Abstract
Purpose
Novel imaging modalities are needed to detect occult metastatic disease in bladder carcinoma. Patients with regional lymphatic spread could be targeted for neoadjuvant chemotherapy, and patients with distant metastatic disease could be spared the unnecessary morbidity of radical cystectomy. Herein, we report a prospective study of positron emission tomography/computed tomography (PET/CT) with [18F]fluorodeoxyglucose (FDG) in patients undergoing radical cystectomy for cT2-3N0M0 urothelial carcinoma of the bladder.
Methods
Forty-three chemotherapy-naïve patients underwent FDG-PET/CT before planned cystectomy. All had negative conventional CT and bone scintigraphy before enrollment. Positive FDG-PET/CT was confirmed by percutaneous biopsy or open surgical exploration, whereas negative FDG-PET/CT was confirmed by complete lymphadenectomy. Recurrence-free survival (RFS), disease-specific survival (DSS), and overall survival (OS) were described using the Kaplan-Meier method and compared using log-rank test.
Results
Median follow-up was 14.9 months (range, 0.4 to 46.1 months). One patient who did not undergo lymphadenectomy was excluded from the pathology data analysis (n = 42), whereas another patient who failed to return for follow-up was excluded from survival analysis (n = 42). FDG-PET/CT demonstrated a positive predictive value of 78% (seven of nine), a negative predictive value of 91% (30 of 33), sensitivity of 70% (seven of 10), and specificity of 94% (30 of 32). RFS, DSS, and OS were all significantly poorer in the patients with positive FDG-PET/CT than in those with negative FDG-PET/CT.
Conclusion
FDG-PET/CT detected occult metastatic disease in seven of 42 patients with negative conventional preoperative evaluations. PET findings were strongly correlated with survival. As such, FDG-PET/CT may help in making treatment decisions before radical cystectomy.
INTRODUCTION
Urothelial carcinoma of the bladder (UCB) is the fifth most common malignancy in the United States, with an estimated 68,810 new cases and 14,100 deaths in 2008.1 The management of UCB is dependent on stage, with more advanced tumors requiring aggressive therapy. For more than 15 years, the standard treatment for advanced disease without metastases has been radical cystectomy, whereas the standard treatment for metastatic disease has been cisplatin-based combination chemotherapy, with surgical removal of the bladder reserved for palliative care.2 Although patients with metastatic disease limited to regional pelvic lymph nodes may be cured with radical cystectomy alone,3 these patients generally undergo neoadjuvant chemotherapy in an effort to improve their relatively poor cure rates.4–7
Accordingly, accurate pretreatment staging of bladder carcinoma is an essential ingredient for improving patient care by allowing better selection of patients needing systemic treatment. However, current imaging methods, including computed tomography (CT) and magnetic resonance imaging (MRI) for staging of bladder carcinoma, have not proven to be highly accurate in identification of patients with metastatic disease.8–10 CT may miss up to 40% of lymph node metastases. CT and MRI have accuracies as low as 55% and 60%, respectively.8,11,12 Novel imaging methods are needed to detect occult metastatic disease.
Positron emission tomography (PET) performed with the radiopharmaceutical [18F]fluorodeoxyglucose (FDG) is a molecular imaging technique that assesses regional glucose metabolism. It is widely used because most cancers exhibit increased glucose utilization compared with normal tissues.13 Often the metabolic changes detectable by PET precede morphologic changes detectable by anatomic imaging methods, leading to greater sensitivity of PET. Conventional PET imaging is limited by a relative paucity of anatomic information, thus making it difficult to localize focally increased tracer uptake accurately and to distinguish pathologic from physiologic tracer uptake. This limitation has largely been overcome by the introduction of combined PET/CT, which provides spatially coregistered functional and anatomic images, resulting in increased sensitivity and specificity by comparison with PET alone for many different tumor types.14 There are currently limited data regarding the utility of FDG-PET for preoperative staging of invasive UCB.15–17 Herein, we report the results of a prospective study of FDG-PET/CT in patients scheduled to undergo radical cystectomy for muscle-invasive UCB with no evidence of metastatic disease by conventional imaging.
