (1·25, 2·01) | 313/1,022 (31) | 1·27 (1·08, 1·49) | |||
| adjusted* | 167/763 (22) | 1·30 (1·05. 1·63) | 95/764 (12) | 1·24 (0·94, 1·64) | 224/767 (29) | 0·98 (0·81, 1·19) |
Model adjusted for maternal age, smoking, parity, prepregnancy BMI, physical activity, breastfeeding, socioeconomic status, maternal history of asthma and allergies, paternal history of asthma and allergies, and child sex.
P-value for trend estimated by modeling exposure as an ordinal variable.
Recurrent wheeze defined as >3 episodes in the past 18 months vs. <=3 episodes or no reported wheeze in the past 18 months.
Ever admitted, prescribed, and current child asthma at 7 years
The ever asthma prevalence was 6% (996/17,399) and 31% (5,360/17,389) by admission and prescription respectively. About 4% (685/17,338) of children were classified with current asthma at age 7. Children whose mothers never consumed fish during pregnancy were more likely to have an ever admitted asthma diagnosis compared to mothers in the highest intake category (OR: 1·46, 95% CI: 0·99, 2·13, P=0·05; P for trend: 0·46) (Table 4). Similarly, we found a direct association for ever prescribed asthma (OR: 1·37, 95% CI: 1·10, 1·71, P=0·01; P for trend: 0·06). The relation for current asthma was in the same direction but did not reach statistical significance.
Table 4.
Association between maternal fish intake and child asthma ascertained with different data sources (patient registry, medication prescription registry, self-report) in the Danish National Birth Cohort
| Categories of fish intake | Cases/N(%) | Ever admitted asthma2 N=17,399/11,631 OR (95% CI) | P for trend1 | Cases/N(%) | Ever prescribed asthma3 N=17,389/11,622 OR (95% CI) | P for trend1 | Cases/N(%) | Current asthma (7 years)4 N=17,338/11,586 OR (95% CI) | P for trend1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Warm meal and sandwich each week, high frequency | crude | 118/2,074 (6) | 0·02 | 591/2,072 (29) | <0·0001 | 87/2,064 (4) | 0·64 | |||
| adjusted* | 79/1,335 (6) | 1·00 (ref.) | 0·46 | 371/1,333 (28) | 1·00 (ref.) | 0·06 | 52/1,327 (4) | 1·00 (ref.) | 0·75 | |
| Warm meal and sandwich each week, low frequency | crude | 239/4,265 (6) | 0·98 (0·78, 1·24) | 1,246/4,263 (29) | 1·04 (0·92, 1·16) | 178/4,2051 (4) | 0·99 (0·76, 1·29) | |||
| adjusted* | 160/2,892 (6) | 0·94 (0·71, 1·25) | 826/2,890 (29) | 1·03 (0·89, 1·20) | 110/2,880 (4) | 0·97 (0·69, 1·37) | ||||
| Warm meal each month, sandwich each week | crude | 333/6,375 (5) | 0·91 (0·74, 1·13) | 1,932/6,372 (30) | 1·09 (0·98, 1·22) | 218/6,354 (3) | 0·81 (0·63, 1·04) | |||
| adjusted* | 229/4,252 (5) | 0·89 (0·68, 1·16) | 1,268/4,250 (30) | 1·05 (0·91, 1·20) | 152/4,241 (4) | 0·89 (0·64, 1·23) | ||||
| Warm meal and sandwich each month / less than each month | crude | 228/3,895 (6) | 1·03 (0·82, 1·30) | 1,267/3,893 (33) | 1·21 (1·08, 1·36) | 155/3,881 (4) | 0·95 (0·72, 1·24) | |||
| adjusted* | 152/2,638 (6) | 0·92 (0·69, 1·22) | 842/2,636 (32) | 1·05 (0·91, 1·23) | 103/2,625 (4) | 0·94 (0·66, 1·34) | ||||
| Zero intake | crude | 78/790 (10) | 1·82 (1·35, 2·45) | 324/789 (41) | 1·75 (1·47, 2·07) | 47/788 (6) | 1·44 (1·00, 2·08) | |||
| adjusted* | 52/514 (10) | 1·46 (0.99, 2·13) | 213/513 (42) | 1·37 (1·10, 1·71) | 31/513 (6) | 1·31 (0·81, 2·11) | ||||
Model adjusted for maternal age, smoking, parity, prepregnancy BMI, physical activity, breastfeeding, socioeconomic status, maternal history of asthma and allergies, paternal history of asthma and allergies, and child sex.
