Abstract
Inflammatory pseudotumor (IPT) of the liver is a rare, benign lesion that may be mistaken for malignancy. IPTs are difficult to diagnose due to non-specific clinical, laboratory and imaging features. We report the case of a 38-year old Asian male who presented with fatigue, weight loss and hepatomegaly. He was found to have a large hepatic IPT and underwent surgical resection; approximately two and a half years later, he developed acute cholangitis secondary to IPT recurrence. We present the imaging features of hepatic IPT using ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). We also review the literature on the diagnosis and management of this disease. The unique features of this case include the IPT’s recurrence following surgical resection, large size and multiple modalities presented.
Keywords: Inflammatory pseudotumor, inflammatory myofibroblastic tumor, liver, ultrasound, computed tomography, magnetic resonance imaging
CASE REPORT
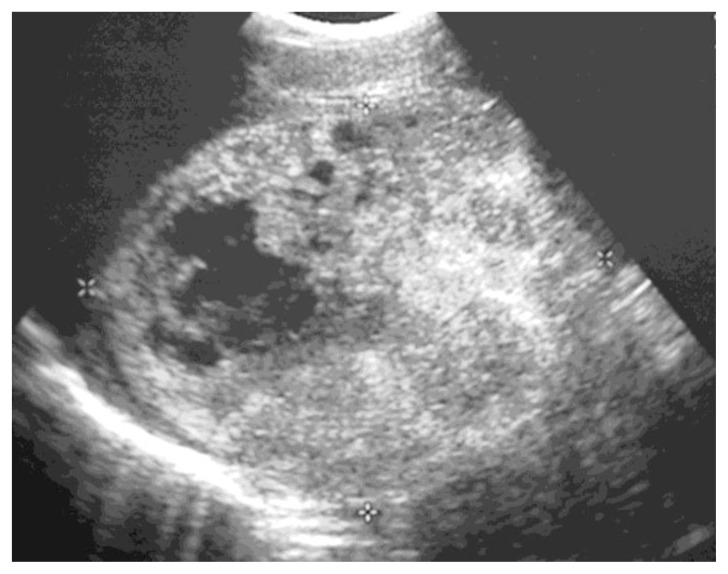
A 38-year old Asian male, originally from Hong Kong, initially presented to his family doctor due to fatigue, abdominal distension and weight loss. On physical exam, he appeared slightly jaundiced and had marked hepatomegaly and bilateral ankle edema. His past medical history was significant for pulmonary tuberculosis as a child and testicular teratoma at age 19, successfully treated with surgery and radiation. Sonography revealed a complex mass, 16.7cm in maximal diameter occupying the right hepatic lobe (Fig. 1). No internal flow was observed on colour Doppler. These findings were concerning for malignancy and CT scan was recommended.
Figure 1.
38-year old male with inflammatory pseudotumor of the liver. Sagittal ultrasound image of the right hepatic lobe demonstrates a large mass (demarcated by cursors) measuring 16.7cm in maximal diameter with cystic and solid components.
Initial bloodwork revealed a normocytic, normochromic anemia (hemoglobin of 93 g/L, normal 133–160 g/L) and thrombocytosis (499 ×109/L, normal 4–10×109/L). Biochemistry revealed abnormal liver function tests with elevated alkaline phosphatase (1367 U/L, normal 48–138 U/L), conjugated bilirubin (33 mol/L, normal <8 mol/L), alanine aminotransferase (62 U/L, normal <60 U/L), aspartate aminotransferase (68 U/L, normal <35 U/L). Albumin was low (28 g/L, normal 35–50 g/L). Erythrocyte sedimentation rate was also elevated (>130mm/h, normal 0–10 mm/h). Hepatitis B surface antigen and hepatitis C antibody were negative; alpha-fetal protein, beta-HCG and CEA were normal.