METHODS
Study Population
This study was approved by the Human Research Protection Office and the Radioactive Drug Research Committee. After obtaining written informed consent, we prospectively enrolled patients with cT2/T3N0M0 UCB in whom radical cystectomy with pelvic lymph node dissection was planned. Patients with primary urothelial carcinomas with squamous or glandular differentiation were included, but those with other histologic subtypes were excluded. All patients were required to have no evidence of locoregional or distant metastatic disease by history and physical examination, conventional abdominopelvic CT, and bone scintigraphy before enrollment onto the trial. As per standard practice at our institution, all imaging studies from outside institutions were reviewed before subject enrollment to ensure that our radiologists agreed there was no metastatic disease. If outside imaging was felt to be inadequate, it was repeated or supplemented with additional tests. Patients were only enrolled after all imaging was completed. Patients who had undergone chemotherapy or were scheduled to receive neoadjuvant chemotherapy were excluded. Patients with poorly controlled diabetes, inability to tolerate PET, or a prior history of malignancy within 5 years were excluded. Patients were followed clinically in accordance with the treating surgeon's standard practice.
PET/CT
PET/CT was performed with a Siemens Biograph LSO 2 scanner (Siemens Medical Solutions, Malvern, PA) approximately 60 minutes after administration of 10 to 15 mCi of FDG. To facilitate clearance of urinary activity, a Foley catheter was placed before injection of FDG, furosemide (20 mg) was administered intravenously approximately 20 minutes after administration of FDG, and the patients were hydrated throughout the study. The CT portion of the study was performed without the administration of intravenous or oral contrast material. CT images (5-mm slices) were obtained from the base of skull through the proximal thighs at 130 kVp and 110 effective mAs. PET images were obtained over the same anatomic extent, with imaging times of 2 to 4 minutes per bed position, depending on patient weight. PET images were corrected for scatter, random coincidences, and attenuation and were reconstructed with an ordered-subset expectation maximization iterative algorithm with the use of a postreconstruction Gaussian filter (5-mm full width at half maximum).
Each PET/CT study was interpreted by one of two experienced nuclear radiologists and by a diagnostic radiologist specializing in genitourinary radiology. Specific attention was directed to uptake of FDG in the primary bladder tumor, pelvic nodes, para-aortic nodes, and distant sites. For the purposes of this study, we used the final consensus interpretation of each PET/CT scan as reflected in the clinical report that became part of each patient's medical record.
In addition to qualitative evaluation of the images, all PET images were evaluated semiquantitatively by determination of the maximum standardized uptake values (SUVmax) of tumor foci.18 Volumes of interest were drawn around the foci of abnormal FDG uptake, with care taken to exclude any residual urine in the catheter-drained bladder from the region, and the SUVmax was obtained.
Identification of Metastatic Disease
The surgical procedure performed included a radical cystoprostatectomy in men or anterior exenteration in women, and bilateral pelvic lymphadenectomy. The lymphadenectomy consisted of bilateral removal of all nodal tissue and skeletonization of all vessels from the bifurcation of the common iliac artery to the genitofemoral nerve, laterally, to the obturator nerve, medially and carried down to the angle between Cooper's ligament and the inferior aspect of the iliac vein. Additional presacral nodes lying medial to the internal iliac artery were also removed. The template was modified only if FDG-PET/CT demonstrated evidence of an intra-abdominal metastasis that was easily accessible during the surgical procedure. If FDG-PET/CT showed lesions not easily accessible surgically, percutaneous biopsy was performed under CT guidance.
Pathologic Assessment of Bladder and Lymph Nodes
Pathologic specimens were processed and reviewed in the standard fashion without modifications by a urologic pathologist unaware of the PET results. Lymph nodes and associated fibroadipose tissue from node dissection were entirely submitted for pathologic evaluation. Lymph nodes smaller than 1.0 cm were bivalved and then entirely submitted for histologic evaluation. Lymph nodes more than 1.0 cm were serially sectioned and then entirely submitted for histologic evaluation. Lymph nodes were grouped as right and left pelvic lymph nodes. Size of metastatic deposit in lymph node was reported as maximum diameter of the largest metastatic lesion.
Statistical Analysis
Data analysis of this pilot study was descriptive in nature. The feasibility of using FDG-PET/CT for detection of metastatic disease in UCB was summarized by sensitivity and specificity, as well as the corresponding exact binomial 95% CIs, treating pathologic and/or biopsy findings as the reference standard. This study also explored the association between FDG-PET/CT and clinical outcomes, including overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS). A positive FDG-PET/CT was defined as evidence of metastatic disease either regionally in the pelvic lymph nodes or distantly to bone, lungs, viscera, or lymph nodes outside of the pelvis. OS was defined as the time from date of PET to the date of death of any cause. DSS was defined as the time from date of PET to the date of death from disease. RFS was defined as the time from date of PET to recurrence or death from disease. Survivors were censored at the date of last contact. OS, DSS, and RFS in FDG-PET/CT-negative and -positive subgroups were estimated using Kaplan-Meier product-limit methods and compared using the log-rank test. All analyses were two-sided, and a P value of .05 was taken to indicate significance. Statistical analyses were performed using SAS (SAS Institute, Cary, NC).