P-value for trend estimated by modeling exposure as an ordinal variable.
Ever admitted asthma: first registered asthma diagnosis in the Danish National Patient Registry.
Ever prescribed asthma: >=2 asthma prescriptions in the Register of Medicinal Product Statistics except for beta-2-agonists as liquid.
Current asthma: self-reported doctor diagnosis of asthma plus wheeze in the past 12 months.
Ever prescribed and reported child allergic rhinitis at 7 years
The prevalence of ever prescribed child allergic rhinitis was 8% (1,332/17,389) and 5% (821/17,269) based on the 7 year questionnaire. Maternal fish intake was not associated with either allergic rhinitis definition in the zero intake category; however, we found that low fish consumers (weekly and monthly) were less likely to report a child allergic rhinitis diagnosis compared to high fish consumers (Supplementary Table S1).
Sensitivity analyses
Given that n-3 fatty acids have been implicated as active agents in the association between fish intake and allergic disease, we examined associations with energy-adjusted n-3 fatty acids assessed by the mid-pregnancy FFQ. We found associations that were inconsistent with the fish results. Higher EPA and ALA intake were protective of wheeze and recurrent wheeze at 18 months respectively, but both became insignificant after further adjustment for dietary confounders. No associations were found for asthma or allergic rhinitis (data not shown).
For the early childhood outcomes we further examined risk of wheeze and asthma at 6 months but found no associations with fish intake (data not shown). Similarly, reporting of any wheeze episodes in the first 18 months of life (>=1 episodes) was not related to higher fish intake (data not shown). To provide a clearer separation for the recurrent wheeze outcome, we excluded children reporting 1-3 wheeze episodes. This did not alter the results (data not shown).
We excluded the first three years of life from the analysis using registry-based outcomes in order to remove potential transient child wheezing. The number of registry cases more than halved and the number of medication-related cases was reduced by close to 80%. The associations with fish weakened slightly and the confidence interval widened for both ever admitted asthma -and ever prescribed asthma (data not shown).
DISCUSSION
In this large prospective study we examined the association between maternal fish intake during pregnancy and child allergic disease. Our results show that fish intake may influence child asthma risk as assessed by parent report of doctor diagnosis in early childhood as well as clinically established asthma using hospital admission and prescription registry data. The increase in risk may be especially relevant for non-consumers of fish. We did not find high fish intake to be protective for allergic rhinitis.
Asthma is a heterogeneous group of conditions and its diagnosis is dependent on the timing of assessment. Wheeze in response to respiratory infections in early life is often transient and resolves as the child grows, while allergic asthma persists and peaks at age 7-9 years. 27 The pathological pathways involved in early and later manifestations of asthmatic symptoms may therefore differ, and the influence of fish on these pathways could vary. Our results suggest that fish intake during pregnancy could influence asthma doctor diagnosis, but not wheeze symptoms, in the first two years of life. This may be because diagnosis of asthma is a more homogenous outcome compared to wheeze (which might include other causes of wheezing such as respiratory infections), or because of more accurate maternal reporting of doctor diagnosis compared to more subjective interpretation of wheeze symptoms. We furthermore found a protective association for ever admitted asthma and ever prescribed asthma, but not self-reported asthma diagnosis. The former outcomes may be better at capturing true asthma compared to the questionnaire. The difference may also indicate a difference in severity of disease in registry vs. self-reported diagnoses.