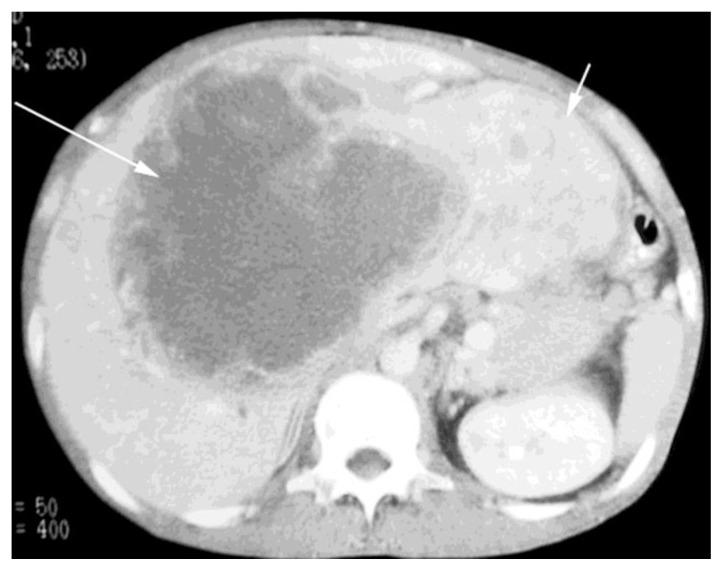
IV contrast enhanced triphasic CT demonstrated a mass involving the anterior segment of the right lobe and the medial segment of the left lobe, with a large cystic or necrotic component. Peripheral enhancement was noted in some areas on the arterial phase. Portal venous and delayed phase imaging showed persistent low attenuation of the cystic portion mass (Fig. 2). The mass was found to partially compress the portal and hepatic veins, which remained patent.
Figure 2.
38-year old male with inflammatory pseudotumor of the liver. IV-contrast enhanced CT scan, portal venous phase, demonstrates a large mass measuring 19.5 by 16.5cm occupying the anterior segments of the right lobe and entire left lobe of the liver (kv 120 mA 180, 150cc of Optiray 380). Most of the cystic/necrotic component is within the anterior and medial segments (long arrow) and the heterogeneous predominantly solid component occupies the lateral segment (short arrow).
Ultrasound-guided fine needle aspirate taken shortly after the CT scan revealed hemorrhage and necrotic tissue. The patient subsequently returned to Hong Kong and elected to undergo additional biopsies, including an open biopsy undertaken during an exploratory laparotomy. These revealed chronic inflammation and no evidence of malignancy. Stains for hepatitis B, bacteria, fungi and mycobacteria were negative.
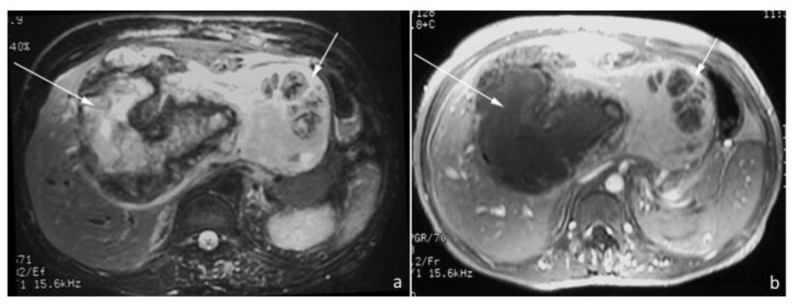
MRI with contrast performed several months after the patients’ initial presentation revealed a complex, heterogeneous mass. It was stable in size compared to previous imaging, measuring approximately 20cm in maximum dimension (Fig. 3a–b). On T2-weighted imaging, the cystic portion of the mass was hyperintense to liver parenchyma and was surrounded by a hypointense rim that lacked gadolinium enhancement. The largely solid portion was T2 hyperintense compared to liver parenchyma and demonstrated gadolinium enhancement.
Figure 3.
38-year old male with inflammatory pseudotumor of the liver. a) Axial FSE T2-weighted (1.5T, TE 102, TR 8571) and b) Axial T1-weighted post-gadolinium (1.5T, TE 4.2, TR 93, 28cc omniscan) MR images demonstrate a heterogeneous, complex mass measuring 20 by 16.5cm, which occupies the anterior segments of the right lobe and entire left lobe. The cystic/necrotic component (long arrow) within the anterior and medial segments demonstrate central high signal intensity surrounded by a thick irregular low signal intensity rim on the T2-weighted images and lack of enhancement following the administration of dynamic gadolinium. The heterogeneous and predominantly solid component of this mass (short arrow) occupies and expands the lateral segments. The solid component is higher in signal intensity compared to normal liver on T2-weighted images and demonstrates enhancement with gadolinium.