RESULTS
Patient Population
From June 2004 to September 2008, 43 patients with cT2/T3N0M0 UCB were prospectively enrolled. Patient characteristics are described in Table 1. Median age was 70 years (range, 32 to 87 years). Eighteen patients were Medicare beneficiaries, and their PET studies were able to be performed via their participation in the National Oncologic PET Registry.19 Median follow-up was 14.9 months (range, 0.4 to 46.1 months). Forty-one patients were taken to surgery for planned cystectomy an average of 8.6 days (range, 2 to 36 days) after FDG-PET/CT. The one patient with no follow-up information was excluded from the survival analysis (n = 42), and the one patient who did not undergo lymphadenectomy was excluded from the pathology data analysis (n = 42). In addition, one patient was found to have widespread metastatic disease by FDG-PET/CT, confirmed by percutaneous biopsy, and was not taken to surgery; one patient was found intraoperatively to have metastatic disease infiltrating the retroperitoneum, and cystectomy was not performed; and one patient had biopsy of a PET-positive pelvic lymph node and received neoadjuvant chemotherapy followed by delayed cystectomy. Median age was 70 years (range, 32 to 87 years). Eighteen patients were Medicare beneficiaries, and their PET studies were able to be performed via their participation in the National Oncologic PET Registry.19 Median follow-up was 14.9 months (range, 0.4 to 46.1 months). Forty-one patients were taken to surgery for planned cystectomy an average of 8.6 days (range, 2 to 36 days) after FDG-PET/CT. The one patient with no follow-up information was excluded from the survival analysis (n = 42), and the one patient who did not undergo lymphadenectomy was excluded from the pathology data analysis (n = 42). In addition, one patient was found to have widespread metastatic disease by FDG-PET/CT, confirmed by percutaneous biopsy, and was not taken to surgery; one patient was found intraoperatively to have metastatic disease infiltrating the retroperitoneum, and cystectomy was not performed; and one patient had biopsy of a PET-positive pelvic lymph node and received neoadjuvant chemotherapy followed by delayed cystectomy.
Table 1.
Demographic and Clinical Characteristics of 43 Patients
| Characteristic | No. of Patients | Total Patients | % |
|---|---|---|---|
| Sex | |||
| Male | 32 | 43 | 74.4 |
| Female | 11 | 43 | 25.6 |
| Race | |||
| White | 41 | 43 | 95.4 |
| Black | 1 | 43 | 2.3 |
| Other | 1 | 43 | 2.3 |
| Age, years | |||
| Median | 70 | ||
| Mean | 68 | ||
| Range | 32-87 | ||
| Clinical stage | |||
| cT2 | 40 | 43 | 93.0 |
| cT3 | 3 | 43 | 7.0 |
| Clinical grade | |||
| 1 | 0 | 0 | |
| 2 | 2 | 43 | 4.7 |
| 3 | 41 | 43 | 95.3 |
| Pathologic stage* | |||
| pT2Nx | 21 | 41 | 51.2 |
| pT3Nx | 16 | 41 | 39.0 |
| pT4Nx | 3 | 41 | 7.3 |
| pTxNxM1 | 2 | 43 | 4.7 |
| pTxN + M0† | 8 | 42 | 19.0 |
| Pathologic grade* | |||
| 1 | 0 | 41 | 0 |
| 2 | 1 | 41 | 2.4 |
| 3 | 40 | 41 | 97.6 |
Pathologic stage reflects the consensus stage from transurethral resection of bladder tumor (TURBT) and cystectomy so as to reflect the true stage of disease before TURBT. As such, patients who demonstrated lower-stage disease at the time of cystectomy were classified on the basis of the TURBT pathology. Cystectomy not performed in two patients with metastatic disease.
One lymph node dissection was not performed.