In our adjusted analyses, breastfeeding accounted for the largest attenuation in effect estimates. The role of breastfeeding in atopic disease is unclear. While some studies showed inverse associations with longer duration/exclusive breastfeeding, 28, 29 others found no 30, 31 or direct 32, 33 relations. In our study, women who tended to breastfeed for longer period of time were less likely to report an early child asthma doctor diagnosis and have a registry-based child diagnosis. Similar results were found for exclusive breastfeeding for >=4 and >=6 months. Women in the higher fish intake categories tended to breastfeed for a longer period of time, yet the correlations were modest for both breastfeeding duration and exclusivity (r=0.04-0.16).We found no statistically significant interaction by either breastfeeding duration or exclusivity.
To date numerous studies have examined the relation between fish intake during pregnancy and allergic outcomes, with inconsistent findings. 9-14 Studies examining intake 15 or blood levels of fatty acids 34, 35 have found protective associations for n-3 fatty acids with early allergic outcomes. These studies have included different allergic disease outcomes and did not have the advantage of our study to separately examine both early and later manifestations of allergic disease. Furthermore, the protective associations were primarily found for eczema, 9-11, 15 wheeze, 9, 15, 36 and atopy, 9, rather than asthma. 13 Randomized clinical trials with fish oil supplementation during pregnancy have found favorable effects on immunological responses and sensitization in the first year of life rather than clinical outcomes. 14, 17, 18, 20 These results are in some accordance with our fatty acid findings for outcomes at 18 months. Our results do not agree with a highly protective effect of EPA+DHA supplementation during pregnancy and asthma registry diagnosis in a Danish trial. 19 Differences in dose and large variation in intake (2·7 g/day in the trial vs. a mean of 0·38±0·29 g/day in our study), and timing of intake (late vs. mid-pregnancy) may account for the discrepancies in results. We are only aware of one previous study looking specifically at fish intake during pregnancy in relation to allergic rhinitis; results from this study were consistent with our results. 11
Our findings provide limited support for the lipid hypothesis proposed by Black and Sharpe3 that lower intake of n-3 fatty acids found in fish may increase risk of allergic disease development However, while the results for asthma are in line with this hypothesis, our findings did not extend to allergic rhinitis; rather we observed lower risks of self-reported allergic rhinitis in the two lower fish intake groups (weekly and monthly), but not in the zero intake group. The differing results for these two outcomes may be explained by failure to capture true allergic rhinitis since the self-reported definition was based on only one question and did not include questions on symptoms, while ever prescribed allergic rhinitis excluded over-the-counter medications like histamines which may have decreased the sensitivity of the outcome measure. Asthma and allergic rhinitis share a genetic predisposition for atopy and a common inflammatory etiology; however, in allergic rhinitis, there is no smooth muscle constriction in the airways. 37Our results could suggest that fish is more strongly related to the tissue, rather than the immune component, of allergic disease, but these cannot be teased apart using the current data.
Furthermore, we did not find any associations with n-3 PUFAs as reported by the FFQ; this may imply that other mechanistic pathways apart from those governed by essential fatty acids and their metabolites are involved. Fish is also an important source for vitamin D, a nutrient that has been implicated in immune function and allergy development.38 Yet, we cannot exclude that poor measurement and low variability of fatty acid intake obscured any potential associations. This may be especially relevant if the association lies in the extreme intake categories.
Our study adds to the current literature on prenatal dietary exposure and child allergic disease development. Our prospective study design allowed us to follow a large cohort for the first 7 years of life. We collected detailed information on maternal fish intake during pregnancy and studied the relation to outcomes at two time points in childhood, allowing for change in risk across time and by disease manifestation. By adjustment for numerous confounders, including a wide range of other maternal dietary factors, we reduced the potential for residual confounding. We employed both self-reported data and national registries. The DNPR has complete follow-up, but includes only hospital cases and the ICD classification could be limited by miscoding. However, a recent validation study in Danish male conscripts against medical examination found that any misclassification in the DNPR was too small to nullify observed associations. 39 Self-report is more useful for outcomes such as allergic rhinitis. Allergic rhinitis is less likely to be captured by the registries as it rarely results in hospitalizations and use of prescription medication because of moderate symptoms and access to over-the-counter drugs.