The patient was initially treated with antibiotics (levofloxacin, roxithromycin and metronidazole) and prednisone. Follow-up CT scan after several weeks of treatment demonstrated no change in the tumor size and treatment was stopped. Shortly thereafter, the patient presented to hospital with acute cholangitis and obstructive jaundice. ERCP revealed distortion of the intrahepatic radicles by the mass. Subsequent diagnostic laparotomy with biopsy was performed, which demonstrated inflammatory pseudotumor.
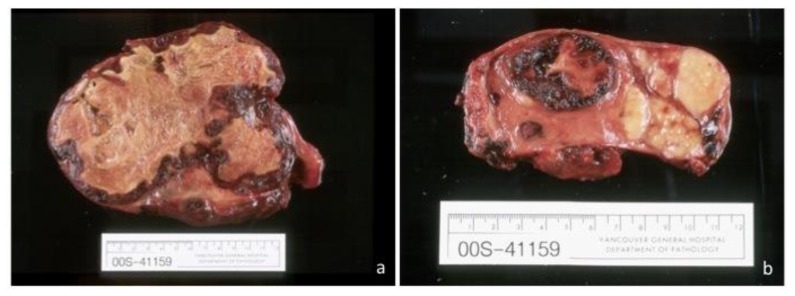
The patient underwent surgical resection of the mass (Fig. 4a–b). The mass extended to the liver capsule and status of resection margins was uncertain. It contained multiple hemorrhagic and necrotic areas. Microscopic features include spindle cell proliferation surrounded by heavy inflammatory infiltrate (Fig. 5). Post-surgically, the patient was monitored with abdominal ultrasound every six months.
Figure 4.
38-year old male with inflammatory pseudotumor of the liver. Gross pathology specimen of the right a) and left b) hepatic lobes measure 17 by 14cm and 9 by 6cm, respectively, and containing large, irregular areas of necrosis and hemorrhage admixed with areas of grey-tan tissue.
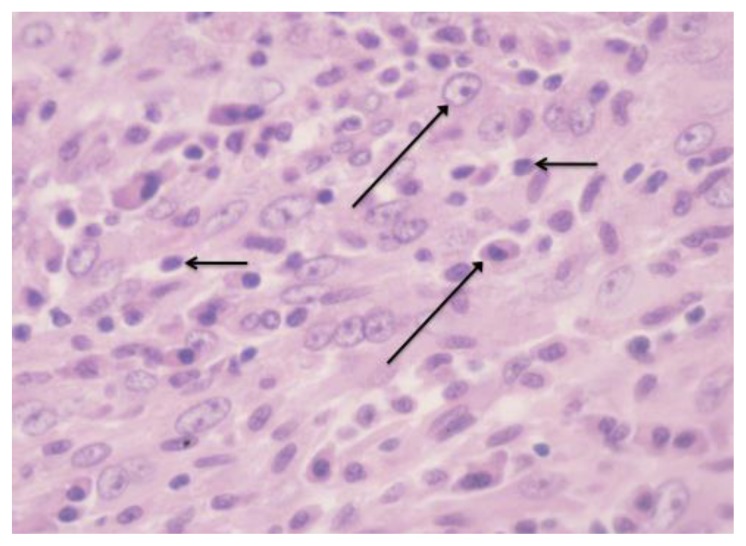
Figure 5.
38-year old male with inflammatory pseudotumor of the liver. High power microscopic view demonstrates a moderately cellular spindle-cell proliferation with heavy inflammatory infiltrate consisting primarily of plasma cells (long arrow) and lymphocytes (short arrow) (40× magnification, hematoxylin and eosin stain).
Approximately two and a half years after surgery, the patient had persistently elevated liver enzymes and intrahepatic duct dilatation was detected on ultrasound. MRCP attributed this dilatation to a new hepatic mass, concerning for recurrence (Fig. 6a–b). The patient presented to hospital a few weeks later with acute cholangitis, most likely secondary to obstruction caused by IPT recurrence. A stent was placed to relieve the obstruction (Fig. 7a–b).