Primary Tumor
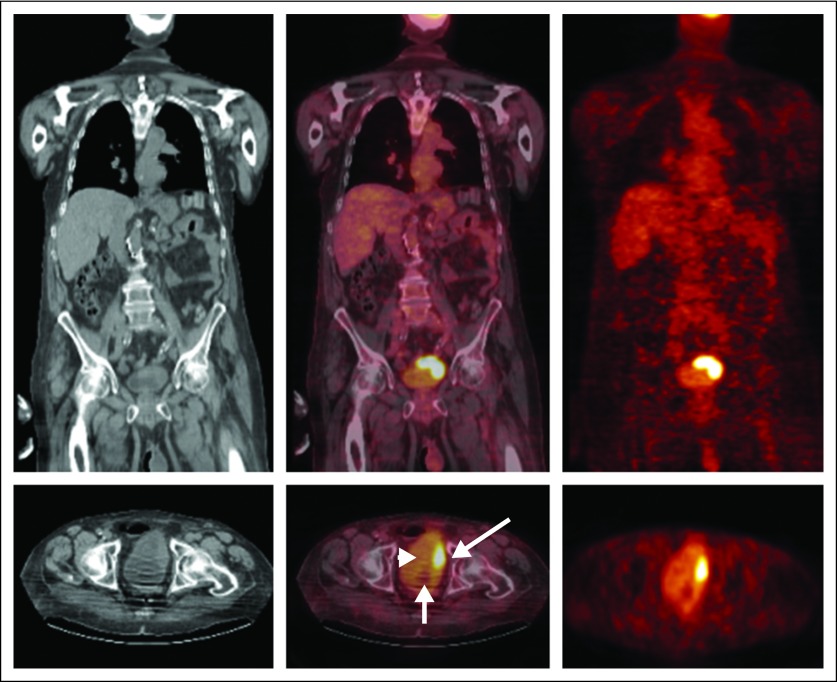
In seven of the 41 patients who underwent cystectomy, there was no residual tumor identified pathologically. A primary tumor focus was identified pathologically in the remaining 34. Increased FDG uptake was seen in the primary tumor in 28 (82%) of 34 patients (Fig 1), and the mean (± standard deviation) SUVmax of the primary lesions was 13.1 (± 10.6).
Fig 1.
Coronal (top row) and transverse (lower row) computed tomography (CT) images (left column), positron emission tomography (PET)/CT fusion images (middle column), and PET images (right column) demonstrate intense [18F]fluorodeoxyglucose uptake in the known primary bladder carcinoma (long arrow). There is mild activity in the urinary bladder (arrowhead). The Foley catheter bulb also is seen (small arrow).
Lymph Node Metastasis
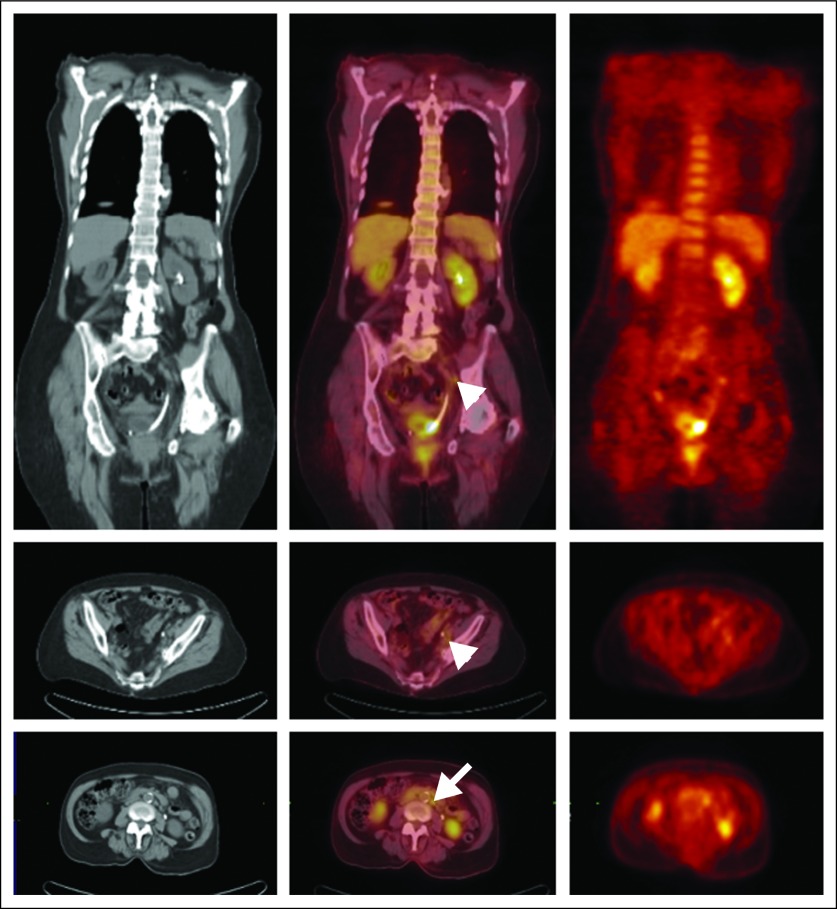
Of the 42 patients available for analysis, FDG-PET/CT demonstrated nodal metastasis in nine patients, seven of whom proved to have nodal metastatic disease by pathologic examination (positive predictive value, 78%; Fig 2). FDG-PET/CT demonstrated no evidence of nodal metastasis in 33 patients, three of whom proved to have metastatic disease at surgery by pathologic examination (negative predictive value, 91%). The corresponding sensitivity and specificity of FDG-PET/CT were thus 70% (seven of 10) and 94% (30 of 32), respectively (Table 2).