The main limitations of this study were self-reporting of exposure and outcomes. We expect any misclassification of fish intake to have been non-differential, underestimating our associations. We cannot exclude misclassification of outcomes assessed by questionnaire; yet, in a recent study 40 we showed that our definition of current asthma has shown high agreement among non-cases (>90%) when compared to the DNPR; suggesting that false positives and biased results were largely avoided. Though associations with all asthma outcomes showed the same directionality, small sample size in the zero intake category may have precluded us from finding an association for current asthma at age 7. Although we did not have full data on child diet, we did have information on early child fish intake, which was only modestly correlated with maternal fish intake. When we included the information on child diet in our analyses there was a slight attenuation of the effect estimates, but this did not alter our conclusions. We find this reassuring for our interpretation of the observed associations as indicative of a beneficial effect of maternal fish intake against child asthma. Moreover, while some studies have found a beneficial association between child fish intake and allergic outcomes, 41, 42 the largest randomized clinical trial did not detect an effect on asthma.43 However, we cannot exclude the possibility, that child dietary factors other than early fish intake were responsible for the associations observed in this study. Also, since the associations we observed seemed to be found particularly among the zero fish consumers, who had a lower SES status and more overall unhealthy lifestyle than the high-fish consumers, we cannot exclude the possibility that unmeasured confounding may have accounted for part of the association.
Lastly, loss to follow-up needs always to be considered in longitudinal studies due to the potential for selection bias. A detailed examination of population characteristics comparing participants and non-participants at 18 months and 7 years of our analysis showed few differences, though participants tended to display healthier life-style habits and higher SES. We also found similar characteristics distributions comparing the full singleton population to populations with outcome data at 18 months (67% of population) and 7 years (55%). However, we cannot exclude residual bias by mismeasured or unmeasured selection variables.
Our results indicate a beneficial relation between a high vs. low maternal fish intake in pregnancy and child asthma during the first 7 years of life. We found no associations for fatty acid intake. While several randomized clinical trials have assessed fish oil supplementation in relation to allergic outcomes, we were able to examine intake of a whole food across a wide range of intake and account for potential synergistic interactions between nutrients. Our results may also be more pertinent in communicating dietary recommendation. Further examination of which nutrients in fish may exert a protection against allergic disease is warranted. Likewise studies are also needed to confirm and investigate potential mechanistic differences between asthma and allergic rhinitis, while also taking into account and further exploring the issue of potential residual confounding by lifestyle factors and SES inherent in observational studies.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Danish Council for Strategic Research (09-067124); the Danish Council for Independent Research | Medical Sciences, Danish Agency for Science, Technology and Innovation (09-063410); the Lundbeck foundation (R13-A907); and the European Union (EU) Integrated Research Project EARNEST (FOOD-CT-2005-007036). The EU project EARNEST (http://www.metabolic-programming.org) receives financial support from the Commission of the European Communities under the FP 6 priority 5: food quality and safety. The Danish National Birth Cohort has been financed by the March of Dimes Birth Defects Foundation, the Danish Heart Association, the Danish Medical Research Council, the Sygekassernes Helsefond, Danish National Research Foundation, Danish Pharmaceutical Association, Ministry of Health, National Board of Health, and Statens Serum Institut. We would also like to acknowledge support from the NIH/NICHD (K24 HD069408).
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors had a conflict of interest. The authors’ responsibilities were as follows – EM and SFO: study concept and design; MS: data preparation; EM, MS: conducted the statistical analyses; EM drafted the manuscript; EM, MS, EO, HC, CL, DG, SFO: contributed critical advice and revisions of the manuscript; SFO: funding; EM and SFO: acquisition of data and responsibility for the entire contents of the manuscript. All authors had full access to study data.