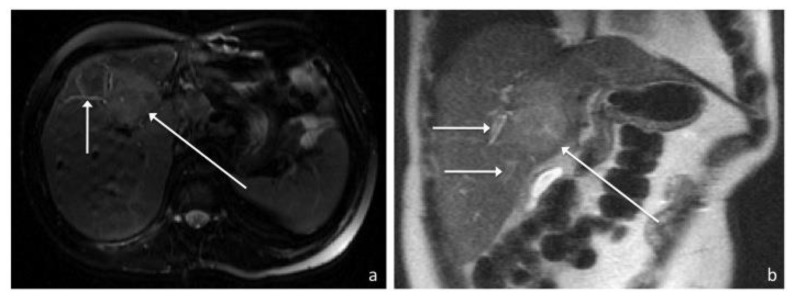
Figure 6.
41-year old male with recurrent inflammatory pseudotumor of the liver. Axial FSE T2-weighted (1.5T, TE 114, TR 2000) (a) and coronal T2-weighted (1.5T, TE 99, TR 1745) (b) MR images in a demonstrate a well-defined hyperintense hepatic mass (long arrow) measuring 5.4 by 3.7cm arising from segment 8 with possible extension into 4A/B. Peripheral biliary ductal dilatation up to 4mm in diameter (short arrows) is also present.
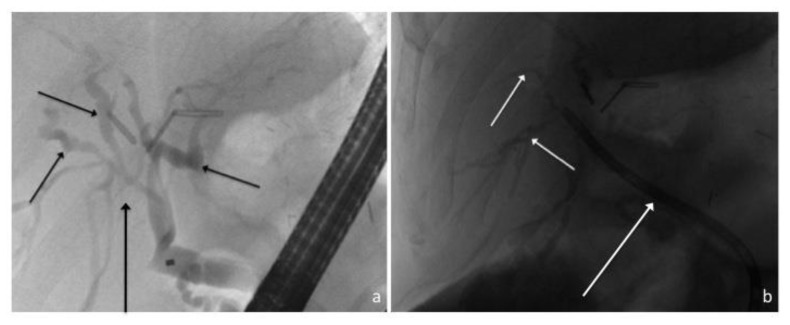
Figure 7.
41-year old male with recurrent inflammatory pseudotumor of the liver. ERCP images (a) demonstrate the recurrent pseudotumor to cause central biliary compression and narrowing (long arrow) with resultant biliary dilatation (short arrows), which was subsequently relieved following stent placement (b, long arrow).
DISCUSSION
Inflammatory pseudotumor (IPT) is a rare lesion of unknown origin, which may occur as a result of infectious, immunologic, allergic or neoplastic mechanisms. Although they occur most commonly in the lung and orbit, IPTs have been reported at nearly all body sites [1]. IPT of the liver was first described in 1953 by Pack and Baker and 289 cases have been reported in the literature to date [2, 3]. IPTs are characterized histologically by a whorl-like pattern of dense collagen bundles infiltrated predominately by plasma cells, lymphocytes and macrophages without cellular atypia or atypical features [4–9]. There is great histologic variation in the composition of IPTs, reflected in the numerous terms have been used to them, including inflammatory myofibroblastic tumor, plasma cell granuloma, histiocytoma and fibroxanthoma [1, 3–4, 8]. Though benign, IPTs may mimic malignancy [5–7, 8–9, 12]. IPTs pose a diagnostic challenge due to their non-specific clinical, laboratory and imaging findings: while some cases may be treated conservatively following diagnosis by precutaneous biopsy, surgical resection has often been the treatment of choice to rule out the possibility of malignancy [4–5, 7–9, 12].
Hepatic IPTs have been reported in all age groups but typically occur in children and young adults, with a reported mean age of presentation of 37 years [5, 7, 8, 10]. Males of non-European background are predominately affected, with a reported male to female ratio of 3:1 up to 8:1 [11, 12]. The incidence of IPT was 0.7% in a retrospective analysis of patients who underwent surgery for focal liver lesions [13].