Fig 2.
Coronal (top row) and transverse (middle and lower rows) computed tomography (CT) images (left column), positron emission tomography (PET)/CT fusion images (middle column), and PET images (right column) demonstrate increased [18F]fluorodeoxyglucose (FDG) uptake in a small left pelvic lymph node (arrowhead) and a small left para-aortic lymph node (arrow). These foci were proved to be sites of nodal metastatic disease at surgery. The PET images also demonstrate focal uptake of FDG in the primary tumor at the left ureterovesical junction (surrounding the stent).
Table 2.
Lymph Node Metastasis: Correlation of PET/CT and Pathologic Results
| PET/CT | Positive Pathology | Negative Pathology | Total |
|---|---|---|---|
| Positive PET/CT | 7 | 2 | 9 |
| Negative PET/CT | 3 | 30 | 33 |
| Total | 10 | 32 | 42 |
NOTE. Sensitivity = 70% (95% CI, 45% to 88%), positive-predictive value = 78% (95% CI, 51% to 94%), specificity = 94% (95% CI, 84% to 98%), negative-predictive value = 91% (95% CI, 81% to 97%).
Abbreviation: PET/CT, positron emission tomography/computed tomography.
The size of the metastatic deposits was related to likelihood of detection on PET images. Of the 10 patients with nodal metastatic disease, eight patients underwent lymphadenectomy. In two patients, biopsy was performed to confirm the diagnosis of metastatic disease, but the size of the metastatic deposits could not be assessed; one did not undergo surgery because of widespread metastatic disease, and the other underwent neoadjuvant chemotherapy. The mean size of the tumor deposits was larger in the five PET-positive pelvic nodes (2.8 cm; range, 0.4 to 6.5 cm) than in the three PET-negative lymph nodes (0.6 cm; range, 0.4 to 0.7 cm), although the difference was not statistically significant (P = .17), likely related to the small number of observations.
In two patients, the treatment approach was altered by the PET/CT. As noted above, one underwent neoadjuvant chemotherapy followed by cystectomy, after nodal metastasis demonstrated by PET was confirmed by biopsy. The metastatic deposit was still present at the time of surgery; however, he remains free of disease 24 months after surgery. The second patient was found to have widespread metastatic disease confirmed by biopsy. Surgery was canceled and palliative chemotherapy was performed. He died of metastatic disease 5 months after PET.
Survival Analysis
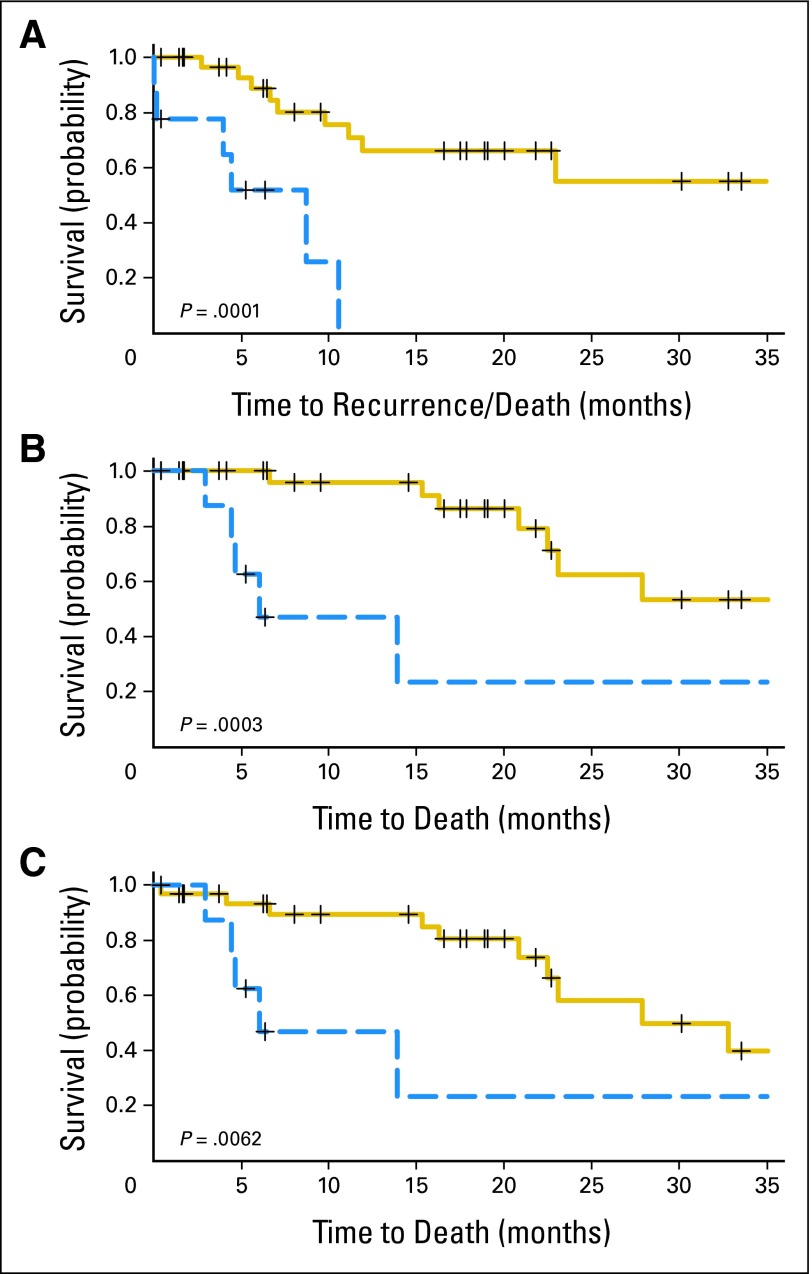