REFERENCES
- 1.Mannino DM, Homa DM, Akinbami LJ, et al. Surveillance for asthma--United States, 1980-1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 2.Thomsen S, Ulrik C, Larsen K, et al. Change in prevalence of asthma in Danish children and adolescents. Annals of Allergy, Asthma and Immunology. 2004;92(5):506–11. doi: 10.1016/S1081-1206(10)61757-7. [DOI] [PubMed] [Google Scholar]
- 3.Black P, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J. 1997;10(1):6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 4.Calder PC, L-S. K, Vlachava M, et al. Is there a role for fatty acids in early life programming of the immune system? Proceedings of the Nutrition Society. 2010;69:373–80. doi: 10.1017/S0029665110001552. [DOI] [PubMed] [Google Scholar]
- 5.Prescott S, Dunstan J. Prenatal Fatty Acid Status and Immune Development: The Pathways and the Evidence. Lipids. 2007;42:801–10. doi: 10.1007/s11745-007-3030-z. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Arita M, Hong S, et al. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39(11):1125–32. doi: 10.1007/s11745-004-1339-7. Epub 2005/02/25. [DOI] [PubMed] [Google Scholar]
- 7.Hodge L, Salome CM, Peat JK, et al. Consumption of oily fish and childhood asthma risk. Med J Aust. 1996;164:137–40. doi: 10.5694/j.1326-5377.1996.tb122010.x. [DOI] [PubMed] [Google Scholar]
- 8.Laerum BN, Wentzel-Larsen T, Gulsvik A, et al. Relationship of fish and cod oil intake with adult asthma. Clin Exp Allergy. 2007;37(11):1616–23. doi: 10.1111/j.1365-2222.2007.02821.x. Epub 2007/09/20. [DOI] [PubMed] [Google Scholar]
- 9.Romieu I, Torrent M, Garcia-Esteban R, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy. 2007;37(4):518–25. doi: 10.1111/j.1365-2222.2007.02685.x. [DOI] [PubMed] [Google Scholar]
- 10.Sausenthaler S, Koletzko S, Schaaf B, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85(2):530–7. doi: 10.1093/ajcn/85.2.530. [DOI] [PubMed] [Google Scholar]
- 11.Willers SM, Devereux G, Craig LCA, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62:773–9. doi: 10.1136/thx.2006.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvani M, Alessandri C, Sopo S, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94–102. doi: 10.1111/j.1399-3038.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 13.Salam M, Li Y, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513–8. doi: 10.1081/JAS-67619. [DOI] [PubMed] [Google Scholar]
- 14.Øien T, Storrø O, Johnsen R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J Epidemiol Community Health. 2010;64(2):124–9. doi: 10.1136/jech.2008.084921. [DOI] [PubMed] [Google Scholar]
- 15.Miyake Y, Sasaki S, Tanaka K, et al. Maternal fat consumption during pregnancy and risk of wheeze and eczema in Japanese infants aged 16-24 months: the Osaka Maternal and Child Health Study. Thorax. 2009;64(9):815–21. doi: 10.1136/thx.2009.115931. [DOI] [PubMed] [Google Scholar]
- 16.Willers SM, Wijga AH, Brunekreef B, et al. Maternal Food Consumption during Pregnancy an Longitudinal Development of Childhood Asthma. Am J Respir Crit Care Med. 2008;178:124–31. doi: 10.1164/rccm.200710-1544OC. [DOI] [PubMed] [Google Scholar]
- 17.Dunstan J, Mori T, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003b;112(6):1178–84. doi: 10.1016/j.jaci.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Furuhjelm C, Warstedt K, Larsson J, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Pædiatrica. 2009;98:1461–7. doi: 10.1111/j.1651-2227.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 19.Olsen S, Østerdal M, Salvig J, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1):167–75. doi: 10.1093/ajcn/88.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Krauss-Etschmann S, Hartl D, Rzehak P, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol. 2008;121:464–70. doi: 10.1016/j.jaci.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Olsen SF, Hansen HS, Sandstrom B, et al. Erythrocyte levels compared with reported dietary intake of marine n-3 fatty acids in pregnant women. British Journal of Nutrition. 1995;73:387–95. doi: 10.1079/bjn19950041. [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen TB, Osler M, Olsen SF. Validity of protein, retinol, folic acid and n–3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutrition. 2005;9(6):771–8. doi: 10.1079/phn2005883. [DOI] [PubMed] [Google Scholar]