Hepatic IPTs are most commonly described as well-defined, solitary, intrahepatic tumors within the right hepatic lobe [5, 7, 14]. Multifocal tumors, concurrent hepatic and splenic IPTs and portal vein occlusion have also been reported [4, 11]. Obstructive jaundice may develop if the bile duct is involved [15]. They vary in size, up to 25cm in diameter [7, 11]. Larger lesions may undergo central necrosis [8]. Short-interval imaging follow-up may reveal a decrease in size of the pseudotumor, suggesting benignity of the lesion [9].
Clinical and laboratory findings of IPT are non-specific. The most common symptoms include fever, epigastric or abdominal pain, malaise and weight loss [3, 6, 7, 11]. Other findings may include obstructive jaundice, hepatomegaly, splenomegaly and portal hypertension [5, 16, 17]. Laboratory findings may suggest an inflammatory process, with marked leukocytosis and elevated ESR and CPR [11]. Anemia, thrombocytosis, and elevated IgG levels have also been reported [4, 5, 7, 9, 11]. Liver enzymes and lactate dehydrogenase may be elevated [4, 5, 8, 9]. Tumour markers are usually normal [4, 7].
Sonographically, hepatic IPTs demonstrate variable echogenicity. While hypoechoic lesions are more common, hyperechoic or complex lesions have been described [5, 10, 18–21]. These may have either ill- or well-defined peripheral margins with homogeneous or heterogeneous internal texture [5, 10, 19]. Internal septations and posterior acoustic enhancement have been described [9, 19, 20]. Contrast enhanced, low mechanical index ultrasonography demonstrated that hepatic pseudotumors lack of enhancement in all phases of contrast [22].
The CT findings of hepatic IPTs are variable. Hypoattenuating, ill-defined masses with a variable degree of contrast enhancement are most common. IPTs generally exhibit delayed phase enhancement, especially on the tumor’s periphery or within internal septae, with hypoattenuating central areas [3–7, 14, 15, 18, 19]. This pattern of delayed enhancement is thought to be related to the tumor’s fibrous components [6, 7, 9]. However, arterial phase enhancement [3, 10, 23], heterogeneous portal venous phase enhancement or lack of any perceptible enhancement has been described [9, 11 18, 19]. Case reports also describe central calcification or necrosis, occlusion of the portal vein on portal venous phase and various degrees of mass effect and dilatation of the intrahepatic biliary tree [3–5, 8, 11, 18, 23].
18F-FDG PET/CT descriptions of hepatic IPTs are limited to case reports [4, 24, 25]. Abnormal metabolic activity as multiple foci or confined to a segment with a high standardized uptake value of 7.1 and 7.3 have been reported [4, 25]. One case describes complete regression of the hypermetabolic foci within six months on follow-up PET/CT [24]. The amount of uptake is thought to vary according to the proportion of fibrosis and inflammatory cell infiltrate, with high FDG uptake among lymphocytes [4, 25].
The MRI findings of hepatic IPTs are variable. They are typically hypointense relative to liver parenchyma on T1-weighted imaging, although iso- and hyperintense T1 lesions have also been described [7, 17, 18, 21, 26]. IPTs are generally iso- or hyperintense on T2-weighted imaging, with some cases demonstrating a T2 hyperintense rim [3, 6, 7, 17, 18, 26, 27]. Patterns of gadolinium contrast enhancement are variable. Peripheral enhancement on the delayed phase is commonly described, attributable to the tumor’s fibrous component [17, 28]. Other patterns include early, intense, central or peripheral enhancement with rapid contrast washout [17, 28, 29].
The lack of characteristic imaging findings of hepatic IPTs has been attributed to the variability in their histologic composition [4]. It is therefore difficult to differentiate between hepatic IPTs and other focal liver lesions, including malignant tumors, based on imaging alone [6, 7]. Hepatic IPTs typically have a pattern of delayed peripheral enhancement on contrast CT, a feature that is also shared by peripheral cholangiocarcinoma and metaststic disease [4, 6, 7]. Some hepatic IPTs exhibit an early enhancement pattern with delayed phase washout, typical of hepatocellular carcinoma [4, 23]. Although IPTs lack the characteristic liquefactive necrosis of a typical abscess, incomplete liver abscess may be considered [7]. The key to differentiating inflammatory pseudotumor from abscess, especially in the presence of cystic or necrotic components, is the replacement of hepatic parenchyma with collagenous tissue and chronic inflammatory infiltrates in IPT compared to the acute inflammatory infiltrates seen in abscess [23]. Histopathologic confirmation via percutaneous biopsy or following surgical resection is often necessary to differentiation between these conditions.