RFS rates at 6 and 24 months for PET-positive patients were 50% (95% CI, 16% to 79%) and 0% (95% CI, not applicable), respectively, compared with 89% (95% CI, 69% to 96%) and 55% (95% CI, 27% to 76%) for PET-negative patients (P = .0001). DSS rates at 6 and 24 months for PET-positive patients were 63% (95% CI, 23% to 86%) and 23% (95% CI, 1% to 62%), respectively, compared with 100% (95% CI, not applicable) and 62% (95% CI, 32% to 82%) for PET-negative patients (P = 0003). Lastly, the corresponding OS at 6 and 24 months for PET-positive patients were 63% (95% CI, 23% to 86%) and 23% (95% CI, 1% to 62%), respectively, compared with 93% (95% CI, 76% to 98%) and 58% (95% CI, 31% to 78%) for PET-negative patients (P = .0062; Fig 3).
Fig 3.
Kaplan-Meier plots of (A) recurrence-free survival, (B) disease-specific survival, and (C) overall survival for nine [18F]fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) –negative patients (solid line) and 33 FDG-PET/CT–positive patients (dashed line).
DISCUSSION
Patients with muscle-invasive UCB have a 10% to 30% risk of lymph node involvement, depending on the primary tumor stage.20–22 Currently, staging of patients with UCB depends on CT or MRI to detect metastatic disease; however, these have limited accuracy,8–12 and thus imaging methods with improved accuracy are clearly needed. Our results demonstrate that FDG-PET/CT provides good sensitivity and excellent specificity for detection of metastatic disease. The extravesical results of FDG-PET/CT were strongly correlated with RFS, DSS, and OS. In addition, we detected residual tumor in the bladder in more than 80% of our cases, demonstrating that UCB is moderately FDG-avid. Identification of both primary tumor and perivesical lymph node metastasis was facilitated by placement of a Foley catheter, the administration of furosemide, and aggressive hydration. These measures decrease the amount of tracer in the bladder lumen, which can obscure important findings.
FDG-PET/CT may have identified an additional four patients with occult metastatic disease. Two patients with pathologically negative lymph nodes had evidence of distal ureteral involvement on FDG-PET/CT. Both developed and died of metastatic disease. Although neither patient was categorized or analyzed as having positive FDG-PET/CT, the fact that they both died of disease raises the possibility that the PET signal identified in the pelvis may have been due to pelvic lymph node metastasis. In addition, two patients with FDG-PET/CT–positive scans for pelvic lymph nodes were found to have pathologically negative lymph nodes. One subsequently died of metastatic bladder cancer, whereas the second has only been observed for 5.2 months. This raises the possibility that lymph node metastasis may have been missed either pathologically or surgically.