- 23.Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 24.Moth G, Vedsted P, Schiøtz PO. National registry diagnoses agree with medical records on hospitalized asthmatic children. Acta Pædiatrica. 2007;96:1470–3. doi: 10.1111/j.1651-2227.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- 25.Moth G, Vedsted P, Schiøtz PO. Identification of asthmatic children using prescription data and diagnosis. Eur J Clin Pharmacol. 2007;63:605–11. doi: 10.1007/s00228-007-0286-4. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York, USA: 1998. [Google Scholar]
- 27.Sporik R, Holgate ST, Cogswell JJ. Natural history of asthma in childhood-a birth cohort study. Archives of Disease in Childhood. 1991;66:1050–3. doi: 10.1136/adc.66.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilbert TW, Stern DA, Morgan WJ, et al. Effect of Breastfeeding on Lung Function in Childhood and Modulation by Maternal Asthma and Atopy. Am J Respir Crit Care Med. 2007;176:843–8. doi: 10.1164/rccm.200610-1507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oddy WH, de Klerk NH, Sly PD, et al. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 30.Burgess SW, Dakin CJ, O'Callaghan MJ. Breastfeeding Does Not Increase the Risk of Asthma at 14 Years. Pediatrics. 2006;117(4):e787–e92. doi: 10.1542/peds.2005-1753. [DOI] [PubMed] [Google Scholar]
- 31.Nagel G, Buchele G, Weinmayr G, et al. Effect of breastfeeding on asthma, lung function and bronchial hyperreactivity in ISAAC Phase II. Eur Respir J. 2009;33:993–1002. doi: 10.1183/09031936.00075708. [DOI] [PubMed] [Google Scholar]
- 32.Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–7. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 33.Fredriksson P, Jaakkola N, Jaakkola JKJ. Breastfeeding and childhood asthma: a six-year population-based cohort study. BMC Pediatrics. 2007;7(39) doi: 10.1186/1471-2431-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newson R, Shaheen S, Henderson A, et al. Umbilical cord and maternal blood red cell fatty acids and early childhood wheezing and eczema. J Allergy Clin Immunol. 2004;114(3):531–7. doi: 10.1016/j.jaci.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Notenboom ML, Mommers M, Jansen EHJM, et al. Maternal fatty acid status in pregnancy and childhood atopic manifestations: KOALA Birth Cohort Study. Clinical & Experiment al Allergy. 2011;41:407–16. doi: 10.1111/j.1365-2222.2010.03672.x. [DOI] [PubMed] [Google Scholar]
- 36.Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507–13. doi: 10.1136/thx.2007.081745. [DOI] [PubMed] [Google Scholar]
- 37.Calder PC, Albers R, Antoine J-M, et al. Inflammatory Disease Processes and Interactions with Nutrition. Br J Nutr. 2009;101(Supplement No. S1) doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- 38.Litonjua AA. Fat-soluble vitamins and atopic disease: what is the evidence? The Proceedings of the Nutrition Society. 2012;71(1):67–74. doi: 10.1017/S002966511100334X. Epub 2011/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Østergaard Jensen A, Lauge Nielsen G, Ehrenstein V. Validity of asthma diagnoses in the Danish National Registry of Patients, including an assessment of impact of misclassification on risk estimates in an actual dataset. Clinical Epi. 2010;2:67–72. doi: 10.2147/clep.s6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen S, Strom M, Maslova E, et al. A comparison of three methods to measure asthma in epidemiologic studies: results from the Danish National Birth Cohort. PloS one. 2012;7(5):e36328. doi: 10.1371/journal.pone.0036328. Epub 2012/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alm B, Aberg N, Erdes L, et al. Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child. 2009;94:11–5. doi: 10.1136/adc.2008.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kull I, Bergstrom A, Lilja G, et al. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. 2006;61:1009–15. doi: 10.1111/j.1398-9995.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 43.Almqvist C, Garden F, Xuan W, et al. Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. J Allergy Clin Immunol. 2007;119:1438–44. doi: 10.1016/j.jaci.2007.01.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