In general, IPTs have a benign clinical course, which has led to the postulation that they may be caused by infectious, immunologic, or allergic mechanisms [26]. Case reports have associated IPTs with a variety of bacterial and viral agents, autoimmune diseases and chronic inflammatory states [1, 3, 5, 6, 16]. While the prognosis of inflammatory pseudotumors is generally favourable, management is controversial [30]. Conservative treatment with antibiotics or nonsteroidal anti- inflammatories with tumor regression has been reported in some cases within 2–6 months [4, 5, 7, 19]. IPTs have also been reported to spontaneously regress [7, 10]. Surgical resection is typically curative and has often been undertaken to rule out malignancy or in those who fail conservative treatment [8]. However, there are case reports of IPT recurrence and malignant transformation several years following resection [31], which is relevant to radiologists reading post-surgical follow-up studies.
In conclusion, this case exhibited many of the previously reported clinical, laboratory and imaging findings of hepatic IPT and demonstrates the diagnostic challenge of this entity. Interesting features of this case include the IPT’s large size, central necrosis and recurrence following surgery. Although rare, hepatic IPT should be considered in the differential diagnosis of focal hepatic lesions. While there are no diagnostic radiologic features, familiarity with the general imaging appearance of hepatic IPTs may lead the radiologist to suspect this benign disease. The decision to pursue histopathologic confirmation through percutaneous biopsy may help to avoid unnecessary surgery.
TEACHING POINT
Hepatic inflammatory pseudotumor is a rare yet benign lesion, which has variable imaging findings that may mimic malignancy. Typically, hepatic inflammatory pseudotumor presents as an ill-defined mass that is hypoechoic on sonography, hypoattenuating on CT and T1 hypointense and T2 hyperintense to liver parenchyma. Delayed phase enhancement is most common, particularly at the lesion’s periphery, on CT and MRI.
Table 1.
| Etiology | The exact etiology and pathogenesis is unknown and may be infectious, immunologic, allergic or neoplastic. Inflammatory pseudotumors are generally considered to be benign. |
| Incidence | The exact incidence is unknown. A retrospective analysis of 403 patients undergoing surgery for focal liver lesions found an incidence of 0.7%. 289 cases have been reported in the literature to date. |
| Gender Ratio | Predominately male, with a male to female ratio of 3:1 to 8:1 |
| Age Predilection | Although inflammatory pseudotumors have been reported across a wide range of ages, they are more common in children and young adults, with a mean age of presentation of 37 years. |
| Risk Factors | Unknown, predominately affects non-Europeans |
| Treatment | Management remains controversial. Surgical resection is curative but conservative treatment, with antibiotics or nonsteroidal anti-inflammatories, has in some cases induced tumor regression. |
| Prognosis | Excellent, with low incidence of local recurrence |
| Findings on Imaging | Imaging findings are non-specific and are most commonly described as well-defined, solitary, intrahepatic masses within the right lobe. Inflammatory pseudotumors are typically hypoechoic on ultrasound, hypoattenuating on CT and T1 hyperintense and T2 iso- or hyperintense of MR imaging. In general, contrast enhancement is present on the delayed phase and confined to the tumor periphery. |
Table 2.