The likelihood of identifying positive lymph nodes at surgery is highly dependent on stage. Lesissner et al20 reported that nodal metastasis was identified at surgery in 12.6% of patients with pT2a, 32% with pT2b, and 49% with pT3 bladder cancers. In addition, patients with node-positive disease have dramatically worse survival. The 5- and 10-year RFS rates of patients with node-negative disease are 85% and 82%, respectively, versus 35% and 34%, respectively, for those with node-positive disease. The corresponding 5- and 10-year OS rates are 78% and 56%, respectively, for node-negative disease and 31% and 23%, respectively, for node-positive disease.3 Even patients with minimal lymph node-positive disease have poor survival. Patients with negative preoperative imaging who are found to have lymph node metastasis pathologically have a median survival of less than 2 years.22
Because of these relatively poor cure rates for patients with regionally advanced disease, neoadjuvant chemotherapy has been explored. Although individual trials have generally failed to demonstrate a survival advantage,4,5 meta-analyses have demonstrated a small, but statistically significant, survival benefit from neoadjuvant chemotherapy.6,7 Although the studies evaluated were not limited to patients with cT3-T4 or N+ disease, the majority of patients receiving neoadjuvant therapy had higher-risk disease. Given the higher cure rates with T1-T2N0 disease and the toxicities associated with neoadjuvant chemotherapy,4,5 many surgeons limit neoadjuvant therapy to patients with more than cT3 disease and N+ disease. Therefore, the ability to identify occult metastatic disease has the potential to allow for better selection of patients who benefit from early systemic therapy.
The value of FDG-PET in assessment of bladder cancer has been examined in three previous studies. Jadvar et al15 retrospectively compared the results of FDG-PET and CT for 35 patients with recurrent or metastatic UCB. PET was discordant with CT in four patients; in two patients, the PET was positive and the CT was negative, and in the remaining two patients, the PET was negative and CT was positive. However, no histologic confirmation was obtained. In the majority of patients, PET confirmed CT findings. Liu et al16 used conventional FDG-PET to detect metastatic disease in 46 patients with UCB. Although all positive findings on FDG-PET scans were confirmed by biopsy, the study was not limited to preoperative staging and, therefore, patients with negative studies did not necessarily have this confirmed histologically. Among patients who had not received previous chemotherapy, the sensitivity was 77% and the specificity was 94%. The most relevant previous trial by Driskens et al17 examined 55 patients with conventional FDG-PET and CT. Of the 55 patients, data on pathologic findings or follow-up were available only in 40 patients (radical cystectomy in 21 patients, biopsy in seven patients, and radiologic follow-up at 12 months to confirm lesions in 12 patients). In these 40 patients, the combination of CT and PET had a sensitivity of 60%, specificity of 88%, positive-predictive value of 75%, and negative-predictive value of 79% for detection of metastatic disease. They also found a statistically significant correlation between overall survival and PET findings.
Our study differs from these three previous studies in that it was limited to patients with muscle-invasive UCB who had no evidence of metastatic disease by conventional staging methods (history and physical examination, abdominopelvic CT, and bone scintigraphy) and were planned to undergo radical cystectomy. In all cases, the results of FDG-PET/CT were confirmed histologically, whether positive or negative, and patients were observed prospectively to determine whether they had disease recurrence. Despite our more strict inclusion criteria, we found the sensitivity, specificity, positive-predictive value, and negative-predictive value of FDG-PET/CT to be better than those reported in previous studies. Although this may be due to patient population, the use of PET/CT may have led to our improved diagnostic accuracy.
There are several limitations to our study. These include the relatively small sample size and the small number of patients with metastatic disease. Our measurements of the SUV of the primary tumor were potentially limited by contamination within the measurement region from residual urine in the catheterized bladder and by the uncertain size of the remaining primary lesions. Additionally, our study design does not permit us to assess whether PET is best employed after conventional staging methods have been done versus earlier in the staging sequence.
In conclusion, combining the anatomic imaging accuracy of CT with functional information provided by PET may lead to improved preoperative staging of invasive UCB. More accurate clinical staging could identify patients most likely to benefit from systemic treatment before definitive surgical intervention and patients with distant metastatic disease who could be saved the unnecessary morbidity and mortality of surgery. We have found that FDG-PET/CT provides good sensitivity and specificity for detecting occult metastatic UCB and accurately predicts RFS, DSS, and OS in patients with clinically localized bladder cancer.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Barry A. Siegel, Radiology Corporation of America (C) Stock Ownership: Barry A. Siegel, Radiology Corporation of America Honoraria: Barry A. Siegel, Radiology Corporation of America, PETNET Solutions, GE Health Care Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Adam S. Kibel, Farrokh Dehdashti, Matthew D. Katz, Robert L. Grubb, Cary Siegel, Barry A. Siegel