| Entity | Ultrasound | CT | MRI |
|---|---|---|---|
| Inflammatory pseudotumor |
|
|
|
| Hepatocellular carcinoma |
|
|
|
| Cholangiocarcinoma (mass-forming intrahepatic) |
|
|
|
| Metastatic disease |
|
|
|
| Liver abscess |
|
|
|
ABBREVIATIONS
- CRP
C-reactive protein
- CT
Computed tomography
- ERCP
Endoscopic retrograde cholangiopancreatography
- ESR
Erythrocyte sedimentation rate
- IPT
Inflammatory pseudotumor
- MRCP
Magnetic resonance cholangiopancreatography
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- US
Ultrasound
REFERENCES
- 1.Das Narla L, Newman B, Spottswood S, Narla S, Kolli R. Inflammatory Pseudotumors. RadioGraphics. 2003;23:719–729. doi: 10.1148/rg.233025073. [DOI] [PubMed] [Google Scholar]
- 2.Pack GT, Baker HW. Total right hepatic lobectomy: report of a case. Ann Surg. 1953;138:253–258. doi: 10.1097/00000658-195308000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ntinas A, Kardassis D, Miliaras D, Tsinoglou K, Dimitriades A, Vrochides D. Inflammatory pseudotumor of the liver: a case report and review of the literature. J Med Case Rep. 2011;5:196. doi: 10.1186/1752-1947-5-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawaguchi T, Mochizuki K, Kizu T, et al. Inflammatory pseudotumor of the liver and spleen diagnosed by percutaneous needle biopsy. World J Gastroenterol. 2012;18:90–95. doi: 10.3748/wjg.v18.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Jabri T, Sanjay P, Shaikh I, Woodward A. Inflammatory myofibroblastic pseudotumor of the liver in association with gall stones - a rare case report and brief review. Diagn Pathol. 2010;5:53. doi: 10.1186/1746-1596-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim K, Kim K, Park S, et al. Unusual mesenchymal liver tumors in adults: radiologic-pathologic correlation. AJR. 2006;187:W481–9. doi: 10.2214/AJR.05.0659. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi J, Sakamoto Y, Sano T, Shimada K, Kosuge T. Spontaneous Regression of Inflammatory Pseudotumor of the Liver: Report of Three Cases. Surg Today. 2007;37:525–529. doi: 10.1007/s00595-006-3433-0. [DOI] [PubMed] [Google Scholar]
- 8.Milias K, Madhavan KK, Bellamy C, Gerden OJ, Parks RW. Inflammatory Pseudotumors of the Liver: Experience of a Specialist Surgical Unit. Journal Gastroenterol and Hepatol. 2009;24:1562–1566. doi: 10.1111/j.1440-1746.2009.05951.x. [DOI] [PubMed] [Google Scholar]
- 9.Nam KJ, Kang HK, Lim JH. Inflammatory Pseudotumor of the Liver: CT and Sonographic Findings. AJR. 1996;167 doi: 10.2214/ajr.167.2.8686633. [DOI] [PubMed] [Google Scholar]
- 10.Zamir D, Jarchowsky J, Singer C, et al. Inflammatory Pseudotumor of the Liver - A Rare Entity and a Diagnostic Challenge. Am J Gastroenterol. 1998;93:1538–1540. doi: 10.1111/j.1572-0241.1998.00476.x. [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi R, Uchida T, Kojima T, Shikita T. Inflammatory pseudotumor of the liver: Clinicopathologic study and review of the literature. Cancer. 1990;65:1583–1590. doi: 10.1002/1097-0142(19900401)65:7<1583::aid-cncr2820650722>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Koea JB, Broadhurst GW, Rodgers MS, McCall JL. Inflammatory Pseudotumor of the Liver: Demographics, Diagnosis, and the Case for Nonoperative Management. J Am Coll Surg. 2003;196:226–235. doi: 10.1016/S1072-7515(02)01495-3. [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Lai EC, Cong WM, et al. Inflammatory myofibroblastic tumor of the liver: a cohort study. World J Surg. 2010;34:309–313. doi: 10.1007/s00268-009-0330-x. [DOI] [PubMed] [Google Scholar]
- 14.Yoon KH, Ha HK, Lee JS, et al. Inflammatory Pseudotumor of the Liver in Patients with Recurrent Pyogenic Cholangitis: CT- Histopathologic Correlation. Radiology. 1999;211:373–379. doi: 10.1148/radiology.211.2.r99ma36373. [DOI] [PubMed] [Google Scholar]