Administrative support: Adam S. Kibel, Aleksandra P. Klim
Provision of study materials or patients: Adam S. Kibel, Robert L. Grubb, Peter A. Humphrey
Collection and assembly of data: Adam S. Kibel, Farrokh Dehdashti, Aleksandra P. Klim, Peter A. Humphrey, Cary Siegel, Dengfeng Cao, Feng Gao, Barry A. Siegel
Data analysis and interpretation: Adam S. Kibel, Farrokh Dehdashti, Matthew D. Katz, Robert L. Grubb, Dengfeng Cao, Feng Gao, Barry A. Siegel
Manuscript writing: Adam S. Kibel, Farrokh Dehdashti, Matthew D. Katz, Robert L. Grubb, Peter A. Humphrey, Dengfeng Cao, Feng Gao, Barry A. Siegel
Final approval of manuscript: Adam S. Kibel, Farrokh Dehdashti, Matthew D. Katz, Aleksandra P. Klim, Robert L. Grubb, Peter A. Humphrey, Cary Siegel, Dengfeng Cao, Feng Gao, Barry A. Siegel
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bellmunt J, Albiol S, Suarez C, et al. Optimizing therapeutic strategies in advanced bladder cancer: Update on chemotherapy and the role of targeted agents. Crit Rev Oncol Hematol. 2009;69:211–222. doi: 10.1016/j.critrevonc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 4.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial—International collaboration of trialists. Lancet. 1999;354:533–540. [PubMed] [Google Scholar]
- 5.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 6.Neoadjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis. Lancet. 2003;361:1927–1934. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 7.Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: A systematic review and meta-analysis. J Urol. 2004;171:561–569. doi: 10.1097/01.ju.0000090967.08622.33. [DOI] [PubMed] [Google Scholar]
- 8.Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163:1693–1696. [PubMed] [Google Scholar]
- 9.Voges GE, Tauschke E, Stockle M, et al. Computerized tomography: An unreliable method for accurate staging of bladder tumors in patients who are candidates for radical cystectomy. J Urol. 1989;142:972–974. doi: 10.1016/s0022-5347(17)38956-5. [DOI] [PubMed] [Google Scholar]
- 10.Yaman O, Baltaci S, Arikan N, et al. Staging with computed tomography, transrectal ultrasonography and transurethral resection of bladder tumour: Comparison with final pathological stage in invasive bladder carcinoma. Br J Urol. 1996;78:197–200. doi: 10.1046/j.1464-410x.1996.01008.x. [DOI] [PubMed] [Google Scholar]
- 11.Lantz EJ, Hattery RR. Diagnostic imaging of urothelial cancer. Urol Clin North Am. 1984;11:567–583. [PubMed] [Google Scholar]
- 12.Buy JN, Moss AA, Guinet C, et al. MR staging of bladder carcinoma: Correlation with pathologic findings. Radiology. 1988;169:695–700. doi: 10.1148/radiology.169.3.3186994. [DOI] [PubMed] [Google Scholar]
- 13.Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 14.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: Current applications and future directions. Radiology. 2006;238:405–422. doi: 10.1148/radiol.2382041977. [DOI] [PubMed] [Google Scholar]
- 15.Jadvar H, Quan V, Henderson RW, et al. [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13:42–47. doi: 10.1007/s10147-007-0720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu IJ, Segall GM, Nino-Murcia M, et al. Fluorodeoxyglucose positron emission tomography studies in the diagnosis and staging of transitional cell carcinoma. Adv Exp Med Biol. 2003;539:129–142. doi: 10.1007/978-1-4419-8889-8_11. [DOI] [PubMed] [Google Scholar]
- 17.Drieskens O, Oyen R, Van Poppel H, et al. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32:1412–1417. doi: 10.1007/s00259-005-1886-9. [DOI] [PubMed] [Google Scholar]
- 18.Hoekstra CJ, Paglianiti I, Hoekstra OS, et al. Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: An overview of different analytical methods. Eur J Nucl Med. 2000;27:731–743. doi: 10.1007/s002590050570. [DOI] [PubMed] [Google Scholar]
- 19.Hillner BE, Liu D, Coleman RE, et al. The National Oncologic PET Registry (NOPR): Design and analysis plan. J Nucl Med. 2007;48:1901–1908. doi: 10.2967/jnumed.107.043687. [DOI] [PubMed] [Google Scholar]
- 20.Leissner J, Hohenfellner R, Thuroff JW, et al. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder: Significance for staging and prognosis. BJU Int. 2000;85:817–823. doi: 10.1046/j.1464-410x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 21.Wishnow KI, Johnson DE, Ro JY, et al. Incidence, extent and location of unsuspected pelvic lymph node metastasis in patients undergoing radical cystectomy for bladder cancer. J Urol. 1987;137:408–410. doi: 10.1016/s0022-5347(17)44050-x. [DOI] [PubMed] [Google Scholar]
- 22.Mills RD, Turner WH, Fleischmann A, et al. Pelvic lymph node metastases from bladder cancer: Outcome in 83 patients after radical cystectomy and pelvic lymphadenectomy. J Urol. 2001;166:19–23. [PubMed] [Google Scholar]