- 15.Fukuya T, Honda H, Matsumata T, et al. Diagnosis of Inflammatory Pseudotumor of the Liver: Value of CT. AJR. 1994;163:1087–1091. doi: 10.2214/ajr.163.5.7976880. [DOI] [PubMed] [Google Scholar]
- 16.Faraj W, Ajouz H, Mukherji D, Kealy G, Shamseddine A, Khalife M. Inflammatory pseudotumor of the liver: a rare pathologic entity. World J Surg Pathol. 2011;9:5. doi: 10.1186/1477-7819-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herek D, Karabulut N. Hepatobiliary and Pancreatic: Inflammatory pseudotumors of the liver. J Gastroenterol and Hepatol. 2011;26:1217. doi: 10.1111/j.1440-1746.2011.06793.x. [DOI] [PubMed] [Google Scholar]
- 18.Abehsera M, Vilgrain V, Belghiti J, Flejou JF, Nahum H. Inflammatory pseudotumor of the liver: radiologic-pathologic correlation. JCAT. 1995;19:80–83. doi: 10.1097/00004728-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Lim JH, Lee JH. Inflammatory pseudotumor of the liver: ultrasound and CT features. Clin Imaging. 1995;19:43–46. doi: 10.1016/0899-7071(94)00020-d. [DOI] [PubMed] [Google Scholar]
- 20.Anderson SW, Kruskal JB, Kane RA. Benign Hepatic tumors and Iatrogenic Pseudotumors. RadioGraphics. 2009;29:211–229. doi: 10.1148/rg.291085099. [DOI] [PubMed] [Google Scholar]
- 21.Caramella T, Novellas S, Foumol M, Saint-Paul MC, Bruneton JN, Chevallier P. Imaging of Inflammatory pseudotumors of the liver. J Radiol. 2007;88:882–888. doi: 10.1016/s0221-0363(07)89890-8. [DOI] [PubMed] [Google Scholar]
- 22.Ding H, Wang WP, Huang BJ, et al. Imaging of Focal Liver Lesions: Low-Mechanical-Index Real-time Ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285–297. doi: 10.7863/jum.2005.24.3.285. [DOI] [PubMed] [Google Scholar]
- 23.Tsou YK, Lin CJ, Liu NJ, Lin CC, Lin CH, Lin SH. Inflammatory pseudotumor of the liver: Report of eight cases, including three unusual cases, and a literature review. J Gastroenterol and Hepatol. 2007;22:2143–2147. doi: 10.1111/j.1440-1746.2006.04514.x. [DOI] [PubMed] [Google Scholar]
- 24.Chong A, Young S, Min JJ. Inflammatory pseudotumors resembling multiple hepatic mestastases and their complete regression as revealed by 18F-FDG PET/CT. E J Nucl Med. 2009;36(7):1199–2000. doi: 10.1007/s00259-009-1132-y. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura E, Habu D, Tsushima H, et al. A case of hepatic inflammatory pseudotumor identified by FDG-PET. Ann Nucl Med. 2006;20:321–323. doi: 10.1007/BF02984650. [DOI] [PubMed] [Google Scholar]
- 26.Yu JS, Park C, Kim JH, Chung JJ, Kim KW. Inflammatory Myofibroblastic Tumors in the Liver: MRI of Two Immunohistochemically-Verified Cases. JMRI. 2007;26:418–421. doi: 10.1002/jmri.21023. [DOI] [PubMed] [Google Scholar]
- 27.Materne R, Van Beers BE, Gigot JF, Horsmans Y, Lacrosse M, Pringot J. Inflammatory pseudotumor of the liver: MRI with mangafodipir trisodium. JCAT. 1998;22:82–84. doi: 10.1097/00004728-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Yan FH, Zhou KR, Jiang YP, Shi WB. Inflammatory pseudotumor of the liver: 13 cases of MRI findings. World J Gastroenterol. 2001;7:422–424. doi: 10.3748/wjg.v7.i3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortele KJ, Wiesner W, de Hemptinne B, Elewaut A, Praet M, Ros PR. Multifocal inflammatory pseudotumor of the liver: dynamic gadolinium-enhanced, ferumoxides-enhanced, and mangafodipir trisodium-enhanced MR imaging findings. Eur Radiol. 2002;12:304–308. doi: 10.1007/s003300101015. [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith PJ, Loganathan A, Jacob M, et al. Inflammatory pseudotumors of the liver: A spectrum of presentation and management options. E J Surg Oncol. 2009;35:1295–1298. doi: 10.1016/j.ejso.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Zavaglia C, Barberis M, Gelosa F, et al. Inflammatory pseudotumor of the liver with malignant transformation: Report of two cases. Ital J Gastroenterol. 1996;28:152–59. [PubMed] [Google Scholar]